Abstract
In this study, we identified a BET bromodomain (BRD) protein, Brd4, not only as a novel epigenetic regulator of autosomal dominant polycystic kidney disease (ADPKD) but also as a novel client protein of Hsp90. We found that Brd4 was upregulated in Pkd1 mutant mouse renal epithelial cells and tissues. This upregulation of Brd4 appears to result from the chaperone activity of Hsp90 and escape proteasomal degradation. We further identify that Brd4 is an upstream regulator of the expression of c-Myc which has been upregulated in all rodent models of PKD and ADPKD patients with unknown mechanism. Inhibition of Brd4 in Pkd1 mutant renal epithelial cells with JQ1, a selective small-molecular inhibitor of BET BRD protein(s), (1) decreased the levels of c-Myc mRNA and protein; (2) increased the levels of p21 mRNA and protein, which was transcriptionally repressed by c-Myc; (3) decreased the phosphorylation of Rb; and (4) decreased cystic epithelial cell proliferation as shown by inhibition of S-phase entry. Most importantly, treatment with JQ1 strikingly delayed cyst growth and kidney enlargement, and preserved renal function in two early stage genetic mouse strains with Pkd1 mutations. This study not only provides one of the mechanisms of how c-Myc is upregulated in PKD but also suggests that targeting Brd4 with JQ1 may function as a novel epigenetic approach in ADPKD. The unraveled link between Brd4 and Hsp90 in ADPKD may also be a general mechanism for the upregulation of Brd4 in cancer cells and opens up avenues for combination therapies against ADPKD and cancer.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is caused by mutations in PKD1 or PKD2, encoding polycystin 1 and polycystin 2, respectively (1). This disease is characterized by progressive enlargement of bilateral kidney cysts and adjacent normal tubular atrophy, leading to end-stage renal disease in more than half of the patients (2). Renal epithelial cells with mutations or absence of polycystins exhibit cellular aberrations including dedifferentiation, increased cell proliferation, loss of cell polarity and altered gene expression, which are associated with cystogenesis and/or cyst expansion. The phenotypic cellular abnormalities are usually used as the therapeutic targets for retarding cyst growth. However, only limited effective clinical therapies for ADPKD are presently available.
The roles of epigenetic modulation of gene expression and protein functions in ADPKD have recently become the focus of scientific investigation (3,4). Our recent studies indicated that targeting histone deacetylases (HDACs) with trichostatin (TSA) or nicotinamide (vitamin B3) delayed cyst growth in Pkd1 knockout mouse models (4,5). Acetylation of histones affects gene expression through direct effect on chromatin structure by neutralizing charges on the histone tails, and/or through recruitment of complexes containing trans factors, including bromodomain (BRD) proteins which specifically bind to acetylated-lysine residues on histone tails through BRDs. Most BRD proteins fall into one of three categories: components of histone acetyltransferase complexes, components of chromatin remodeling complexes, and bromodomain-extraterminal (BET) proteins. The BRD and BET family proteins (Brd2, Brd3, Brd4 and Brdt), which consist of two highly conserved amino-terminal BRDs, can recognize acetylated-lysine residues in histone tails to regulate the expression of numerous genes associated with cell cycle, cell growth, inflammation and cancer (6–11).
c-Myc has been suggested to play an important role in the pathogenesis of ADPKD over the past two decades. It has been reported that (1) c-Myc mRNA is overexpressed in kidneys from human ADPKD and murine autosomal recessive PKD (ARPKD) models (12–16); (2) c-Myc transgenic mice represent a genetic model of PKD similar to human ADPKD (15,17); and (3) c-Myc antisense oligonucleotide treatment has been shown to ameliorate cyst growth in ARPKD (18). These studies make c-Myc an attractive pharmacological target for treating PKD. However, the mechanism leading to c-Myc upregulation in PKD remains unknown.
It has been reported that upregulation of Brd4 plays a critical role in the development of several hematopoietic and somatic cancers via regulating the transcription of c-Myc (19–21). A potent Brd4 inhibitor named JQ1 (a thieno-triazolo-1,4-diazapine), which competitively occupies the acetyl-lysine recognition motifs of BET family proteins, resulting in release of BET family proteins from active chromatin and suppression of mRNA transcription and elongation (10,22), has been developed and pharmacologically modulates c-Myc transcriptional function in cancer cells (10,23–26). In particular, JQ1 is highly effective against NUT midline carcinoma (NMC) xenografts in vivo and promotes both growth arrest and differentiation of NMC cells in vitro through targeting BRD4 (22). JQ1 also inhibits the activity of cell proliferation in a range of cell lines derived from hematological malignancies, including multiple myeloma (10), acute myeloid leukemia (AML), Burkitt's lymphoma (BL) (23), primary effusion lymphoma (27) and B-Cell acute lymphoblastic leukemia (28). However, the mechanism(s) for the upregulation of Brd4 in cancer cells remains elusive.
In this study, we identified Brd4 not only as a novel epigenetic regulator of ADPKD but also as a novel Hsp90 client protein. Brd4 is upregulated in Pkd1 mutant renal epithelial cells and tissues and is able to form a complex with Hsp90. Hsp90 chaperone complex protects Brd4 from degradation since pharmacological inhibition of Hsp90 activity destabilizes Brd4 in Pkd1 mutant renal epithelial cells. Further, we showed that increased Brd4 expression in Pkd1 mutant renal epithelial cells and tissues is responsible for the upregulation of c-Myc through transcriptional regulation that revealed a mechanism of c-Myc upregulation in PKD. Targeting Brd4 with JQ1 slows renal cyst growth, which suggests that JQ1 treatment may function as a novel therapeutic strategy in ADPKD. The findings of a regulatory network by the association of Brd4 with Hsp90 complex that stimulate c-Myc regulation suggest targeting synergistically Brd4 and Hsp90 as a therapeutic combination in ADPKD and cancer.
Results
Brd4 is upregulated in Pkd1 mutant renal epithelial cells and tissues
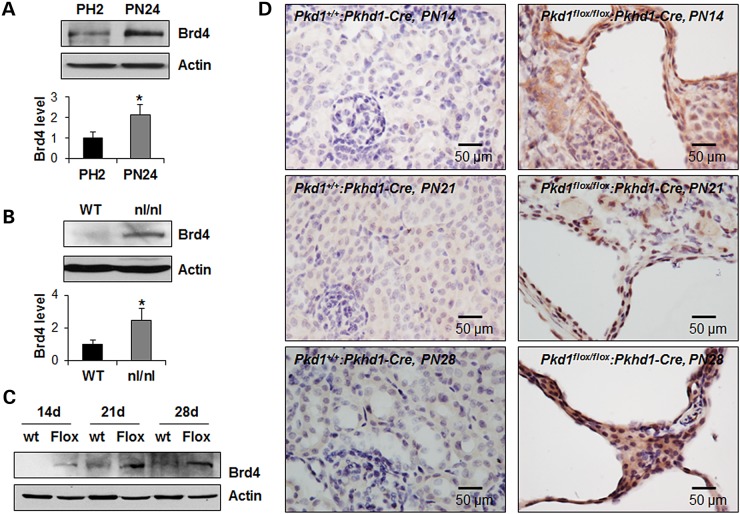
We first evaluated the levels of BET BRD protein Brd4 in Pkd1 mutant renal epithelial cells and kidneys. We found that the mRNA and protein expression of Brd4, but not other BET family proteins, including Brd2 or Brd3, were upregulated in postnatal Pkd1 mutant PN24 cells compared with that in postnatal Pkd1 heterozygous PH2 cells as analyzed by quantitative reverse-transcription PCR (qRT-PCR) and western blot (Fig. 1A and Supplementary Material, Fig. S1A). However, only Brd4 protein but not Brd4 mRNA was upregulated in the kidneys from postnatal day 28 Pkd1nl/nl mice demonstrated by western blot and qRT-PCR (Fig. 1B and Supplementary Material, Fig. S1B). In addition, the expression of Brd4 protein but not Brd4 mRNA was upregulated in the postnatal day 14, 21 and 28 kidneys from Pkd1flox/flox:Pkhd1-Cre mice compared with that of age-matched wild-type mice demonstrated by western blot, immunohistochemistry staining and qRT-PCR, respectively (Fig. 1C and D and Supplementary Material, Fig. S1C). These results suggest that the increased expression of Brd4 in renal epithelial cells and tissues is caused by loss or mutation of Pkd1.
Figure 1.
Brd4 is upregulated in cystic renal epithelial cells. (A–C) Western blot analysis of the expression of Brd4 protein in postnatal Pkd1 heterozygous PH2 (PH2) cells and Pkd1 homozygous PN24 (PN24) cells (A), in postnatal day 28 kidneys from Pkd1+/+ (WT) mice and Pkd1nl/nl (nl/nl) mice (B), and in postnatal day 14, 21 and 28 kidneys from Pkd1+/+:Pkhd1-Cre (WT) and Pkd1flox/flox:Pkhd1-Cre (Flox) mice (C), respectively. In A and B, the expression of Brd4 was quantified from three independent immunoblots and was presented as the relative expression level of Brd4 standardized to actin in the bottom panel. *P < 0.05. (D) The immunohistochemistry staining of Brd4 in the postnatal day 14, 21 and 28 kidneys from Pkd1+/+:Pkhd1-Cre and Pkd1flox/flox:Pkhd1-Cre mice, respectively. Scale bar, 50 µm.
Brd4 is a novel client protein of heat shock protein 90 (Hsp90) which protects Brd4 from proteasomal degradation in cystic renal epithelial cells
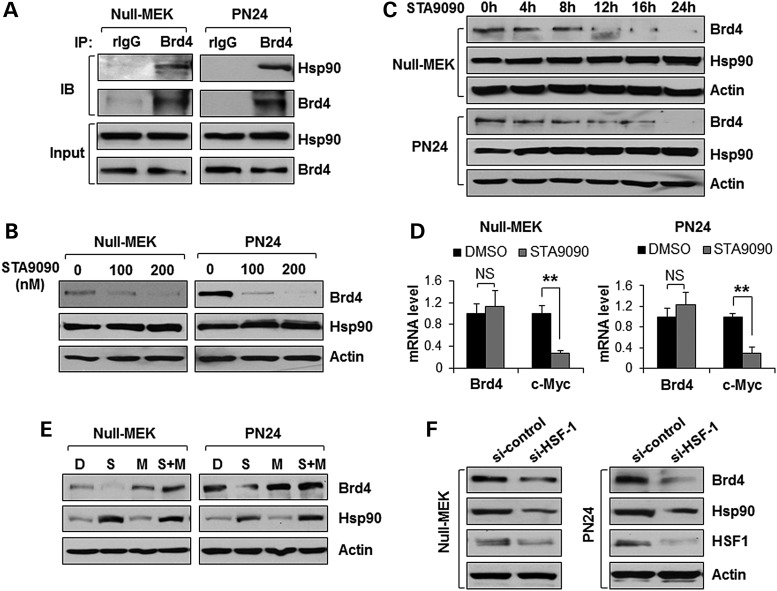
It has been reported that the expression of Hsp90 was upregulated in Pkd1 mutant renal epithelial cells and tissues and inhibition of Hsp90 slowed cyst growth in an ADPKD mouse model (29). We found that Brd4 antibody could pull down endogenous Hsp90 in the Pkd1 mutant mouse embryonic kidney (MEK) cells and PN24 cells (Fig. 2A). Importantly, treatment with STA9090, a second generation Hsp90 inhibitor that binds to ATP binding domain at the N-terminal of Hsp90, decreased the levels of Brd4 protein (Fig. 2B and C) but not Brd4 mRNA (Fig. 2D) in Pkd1 mutant MEK cells and PN24 cells in a dose- and time-dependent manner. STA9090 binds and inhibits Hsp90, resulting in the proteasomal degradation of client proteins (30). We further found that STA9090-induced downregulation of Brd4 was reverted by the proteasomal inhibitor MG132 in Pkd1 mutant MEK cells and PN24 cells (Fig. 2E), indicating that the reduction of Brd4 protein induced by STA9090 were mediated by proteasomal degradation. Heat shock factor 1 (HSF1) is a transcriptional regulator of the inducible heat shock response, which increases the Hsp90 transcription (31). We found that knockdown of HSF-1 with siRNA decreased the levels of Hsp90 and Brd4 in Pkd1 mutant MEK cells and PN24 cells (Fig. 2F). These results support that Brd4 is a novel client protein of Hsp90 and the upregulation of Brd4 may be a result of protection from degradation by HSF1-Hsp90 signaling in cystic renal epithelial cells.
Figure 2.
Hsp90 regulates the expression of Brd4 in cystic renal epithelial cells. (A) Interaction between Brd4 and Hsp90 in Pkd1 null MEK (Null) cells and Pkd1 mutant PN24 cells, examined by IP with anti-Brd4 antibody and then blotting with anti-Hsp90 antibody. Normal rabbit IgG (rIgG) was used as a NC. (B and C) Western blot analysis of the expression of Brd4 and Hsp90 from whole-cell lysates of Pkd1 null MEK (Null) cells and Pkd1 mutant PN24 cells treated with STA9090 at indicated concentrations for 24 h (B), and in Pkd1 null MEK (Null) cells and Pkd1 mutant PN24 cells treated with STA9090 (200 nm) at indicated time points (C). (D) qRT-PCR analysis of the expression of Brd4 and c-Myc from Pkd1 null MEK (Null) cells and Pkd1 mutant PN24 cells treated with STA9090 (200 nm) for 24 h. (E) Western blot analysis of the expression of Brd4 and Hsp90 from whole-cell lysates of Pkd1 null MEK (Null) cells treated with DMSO (D), 200 nm STA9090 (S), 1 µm MG132 (M) and 200 nm STA9090 plus 1 µm MG132 (S + M) for 24 h, as well as from Pkd1 mutant PN24 cells treated with DMSO (D), 200 nm STA9090 (S), 5 µm MG132 (M), and 200 nm STA9090 plus 5 µm MG132 (S + M) for 24 h. (F) Western blot analysis of the expression of Brd4, Hsp90 and HSF1 from whole-cell lysates of Pkd1 null MEK (Null) cells and Pkd1 mutant PN24 cells transfected with control siRNA or HSF1 siRNA for 48 h.
Brd4 regulates c-Myc transcription in renal epithelial cells
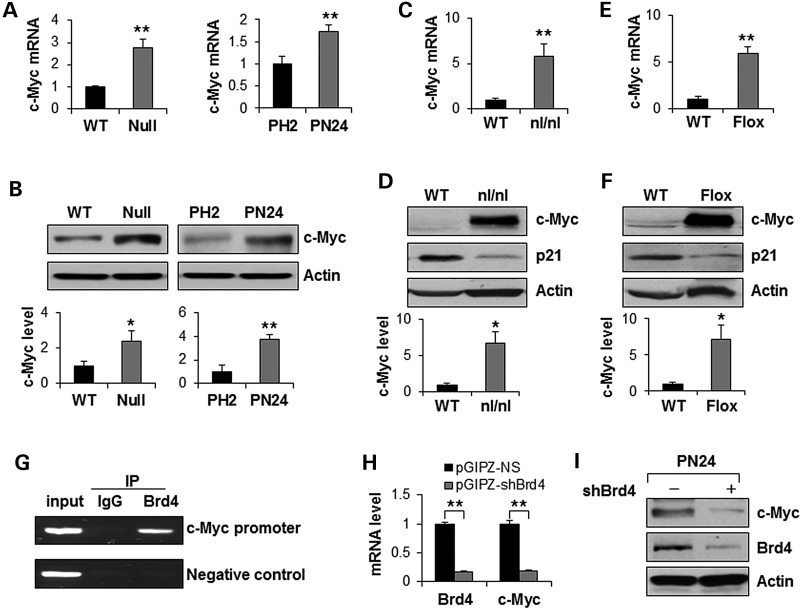
Overexpression of c-Myc has been reported in kidneys from murine autosomal recessive PKD (ARPKD) models and human ADPKD (4,12–16) with unknown mechanism. It has been reported that BET BRD protein Brd4, is enriched in the upstream and downstream of the transcription start site of c-Myc promoter, which acts as a transcriptional coactivator that drives c-Myc expression in LP-1 cells (23). We found that the expression levels of c-Myc mRNA and protein were increased in Pkd1 mutant MEK cells and postnatal Pkd1 homozygous PN24 cells compared with Pkd1 wild-type MEK cells and postnatal Pkd1 heterozygous PH2 cells, respectively, as analyzed by qRT-PCR and western blot (Fig. 3A and B). The expression levels of c-Myc mRNA and protein were also increased in the postnatal day 28 kidneys from Pkd1nl/nl mice (Fig. 3C and D) and in the postnatal day 14, 21 and 28 kidneys (Fig 3E and 3F and Supplementary Material, Fig. S2A) from Pkd1flox/flox:Pkhd1-Cre mice compared with that in the age-matched wild-type kidneys, respectively. These results suggest that the increased expression of c-Myc in renal epithelial cells and tissues is caused by loss or mutation of Pkd1.
Figure 3.
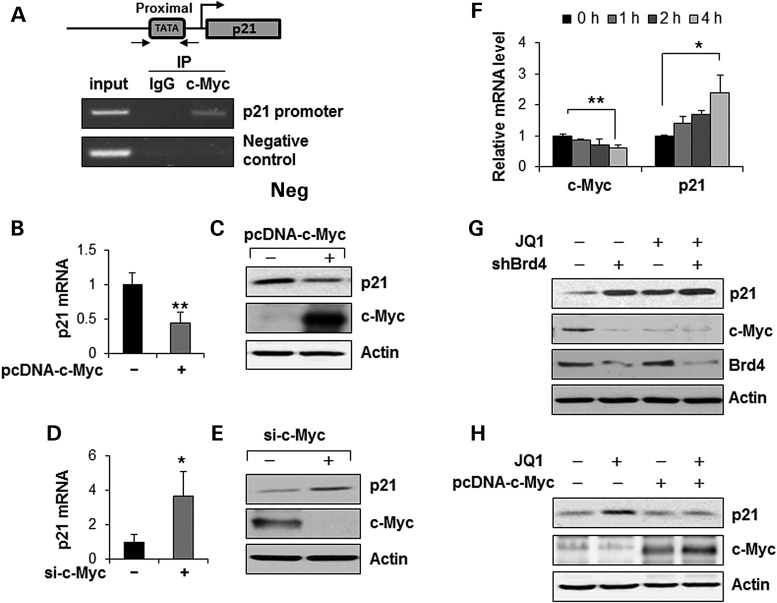
Brd4 regulates the transcription of c-Myc in cystic renal epithelial cells. (A) qRT-PCR analysis of the expression of c-Myc mRNA in Pkd1 wild-type (WT) and Pkd1null/null (Null) MEK cells as well as in postnatal Pkd1 heterozygous PH2 (PH2) cells and Pkd1 homozygous PN24 (PN24) cells. **P < 0.01. (B) Western blot analysis of the expression of c-Myc from whole-cell lysates of the above cells. The expression of c-Myc was quantified from three independent immunoblots and was presented as the relative expression level of c-Myc standardized to actin in the bottom panel. *P < 0.05, **P < 0.01. (C and D) qRT-PCR analysis of the expression of c-Myc mRNA (C) and western blot analysis of the expression of c-Myc and p21 protein (D) in postnatal day 28 kidneys from Pkd1+/+ (WT) and Pkd1nl/nl (nl/nl) mice. n = 4, *P < 0.05, **P < 0.01. (E and F) qRT-PCR analysis of the expression of c-Myc mRNA (E) and western blot analysis of the expression of c-Myc and p21 protein (F) in postnatal day 28 kidneys from Pkd1+/+:Pkhd1-Cre (WT) and Pkd1flox/flox:Pkhd1-Cre (Flox) neonates. n = 4, *P < 0.05, **P < 0.01. (G) Brd4 binds to the promoter of c-Myc. CHIP assay was performed with anti-Brd4 antibody or normal rabbit IgG in Pkd1 mutant renal epithelial cells. The precipitated chromatin DNA was analyzed by PCR with primers that amplified from −613 and −374 bp upstream of the c-Myc ATG start codon. The PCR amplification for c-Myc coding regions was used as a NC. (H) qRT-PCR analysis of the mRNA level of Brd4 and c-Myc in Pkd1 homozygous PN24 (PN24) cells transduced with pGIPZ-shBrd4 compared with that in the cells transduced with the control vector pGIPZ-NS. n = 3, ** P < 0.01. (I) Western blot analysis of the expression of c-Myc and Brd4 in Pkd1 homozygous PN24 (PN24) cells transduced with Brd4 shRNA.
We further found that, (1) Brd4 bound directly to the c-Myc promoter near the transcription start site in postnatal Pkd1 mutant PN24 cells as analyzed by chromatin immunoprecipitation (CHIP) assay with antibody against Brd4 (Fig. 3G); (2) knockdown of Brd4 with lentivirus mediated Brd4 shRNA decreased the levels of c-Myc mRNA (Fig. 3H) and protein (Fig. 3I) in postnatal Pkd1 mutant PN24 cells; and (3) inhibition of Hsp90 with STA9090 decreased Brd4 protein and decreased the levels of c-Myc mRNA (Fig. 2D) and protein (Supplementary Material, Fig S2B) in Pkd1 mutant MEK cells and PN24 cells. These results suggested that Brd4 regulated c-Myc expression in the Pkd1 mutant renal epithelial cells.
JQ1, a BET BRD inhibitior, suppresses c-Myc gene expression in cystic renal epithelial cells
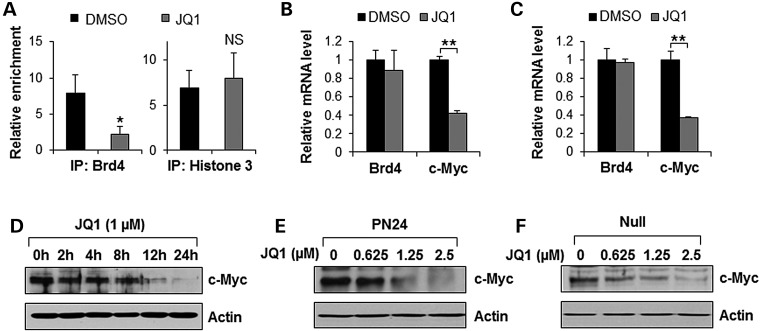
It has been reported that transcription of c-Myc can be pharmacologically modulated by JQ1, which binds competitively to acetyl-lysine recognition motifs or BRDs (10,22). We found that treatment with JQ1 induced the release of Brd4 from the promoter of c-Myc in postnatal Pkd1 mutant PN24 cells (Fig. 4A). However, as a control, the levels of histone H3 was unaffected upon JQ1 treatment (Fig. 4A). We further found that treatment with JQ1 decreased the level of c-Myc mRNA but not Brd4 mRNA in both embryonic and postnatal Pkd1 mutant cells (Fig. 4B and C). Treatment with JQ1 also decreased the protein level of c-Myc in a dose-dependent and time-dependent manner in these cells (Fig. 4D–F). These results indicated that release of Brd4 from chromatin by JQ1 suppressed the transcription of c-Myc in Pkd1 mutant renal epithelial cells.
Figure 4.
BET-BRD inhibition with JQ1 potently suppresses c-Myc gene expression in Pkd1 mutant renal epithelial cells. (A) CHIP assay was performed with anti-Brd4 antibody or anti-histone 3 antibody in Pkd1 mutant PN24 cells treated with DMSO or JQ1 (1 µm) for 4 h, respectively. The precipitated chromatin DNA was analyzed by quantitative PCR with primers that amplified from −613 and −374 bp upstream of the c-Myc ATG start codon. Enrichment relative to no antibody control was shown. n = 3, *P < 0.05. (B and C) qRT-PCR analysis of the expression of Brd4 and c-Myc mRNA in postnatal Pkd1 mutant PN24 cells (B) and Pkd1 null MEK cells (C) treated with 1 µm JQ1 for 24 h. n = 3, **P < 0.01. (D) Western blot analysis of the expression of c-Myc in Pkd1 mutant PN24 cells treated with 1 µm JQ1 at indicated time points. (E and F) Western blot analysis of the expression of c-Myc in Pkd1 mutant PN24 cells (E) and Pkd1 null MEK cells (F) treated with JQ1 with indicated concentrations.
JQ1 decreased cystic renal epithelial cell proliferation through c-Myc–p21 signaling
Previous studies showed that JQ1 treatment could induce cell cycle arrest and apoptosis in different cancer cells (23). We found that treatment with JQ1 significantly inhibited the S-phase entry and induced the G1-phase arrest in Pkd1 mutant PN24 cells (Supplementary Material, Fig. S3A), but not that in postnatal Pkd1 heterozygous PH2 cells (Supplementary Material, Fig. S3B). Next, we investigated the JQ1-c-Myc mediated downstream signaling in Pkd1 mutant renal epithelial cells. It has been reported that c-Myc represses the transcription of cyclin-dependent kinase (CDK) inhibitor p21 through its physical association with MIZ1 in cancer cells (32,33). Our previous studies found that the expression of p21 was down-regulated in the Pkd1 mutant renal epithelial cells (5,34). We further found that the expression of p21 was also decreased in kidneys from the postnatal day 28 Pkd1nl/nl mice (Fig. 3D) and Pkd1flox/flox:Pkhd1-Cre mice (Fig. 3F) compared with that in kidneys from the age-matched wild-type mice, respectively. To support that c-Myc regulated the expression of p21 in renal epithelial cells, we found that, (i) c-Myc bound to the core promoter region of the p21 promoter by ChIP assay with anti-c-Myc antibody (Fig. 5A); (ii) overexpression of c-Myc decreased the mRNA (Fig. 5B) and protein (Fig. 5C) levels of p21 in Pkd1 wild-type MEK cells; (iii) knockdown of c-Myc with siRNA increased the mRNA (Fig. 5D) and protein (Fig. 5E) levels of p21 in Pkd1 null MEK cells. We further found that JQ1 treatment increased p21 mRNA but decreased c-Myc mRNA in Pkd1 null MEK cells in a time-dependent manner (Fig. 5F). In addition, we found that knockdown of Brd4 with shRNA or inhibition of Brd4 with JQ1 also increased the expression of p21 protein in Pkd1 mutant MEK cells and PN24 cells (Supplementary Material, Fig. S4).
Figure 5.
c-Myc transcriptionally represses p21 in renal epithelial cells. (A) c-Myc binds to the promoter of p21. CHIP assay was performed with anti-c-Myc antibody or normal rabbit IgG in Pkd1 mutant cells. The precipitated chromatin DNA was analyzed by PCR with primers that amplified from −296 and −51 bp upstream of the p21 ATG start codon. The PCR amplification for p21 coding regions was used as a NC. (B and C) Overexpression of c-Myc decreased the levels of p21 mRNA as analyzed by qRT-PCR (B) and p21 protein as analyzed by western blot (C) in Pkd1 wild-type MEK cells transfected with pcDNA3-c-Myc for 48 h compared with empty vector transfected cells. **P < 0.01. (D and E) Knockdown of c-Myc with siRNA increased the levels of p21 mRNA as analyzed by qRT-PCR (D) and p21 protein as analyzed by western blot (E) in Pkd1 null MEK cells transfected with c-Myc siRNA for 48 h compared with control siRNA transfected cells. *P < 0.05. (F) qRT-PCR analysis of the expression of c-Myc and p21 mRNA in PN24 cells upon treatment with JQ1 for the indicated time points. The mRNA expression level was shown relative to DMSO control, where both c-Myc and p21 were set to 1. n = 3, *P < 0.05, **P < 0.01. (G) Western blot analysis of the expression of c-Myc and p21 in Pkd1 homozygous PN24 (PN24) cells transduced with lentivirus mediated Brd4 shRNA or control vector upon treatment with 1 µm JQ1 for 24 h. (H) Western blot analysis of the expression of c-Myc and p21 in PN24 cells transfected with pcDNA-c-Myc for 24 h and followed by treatment with 1 µm JQ1 for 24 h.
To investigate whether JQ1 induced downregulation of c-Myc and upregulation of p21 was through targeting Brd4, PN24 cells stably infected with Brd4 shRNA or control empty lentivirus were treated with JQ1. We found that JQ1 treatment strikingly decreased the expression of c-Myc and increased the p21 expression in the PN24 cells stably infected with control empty lentivirus (Fig. 5G). However, JQ1 treatment only slightly increased the expression of p21 in the PN24 cells stably transduced with Brd4 shRNA (Fig. 5G), suggesting JQ1 regulates the expression of c-Myc and p21 through specifically targeting Brd4. Next, to confirm that JQ1 regulates p21 expression through c-Myc, we overexpressed the exogenous c-Myc in the Pkd1 mutant cells and then treated the cells with JQ1. We found that the induction of p21 by JQ1 could be strongly attenuated in the presence of exogenous c-Myc (Fig. 5H). The addition of ectopic c-Myc to PN24 cells also rescued JQ1 induced G0/G1 arrest in these cells as determined by flow cytometry analysis (Supplementary Material, Fig. S5). These results suggested that treatment with JQ1 inhibited cystic epithelial cell proliferation through targeting Brd4 and its downstream c-Myc/p21 pathway.
BET inhibitor, JQ1 delays renal cyst growth in Pkd1 knockout mouse models
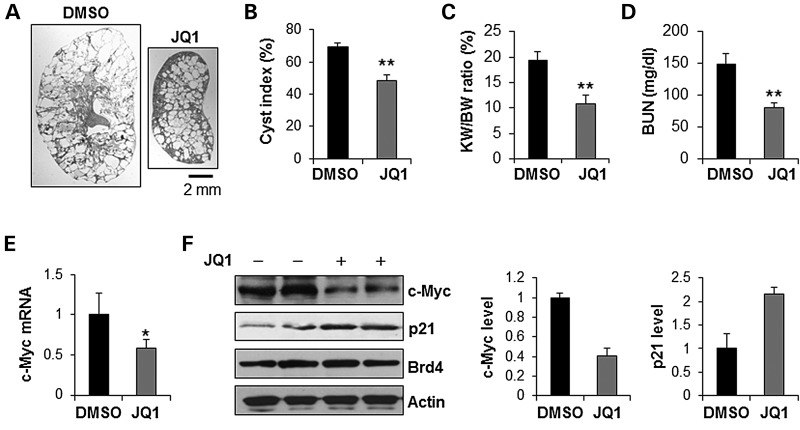
Given the robust evidence that JQ1 could decrease cystic epithelial cell growth through inhibiting Brd4 and its downstream target c-Myc, we examined the effect of JQ1 on cyst growth in vivo utilizing animal models bearing Pkd1 mutations. First, we determined if JQ1 reduced cyst growth in Pkd1 conditional knockout Pkd1flox/flox:Pkhd1-Cre mice. The cyst growth in Pkd1flox/flox:Pkhd1-Cre mice begins around postnatal day 10 (PN10), followed by rapidly cyst development over the subsequent 2 weeks (35). We treated Pkd1+/+:Pkhd1-Cre and Pkd1flox/flox:Pkhd1-Cre pups with daily intraperitoneal injection of JQ1 (5 mg/kg) or dimethyl sulfoxide (DMSO) (control), respectively, from postnatal day 8 (PN8) to postnatal day 24 (PN24). The kidneys were harvested and analyzed at postnatal day 25 (PN25). No animal death was observed during the treatment. JQ1 treatment did not affect the kidney weight (KW) and body weight (BW) of Pkd1+/+:Pkhd1-Cre mice, suggesting that JQ1 treatment did not affect the normal mice growth and development (Supplementary Material, Fig. S6). We found that administration of JQ1 delayed cyst growth characterized by partial preservation of renal parenchyma (Fig. 6A) and a significant decrease in cystic index (Fig. 6B). JQ1 treatment also significantly decreased the KW/BW ratios in the Pkd1flox/flox:Pkhd1-Cre mice compared with that in DMSO-injected Pkd1flox/flox:Pkhd1-Cre mice (Fig. 6C). Reduction in cyst growth correlated with significantly improved renal function as indicated by lower blood urea nitrogen (BUN) levels in JQ1 treated mice (Fig. 6D). We also found that JQ1 treatment decreased the expression of c-Myc mRNA (Fig. 6E) but not Brd4 mRNA (Supplementary Material, Fig. S7A), as well as the expression level of c-Myc protein in the kidneys of Pkd1flox/flox:Pkhd1-Cre mice (Fig. 6F). JQ1 treatment also increased the expression of p21 in the kidneys of Pkd1flox/flox:Pkhd1-Cre mice (Fig. 6F). In addition, the proliferation of the cyst-lining epithelial cells was significantly decreased in kidneys of JQ1 treated Pkd1flox/flox:Pkhd1-Cre mice compared with that in the DMSO treated mice as analyzed by proliferating cell nuclear antigen (PCNA) staining (Supplementary Material, Fig. S7B).
Figure 6.
Treatment with JQ1 delayed cyst growth in Pkd1flox/flox:Pkhd1-Cre mice. (A) Gross and histologic examination of PN25 kidneys from Pkd1flox/flox:Pkhd1-Cre neonates treated with vehicle (DMSO) (n = 7) or JQ1 (n = 7), respectively. Scale bar, 2 mm. (B) Quantification of the percentage of cystic areas over total kidney section areas of PN25 kidney sections from Pkd1flox/flox:Pkhd1-Cre neonates treated as in (A). Shown is mean ± SEM of all sections quantified for each condition. **P < 0.01. (C and D) KW/BW ratios (C) and BUN levels (D) were decreased in PN25 Pkd1flox/flox:Pkhd1-Cre mice treated with JQ1 compared with that treated with DMSO (control). **P < 0.01. (E) qRT-PCR analysis mRNA level of c-Myc in kidneys from Pkd1flox/flox:Pkhd1-Cre mice treated with JQ1 or DMSO, respectively. c-Myc mRNA was decreased in kidneys of JQ1 treated Pkd1flox/flox:Pkhd1-Cre mice versus DMSO treated Pkd1flox/flox:Pkhd1-Cre mice. n = 3, *P < 0.05. (F) The expression of c-Myc protein was decreased and the expression of p21 was increased in kidneys of JQ1 treated Pkd1flox/flox:Pkhd1-Cre mice versus DMSO treated Pkd1flox/flox:Pkhd1-Cre mice. The expression of c-Myc and p21 was quantified by Image J and was presented as the relative expression level standardized to actin.
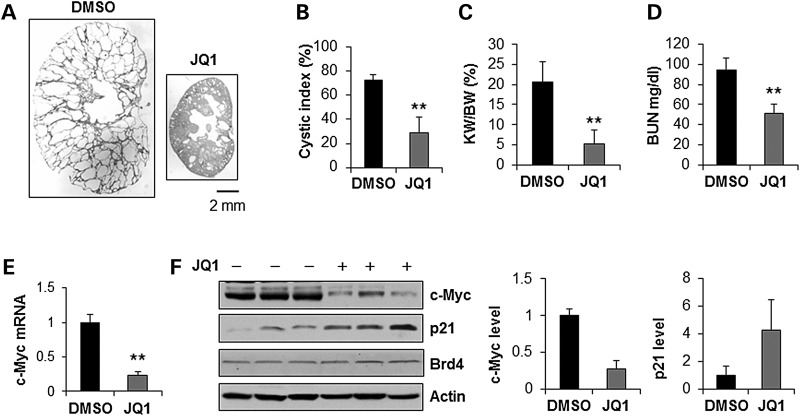
Next, we examined whether JQ1 could delay cyst growth in a milder hypomorphic Pkd1nl/nl mouse model that closely resembles human ADPKD (36). We treated Pkd1+/+ and Pkd1nllnl pups with daily intraperitoneal injection of JQ1 (5 mg/kg) or DMSO (control) from postnatal day 5 (PN5) to postnatal day 27 (PN27). The kidneys were harvested and analyzed at postnatal day 28 (PN28). JQ1 treatment did not induce animal death and did not affect the kidney weight and body weight in the Pkd1+/+ mice (Supplementary Material, Fig. S8A). We found that administration of JQ1 delayed renal cyst growth (Fig. 7A) characterized by significantly decreasing cyst index (Fig. 7B), the KW/BW ratios (Fig. 7C) and BUN levels (Fig. 7D) as well as by inhibiting cystic epithelial cell proliferation as analyzed with PCNA staining (Supplementary Material, Fig. S8B) in kidneys from Pkd1nl/nl mice compared to that in the aged matched kidneys from DMSO-injected Pkd1nl/nl mice. JQ1 treatment decreased the expression of c-Myc mRNA (Fig. 7E) and protein (Fig. 7F) but increased the expression of p21 protein (Fig. 7F) whereas had no effect on Brd4 mRNA and protein in the kidneys of Pkd1nllnl mice (Supplementary Material, Fig. S8C and Fig. 7F).
Figure 7.
Treatment with JQ1 delayed cyst growth in Pkd1nl/nl mice. (A) Gross and histologic examination of PN28 kidneys from Pkd1nl/nl neonates treated with vehicle (DMSO) (n = 8) or JQ1 (n = 7), respectively. Scale bar, 2 mm. (B) Quantification of the percentage of cystic areas over total kidney section areas of PN28 kidney sections from Pkd1nl/nl neonates treated as in (A). Shown is mean ± SEM of all sections quantified for each condition. **P < 0.01. (C and D) KW/BW ratios (C) and BUN levels (D) were decreased in PN28 Pkd1nl/nl mice treated with JQ1 compared with that treated with DMSO (control). **P < 0.01. (E) qRT-PCR analysis mRNA level of c-Myc in kidneys from Pkd1nl/nl mice treated with JQ1 or DMSO, respectively. c-Myc mRNA was decreased in kidneys of JQ1 treated Pkd1nl/nl mice versus DMSO treated Pkd1nl/nl mice. n = 4, **P < 0.01. (F) The expression of c-Myc protein was decreased and the expression of p21 was increased in kidneys of JQ1 treated Pkd1nl/nl mice versus DMSO treated Pkd1nl/nl mice. The expression of c-Myc and p21 was quantified by Image J and was presented as the relative expression level standardized to actin.
Discussion
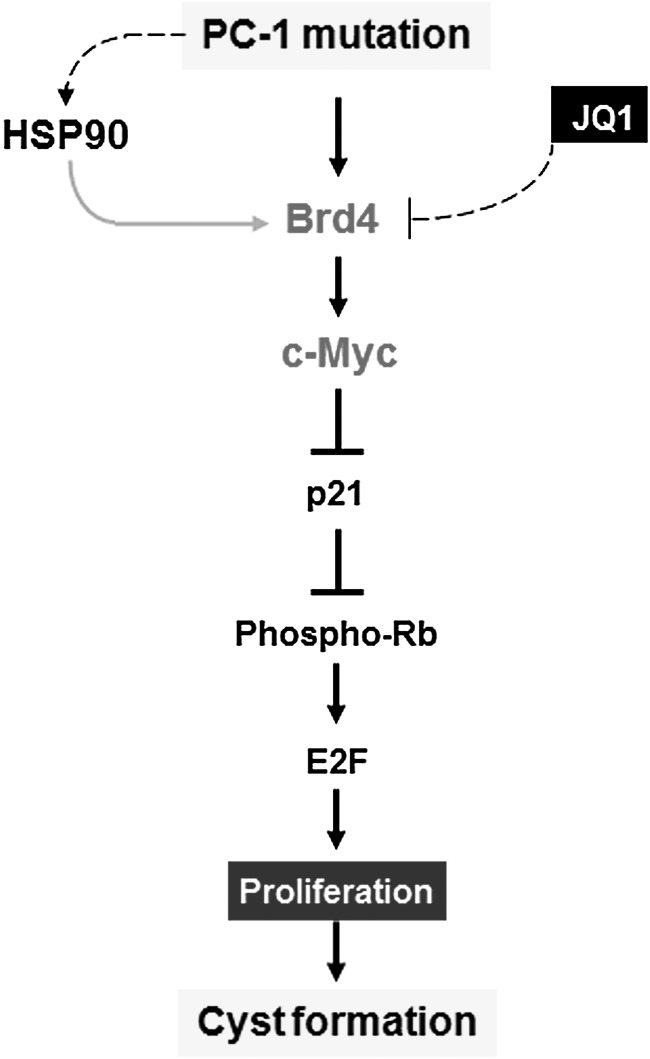
In this study, we demonstrate a novel functional role of BRD protein in ADPKD, which provides a molecular basis for using BRD protein inhibitor to delay cyst growth. We also identify that Brd4 is a novel client protein of Hsp90, which protects Brd4 from degradation and contributes to the upregulation of Brd4 in cystic renal epithelial cells and tissues. The upregulation of Brd4 regulates cystic renal epithelial cell proliferation through affecting the transcription of c-Myc and c-Myc mediated p21-Rb signaling (Fig. 8). Inhibition of Brd4 with the first-in-class small-molecular BRD prototype drug, JQ1, delayed renal cyst growth at early stage of two Pkd1 conditional knockout mouse models. Since targeting Hsp90 has been proved to delay cyst growth in late stage ADPKD animals (29), thus the association between Brd4 and Hsp90 suggests synergistic and therapeutic combinations by targeting Brd4 and Hsp90 in all stages of ADPKD and cancer development.
Figure 8.
A schematic diagram depicting Brd4 mediated signaling pathway in Pkd1 mutant renal epithelial cells. Knockout or mutation of Pkd1 results in the upregulation of Hsp90, Hsp90 mediated upregulation of Brd4 and Brd4 mediated upregulation of c-Myc. Upregulated c-Myc represses the expression of p21 in Pkd1 mutant renal epithelial cells, which increases the phosphorylation of Rb and leads to release E2F1 from Rb-E2F1 complex to increase S-phase entry. Inhibition of Brd4 with JQ1 decreases cystic renal epithelial cell proliferation to delay cyst growth in Pkd1 knockout mouse kidneys.
Brd4 is aberrantly upregulated in some cancer models with unknown mechanism (6,19). We found that Brd4 was also upregulated in Pkd1 mutant renal epithelial cells and tissues (Fig. 1) and formed a complex with Hsp90 (Fig. 2A), which had been reported to be upregulated in Pkd1 mutant renal epithelial cells and tissues as well as in cancer cells (29). Inhibition of Hsp90 decreases the levels of Brd4 in Pkd1 mutant renal epithelial cells (Fig. 2B), suggesting that Brd4 is a novel client protein of Hsp90 and Hsp90 chaperone complex protects Brd4 from degradation. The crosstalk between Brd4 and Hsp90 in the upregulation of Brd4 in cystic renal epithelial cells and tissues may also be a potential mechanism for the upregulation of Brd4 in cancer cells, as Hsp90 is highly expressed and activated in various cancers (37,38). However, the observation that both Brd4 mRNA and protein levels are upregulated in the Pkd1 mutant PN24 cells suggests that in addition to Hsp90 mediated stabilization of Brd4, other factor(s) regulating Brd4 transcription are implicated.
Similar to overexpression of c-Myc in different cancers, c-Myc mRNA was upregulated in kidneys from variety of PKD animal models, including C57BL/6J (cpk/cpk) mice (12), BALB/c-cpk/cpk mice (39), CD-1 pcy/pcy mice (14), Han:SPRD-cy/cy rats (13) and in human ADPKD kidneys (15,16). c-Myc transgenic mice developed polycystic kidney disease, which resulted from overexpression of c-Myc in the renal tubular epithelium and subsequent abnormal cell proliferation (17). However, the mechanism(s) controlling c-Myc upregulation in polycystic kidney disease was unclear. In this study, we confirm that c-Myc is upregulated in kidneys of two Pkd1 conditional knockout mouse models. Significantly, we identify that the only upregulated BET BRD protein, Brd4, is one of the upstream regulators of c-Myc in cystic renal epithelial cells (Fig. 3). These results provide a likely mechanism of how c-Myc is upregulated in PKD.
It has been reported that c-Myc suppresses the transcription of p21 either through directly binding to the promoter of p21 via the DNA-binding protein Miz-1 or through indirectly sequestrating the transcription factors Sp1/Sp3 away from the p21 promoter (32,40). We demonstrate that c-Myc binds to the promoter of p21 and regulates the transcription of p21 in cystic renal epithelial cells (Fig. 5). p21 functions as a regulator of cell cycle progression at G1 phase through binding and inhibiting the cyclin-CDK complexes, which can phosphorylate and inactivate Rb (41,42). It is reported that the hypo-phosphorylated Rb (active) associates with E2F1 to form Rb-E2F1 complex, which represses the cell cycle (43), whereas the hyper-phosphorylation of Rb (inactive) releases E2F1 from Rb-E2F1 complex, which increases E2F1 mediated S-phase entry (44). Our results that knockdown of Brd4 with siRNA or inhibition of Brd4 with JQ1 not only increased the expression of p21 but also decreased the phosphorylation of Rb in cystic renal epithelial cells (Supplemental Material, Fig 4) suggested that Brd4 might regulate cystic renal epithelial cell proliferation through c-Myc mediated p21 and Rb signaling pathways (Fig. 8). However, JQ1 can also regulate other genes than c-Myc through targeting BET proteins (28) and thereby, modulates additional mechanism(s).
The findings that JQ1 shows anti-tumor activity in several cancer types through repressing c-Myc transcription (10,23) by competitively occupying the acetyl-lysine recognition motifs of BET family proteins on the promoter of c-Myc (22) provide the rationale to test whether administration of JQ1 delays renal cyst growth. Our results strongly support that JQ1 is able to delay early stage renal cyst growth and preserve renal function in Pkd1 conditional knockout mouse models (Figs 6 and 7), which suggests that BET BRD protein, Brd4, is a novel epigenetic regulator of ADPKD and JQ1 may be a novel therapeutic strategy for ADPKD treatment.
Since Brd4 regulates gene transcription through binding to acetylated histones on gene promoters, a concern may be raised on how JQ1 treatment that removes Brd4 results in similar beneficiary effects in PKD as the pan-HDAC inhibitor, TSA. While TSA may increase binding of Brd4, it could as well modulate binding of several other proteins to chromatin with various responses. Of interest, co-treatment with JQ1 and a pan-HDAC inhibitor, panobinostat, further decreased the expression of c-Myc and increased the expression of p21 in human AML cells, supporting an additive effect (45). In a recent study, BET and HDAC inhibitors suppress the expression of different genes in Myc-induced murine lymphoma, which suggested that suppression of gene transcription occurred through different mechanisms (26). Based on these reports, we assume that JQ1 and TSA suppress transcription of genes, including c-Myc and other unique target genes, through different mechanisms in cystic renal epithelial cells and result in a similar beneficiary effect in PKD.
The findings that inhibition of Brd4 with JQ1 not only decreased cell proliferation but also induced apoptosis of different cancer cells (23,27) imply that JQ1 treatment would delay cyst growth through inhibition of cystic renal epithelial cell proliferation and inducing cyst-lining epithelial cell death in ADPKD as we have reported recently (4,46). However, our results that JQ1 treatment only decreased cystic renal epithelial cell proliferation but did not induce cyst lining epithelial cell apoptosis in kidneys of Pkd1 conditional knockout mouse models (data not shown) suggested a novel mechanism for Brd4 signaling in Pkd1 disease progression.
In summary, we present for the first time that a BET BRD protein, Brd4, is a novel epigenetic regulator of ADPKD, and inhibition of Brd4 with JQ1, a first-in-class BET BRD inhibitor, delays cyst growth in two Pkd1 conditional knockout mouse models. We further identify that Brd4 is a novel client protein of Hsp90 and the Hsp90 chaperone activity protects Brd4 from proteasomal degradation in cystic renal epithelial cells, which may also be a general mechanism for the upregulation of Brd4 in cancer cells. Since targeting Hsp90 was shown to delay cyst growth in late stage ADPKD animals (29), the combined treatment of JQ1 and Hsp90 inhibitor(s) could be a promising therapeutic strategy for ADPKD.
Materials and Methods
Cell culture and reagents
HEK293T cells were maintained at 37°C in 5% CO2 in DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS). Pkd1 wild-type and Pkd1 null MEK cells, which were generated from Dr Jing Zhou's laboratory at Harvard and were used in our recent publications, were maintained as previously described (47). Pkd1 heterozygous PH2 cells and homozygous PN24 cells, kindly provided by Dr Stefan Somlo through the George M O’Brien Kidney Center at Yale University, were cultured as described (48,49). STA9090 was purchased from Selleckchem.
Immunoprecipitation and western blot
We performed immunoprecipitation and western blotting on whole-cell lysates as previous described (4). The antibodies used for immunoprecipitation and western analysis included: anti-c-Myc (Millipore and Santa Cruz), anti-Brd4 (Abcam, Bethyl Laboratory and Santa Cruz), anti-Hsp90 (BD Biosciences), anti-HSF1 (Cell Signaling Technologies), anti-phospho-Rb (Cell Signaling Technologies), anti-Rb (Santa Cruz), anti-p21 (Santa Cruz), anti-actin and anti-α-tubulin antibodies (Sigma). Donkey-anti-rabbit IgG-horseradish peroxidase and Donkey-anti-mouse IgG-horseradish peroxidase (Santa Cruz) were used as secondary antibodies. The band density was quantified by software ImageJ (NIH).
Flow cytometry
PH2 and PN24 cells were seeded in the 60 mm dish in growth medium with 10% FBS for 16 h and then were cultured in the serum free medium for 24 h. After serum starvation, the cells were released into cell cycle by culturing in the growth medium with 10% FBS plus JQ1 (1 µM) or vehicle (DMSO) for 24 h. For cell cycle analysis, cells were washed with phosphate buffered saline, fixed with 70% ethanol overnight. RNA was degraded with RNase A (Invitrogen), and DNA was stained with propidium iodide (Sigma). All samples were analyzed on a BD FACSCanto II.
Pkd1 knockdown by lentivirus carrying Brd4 shRNA
The packaging of Lentivirus with Brd4 shRNA was performed as described previously (50). Briefly, HEK293T cells were transfected with either lentiviral plasmid pGIPZ-shBrd4 (Open Biosystems) carrying Brd4 shRNA or control empty vector pGIPZ-NS, plus psPAX2 packaging plasmid and pMD2.G envelope plasmid using calcium phosphate. The lentiviral particles were harvested from HEK293T cells after 48 h. PN24 cells were infected with appropriate amounts of lentiviral particles together with 5 µg/ml polybrene (Sigma) for 24 h. Virus-containing medium was removed and replaced with fresh medium plus 5 µg/ml puromycin. After 48 h puromycin selection, all the remaining cells were GFP positive as detected by the microscope. The cells were harvested after lentiviral particles infection for 5 days and analyzed by RT-PCR and western blot.
Quantitative reverse-transcription polymerase chain reaction
Total RNA was extracted using the RNeasy plus mini kit (Qiagen). One microgram of total RNA was used for reverse-transcription reactions in a 20 µl reaction to synthesize cDNA using Iscript cDNA Synthesis Kit (BioRad). RNA expression profiles were analyzed by real-time PCR using iTaq SYBER Green Supermix (BioRad) in a CFX Connect System. Genes were amplified using the following primers. c-Myc-F: 5′-GCTGTTTGAAGGCTGGATTTC-3′; c-Myc-R, 5′-GATGAAATAGGGCTGTACGGAG-3′; Brd4-F: 5′-AAAACTCCAACCCCGATGAG-3′; Brd4-R: 5′-GAACCAGCAATCACGTCAAC-3′; Brd3-F: 5′-AGATGGATAGCCGAGAGTACC-3′; Brd3-R: 5′-GCAAACCTCATCTCAAACACATC-3′; Brd2-F: 5′-GGTTCCCTGAGGTCAAGATG-3′; Brd2-R: 5′-GGCTCTCAAATCCCTCATAGAG-3′; p21-F: 5′-CAGATCCACAGCGATATCCAG-3′; p21-R: 5′-AGAGACAACGGCACACTTTG-3′; Actin-F: 5′-AAGAGCTATGAGCTGCCTGA-3′; Actin-R: 5′-TACGGATGTCAACGTCACAC-3′. The complete reactions were subjected to the following program of thermal cycling: 40 cycles of 10 s at 95°C and 20 s at 61°C, a melting curve was run after the PCR cycles, followed by a cooling step. Each sample was run in triplicate in each experiment, and each experiment was repeated three times. The expression level of c-Myc, Brd4, Brd3, Brd2 and p21 was normalized to the expression level of actin.
RNA interference
The RNA oligonucleotides that specifically targeted mouse c-Myc and mouse HSF-1 were purchased from Thermo Dharmacon. The RNA oligonucleotides were transfected with the DharmaFECT siRNA transfection reagent (Dharmacon). Forty-eight hours after transfection, cells were harvested and analyzed by western blotting.
CHIP assay
CHIP assay was performed according to the manufacturer's protocol (EZ CHIPTM Chromatin Immunoprecipitation Kit, Upstate Biotechnology). Chromatin DNA was immunoprecipitated with anti-c-Myc antibody (SC-764, Santa Cruz) or normal rabbit IgG, washed, and then the DNA-protein cross-links were reversed. The recovered DNA was analyzed by PCR for the core p21 promoter region between −296 and −51 bp upstream of the p21 ATG start codon. The primers for PCR were 5′-CGCTGCGTGACAAGAGAATA-3′ and 5′-TGTCTGGATATC GCTGTGGA-3′. A pair of primers in the p21 coding region (5′-AATTTTCACCACCCCTCCTC-3′ and 5′-GACAGAGGCAGGTGGATCTC-3′) was used as negative control (NC). Chromatin DNA was also immunoprecipitated with anti-Brd4 antibody (Abcam) and anti-Histone 3 antibody, respectively. The recovered DNA was analyzed by PCR for the c-Myc promoter region between −613 and −374 bp upstream of the c-Myc ATG start codon. The primers for PCR were 5′-CCTTTATATTCCGGGGGTCT-3′ and 5′-CGCTCACTCCCTCTGTCTCT-3′. A pair of primers in the c-Myc coding region (5′-CCACTAGGGCACATCCATTC-3′ and 5′-TAGATGAGGGCGAGGACAAC-3′) was used as NC.
Immunohistochemistry
Kidneys were fixed with 4% paraformaldehyde (pH 7.4). For PCNA staining, a monoclonal mouse anti-PCNA antibody (Cell Signaling Technologies, 1:1000 dilution), a biotinylated secondary antibody (Sigma, 1:100 dilution), and DAB (3, 3′-diaminabenzidine tetrahydrochloride) substrate system were used. The rabbit anti-Brd4 antibody (Abcam, 1:100 dilution) was used for the Brd4 staining. The kidney sections were counterstained by hematoxylin. Images were analyzed with a NIKON ECLIPSE 80i Microscope.
Cystic index
The cyst formation was quantified from sagittal sections of whole kidneys. Whole-kidney images were acquired and total kidney area, cystic area and noncystic area were measured. Cystic index = (total cystic area/total kidney area) × 100 and is expressed as a percent.
Mouse strain and treatment
All animal protocols were approved and conducted in accordance with Laboratory Animal Resources of University of Kansas Medical Center and Institutional Animal Care and Use Committee regulations. Pkd1flox/flox:Pkhd1-Cre mice were used to test the effect of JQ1 on cyst progression at postnatal day 25 (PN25). Pkhd1-Cre transgenic mice were kindly provided by Dr Peter Igarashi from UT Southwestern Medical Center (51). Pkd1flox/flox:Pkhd1-Cre mice were generated by cross-breeding Pkd1flox/+:Pkhd1-Cre female mice with Pkd1flox/+:Pkhd1-Cre male mice. Pkd1+/+:Pkhd1-Cre and Pkd1flox/flox:Pkhd1-Cre neonate was intraperitoneally injected daily with 5 mg/kg JQ1 or DMSO from postnatal day 8 (PN8) to postnatal day 24 (PN24). All the kidneys were harvested and analyzed at postnatal day 25.
Hypomorphic Pkd1nl/nl mice, generated by cross-breeding Pkd1nl/+ females with Pkd1nl/+ male, were used to test the effect of JQ1 on cyst growth at PN28. Each neonate was injected i.p. daily with 5 mg/kg JQ1 or DMSO from PN5 to PN27, and the kidneys were harvested at PN28 for further analysis.
The time points of administration of JQ1 to animals were based on the studies using different drugs to treat Pkd1flox/flox:Pkhd1-Cre mice (35) and prior observations of cyst development in the Pkd1nl/nl mice (36).
Statistics
Data are presented as mean ± SEM. An unpaired two-tailed student's t test was used to determine the significant differences. A P value < 0.05 is considered significant.
Supplementary Material
Funding
X.L. is supported by an internal grant from the KUMC Kidney Institute and NIH grant R01DK084097. M.T. is supported by grants from the CIHR and the PKD Foundation.
Supplementary Material
Acknowledgements
We are grateful for cell lines PH2 and PN24 provided by Dr S. Somlo through the George M O'Brien Kidney Center at Yale University. We are also grateful for Pkhd1-Cre mice provided by Dr Peter Igarashi through the George M. O'Brien Kidney Research Core Center at UT Southwestern Medical Center.
Conflict of Interest statement. None declared.
References
- 1.Torres V.E., Harris P.C. (2009) Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int., 76, 149–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris P.C., Torres V.E. (2009) Polycystic kidney disease. Annu. Rev. Med., 60, 321–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li X. (2011) Epigenetics and autosomal dominant polycystic kidney disease. Biochim. Biophys. Acta, 1812, 1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou X., Fan L.X., Sweeney W.E., Jr, Denu J.M., Avner E.D., Li X. (2013) Sirtuin 1 inhibition delays cyst formation in autosomal-dominant polycystic kidney disease. J. Clin. Invest., 123, 3084–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fan L.X., Li X., Magenheimer B., Calvet J.P., Li X. (2012) Inhibition of histone deacetylases targets the transcription regulator Id2 to attenuate cystic epithelial cell proliferation. Kidney Int., 81, 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belkina A.C., Denis G.V. (2012) BET domain co-regulators in obesity, inflammation and cancer. Nat. Rev. Cancer, 12, 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey A., Nishiyama A., Karpova T., McNally J., Ozato K. (2009) Brd4 marks select genes on mitotic chromatin and directs postmitotic transcription. Mol. Biol. Cell, 20, 4899–4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LeRoy G., Rickards B., Flint S.J. (2008) The double bromodomain proteins Brd2 and Brd3 couple histone acetylation to transcription. Mol. Cell, 30, 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicodeme E., Jeffrey K.L., Schaefer U., Beinke S., Dewell S., Chung C.W., Chandwani R., Marazzi I., Wilson P., Coste H., et al. (2010) Suppression of inflammation by a synthetic histone mimic. Nature, 468, 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delmore J.E., Issa G.C., Lemieux M.E., Rahl P.B., Shi J., Jacobs H.M., Kastritis E., Gilpatrick T., Paranal R.M., Qi J., et al. (2011) BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell, 146, 904–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu S.Y., Chiang C.M. (2007) The double bromodomain-containing chromatin adaptor Brd4 and transcriptional regulation. J. Biol. Chem., 282, 13141–13145. [DOI] [PubMed] [Google Scholar]
- 12.Cowley B.D., Jr, Smardo F.L., Jr, Grantham J.J., Calvet J.P. (1987) Elevated c-myc protooncogene expression in autosomal recessive polycystic kidney disease. Proc. Natl. Acad. Sci.U. S. A., 84, 8394–8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowley B.D., Jr, Gudapaty S., Kraybill A.L., Barash B.D., Harding M.A., Calvet J.P., Gattone V.H., 2nd (1993) Autosomal-dominant polycystic kidney disease in the rat. Kidney Int., 43, 522–534. [DOI] [PubMed] [Google Scholar]
- 14.Gattone V.H., 2nd, Kuenstler K.A., Lindemann G.W., Lu X., Cowley B.D., Jr, Rankin C.A., Calvet J.P. (1996) Renal expression of a transforming growth factor-alpha transgene accelerates the progression of inherited, slowly progressive polycystic kidney disease in the mouse. J. Lab. Clin. Med., 127, 214–222. [DOI] [PubMed] [Google Scholar]
- 15.Lanoix J., D'Agati V., Szabolcs M., Trudel M. (1996) Dysregulation of cellular proliferation and apoptosis mediates human autosomal dominant polycystic kidney disease (ADPKD). Oncogene, 13, 1153–1160. [PubMed] [Google Scholar]
- 16.Song X., Di Giovanni V., He N., Wang K., Ingram A., Rosenblum N.D., Pei Y. (2009) Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet., 18, 2328–2343. [DOI] [PubMed] [Google Scholar]
- 17.Trudel M., D'Agati V., Costantini F. (1991) C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int., 39, 665–671. [DOI] [PubMed] [Google Scholar]
- 18.Ricker J.L., Mata J.E., Iversen P.L., Gattone V.H. (2002) c-myc antisense oligonucleotide treatment ameliorates murine ARPKD. Kidney Int., 61, S125–S131. [DOI] [PubMed] [Google Scholar]
- 19.Chen R., Yik J.H., Lew Q.J., Chao S.H. (2014) Brd4 and HEXIM1: multiple roles in P-TEFb regulation and cancer. BioMed Res. Int., 2014, 232870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang Y., Gholamin S., Schubert S., Willardson M.I., Lee A., Bandopadhayay P., Bergthold G., Masoud S., Nguyen B., Vue N., et al. (2014) Epigenetic targeting of Hedgehog pathway transcriptional output through BET bromodomain inhibition. Nat. Med., 20, 732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J., Wang Y., Zeng L., Wu Y., Deng J., Zhang Q., Lin Y., Li J., Kang T., Tao M., et al. (2014) Disrupting the interaction of BRD4 with diacetylated Twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell, 25, 210–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Filippakopoulos P., Qi J., Picaud S., Shen Y., Smith W.B., Fedorov O., Morse E.M., Keates T., Hickman T.T., Felletar I., et al. (2010) Selective inhibition of BET bromodomains. Nature, 468, 1067–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mertz J.A., Conery A.R., Bryant B.M., Sandy P., Balasubramanian S., Mele D.A., Bergeron L., Sims R.J., 3rd (2011) Targeting MYC dependence in cancer by inhibiting BET bromodomains. Proc. Natl. Acad. Sci. U. S. A., 108, 16669–16674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamoureux F., Baud'huin M., Rodriguez Calleja L., Jacques C., Berreur M., Redini F., Lecanda F., Bradner J.E., Heymann D., Ory B. (2014) Selective inhibition of BET bromodomain epigenetic signalling interferes with the bone-associated tumour vicious cycle. Nat. Commun., 5, 3511. [DOI] [PubMed] [Google Scholar]
- 25.Knoechel B., Roderick J.E., Williamson K.E., Zhu J., Lohr J.G., Cotton M.J., Gillespie S.M., Fernandez D., Ku M., Wang H., et al. (2014) An epigenetic mechanism of resistance to targeted therapy in T cell acute lymphoblastic leukemia. Nat. Genet., 46, 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhadury J., Nilsson L.M., Muralidharan S.V., Green L.C., Li Z., Gesner E.M., Hansen H.C., Keller U.B., McLure K.G., Nilsson J.A. (2014) BET and HDAC inhibitors induce similar genes and biological effects and synergize to kill in Myc-induced murine lymphoma. Proc. Natl. Acad. Sci. U. S. A., 111, E2721–E2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tolani B., Gopalakrishnan R., Punj V., Matta H., Chaudhary P.M. (2013) Targeting Myc in KSHV-associated primary effusion lymphoma with BET bromodomain inhibitors. Oncogene, 33, 2928–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott C.J., Kopp N., Bird L., Paranal R.M., Qi J., Bowman T., Rodig S.J., Kung A.L., Bradner J.E., Weinstock D.M. (2012) BET bromodomain inhibition targets both c-Myc and IL7R in high-risk acute lymphoblastic leukemia. Blood, 120, 2843–2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seeger-Nukpezah T., Proia D.A., Egleston B.L., Nikonova A.S., Kent T., Cai K.Q., Hensley H.H., Ying W., Chimmanamada D., Serebriiskii I.G., et al. (2013) Inhibiting the HSP90 chaperone slows cyst growth in a mouse model of autosomal dominant polycystic kidney disease. Proc. Natl. Acad. Sci. U. S. A., 110, 12786–12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y., Trepel J.B., Neckers L.M., Giaccone G. (2010) STA-9090, a small-molecule Hsp90 inhibitor for the potential treatment of cancer. Curr. Opin. Investig. Drugs, 11, 1466–1476. [PubMed] [Google Scholar]
- 31.Xiao X., Zuo X., Davis A.A., McMillan D.R., Curry B.B., Richardson J.A., Benjamin I.J. (1999) HSF1 is required for extra-embryonic development, postnatal growth and protection during inflammatory responses in mice. EMBO J., 18, 5943–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herold S., Wanzel M., Beuger V., Frohme C., Beul D., Hillukkala T., Syvaoja J., Saluz H.P., Haenel F., Eilers M. (2002) Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol. Cell, 10, 509–521. [DOI] [PubMed] [Google Scholar]
- 33.Seoane J., Le H.V., Massague J. (2002) Myc suppression of the p21(Cip1) Cdk inhibitor influences the outcome of the p53 response to DNA damage. Nature, 419, 729–734. [DOI] [PubMed] [Google Scholar]
- 34.Li X., Luo Y., Starremans P.G., McNamara C.A., Pei Y., Zhou J. (2005) Polycystin-1 and polycystin-2 regulate the cell cycle through the helix-loop-helix inhibitor Id2. Nat. Cell Biol., 7, 1202–1212. [DOI] [PubMed] [Google Scholar]
- 35.Karihaloo A., Koraishy F., Huen S.C., Lee Y., Merrick D., Caplan M.J., Somlo S., Cantley L.G. (2011) Macrophages promote cyst growth in polycystic kidney disease. J. Am. Soc. Nephrol., 22, 1809–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lantinga-van Leeuwen I.S., Dauwerse J.G., Baelde H.J., Leonhard W.N., van de Wal A., Ward C.J., Verbeek S., Deruiter M.C., Breuning M.H., de Heer E., et al. (2004) Lowering of Pkd1 expression is sufficient to cause polycystic kidney disease. Hum Mol. Genet., 13, 3069–3077. [DOI] [PubMed] [Google Scholar]
- 37.Jensen M.R., Schoepfer J., Radimerski T., Massey A., Guy C.T., Brueggen J., Quadt C., Buckler A., Cozens R., Drysdale M.J., et al. (2008) NVP-AUY922: a small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res., 10, R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheung C.H., Chen H.H., Cheng L.T., Lyu K.W., Kanwar J.R., Chang J.Y. (2010) Targeting Hsp90 with small molecule inhibitors induces the over-expression of the anti-apoptotic molecule, survivin, in human A549, HONE-1 and HT-29 cancer cells. Mol. Cancer, 9, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricker J.L., Gattone V.H., 2nd, Calvet J.P., Rankin C.A. (2000) Development of autosomal recessive polycystic kidney disease in BALB/c-cpk/cpk mice. J. Am. Soc. Nephrol., 11, 1837–1847. [DOI] [PubMed] [Google Scholar]
- 40.Gartel A.L., Ye X., Goufman E., Shianov P., Hay N., Najmabadi F., Tyner A.L. (2001) Myc represses the p21(WAF1/CIP1) promoter and interacts with Sp1/Sp3. Proc. Natl. Acad. Sci. U. S. A., 98, 4510–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H.S., Gavin M., Dahiya A., Postigo A.A., Ma D., Luo R.X., Harbour J.W., Dean D.C. (2000) Exit from G1 and S phase of the cell cycle is regulated by repressor complexes containing HDAC-Rb-hSWI/SNF and Rb-hSWI/SNF. Cell, 101, 79–89. [DOI] [PubMed] [Google Scholar]
- 42.Harper J.W., Adami G.R., Wei N., Keyomarsi K., Elledge S.J. (1993) The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell, 75, 805–816. [DOI] [PubMed] [Google Scholar]
- 43.Dimova D.K., Dyson N.J. (2005) The E2F transcriptional network: old acquaintances with new faces. Oncogene, 24, 2810–2826. [DOI] [PubMed] [Google Scholar]
- 44.Dyson N. (1998) The regulation of E2F by pRB-family proteins. Genes Dev., 12, 2245–2262. [DOI] [PubMed] [Google Scholar]
- 45.Fiskus W., Sharma S., Qi J., Valenta J.A., Schaub L.J., Shah B., Peth K., Portier B.P., Rodriguez M., Devaraj S.G., et al. (2014) Highly active combination of BRD4 antagonist and histone deacetylase inhibitor against human acute myelogenous leukemia cells. Mol. Cancer Ther., 13, 1142–1154. [DOI] [PubMed] [Google Scholar]
- 46.Fan L.X., Zhou X., Sweeney W.E., Jr, Wallace D.P., Avner E.D., Grantham J.J., Li X. (2013) Smac-mimetic-induced epithelial cell death reduces the growth of renal cysts. J. Am. Soc. Nephrol., 24, 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nauli S.M., Alenghat F.J., Luo Y., Williams E., Vassilev P., Li X., Elia A.E., Lu W., Brown E.M., Quinn S.J., et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet., 33, 129–137. [DOI] [PubMed] [Google Scholar]
- 48.Shibazaki S., Yu Z., Nishio S., Tian X., Thomson R.B., Mitobe M., Louvi A., Velazquez H., Ishibe S., Cantley L.G., et al. (2008) Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet., 17, 1505–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wei F., Karihaloo A., Yu Z., Marlier A., Seth P., Shibazaki S., Wang T., Sukhatme V.P., Somlo S., Cantley L.G. (2008) Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int., 74, 1310–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou X., Fan L.X., Li K., Ramchandran R., Calvet J.P., Li X. (2014) SIRT2 regulates ciliogenesis and contributes to abnormal centrosome amplification caused by loss of polycystin-1. Hum. Mol. Genet., 23, 1644–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel V., Li L., Cobo-Stark P., Shao X., Somlo S., Lin F., Igarashi P. (2008) Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet., 17, 1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.