Abstract
Pseudoachondroplasia (PSACH), a severe short-limb dwarfing condition, results from mutations that cause misfolding of the cartilage oligomeric matrix protein (COMP). Accumulated COMP in growth plate chondrocytes activates endoplasmic reticulum stress, leading to inflammation and chondrocyte death. Using a MT-COMP mouse model of PSACH that recapitulates the molecular and clinical PSACH phenotype, we previously reported that oxidative stress and inflammation play important and unappreciated roles in PSACH pathology. In this study, we assessed the ability of antioxidant and anti-inflammatory agents to affect skeletal and cellular pathology in our mouse model of PSACH. Treatment of MT-COMP mice with aspirin or resveratrol from birth to P28 decreased mutant COMP intracellular retention and chondrocyte cell death, and restored chondrocyte proliferation. Inflammatory markers associated with cartilage degradation and eosinophils were present in the joints of untreated juvenile MT-COMP mice, but were undetectable in treated mice. Most importantly, these treatments resulted in significantly increased femur length. This is the first and only therapeutic approach shown to mitigate both the chondrocyte and long-bone pathology of PSACH in a mouse model and suggests that reducing inflammation and oxidative stress early in the disease process may be a novel approach to treat this disorder.
Introduction
Cartilage oligomeric matrix protein (COMP) is a non-collagenous extracellular matrix (ECM) glycoprotein that is most abundant in musculoskeletal tissues (1–3). In the ECM, COMP interacts with other ECM proteins such as collagen type II, collagen type IX, matrilin 3 and SPARC (4,5). Functionally, COMP participates in type II collagen fibril assembly, enhances chondrocyte attachment and proliferation (6–10). Mutations that result in misfolding of COMP cause two skeletal dysplasias, pseudoachondroplasia (PSACH) and multiple epiphyseal dysplasia (MED/EDM1) (11). Clinically, short stature, rhizomelic shortening of the limbs and shortened fingers (brachydactyly) are classic features of PSACH (11,12). Molecularly, PSACH is associated with massive intracellular retention of COMP and other ECM proteins in the endoplasmic reticulum (ER) of growth plate chondrocytes, leading to premature chondrocyte death before complete skeletal maturation (4,13–15). Newborns with PSACH are indistinguishable from unaffected babies; diagnosis is typically made around 2–3 years of age when a waddling gait develops and linear growth slows (11,12). The most debilitating complication is early onset joint pain generally attributed to joint erosion and osteoarthritis (OA) (11,16).
Previously, we developed an inducible MT-COMP mouse as a model of PSACH through chondrocyte-specific expression of the human COMP with a mutation, D469del, which is found in approximately 30% of PSACH cases (17,18). We have shown that critical cellular and clinical features of PSACH are recapitulated when transgenic human MT-COMP is expressed during growth plate development and maturation, allowing for identification of pathological events and characterization of molecular mechanisms that develop early in the pathogenesis of PSACH (19–21) Using transcriptome analysis and immunostaining of growth plates, we found that a proapoptotic environment was established early in development, from E15 to P1. After birth, cellular mechanisms that cope with ER stress resulting from misfolded proteins can prevent substantial chondrocyte death until 3 weeks of age, at which time the majority of chondrocytes retain MT-COMP and necroptosis is triggered by persistent ER stress and DNA damage (20).
Under conditions of unremitting ER stress, inflammation and oxidative stress are stimulated which in turn can exacerbate ER stress, thereby stimulating a self-perpetuating pathological loop between ER stress, inflammation and oxidative stress process (22–26). Consistent with this idea, antioxidant therapies have shown improved secretion of the protein and decreased ER stress resulting from misfolded coagulation factor VIII (27). Similarly, non-steroidal anti-inflammatory drugs (NSAIDs) reduce ER stress in a variety of cell types (28–31). In our MT-COMP mouse, we found that intracellular retention of misfolded mutant COMP drives an inflammatory and oxidative stress response, suggesting that oxidative stress and inflammation feedback generates additional ER stress, which leads to more mutant COMP accumulation and the co-retention of other ECM proteins that typically interact with COMP in the matrix (4,5,8). This vicious cycle of ER stress resulting from intracellular retention in MT-COMP growth plate chondrocytes initiates inflammatory responses and production of reactive oxygen species (ROS) that overwhelm the cellular stress coping mechanisms and cause premature chondrocyte death (20). MT-COMP-induced chondrocyte death occurs through caspase-independent necroptosis (20,32). The intracellular retention of MT-COMP increases CHOP and GADD34 expression, which reactivates protein translation exacerbating intracellular retention of MT-COMP (32). Reactive oxygen species are generated by increases in Nox4 and ER receptor stress-inducible, Ero1β (32). Oxidative stress leads to DNA damage as indicated by increased expression of growth arrest and DNA damage (GADD) genes (32). The presence of cleaved apoptosis inducing factor (tAIF) in the absence of activated caspases (3, 8, 9 and 12) indicates that MT-COMP-induced premature chondrocyte death occurs through necroptosis (32).
In our previous work, we showed that interrupting the ER-stress sensing/signaling mechanisms through the loss of CHOP reduces intracellular retention, inflammatory marker expression and premature chondrocyte cell death and partially restores growth plate chondrocyte proliferation (20). Inflammation and oxidative stress in the MT-COMP growth plate coincides with maximal MT-COMP intracellular retention and chondrocyte death, leading us to hypothesize that suppressing inflammation or oxidative stress may extend chondrocyte longevity. To test this hypothesis, we treated MT-COMP mice from birth to P28 with antioxidants or anti-inflammatory agents (aspirin, ibuprofen, resveratrol, grape seed extract (GSE), turmeric, CoQ10) and assessed intracellular retention, chondrocyte death, inflammation markers and femur lengths.
Results
MT-COMP growth plate organization is improved by anti-inflammatory and antioxidant treatment
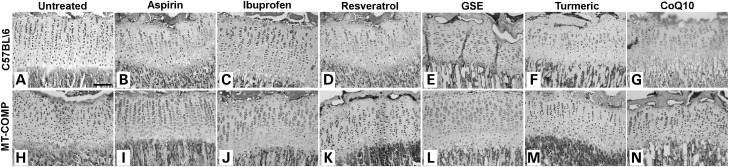
In previous work, we showed that inflammation and oxidative stress play a role in MT-COMP growth plate chondrocyte pathology (20,21). To determine if reducing inflammation and ROS would ameliorate the disruptive effects of mutant COMP expression on growth plate organization, MT-COMP mice were treated with a variety of over-the-counter antioxidant and anti-inflammatory agents and their effects on growth plate assessed. Expression of MT-COMP was induced using DOX from conception to P28. Experimental and control dams and offspring were treated from P0 to P28 with aspirin, ibuprofen, resveratrol, GSE, turmeric or CoQ10 continuously in the drinking water at concentrations shown in Table 1. C57\BL6 mice were used as controls as we had previously shown that expression of wild-type COMP did not affect growth plate chondrocytes, fertility or longevity (19). At P28, hind limbs were collected, the tibial growth plates sectioned and visualized by H&E staining. As seen previously and shown in Figure 1, chondrocyte-specific induction of MT-COMP expression in mice from conception to P28 disrupts the expected organized columnar arrangement of growth plate chondrocytes. The growth plate from mice expressing MT-COMP is more disorganized with fewer chondrocytes organized into columns (Fig. 1H) compared with the highly organized C57BL\6 growth plate (Fig. 1A) (19,20). In comparison, treatment of mice expressing MT-COMP with any of the antioxidant and anti-inflammatory agents in Table 1 improved growth plate organization with varying degrees of efficacy. Compared with untreated mice expressing MT-COMP (Fig. 1H), columnar organization was fully restored with aspirin (Fig. 1I) and GSE treatment (Fig. 1L) and partially restored by treatment with resveratrol (Fig. 1K), turmeric (Fig. 1M) and CoQ10 (Fig. 1N). However, GSE, turmeric and CoQ10 treated control growth plates showed varying degrees of disorganization as compared with untreated controls (Fig. 1A compared with E–G). This suggests that aspirin and resveratrol may be the best agents to improve growth plate organization because they improve organization in the MT-COMP growth plate and do not negatively impact the control growth plate.
Table 1.
Treatment drugs and dosages
| Drug | Dosage in drinking water | Source | Equivalent human dosage for 26 kg child | Equivalent human dosage for 70 kg adult |
|---|---|---|---|---|
| Aspirin | 0.3 g/l | Sigma, St Louis, MO, USA | 64 mg/daily | 166 mg/daily |
| Ibuprofen | 0.08 g/l | Sigma, St Louis, MO, USA | 17 mg/daily | 45 mg/daily |
| Resveratrola | 0.25 g/l | Reserveages Organics, Gainesville, FL, USA | 53 mg/daily | 140 mg/daily |
| Turmeric | 20 g/l | Planetary Herbals, Soquel, CA, USA | 4 g/daily | 11 g/daily |
| CoQ10 | 0.001 g/l | Liqsorb, Smithtown, NY, USA | 0.2 mg/daily | 0.6 mg/daily |
| GSE | 0.666 g/l | Healthy Origins, Pittsburgh, PA, USA | 140 mg/daily | 370 mg/daily |
aReserveages Organics liquid resveratrol also contains a mixed berry extract which contains 760 oxygen radical absorbance capacity units (ORAC)/ml.
Figure 1.
Treatment with aspirin or antioxidants improves growth plate organization in MT-COMP mice at P28. H & E staining of representative P28 growth plates from control (C57BL\6) and MT-COMP mice treated with aspirin, ibuprofen, resveratrol, GSE, turmeric or CoQ10 from P0 to P28. Representative growth plates are shown from examination of at least 8 mice. Bar = 500 µm.
Antioxidant and anti-inflammatory agents reduce intracellular retention of MT-COMP
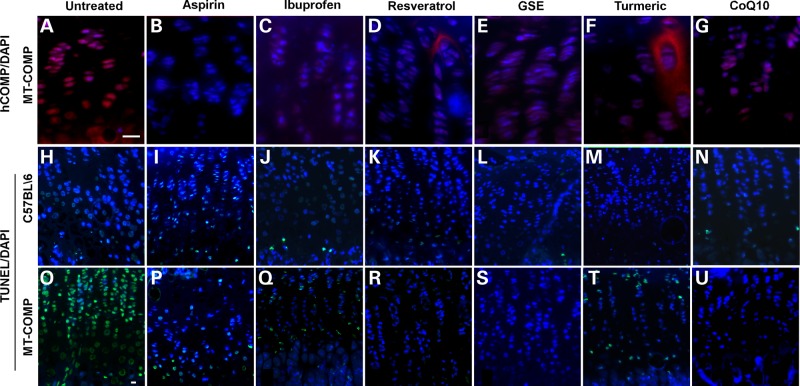
Intracellular retention of misfolded mutant COMP in the ER of growth plate chondrocytes is a distinctive feature of PSACH, first documented in 1973 (33). To evaluate intracellular retention of human MT-COMP in treated and untreated transgenic mice, we used an antibody that specifically recognizes human COMP and does not cross react with the endogenous mouse COMP. Since non-transgenic control mice do not express human COMP, no intracellular (or extracellular) MT-COMP was observed (data not shown) (20). MT-COMP intracellular retention was present in untreated MT-COMP chondrocytes throughout the growth plate (Fig. 2A). Administration of aspirin, ibuprofen and resveratrol from P0-P28 dramatically reduced intracellular retention of MT-COMP in growth plate chondrocytes compared with untreated MT-COMP mice (Fig. 2B–D compared with A). GSE, turmeric and CoQ10 treatment decreased intracellular retention of MT-COMP as well, but to a lesser extent than the other drugs treatments (Fig. 2E–G compared with A). Interestingly, some extracellular MT-COMP was observed with resveratrol and turmeric treatments (Fig. 2D and F). Previously, we observed the export of mutant COMP when the protein was expressed in a CHOP null background, suggesting that interrupting CHOP signaling permits the misfolded protein to bypass the ER quality control systems (20). The finding that resveratrol, an antioxidant, can allow some MT-COMP to be exported to the ECM suggests that oxidative stress and ER stress may be closely linked in growth plate chondrocytes expressing high levels of misfolded proteins.
Figure 2.
Intracellular retention of MT-COMP and chondrocyte death were decreased by treatment with aspirin or antioxidants. TUNEL and human COMP immunostaining of P28 growth plates from control (C57BL\6) and MT-COMP mice treated with aspirin, ibuprofen, resveratrol, GSE, turmeric or Coq10 from P0 to P28. TUNEL staining is present in only the hypertrophic zone of wild-type mice (C57BL\6) (H), in comparison, most chondrocytes are TUNEL positive in the MT-COMP growth plate (O). TUNEL staining is markedly decreased in MT-COMP chondrocytes with aspirin, ibuprofen, resveratrol, GSE, turmeric or CoQ10 treatment (P–U compared with O). Drug therapy does not change TUNEL staining in control chondrocytes (I–N compared with H). Growth plates from at least 8 mice were examined for each treatment. Bar = 100 µm.
Cell death is decreased in MT-COMP growth plates treated with antioxidant and anti-inflammatories
Intracellular MT-COMP retention is associated with chondrocyte death in our mouse model and chondrocyte death has been reported in human PSACH growth plates (1,19,20). Since intracellular retention of mutant COMP is decreased by antioxidant/anti-inflammatory treatments in MT-COMP growth plates (Fig. 2), we next assessed efficacy of anti-inflammatories and antioxidants on chondrocyte death. As expected, few TUNEL positive chondrocytes were observed in control growth plates and the majority of these were restricted to the hypertrophic zone where chondrocyte death normally occurs (Fig. 2H–N). In contrast, there is widespread chondrocyte death in the growth plate at P28 in the untreated growth plate expressing MT-COMP (Fig. 2O). Previously, we showed that over 80% of MT-COMP expressing chondrocytes are TUNEL positive (20). Aspirin, ibuprofen, resveratrol, GSE and CoQ10, and to a lesser extent with turmeric, treatment reduced TUNEL staining in the MT-COMP growth plates (Fig. 2P–U compared with O). Resveratrol, GSE and CoQ10 treatment was associated with the lowest level of TUNEL staining in the MT-COMP mice (Fig. 2R, S and U).
Chondrocyte proliferation is restored in the MT-COMP growth plates treated with aspirin or resveratrol
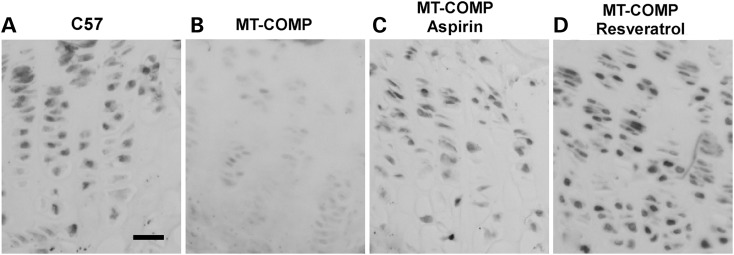
In our previous work, using proliferating cell nuclear antigen (PCNA) immunostaining, we found that the level of growth plate chondrocyte proliferation was decreased in MT-COMP mice relative to controls (Fig. 3A and B) (20). Additionally, we have reported that the absence of CHOP in mice expressing MT-COMP reduced retention of MT-COMP in the ER and partially restored chondrocyte proliferation (20). Since our current work indicates that antioxidant or anti-inflammatory treatment of mice expressing MT-COMP reduces retention of MT-COMP, we evaluated whether these treatments would prevent the MT-COMP-induced decrease in chondrocyte proliferation. As seen in Figure 3, treatment of mice expressing MT-COMP with resveratrol and aspirin fully restores PCNA immunostaining in the growth plate as compared with levels seen in control growth plates (Fig. 3B compared with C and D). This suggests that suppressing inflammation or oxidative stress not only preserves growth plate chondrocyte viability but also restores some functions such as cell proliferation.
Figure 3.
Growth plate chondrocyte proliferation in MT-COMP mice was restored by treatment with aspirin or antioxidants. Proliferating cell nuclear antigen immunostaining of P28 growth plates from control (C57BL\6) and MT-COMP mice treated with aspirin or resveratrol from P0 to P28. Proliferating cell nuclear antigen staining is present throughout the wild-type growth plate (C57BL\6) (A), in comparison; less PCNA immunostaining is present in the MT-COMP growth plate (B). Proliferating cell nuclear antigen staining is markedly increased in MT-COMP chondrocytes with aspirin or resveratrol treatments (B compared with C and D). Growth plates from at least 8 mice were examined for each treatment. Bar = 100µm.
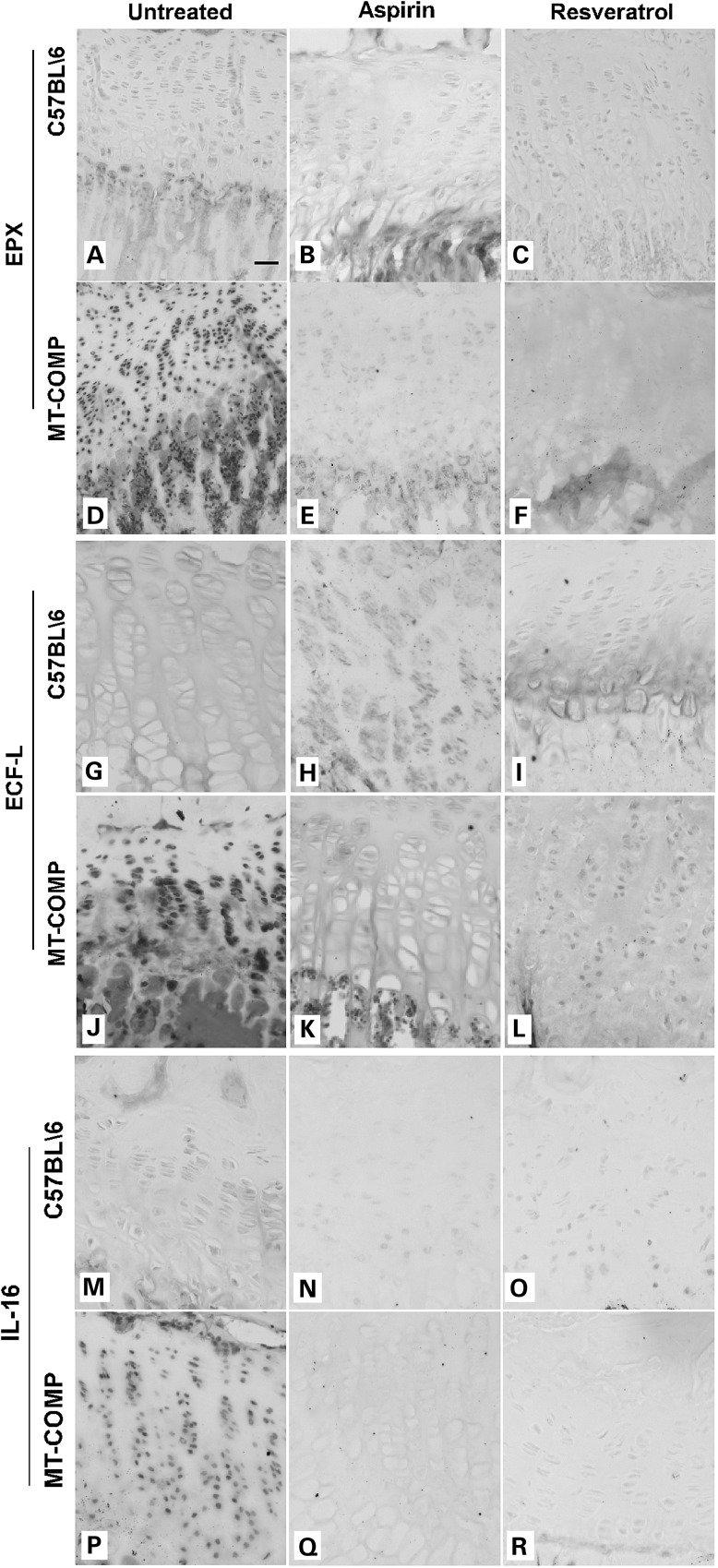
Antioxidant and anti-inflammatory therapies decrease markers of inflammation in MT-COMP growth plate and articular cartilage chondrocytes
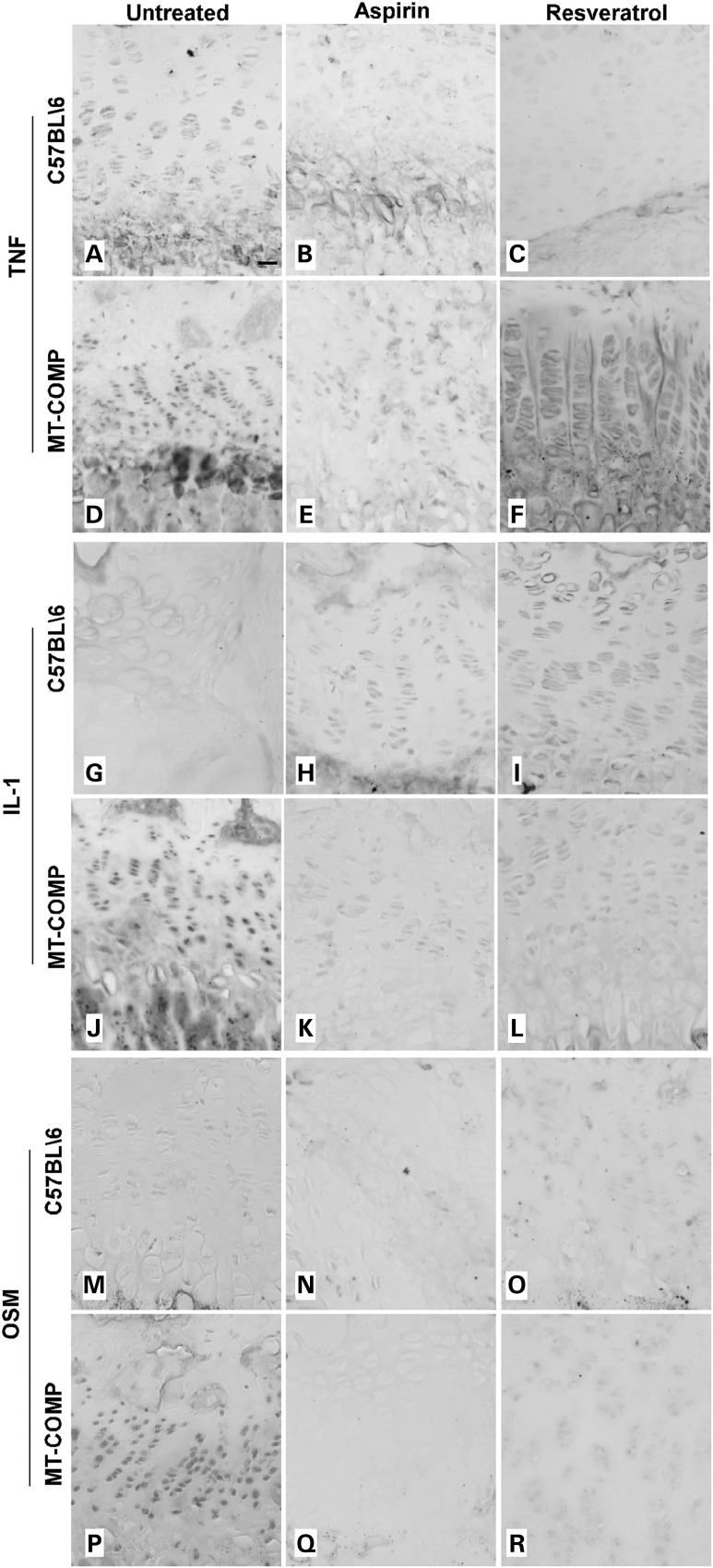
Previously, we found that a variety of inflammatory-related proteins [EPX (eosinophil peroxidase), CCR5 (C-C chemokine receptor type 5), IL-16 (interleukin 16), ECF-L (eosinophil chemotactic factor-lymphocyte also known as YM1), IL-18 (interleukin 18), TNFα (tumor necrosis factor alpha) and IL-1 (interleukin 1)] were increased in untreated MT-COMP mouse growth plate (20,21). We next assessed the effect of aspirin and resveratrol treatments on inflammatory markers present in growth plates expressing MT-COMP (21). All of the inflammatory markers evaluated were decreased by resveratrol and aspirin treatments (Figs 4 and 5). As shown in Figure 4, TNFα, IL-1 and oncostatin (OSM) were markedly reduced by aspirin or resveratrol treatment (Fig. 4E–F compared with D; K–L compared with J; Q–R compared with P) and were similar to the untreated and treated controls (Fig. 4A–C, G–I and M–O) but distinct from the marked expression of TNFα, IL-1 and OSM in MT-COMP growth plate (Fig. 4D, J and P). Ibuprofen treated growth plate chondrocytes showed similar results (data not shown). Likewise, EPX, ECF-L and IL-16 showed similar pattern of expression. As shown in Figure 5, all were decreased in the MT-COMP treated growth plate chondrocytes (E and F, K and L, Q and R) compared with the untreated MT-COMP mice (D, J and P) and similar to the controls (A–C, G–I, M–O).
Figure 4.
Aspirin or resveratrol treatments decrease expression of cartilage degradation inflammation markers in MT-COMP growth plate chondrocytes. Immunostaining of P28 growth plates from control (C57BL\6) and MT-COMP mice treated with either aspirin or resveratrol from P0 to P28. TNF, IL-1 and OSM immunostaining is shown for control mice (A–C, G–I, M–O) and for MT-COMP mice (D–F, J–L, P–R). Growth plates from at least 8 mice were examined for each treatment. Bar = 100 µm.
Figure 5.
Aspirin or resveratrol treatment decreased expression of eosinophil-associated inflammation markers in MT-COMP growth plate chondrocytes. Immunostaining of P28 growth plates from control and MT-COMP mice treated with either aspirin or resveratrol from P0 to P28. EPX, ECF-L and IL16 immunostaining is shown for control mice (A–C, G–I, M–O) and for MT-COMP mice (D–F, J–L, P–R). Growth plates from at least 8 mice were examined for each treatment. Bar = 100 µm.
Femur length in MT-COMP mice is increased by antioxidant and anti-inflammatories treatments
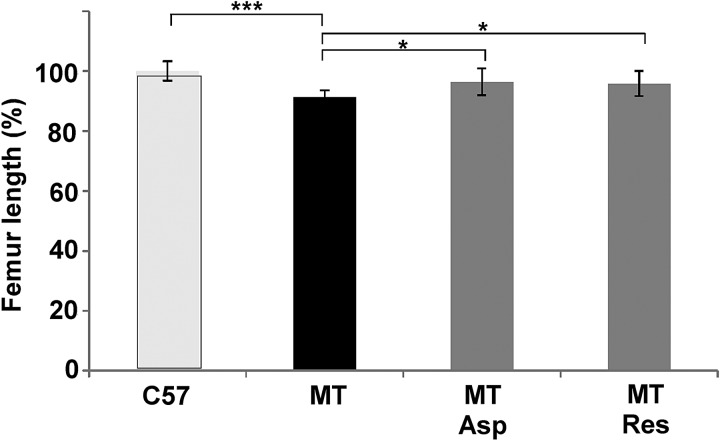
In previous work, we demonstrated that MT-COMP reduces the hind limb length by 12% in mice at 1 month of age (20). Since treatment of mice expressing MT-COMP with these antioxidant and anti-inflammatory agents reverses the cellular pathology associated with expression, we used femoral length to determine whether aspirin or resveratrol treatment improved limb growth in the MT-COMP mice. Mice were treated from P0 to P28 with aspirin or resveratrol and then femurs were measured on microCT images. As shown in Figure 6, the femurs of the MT-COMP mice were shorter than the control femurs consistent with our previous findings (20). Importantly, treatment with resveratrol or aspirin significantly increased femoral length and partially rescues the long-bone phenotype. The treated femur lengths (11.0 ± 0.50 mm aspirin and 10.9 ± 0.47 mm resveratrol) were between those of the control (11.4 ± 0.37 mm) and untreated MT-COMP (10.4 ± 0.25 mm) mice (Fig. 6).
Figure 6.
Femur length increased in aspirin or resveratrol treated MT-COMP mice. Femurs were collected at P28 from control (C57BL\6) and MT-COMP after aspirin or resveratrol treatments from P0-P28. Measurements were obtained as described in the methods. Both aspirin and resveratrol treatments resulted in significantly increased femoral lengths. C57 = C57BL\6, MT = MT-COMP, Asp = aspirin and Res = resveratrol. C57BL\6 N = 10, MT-COMP N = 10, MT-COMP aspirin treated N = 5 and MT-COMP resveratrol treated N = 5. ***P > 0.0005, *P > 0.05.
Discussion
We generated the inducible MT-COMP mouse to study the pathologic processes in chondrocytes resulting from mutations in COMP. Using a phenotype-driven approach, we found that misfolded retained mutant COMP activates ER stress through CHOP signaling, resulting in inflammation and oxidative stress (20). This was an unappreciated consequence of mutant COMP expression, but importantly led to identification of potential antioxidant and anti-inflammatory therapeutic approaches. Using, the MT-COMP mouse, we assessed aspirin, ibuprofen, resveratrol, GSE, turmeric and CoQ10, all readily available over-the-counter antioxidant and anti-inflammatory therapeutics for the ability to dampen or prevent the PSACH clinical and chondrocyte phenotypes in mice expressing MT-COMP. These agents were selected based upon a long history of safe usage in humans and their ability to reduce cellular stress, which we have shown to be a hallmark of PSACH chondrocytes expressing mutant COMP (19–21). Remarkably, these therapies produced significant amelioration of the short-limb phenotype and resolution of the chondrocyte-specific pathology.
We found by 1 month of age that all of these therapeutic agents reduced intracellular accumulation of COMP, inflammation markers and chondrocyte death throughout the MT-COMP growth plate. However, resveratrol and aspirin were most effective at reducing the pathology and thus we focused our studies on these agents. The most important finding and the gold standard resulting from treatment was rescue of limb growth by 51–58% compared with the untreated MT-COMP mouse (Fig. 6). This is the first report of a therapy that not only suppresses the mutant COMP pathology in growth plate chondrocyte but also translates into an increase in murine limb length. This sets the stage for development of therapies for PSACH, which were identified using chondrocyte-specific phenotype-driven therapeutic discovery (20).
Until recently, only symptomatic treatments and/or interventions have been available for skeletal dysplasias because little was known about the underlying molecular defects. More recently, researchers have had great success in delineating the genetic mutations that cause skeletal dysplasias with more than 140 genes involved in 372 short stature disorders (34). However, approaches to treat these disorders have been limited by the lack of animal models to delineate the molecular and cellular pathologies and to test therapeutics. To circumvent this problem in PSACH, we generated the MT-COMP mouse with the D469del mutation [the most common mutation that we found accounts for 30% of cases (17,18,35)] and demonstrated that it recapitulates the clinical and growth plate phenotypes (19,20). Importantly, we made the novel observation that severe inflammatory and oxidative responses mediated through CHOP results in chondrocyte death and depletion of the growth plate chondrocytes (20). This observation opened the door for treatment approaches in the MT-COMP mice using a variety of anti-inflammatory and antioxidant therapies.
Intracellular accumulation of MT-COMP in the ER of growth plate chondrocytes is a well-established and characteristic feature of the PSACH chondrocyte pathology (16,33,36). If inflammation, oxidative stress and ER stress are interconnected with each perpetuating the other, then reducing one process should lessen the others. We tested this hypothesis by administering antioxidants or anti-inflammatories and evaluating intracellular accumulation of MT-COMP. Consistent with this proposed mechanism, aspirin, resveratrol, GSE, turmeric and CoQ10 all reduced intracellular retention of MT-COMP and inflammatory marker expression with aspirin and resveratrol having the greatest effect (Figs 2, 4 and 5). This finding is similar to butylated hydroxyanisole (BHA), and antioxidant therapy that improved secretion of misfolded coagulation factor VIII protein and decreased ER stress and apoptosis in mice (27). Additionally, several studies have shown that NSAIDS can reduce ER stress in mice, rats and cell culture (28–31). For example, in SH-SY5Y neuronal cells in vitro, thapsigargin/tunicamycin driven ER-stress-induced-apoptosis was blunted by diclofenac, indomethacin, ibuprofen, aspirin or ketoprofen, all of which are NSAIDS (28). Our findings and treatment approach may have implications for other ER storage/stress disorders, such as Alzheimer disease and type II diabetes, which also result from cross talk between oxidative and inflammation ER stresses (24,37).
The treatments in this study were chosen based upon tolerance/side-effects and their anti-inflammatory and antioxidant properties. Aspirin and ibuprofen work primarily by inhibiting cyclooxygenase (COX1 > COX2), which decreases prostaglandin and thromboxane synthesis, thereby reducing inflammation (http://en.wikipedia.org/wiki/Mechanism_of_action_of_aspirin; http://www.drugbank.ca/drugs/DB01050). Both aspirin and ibuprofen are NSAIDs (non-steroidal anti-inflammatory drugs) but aspirin's irreversible inhibition of COX leads to greater gastrointestinal complications. Interestingly, resveratrol, GSE and curcumin (active ingredient in turmeric) reportedly are natural COX2 inhibitors (38,39). While COX2 inhibition has been reported to decrease chondrocyte progression to hypertrophy and endochondral ossification (40,41), we found that MT-COMP mice treated with either aspirin or resveratrol partially recover limb length. On-going studies will determine whether aspirin treatment/COX2 inhibition permits only partial limb length rescue or whether longer treatment (10 weeks) will provide a more complete rescue. Nevertheless, we clearly demonstrate that these treatments have a positive effect on chondrocyte viability, allowing for at least a partial limb length rescue. This important outcome would make a profound difference in quality of life issues for PSACH patients.
Resveratrol is best known for its ability to increase Sirt1 expression, which has been shown to increase life span of some organisms in response to caloric restriction (42). We found that resveratrol modestly increases Sirt1 immunostaining in C57BL\6 but not in MT-COMP growth plates (Supplementary Material, Fig. S1). Therefore, resveratrol may be acting as both an antioxidant and an anti-inflammatory to dampen the MT-COMP chondrocyte phenotype through a Sirt1 independent mechanism. Resveratrol is known to enhance endogenous antioxidant defenses and inhibit phosphodiesterases (PDEs) (43,44). PDE4 is a direct target of resveratrol and inhibition of PDE4 results in dampening of inflammation through TNFα and interleukins, which we observe. Grape seed extract (proanthocyanidin active compound) has multiple effects on cells including: (1) free radical scavenging, (2) inhibition of COX and 5-LOX that control prostaglandin synthesis and (3) reduction of nitrogen oxide synthase (NOS) activity (38). Tumeric inhibits COX and prevents NF-κB activation (45);(39). CoQ10 is an electron transport carrier used in ATP synthesis, a free radical scavenger and may alter prostaglandin synthesis (http://s406515300.onlinehome.us/coq10morfrom.html). There is little information about the mechanism(s) of action of GSE, turmeric and CoQ10, limiting our ability to explain the observed growth plate perturbations.
The most important outcome with respect to PSACH morbidity was increased femoral lengths with both resveratrol and aspirin treatments (Fig. 6). While the modes of action are different for these agents, both interrupt the lethal chondrocyte pathological process induced by mutant COMP retention, thereby improving chondrocyte function and longevity. As seen in Figure 2, apoptosis/cell death in treated murine growth plate chondrocytes is dramatically reduced and chondrocyte proliferation was restored (Fig. 3). This resulted in a significant increase in limb length but did not completely restore full limb length, (Fig. 6) suggesting that there is still compromise of chondrocyte function. Therefore, combining therapies may provide a more efficient rescue and are being tested.
While these therapeutics are readily available and widely used, there are medical concerns especially with long-term aspirin use (46,47). Gastrointestinal irritation and bleeding can occur at any age and aspirin is generally not recommended for children/teenagers with viral illnesses because of the association with Reyes syndrome (http://www.mayoclinic.org/diseases-conditions/reyes-syndrome/basics/definition/con-20020083). To circumvent the risk of Reyes syndrome in children on long-term aspirin therapy, annual flu vaccines and two doses of the varicella (chickenpox) vaccine are given. While aspirin is not administered to children less than 2 years, importantly, most PSACH patients are not diagnosed prior to 2–3 years of age allowing these treatments to fit into the appropriate time frame for therapeutic intervention. Moreover, antioxidants have fewer side-effects and appear to be as effective as aspirin.
Animal studies demonstrate that resveratrol improves metabolic health (48–53). More importantly in the context of this work, resveratrol has been shown to preserve articular cartilage health in a rabbit model of OA (54,55). Consistent with the findings in the OA model, resveratrol decreased inflammation and lengthened femurs in the MT-COMP mouse. There have been several promising clinical trials involving resveratrol (8,56), however the interpretation of these studies is complicated by differences in dosages, delivery method (wine, juice, supplements, etc.) and compound preparation including/excluding synergistic molecules (56). More studies with consistent and rigorous dosing, delivery and preparation of resveratrol are needed to evaluate the potential health benefits of resveratrol in humans.
Inflammatory processes are linked to painful sequelae, and n PSACH, childhood joint pain is a frequent presenting symptom and complaint and is the least understood medical problem with this short stature disorder (11,36,57,58). Our observation of the inflammatory process in growth plate chondrocytes starting at P14 (translates to approximately 4 years in humans) may explain the childhood pain reported in PSACH, which was previously considered to result from abnormal bony/joint architectures. The inflammatory pathologic process in PSACH is an important and novel finding that should be considered in pain management of this condition using appropriate NSAID or antioxidant treatments.
Another potential benefit of NSAID and antioxidant therapies in PSACH may be an amelioration of OA. Osteoarthritis is a common co-morbidity in young adults with PSACH and causes pain and decreased mobility. We demonstrate that NSAID and antioxidant therapies reduce TNFα, IL-1β and OSM markers, all of which are often seen in OA and have been implicated in cartilage degradation in vivo (59–61). To validate that the presence of inflammatory-related proteins is specific to MT-COMP accumulation in the ER and not an immune reaction from human COMP expression in a mouse, growth plates from mice expressing transgenic human wild-type (WT-) COMP were evaluated for the presence of EPX and IL-16. As shown in Supplementary Material, Fig. S2, EPX and IL-16 immunostaining is increased in the MT-COMP mice (B and E) compared with control C57BL\6 (A and D) and WT-COMP (C and F) mice. The inflammatory process therefore is specific to the intracellular retention of MT-COMP and is not a consequence of human COMP expression (Supplementary Material, Fig. S2). For PSACH, it is possible that by decreasing the activity of cytokines associated with OA using antioxidant or anti-inflammatory treatment(s) may decrease joint damage by delaying or diminishing OA (36). This outcome would also dramatically improve the quality of life for individuals with PSACH.
Translation of drug dosages used in MT-COMP mice suggests that aspirin, ibuprofen and resveratrol could be used to treat PSACH. Human equivalent dosage is calculated based on body surface and dosage administered in mg/kg (Human Equivalent Dose = animal dosage mg/kg × animal km/human km) (62). Table 1 lists the pediatric and adult equivalent dosages of the drugs used to treat MT-COMP mice. For juvenile rheumatoid arthritis, pediatric dosages of aspirin 60–90 mg/kg/day are used to initially treat followed by maintenance dosage of 80–100 mg/kg/day (not to exceed 5.4 g/day) for 2- to 11-year olds and 2.4–3.6 g/daily (initially) and maintenance 3.6–5.4 g/daily for children 12 years and over (http://www.drugs.com/dosage/aspirin.html). These dosages are often associated with gastrointestinal complications and are well above the equivalent dosage used to treat MT-COMP mice (Table 1). The pediatric human equivalent of the ibuprofen dosage used in our mice (pediatric 10–30 mg/daily) is below the daily dosages administered to pediatric patients with rheumatoid arthritis (30–50 mg/kg/day). Resveratrol human equivalent dosages are 120–170 mg/daily for adults and 25–75 for children and supplementation of resveratrol to improve cerebral blood flow requires 250–500 mg/daily (http://examine.com/supplements/Resveratrol/). Given that there have been very few side-effects associated with resveratrol supplementation; we expect that 120–170 mg would be easily tolerated. Long-term resveratrol pediatric supplementation studies have not been conducted on a large scale and with little information on pediatric use we are unable to predict tolerance to long-term administration. In addition, human clinical trials will be needed to establish effective dosing parameters.
There have been few treatments for skeletal dysplasias because the growth plate chondrocytes have been considered inaccessible for delivery of drug treatments due to the avascular nature of cartilage. Recently, long-bone growth in an achondroplasia mouse model was rescued by injection of a FGFR3 decoy (63). The mechanism of disease pathology in the achondroplasia and PSACH mice are very different but systemic treatments are able to reach the growth plate chondrocytes and affect a resolution of the disease processes. We now demonstrate that readily available antioxidant and anti-inflammatory therapeutics partially rescue limb growth in the MT-COMP mouse model of PSACH. Longer administration of antioxidants and anti-inflammatories in MT-COMP mice may generate complete rescue of long-bone growth. This opens the door for innovative approaches to the different types of pathologic processes underlying the skeletal dysplasias and offers potential treatments that may ameliorate the associated morbidities. From a clinical perspective, even partial rescue of limb length and decrease in joint pain would significantly increase functionality for PSACH individuals.
Material and Methods
Generation of transgenic mutant MT-COMP mice
The Animal Welfare Committee at the University of Texas Medical School at Houston approved these studies and all experiments comply with Guide for the Care and Use of Laboratory Animals: Eighth Edition, ISBN-10: 0-309-15396-4. Mutant MT-COMP mice were produced by introducing DNA containing expression cassettes derived from two plasmids, pTRE-MT-COMP (D469del-COMP mutation) and pTET-On-Col II as previously described (19). Mice were administered doxycycline (DOX) (500 ng/ml) through drinking water (with 5% wt/vol sucrose) pre- and postnatally. C57BL/6 mice were used as controls since the wild-type (WT)-COMP mice showed no phenotypic differences in our previous studies (19).
Drug administration
Each drug was administered from P0 to P28 through drinking water containing DOX at dosages listed in Table 1. Concentrations were used based upon published mouse studies (50,64–70). In our previous studies, we found newborn mice had adverse outcomes to lithium, phenyl butyrate and valproate drugs that reduce ER stress that are well tolerated in adult mice (21). Additionally, prenatal administration of aspirin causes birth defects (71). Therefore, drugs were only administered postnatally.
Immunostaining
Hind limbs from 1-month old MT-COMP and C57BL/6 control mice were collected and tibial growth plates were analyzed as previously described (19). Briefly, the limbs were fixed in 95% vol/vol ethanol for immunostaining for COMP (Abcam Cambridge, MA ab11056-rat 1:100), eosinophil peroxidase (EPX) (Santa Cruz Biotechnology, Santa Cruz, CA, USA; sc-19147, 1:50), YM1/ECF-L (Stem Cell Technologies; 01404, 1:100), interleukin 1α (IL-1α) (Abcam; ab7632, 1:200), interleukin 16 (IL-16) (Santa Cruz Biotechnology; sc-7902, 1:100), oncostatin (OSM) (Santa Cruz Biotechnology;sc-5488, 1:100), or tumor necrosis factor α (TNF-α) (Abcam; ab6671, 1:200), PCNA staining kit (Invitrogen, Frederick, MD 93-1143) or in 10% wt/vol formalin for terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) staining. The COMP rat antibody does not cross-reacts with both endogenous mouse COMP. At least 8 C57BL/6 and 8 D469del-COMP mice were examined for each treatment.
Limb length measurements
Hind limbs were obtained from 8 control and 8 MT-COMP mice and the soft tissue was removed. The skeleton was stored in 70% ethanol and then subjected to radiographic examination with an Explore Locus RS microCT (GE Medical Systems, London Ontario). The limbs were attached to a grid to ensure consistency and accuracy of measurements. Measurements were made from end-to-end on femurs using MicroView software (GE Healthcare) as previously described (72). T-test was used to compare the femoral measurements from control and MT-COMP mice limbs.
Supplementary Material
Funding
This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number #1R01AR057117, Shriners Hospital for Children grant to J.T.H. and the Leah Lewis Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Supplementary Material
Acknowledgements
We thank Seenya Vincent and Joanna Espinosa for technical assistance.
Conflict of Interest statement. None declared.
References
- 1.Hecht J.T., Deere M., Putnam E., Cole W., Vertel B., Chen H., Lawler J. (1998) Characterization of cartilage oligomeric matrix protein (COMP) in human normal and pseudoachondroplasia musculoskeletal tissues. Matrix Biol., 17, 269–278. [DOI] [PubMed] [Google Scholar]
- 2.Hedbom E., Antonsson P., Hjerpe A., Aeschlimann D., Paulsson M., Rosa-Pimentel E., Sommarin Y., Wendel M., Oldberg A., Heinegard D. (1992) Cartilage matrix proteins. An acidic oligomeric protein (COMP) detected only in cartilage. J. Biol. Chem., 267, 6132–6136. [PubMed] [Google Scholar]
- 3.Fang C., Carlson C.S., Leslie M.P., Tulli H., Stolerman E., Perris R., Ni L., Di Cesare P.E. (2000) Molecular cloning, sequencing, and tissue and developmental expression of mouse cartilage oligomeric matrix protein (COMP). J. Orthop. Res., 18, 593–603. [DOI] [PubMed] [Google Scholar]
- 4.Merritt T.M., Bick R., Poindexter B.J., Alcorn J.L., Hecht J.T. (2007) Unique matrix structure in the rough endoplasmic reticulum cisternae of pseudoachondroplasia chondrocytes. Am. J. Pathol., 170, 293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thur J., Rosenberg K., Nitsche D.P., Pihlajamaa T., Ala-Kokko L., Heinegard D., Paulsson M., Maurer P. (2001) Mutations in cartilage oligomeric matrix protein causing pseudoachondroplasia and multiple epiphyseal dysplasia affect binding of calcium and collagen I, II, and IX. J. Biol. Chem., 276, 6083–6092. [DOI] [PubMed] [Google Scholar]
- 6.Bleasel J.F., Poole A.R., Heinegard D., Saxne T., Holderbaum D., Ionescu M., Jones P., Moskowitz R.W. (1999) Changes in serum cartilage marker levels indicate altered cartilage metabolism in families with the osteoarthritis-related type II collagen gene COL2A1 mutation. Arthritis Rheum., 42, 39–45. [DOI] [PubMed] [Google Scholar]
- 7.Lohmander L.S., Saxne T., Heinegard D.K. (1994) Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann. Rheum. Dis., 53, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mann H.H., Ozbek S., Engel J., Paulsson M., Wagener R. (2004) Interactions between the cartilage oligomeric matrix protein and matrilins. Implications for matrix assembly and the pathogenesis of chondrodysplasias. J. Biol. Chem., 279, 25294–25298. [DOI] [PubMed] [Google Scholar]
- 9.Xu K., Zhang Y., Ilalov K., Carlson C.S., Feng J.Q., Di Cesare P.E., Liu C.J. (2007) Cartilage oligomeric matrix protein associates with granulin-epithelin precursor (GEP) and potentiates GEP-stimulated chondrocyte proliferation. J. Biol. Chem., 282, 11347–11355. [DOI] [PubMed] [Google Scholar]
- 10.Chen F.H., Thomas A.O., Hecht J.T., Goldring M.B., Lawler J. (2005) Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J. Biol. Chem., 280, 32655–32661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger S., Hecht J.T. (2001) Pseudoachondroplasia and multiple epiphyseal dysplasia: new etiologic developments. Am. J. Med. Genet., 106, 244–250. [PubMed] [Google Scholar]
- 12.McKeand J., Rotta J., Hecht J.T. (1996) Natural history study of pseudoachondroplasia. Am. J. Med. Genet., 63, 406–410. [DOI] [PubMed] [Google Scholar]
- 13.Hecht J.T., Makitie O., Hayes E., Haynes R., Susic M., Montufar-Solis D., Duke P.J., Cole W.G. (2004) Chondrocyte cell death and intracellular distribution of COMP and type IX collagen in the pseudoachondroplasia growth plate. J. Orthop. Res., 22, 759–767. [DOI] [PubMed] [Google Scholar]
- 14.Hecht J.T., Montufar-Solis D., Decker G., Lawler J., Daniels K., Duke P.J. (1998) Retention of cartilage oligomeric matrix protein (COMP) and cell death in redifferentiated pseudoachondroplasia chondrocytes. Matrix Biol., 17, 625–633. [DOI] [PubMed] [Google Scholar]
- 15.Merritt T.M., Alcorn J.L., Haynes R., Hecht J.T. (2006) Expression of mutant cartilage oligomeric matrix protein in human chondrocytes induces the pseudoachondroplasia phenotype. J. Orthop. Res., 24, 700–707. [DOI] [PubMed] [Google Scholar]
- 16.Posey K.L., Hecht J.T. (2008) The role of cartilage oligomeric matrix protein (COMP) in skeletal disease. Curr. Drug Targets, 9, 869–877. [DOI] [PubMed] [Google Scholar]
- 17.Deere M., Sanford T., Ferguson H.L., Daniels K., Hecht J.T. (1998) Identification of twelve mutations in cartilage oligomeric matrix protein (COMP) in patients with pseudoachondroplasia. Am. J. Med. Genet., 80, 510–513. [DOI] [PubMed] [Google Scholar]
- 18.Deere M., Sanford T., Francomano C.A., Daniels K., Hecht J.T. (1999) Identification of nine novel mutations in cartilage oligomeric matrix protein in patients with pseudoachondroplasia and multiple epiphyseal dysplasia. Am. J. Med. Genet., 85, 486–490. [DOI] [PubMed] [Google Scholar]
- 19.Posey K.L., Veerisetty A.C., Liu P., Wang H.R., Poindexter B.J., Bick R., Alcorn J.L., Hecht J.T. (2009) An inducible cartilage oligomeric matrix protein mouse model recapitulates human pseudoachondroplasia phenotype. Am. J. Pathol., 175, 1555–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. (2012) Chop (Ddit3) is essential for D469del-COMP retention and cell death in chondrocytes in an inducible transgenic mouse model of pseudoachondroplasia. Am. J. Pathol., 180, 727–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posey K.L., Coustry F., Veerisetty A.C., Liu P., Alcorn J.L., Hecht J.T. (2014) Chondrocyte-specific pathology during skeletal growth and therapeutics in a murine model of pseudoachondroplasia. J. Bone Miner. Res., 29, 1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhotra J.D., Kaufman R.J. (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid. Redox. Signal, 9, 2277–2293. [DOI] [PubMed] [Google Scholar]
- 23.Gotoh T., Endo M., Oike Y. (2011) Endoplasmic reticulum stress-related inflammation and cardiovascular diseases. Int. J. Inflam., 2011, 259462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. (2009) ER stress in Alzheimer's disease: a novel neuronal trigger for inflammation and Alzheimer's pathology. J. Neuroinflammation, 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cullinan S.B., Diehl J.A. (2006) Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell. Biol., 38, 317–332. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil G.S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell, 140, 900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. U. S. A., 105, 18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamazaki T., Muramoto M., Oe T., Morikawa N., Okitsu O., Nagashima T., Nishimura S., Katayama Y., Kita Y. (2006) Diclofenac, a non-steroidal anti-inflammatory drug, suppresses apoptosis induced by endoplasmic reticulum stresses by inhibiting caspase signaling. Neuropharmacology, 50, 558–567. [DOI] [PubMed] [Google Scholar]
- 29.Hosoi T., Yamaguchi R., Noji K., Matsuo S., Baba S., Toyoda K., Suezawa T., Kayano T., Tanaka S., Ozawa K. (2014) Flurbiprofen ameliorated obesity by attenuating leptin resistance induced by endoplasmic reticulum stress. EMBO Mol. Med., 6, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hosoi T., Sasaki M., Baba S., Ozawa K. (2009) Effect of pranoprofen on endoplasmic reticulum stress in the primary cultured glial cells. Neurochem. Int., 54, 1–6. [DOI] [PubMed] [Google Scholar]
- 31.Cong X.D., Ding M.J., Dai D.Z., Wu Y., Zhang Y., Dai Y. (2012) ER stress, p66shc, and p-Akt/Akt mediate adjuvant-induced inflammation, which is blunted by argirein, a supermolecule and rhein in rats. Inflammation, 35, 1031–1040. [DOI] [PubMed] [Google Scholar]
- 32.Coustry F., Posey K.L., Liu P., Alcorn J.L., Hecht J.T. (2012) D469del-COMP retention in chondrocytes stimulates caspase-independent necroptosis. Am. J. Pathol., 180, 738–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper R.R., Ponseti I.V., Maynard J.A. (1973) Pseudoachondroplastic dwarfism. A rough-surfaced endoplasmic reticulum storage disorder. J. Bone Joint Surg., 55, 475–484. [PubMed] [Google Scholar]
- 34.Warman M.L., Cormier-Daire V., Hall C., Krakow D., Lachman R., LeMerrer M., Mortier G., Mundlos S., Nishimura G., Rimoin D.L., et al. (2011) Nosology and classification of genetic skeletal disorders: 2010 revision. Am. J. Med. Genet. A, 155A, 943–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hecht J.T., Nelson L.D., Crowder E., Wang Y., Elder F.F., Harrison W.R., Francomano C.A., Prange C.K., Lennon G.G., Deere M., et al. (1995) Mutations in exon 17B of cartilage oligomeric matrix protein (COMP) cause pseudoachondroplasia. Nat. Genet., 10, 325–329. [DOI] [PubMed] [Google Scholar]
- 36.Posey K.L., Hayes E., Haynes R., Hecht J.T. (2004) Role of TSP-5/COMP in pseudoachondroplasia. Int. J. Biochem. Cell Biol., 36, 1005–1012. [DOI] [PubMed] [Google Scholar]
- 37.Allen J.R., Nguyen L.X., Sargent K.E., Lipson K.L., Hackett A., Urano F. (2004) High ER stress in beta-cells stimulates intracellular degradation of misfolded insulin. Biochem. Biophys. Res. Commun., 324, 166–170. [DOI] [PubMed] [Google Scholar]
- 38.Katiyar S.K. (2008) Grape seed proanthocyanidines and skin cancer prevention: inhibition of oxidative stress and protection of immune system. Mol. Nutr. Food Res., 52(Suppl 1), S71–S76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rao C.V. (2007) Regulation of COX and LOX by curcumin. Adv. Exp. Med. Biol., 595, 213–226. [DOI] [PubMed] [Google Scholar]
- 40.Welting T.J., Caron M.M., Emans P.J., Janssen M.P., Sanen K., Coolsen M.M., Voss L., Surtel D.A., Cremers A., Voncken J.W., et al. (2011) Inhibition of cyclooxygenase-2 impacts chondrocyte hypertrophic differentiation during endochondral ossification. Eur. Cell. Mater., 22, 420–436; discussion 436–427. [DOI] [PubMed] [Google Scholar]
- 41.Hutchison M.R., White P.C. (2015) Prostacyclin regulates bone growth via the Epac/Rap1 pathway. Endocrinology, 156, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulkarni S.S., Canto C. (2015) The molecular targets of resveratrol. Biochim. Biophys. Acta, 1852, 1114–1123. [DOI] [PubMed] [Google Scholar]
- 43.Park S.J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., et al. (2012) Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell, 148, 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh N., Agrawal M., Dore S. (2013) Neuroprotective properties and mechanisms of resveratrol in in vitro and in vivo experimental cerebral stroke models. ACS Chem. Neurosci., 4, 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Funk J.L., Frye J.B., Oyarzo J.N., Kuscuoglu N., Wilson J., McCaffrey G., Stafford G., Chen G., Lantz R.C., Jolad S.D., et al. (2006) Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum., 54, 3452–3464. [DOI] [PubMed] [Google Scholar]
- 46.Garcia Rodriguez L.A., Hernandez-Diaz S., de Abajo F.J. (2001) Association between aspirin and upper gastrointestinal complications: systematic review of epidemiologic studies. Br. J. Clin. Pharmacol., 52, 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia Rodriguez L.A., Hernandez-Diaz S. (2001) Relative risk of upper gastrointestinal complications among users of acetaminophen and nonsteroidal anti-inflammatory drugs. Epidemiology, 12, 570–576. [DOI] [PubMed] [Google Scholar]
- 48.Jang M., Cai L., Udeani G.O., Slowing K.V., Thomas C.F., Beecher C.W., Fong H.H., Farnsworth N.R., Kinghorn A.D., Mehta R.G., et al. (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science, 275, 218–220. [DOI] [PubMed] [Google Scholar]
- 49.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. (2006) Resveratrol improves health and survival of mice on a high-calorie diet. Nature, 444, 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baur J.A., Sinclair D.A. (2006) Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Dis., 5, 493–506. [DOI] [PubMed] [Google Scholar]
- 51.Pearson K.J., Baur J.A., Lewis K.N., Peshkin L., Price N.L., Labinskyy N., Swindell W.R., Kamara D., Minor R.K., Perez E., et al. (2008) Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab., 8, 157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barger J.L., Kayo T., Pugh T.D., Prolla T.A., Weindruch R. (2008) Short-term consumption of a resveratrol-containing nutraceutical mixture mimics gene expression of long-term caloric restriction in mouse heart. Exp. Gerontol., 43, 859–866. [DOI] [PubMed] [Google Scholar]
- 53.Barger J.L., Kayo T., Vann J.M., Arias E.B., Wang J., Hacker T.A., Wang Y., Raederstorff D., Morrow J.D., Leeuwenburgh C., et al. (2008) A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PloS One, 3, e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elmali N., Esenkaya I., Harma A., Ertem K., Turkoz Y., Mizrak B. (2005) Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res., 54, 158–162. [DOI] [PubMed] [Google Scholar]
- 55.Wang J., Gao J.S., Chen J.W., Li F., Tian J. (2012) Effect of resveratrol on cartilage protection and apoptosis inhibition in experimental osteoarthritis of rabbit. Rheumatol. Int., 32, 1541–1548. [DOI] [PubMed] [Google Scholar]
- 56.Smoliga J.M., Baur J.A., Hausenblas H.A. (2011) Resveratrol and health—a comprehensive review of human clinical trials. Mol. Nutr. Food Res., 55, 1129–1141. [DOI] [PubMed] [Google Scholar]
- 57.Kidd B.L., Urban L.A. (2001) Mechanisms of inflammatory pain. Br. J. Anaesth., 87, 3–11. [DOI] [PubMed] [Google Scholar]
- 58.Mackey S. (2004) Mechanisms of inflammatory pain: therapeutic implications. J. Clin. Rheumatol., 10, S5–S11. [DOI] [PubMed] [Google Scholar]
- 59.Hui W., Rowan A.D., Richards C.D., Cawston T.E. (2003) Oncostatin M in combination with tumor necrosis factor alpha induces cartilage damage and matrix metalloproteinase expression in vitro and in vivo. Arthritis Rheum., 48, 3404–3418. [DOI] [PubMed] [Google Scholar]
- 60.Kobayashi M., Squires G.R., Mousa A., Tanzer M., Zukor D.J., Antoniou J., Feige U., Poole A.R. (2005) Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum., 52, 128–135. [DOI] [PubMed] [Google Scholar]
- 61.Westacott C.I., Barakat A.F., Wood L., Perry M.J., Neison P., Bisbinas I., Armstrong L., Millar A.B., Elson C.J. (2000) Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteo. Cart., 8, 213–221. [DOI] [PubMed] [Google Scholar]
- 62.Reagan-Shaw S., Nihal M., Ahmad N. (2008) Dose translation from animal to human studies revisited. FASEB J., 22, 659–661. [DOI] [PubMed] [Google Scholar]
- 63.Garcia S., Dirat B., Tognacci T., Rochet N., Mouska X., Bonnafous S., Patouraux S., Tran A., Gual P., Le Marchand-Brustel Y., et al. (2013) Postnatal soluble FGFR3 therapy rescues achondroplasia symptoms and restores bone growth in mice. Sci. Transl. Med., 5, 203ra124. [DOI] [PubMed] [Google Scholar]
- 64.Lichtenberger L.M., Ulloa C., Romero J.J., Vanous A.L., Illich P.A., Dial E.J. (1996) Nonsteroidal anti-inflammatory drug and phospholipid prodrugs: combination therapy with antisecretory agents in rats. Gastroenterology, 111, 990–995. [DOI] [PubMed] [Google Scholar]
- 65.Obeid G., Zhang X., Wang X. (1992) Effect of ibuprofen on the healing and remodeling of bone and articular cartilage in the rabbit temporomandibular joint. J. Oral Maxillofac. Surg., 50, 843–849; discussion 849–850. [DOI] [PubMed] [Google Scholar]
- 66.Elmali N., Baysal O., Harma A., Esenkaya I., Mizrak B. (2007) Effects of resveratrol in inflammatory arthritis. Inflammation, 30, 1–6. [DOI] [PubMed] [Google Scholar]
- 67.Mao Q.Q., Bai Y., Lin Y.W., Zheng X.Y., Qin J., Yang K., Xie L.P. (2010) Resveratrol confers resistance against taxol via induction of cell cycle arrest in human cancer cell lines. Mol. Nutr. Food Res., 54, 1574–1584. [DOI] [PubMed] [Google Scholar]
- 68.Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. (2008) Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol., 75, 677–687. [DOI] [PubMed] [Google Scholar]
- 69.Srinivasan K. (2005) Plant foods in the management of diabetes mellitus: spices as beneficial antidiabetic food adjuncts. Int. J. Food Sci. Nutr., 56, 399–414. [DOI] [PubMed] [Google Scholar]
- 70.Beal M.F., Shults C.W. (2003) Effects of Coenzyme Q10 in Huntington's disease and early Parkinson's disease. Biofactors, 18, 153–161. [DOI] [PubMed] [Google Scholar]
- 71.Burdan F., Staroslawska E., Szumilo J. (2012) Prenatal tolerability of acetaminophen and other over-the-counter non-selective cyclooxygenase inhibitors. Pharmacol. Rep., 64, 521–527. [DOI] [PubMed] [Google Scholar]
- 72.Posey K.L., Hankenson K., Veerisetty A.C., Bornstein P., Lawler J., Hecht J.T. (2008) Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am. J. Pathol., 172, 1664–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.