Abstract
Background
Osteosarcoma is a rare bone tumor that is the most frequently diagnosed among children and adolescents, although this cancer affects people of all ages. This study aims to augment the current literature by examining the incidence of osteosarcoma by its subsites on a national level.
Methods
Data from central cancer registries in the National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) programs for diagnosis years 1999-2008 and covering 90.1% of the US population were analyzed. Analyses included cases of malignant primary osteosarcomas, which were further segmented by topography, appendicular (C40) and axial (C41), to assess differences between these sites. Descriptive statistics, including estimated age-adjusted incidence rates standardized to the 2000 US standard population, were calculated using SEER*Stat 7.0.5 software.
Results
Approximately 7,104 cases of malignant primary osteosarcomas were identified during 1999-2008, of which 5,379 were appendicular and 1,725 were axial The incidence of malignant primary osteosarcomas differed by age, gender, race, ethnicity, region, grade, and stage. These differences in incidence persisted when malignant primary osteosarcomas were categorized by topography codes.
Conclusions
These analyses provide a better understanding of the incidence of malignant osteosarcoma which cover 90.1 % of the US population from 1999-2008. This study provides a more detailed understanding of age, gender, race, and ethnicity by primary site for malignant osteosarcoma incidence on a national level in the United States. More importantly, differences between appendicular and axial sites were observed overall by selected demographic characteristics, in particular regional variations.
Keywords: osteosarcoma, epidemiology, malignant, incidence, primary site
Introduction
Osteosarcoma is a rare bone tumor that is most frequently diagnosed among children and adolescents, although this cancer affects people of all ages.1-5 While the incidence of primary osteosarcoma peaks during adolescence, a second incidence peak occurs among individuals over age 60, most commonly in the seventh or eighth decades of life,3,5,6 Among 15-29 year olds, most osteosarcomas occurred in the metaphyses of long bones and particularly the distal femur, proximal humerus, and the proximal tibia.1 Among older adults, osteosarcomas are more likely to occur in the axial skeleton locations and in areas that have been previously irradiated or have underlying bone abnormalities.7 Males are affected more frequently than females except during the first decade of life.6
Currently, there is a paucity of information on osteosarcoma in the US population.3,5 In order to understand the burden of osteosarcoma, this study will analyze the incidence of osteosarcoma by topography (primary site) and demographic characteristics, including age groups, race, ethnicity, and gender.
Materials and Methods
Microscopically confirmed incident cases of invasive primary osteosarcoma were identified from population-based cancer registries that participated in the Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries (NPCR) or the National Cancer Institutes’ (NCI) Surveillance, Epidemiology, and End Results (SEER) Program. NPCR supports central cancer registries (CCRs) in 45 states, the District of Columbia, Puerto Rico, and US Pacific Island jurisdictions, which cover approximately 96% of the US population.8 Together, NPCR and SEER collect cancer incidence data for the entire US population.8,9 Cancer incidence data for this study included those from the NPCR-Cancer Surveillance System (NPCR-CSS) November 2010 submission and the November 2010 SEER submission. The analyses focused on microscopically confirmed incident malignant primary osteosarcoma cases diagnosed between 1999-2008 from CCRs in 44 states that met case ascertainment and quality criteria for all 10 years.9 These data cover approximately 90.1% of the US population during these 10 years. Data from 6 states and the District of Columbia (7 CCRs) were excluded because case ascertainment and quality criteria standards were not met for the entire time period.
Tumors were included in the study if they were the only primary tumor or the first of 2 or more independent primary tumors for the patient. All malignant primary osteosarcomas were classified according to the International Classification of Diseases for Oncology (ICD-O-3).10 Since the International Classification of Childhood Cancers (ICCC) is similar to ICD-O-3 for osteosarcomas, it was decided to use the classification schemes from ICD-O-3 only. We included all malignant primary osteosarcomas (primary site code C40.0-40.9 and C41.0-41.9 and histology codes 9180/3-9187/3, 9192/3-9195/3, 9200/3) in these analyses. Table 1 shows the topography codes (primary site) used to define appendicular and axial sites for malignant primary osteosarcomas.11 Osteosarcoma histologic groupings were coded according to the version of the International Classification of Diseases for Oncology (ICD-O) used at the time of diagnosis.10, 12 The osteosarcoma cases diagnosed from 1999 to 2000 used the ICD-O second edition (ICD-O-2), and those from 2001 to 2008 used the ICD-O third edition (ICD-O-3); data from the 1999-2000 diagnosis years were converted to the ICD-O-3 codes. Table 2 shows the histologic codes used to define malignant primary osteosarcomas in these analyses.
Table 1. Topography Codes* Used to Define Malignant Primary Osteosarcomas† by Appendicular and Axial Sites, United States, 1999-2008.
|
Topography Definitions for Malignant
Primary Osteosarcomas |
Topography
Codes |
|---|---|
| Appendicular | C40 |
| Long bones of upper limb, scapula, and associated joints |
C40.0 |
| Short bones of upper limb and associated joints | C40.1 |
| Long bones of lower limb and associated joints | C40.2 |
| Short bones of lower limb and associated joints | C40.3 |
| Overlapping lesion of bones, joints, and articular cartilage of limbs |
C40.8 |
| Bone of limb, NOS‡ | C40.9 |
| Axial | C41 |
| Bones of skull and face and associated joints | C41.0 |
| Mandible | C41.1 |
| Vertebral column | C41.2 |
| Rib, sternum, clavicle, and associated joints | C41.3 |
| Pelvic bones, sacrum, coccyx, and associated joints |
C41.4 |
| Overlapping lesion of bones, joints, and articular cartilage |
C41.8 |
| Bone, NOS | C41.9 |
Topography codes from the American Joint Committee on Cancer (AJCC) Cancer Staging Handbook, 6th edition.
The analyses were limited to microscopically confirmed osteosarcomas.
NOS, not otherwise specified.
Table 2. Histology Categories and ICD-O-3,* Codes Used to Define Malignant Primary Osteosarcomas† United States, 1999-2008.
|
Histology Definitions for Primary
Malignant Osteosarcomas |
ICD-O-3 Codes |
|---|---|
| Osteosarcoma, NOS‡ | 9180/3 |
| Chondroblastic osteosarcoma | 9181/3 |
| Fibroblastic osteosarcoma | 9182/3 |
| Telangiectatic osteosarcoma | 9183/3 |
| Osteosarcoma in Paget disease | 9184/3 |
| Small cell osteosarcoma | 9185/3 |
| Central osteosarcoma | 9186/3 |
| Intraosseous well differentiated osteosarcoma | 9187/3 |
| Paraosteal osteosarcoma | 9192/3 |
| Periosteal osteosarcoma | 9193/3 |
| High grade surface osteosarcoma | 9194/3 |
| Intracortical osteosarcoma | 9195/3 |
| Malignant osteosarcoma | 9200/3 |
ICD-O-3 indicates International Classification of Diseases for Oncology-3rd Edition
The analyses were limited to microscopically confirmed osteosarcomas.
NOS, not otherwise specified.
Cancer stage was assigned SEER summary stage 1977 (SS77) for cases diagnosed before 2001, SEER summary stage 2000 (SS2000) for cases diagnosed during 2001-2003, and derived summary stage using the collaborative stage algorithm for cases diagnosed in 2004 or later.13 These data were then aggregated to obtain an overall frequency count for stage. Stage was categorized as in situ, localized, regional, distant, or unstaged. Grade was categorized as I (well differentiated), II (moderately differentiated), III (poorly differentiated), IV (undifferentiated/anaplastic), or unknown.
Race was categorized as white, black, American Indian/Alaska Native (AI/AN), Asian/Pacific Islander (API), or other/unknown race. To increase the accuracy of the AI/AN designation, linkages between the CCRs and the Indian Health Service (IHS) database were completed before data submission to NPCR and SEER. Ethnicity was categorized as Hispanic or non-Hispanic based on the North American Association of Central Cancer Registries’ (NAACCR) Hispanic/Latino Identification Algorithm (NHIA) which employs a combination of variables to assign Hispanic/Latino classification to cancer cases.14 Cases that are not classified as Hispanic by NHIA are classified as non-Hispanic which leaves no cases with an unknown Hispanic status.15 Nearly all NPCR and SEER registries assign Hispanic ethnicity through the standardized use of the NHIA.15 Race and ethnicity are not mutually exclusive.
Frequencies by demographic and tumor characteristics for all malignant primary osteosarcomas were calculated. Age-adjusted incidence rates with 95% confidence intervals (95% CI) for all malignant primary osteosarcomas were calculated overall, and by gender, race, ethnicity, US Census regions (Northeast, Midwest, South, and West), grade, and stage. Rates were expressed per 1,000,000 persons and age-adjusted using the US 2000 population standard (19 age groups—Census P25-1130). Counts and rates based on fewer than 16 cases were not presented to ensure rate stability and confidentiality. All data analyses for this study were performed using SEER*Stat version 7.0.5.16
Results
Table 3 shows demographic and tumor characteristics for malignant osteosarcomas by topography (primary site) for cases diagnosed during the 1999-2008 period. Of the reported 7,104 malignant osteosarcoma incident cases diagnosed from 1999 to 2008,5,379 (76%) were appendicular and 1,725 (24%) were axial. The age-adjusted incidence rate for appendicular malignant osteosarcomas (2.06 per 1,000,000) was higher than axial (0.65 per 1,000,000).
Table 3. Description of Primary Osteosarcoma Incidence by Selected Characteristics, United States, 1999-2008.
| Appendicular (C40) | Axial (C41) | Total | ||||
|---|---|---|---|---|---|---|
| Count* (%) | Rate† (95% CI)‡ | Count (%) | Rate (95% CI) | Count (%) | Rate (95% CI) | |
| Total | 5, 379 (100.0%) | 2.06 (2.01, 2.12) | 1,725 (100.0%) | 0.65 (0.62, 0.69) | 7,104 (100.0%) | 2.71 (2.65, 2.78) |
| Age at diagnosis, years | ||||||
| 00-09 | 526 (9.8%) | 1.48 (1.36, 1.61) | 40 (2.3%) | 0.11 (0.08, 0.15) | 566 (8.0%) | 1.59 (1.46, 1.73) |
| 10-19 | 2,655 (49.4%) | 7.08 (6.81, 7.35) | 316 (18.3%) | 0.84 (0.75, 0.94) | 2,971 (41.8%) | 7.92 (7.64, 8.21) |
| 20-29 | 780 (14.5%) | 2.16 (2.01, 2.31) | 261 (15.1%) | 0.72 (0.64, 0.82) | 1,041 (14.7%) | 2.88 (2.71, 3.06) |
| 30-39 | 403 (7.5%) | 1.07 (0.97, 1.18) | 198 (11.5%) | 0.53 (0.46, 0.61) | 601 (8.5%) | 1.60 (1.47, 1.73) |
| 40-49 | 342 (6.4%) | 0.86 (0.77, 0.95) | 269 (15.6%) | 0.68 (0.60, 0.76) | 611 (8.6%) | 1.54 (1.42, 1.66) |
| 50-59 | 249 (4.6%) | 0.78 (0.69, 0.89) | 199 (11.5%) | 0.63 (0.54, 0.72) | 448 (6.3%) | 1.41 (1.28, 1.55) |
| 60-69 | 187 (3.5%) | 0.93 (0.80, 1.07) | 173 (10.0%) | 0.87 (0.74, 1.01) | 360 (5.1%) | 1.80 (1.61, 1.99) |
| 70-79 | 142 (2.6%) | 0.97 (0.82, 1.15) | 161 (9.3%) | 1.10 (0.94, 1.29) | 303 (4.3%) | 2.08 (1.85, 2.32) |
| 80+ | 95 (1.8%) | 1.03 (0.83, 1.26) | 108 (6.3%) | 1.17 (0.96, 1.41) | 203 (2.9%) | 2.20 (1.91, 2.53) |
| Gender | ||||||
| Male | 3,057 (56.8%) | 2.32 (2.24, 2.41) | 885 (51.3%) | 0.70 (0.65, 0.74) | 3,942 (55.5%) | 3.02 (2.93, 3.12) |
| Female | 2,322 (43.2%) | 1.79 (1.72, 1.87) | 840 (48.7%) | 0.62 (0.58, 0.66) | 3,162 (44.5%) | 2.41 (2.33, 2.50) |
| Race§ | ||||||
| White | 4,176 (77.6%) | 1.99 (1.93, 2.05) | 1,387 (80.4%) | 0.64 (0.60, 0.67) | 5,563 (78.3%) | 2.63 (2.56, 2.70) |
| Black | 856 (15.9%) | 2.42 (2.26, 2.59) | 252 (14.6%) | 0.81 (0.71, 0.92) | 1,108 (15.6%) | 3.23 (3.03, 3.43) |
| AI/AN | # | 1.34 (0.97, 1.83) | # | # | 54 (0.8%) | 1.87 (1.36, 2.51) |
| API | 220 (4.1%) | 1.75 (1.52, 2.00) | 53 (3.1%) | 0.45 (0.33, 0.59) | 273 (3.8%) | 2.19 (1.94, 2.48) |
| Ethnicity∥ | ||||||
| Hispanic | 1,037 (19.3%) | 2.37 (2.22, 2.54). | 222 (12.9%) | 0.64 (0.55, 0.74) | 1,259 (17.7%) | 3.01 (2.83, 3.20) |
| Non-Hispanic | 4,342 (80.7%) | 2.00 (1.94, 2.06) | 1,503 (87.1%) | 0.66 (0.62, 0.69) | 5,845 (82.3%) | 2.66 (2.59, 2.73) |
| Region | ||||||
| Northeast | 1,085 (20.2%) | 2.06 (1.94, 2.19) | 384 (22.3%) | 0.70 (0.63, 0.77) | 1,469 (20.7%) | 2.76 (2.62, 2.90) |
| Midwest | 1,310 (24.4%) | 2.03 (1.92, 2.14) | 417 (24.2%) | 0.64 (0.58, 0.70) | 1,727 (24.3%) | 2.67 (2.54, 2.79) |
| South | 1,571 (29.2%) | 2.05 (1.95, 2.15) | 545 (31.6%) | 0.70 (0.65, 0.77) | 2,116 (29.8%) | 2.75 (2.64, 2.87) |
| West | 1,413 (26.3%) | 2.10 (1.99, 2.21) | 379 (22.0%) | 0.57 (0.52, 0.64) | 1,792 (25.2%) | 2.68 (2.55, 2.80) |
| Grade | ||||||
| I | 220 (4.1%) | 0.08 (0.07, 0.09) | 95 (5.5%) | 0.04 (0.03, 0.04) | 315 (4.4%) | 0.12 (0.11, 0.13) |
| II | 261 (4.9%) | 0.10 (0.09, 0.11) | 153 (8.9%) | 0.06 (0.05, 0.07) | 414 (5.8%) | 0.16 (0.14, 0.17) |
| III | 1,350 (25.1%) | 0.52 (0.49, 0.54) | 462 (26.8%) | 0.17 (0.16, 0.19) | 1,812 (25.5%) | 0.69 (0.66, 0.72) |
| IV | 2,012 (37.4%) | 0.77 (0.74, 0.81) | 439 (25.4%) | 0.17 (0.15, 0.18) | 2,451 (34.5%) | 0.94 (0.90, 0.98) |
| Unknown | 1,536 (28.6%) | 0.59 (0.56, 0.62) | 576 (33.4%) | 0.22 (0.20, 0.24) | 2,112 (29.7%) | 0.81 (0.78, 0.85) |
| Stage at diagnosis¶ | ||||||
| In situ | 0 (0.0%) | 0.00 (0.00, 0.00) | 0 (0.0%) | 0.00 (0.00, 0.00) | 0 (0.0%) | 0.00 (0.00, 0.00) |
| Localized | 2,181 (40.5%) | 0.84 (0.80, 0.87) | 472 (27.4%) | 0.18 (0.16, 0.20) | 2,653 (37.3%) | 1.01 (0.98, 1.05) |
| Regional | 1,837 (34.2%) | 0.70 (0.67, 0.74) | 630 (36.5%) | 0.24 (0.22, 0.26) | 2,467 (34.7%) | 0.94 (0.91, 0.98) |
| Distant | 938 (17.4%) | 0.36 (0.34, 0.38) | 393 (22.8%) | 0.15 (0.13, 0.16) | 1,331 (18.7%) | 0.51 (0.48, 0.54) |
| Unstaged | 423 (7.9%) | 0.16 (0.15, 0.18) | 230 (13.3%) | 0.09 (0.08, 0.10) | 653 (9.2%) | 0.25 (0.23, 0.27) |
Counts pertain to 90.1% of U.S population covered by eligible cancer registries. Data from 7 central cancer registries are not included.
Rates are per 1,000,000 persons and age-adjusted to the 2000 US standard population (19 age groups - Census P25-1130) except age-specific rates which were not age-adjusted.
95% CI indicates 95% confidence interval and were calculated using the Tiwari modification.
Other unspecified/unknown race accounted for 106 (1.5%) cases of primary osteosarcomas.
Race and ethnicity are not mutually exclusive.
SEER summary stage 1977 was used for 1999–2000 cases, SEER summary stage 2000 was used for 2001–2003 cases, and derived summary stage 2000 [also known as collaborative stage) was used for 2004–2008 cases.
Not presented due to count or rate instability or complementarity suppressed for confidentiality.
AI/AN, American Indian/Alaska Native; API, Asian/Pacific Islander; SEER, National Cancer Institute’s Surveillance, Epidemiology, and End Results Program.
Percentages may not add to 100% because of rounding. The analyses were limited to microscopically confirmed osteosarcomas.
Rates for appendicular malignant osteosarcoma peaked for 2 age groups: 10-19 years and 80+ years. However, for axial malignant osteosarcomas, incidence rates were much lower and the peaks varied more with peaks at 10-19 years of age, at 40-49 years of age, and 80+ years. The age-specific incidence rate for appendicular malignant osteosarcomas was lowest among adults aged 50-59 years (0.78 per 1,000,000) and was highest among adolescents aged 10-19 years (7.08 per 1,000,000), However, the age-specific incidence rate for axial malignant osteosarcoma was lowest among children aged less than 10 years (0.11 per 1,000,000) and highest among adults aged 80+ years (1.17 per 1,000,000).
Moreover, differences in malignant osteosarcomas by gender were also observed. Among axial malignant osteosarcoma, the frequency was distributed almost equally by gender, whereas among appendicular malignant osteosarcoma cases, the distribution was slightly higher among males (57%) compared with females (43%). In addition, the appendicular malignant osteosarcoma age-adjusted rates among males (2.32 per 1,000,000) were higher than those observed among females (1.79 per 1,000,000) but no difference was seen in axial malignant osteosarcomas age-adjusted incidence rates by gender.
Among appendicular and axial cases, the predominant race group was whites (approximately 80%) followed by blacks (16%). Age-adjusted rates for appendicular and axial malignant osteosarcomas were higher among blacks than whites, American Indian/Alaska Natives, or Asian/Pacific Islanders. With respect to ethnicity, non-Hispanics accounted for approximately 81% of the reported cases among appendicular malignant osteosarcomas but almost 90% among axial malignant osteosarcomas. Moreover, Hispanics age-adjusted incidence for appendicular malignant osteosarcomas was higher than non-Hispanics.
Among appendicular and axial cases the geographic region with the largest proportion of cases (approximately 30%) occurred in the South with about 20%-26% of the remaining cases distributed in each of the remaining 3 regions (Northeast, Midwest, and West). Appendicular and axial rates among the 4 geographic regions were not different.
More than one third of appendicular cases were grade IV (37%), followed by unknown grade (29%) and grade III (25%). For the axial site, unknown grade contributed 33% of cases followed by grade III (27%), and grade IV (25%). The age-adjusted incidence rates were higher among appendicular cases for grade IV (0.77 per 1,000,000) when compared with other grades, whereas unknown cases accounted for the highest incidence rate (0.22 per 1,000,000) for axial cases.
For appendicular osteosarcomas, the highest proportion of cases were localized (41%). Most axial cases were diagnosed with regional stage (37%). The age-adjusted rates were higher among appendicular cases for localized stage (0.84 per 1,000,000), whereas regional stage (0.24 per 1,000,000) was higher for axial cases. Total malignant osteosarcomas had the highest age-adjusted incidence rates in localized stage (1.01 per 1,000,000) compared with other stages at diagnosis. Appendicular osteosarcomas had the highest age-adjusted incidence rates in the localized stage (0.84 per 1,000,000) compared with regional stage (0.24 per 1,000,000) among axial osteosarcomas.
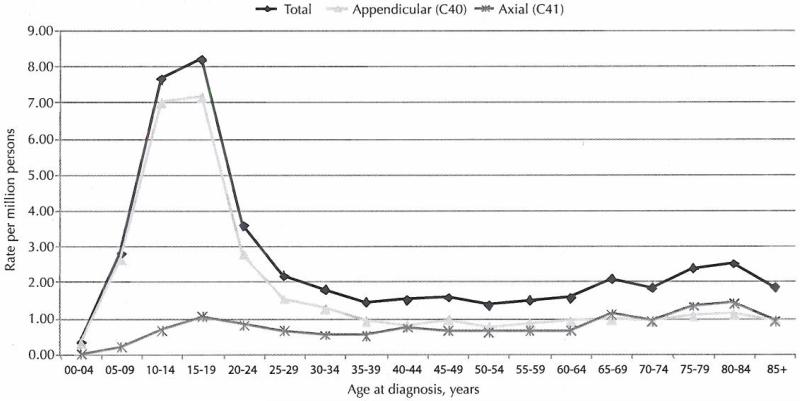
Figure 1 presents the incidence of malignant osteosarcomas by primary site and age (5-year intervals). Incidence rates peaked among 2 age groups: 15-19 year-olds (adolescence) and 80-84 year-olds (elderly).
Figure 1. Incidence of Primary Malignant Osteosarcomas, United States, 1999-2008.
Rates are per million persons and age-adjusted to the 2000 US standard population (19 age groups—Census P25-1130). Rates pertain to 90.1% of the US population covered by eligible cancer registries. Data from 7 central cancer registries are not included.
Discussion
These analyses provide detailed information on the descriptive epidemiology of malignant osteosarcomas complementing 2 recent studies published by Mirabello et al which reported the incidence and survival rates of osteosarcoma from 1973 to 2004 using SEER data in the United States, as well as the international osteosarcoma incidence patterns among children and adolescents, and middle-aged and elderly persons.3,17 The current study covers more than 90% of the US population. Together, these studies provide a detailed assessment of the burden of primary osteosarcomas.
Our results confirms previous findings of a bimodal frequency distribution for osteosarcoma incidence by age with the first peak occurring during adolescence and the second peak among adults over 60 years of age in the United States.3,6,17 Anfinsen et al reported a bimodal distribution with the highest incidence rate occurring in the second decade of life, with a decline between 30 and 50 years of age, followed by a second peak among those aged 75-79.6 Mirabello et al study reported an observed bimodal osteosarcoma incidence pattern.3
Differences in incidence rates by gender were found overall and by primary site. Among axial osteosarcoma, the frequency was distributed almost equally by gender, whereas among total and appendicular malignant osteosarcoma cases, the distribution was higher among males compared with females. An international study by Mirabello et al on osteosarcomas also reported that osteosarcoma was more common in males than females in most countries.17 Bleyer et al reported a higher incidence of malignant osteosarcomas among males than females among the adolescent and young adult population in the United States.1 Anfinsen et al reported that male predominance was generally apparent at the older ages and at ages less than 30 years, in contrast to modest and inconsistent sex differences among the middle age groups in the United States.6 A possible explanation for these observed sex differences in osteosarcoma incidence rates may be attributed to hormonal differences between genders. The effects of these sex steroids on osteoblasts have been well-documented both in vivo and in vitro.18-21 However, there is limited research on the possible effects of sex steroids in human osteosarcomas. Study findings by Dohi et al, however, suggest that inhibitors of sex steroid actions could be a potential treatment for osteosarcoma in the future.22 A study in England by Arora et al reported that a key factor in osteosarcoma development may be related to pubertal bone growth due to observed variations in incidence patterns by age and site.23 However, Arora et al stated that although pubertal growth may be a biological plausibility, further research may be required to establish this link.23
Differences were observed in our study by race and ethnicity separately. Among appendicular and axial cases, approximately 80% were found among whites followed by blacks (16%). In contrast, age-adjusted incidence rates for appendicular and axial malignant osteosarcomas were higher among blacks compared with other racial groups. Mirabello et al have reported similar results using SEER data.3 With respect to ethnicity, non-Hispanics accounted for approximately 81% of the reported cases among total and appendicular malignant osteosarcomas but almost 90% among axial malignant osteosarcomas. Moreover, Hispanics had a higher age-adjusted incidence rate than non-Hispanics for appendicular but a similar rate for axial cases. Bleyer et al reported that Hispanics had the highest incidence of osteosarcoma among those aged 15-29 in the United States.1
There were no differences observed in the regional data within subsites we analyzed. The Northeast, Midwest, South, and West all had similar incidence rates within their respective subsites. However, differences were observed between subsites. The incidence of osteosarcoma in the appendicular sites tended to be higher (ranged between 2.03-2.10 per 1,000,000) in these regions than axial (ranged between 0.57-0.70 per 1,000,000), An international study by Mirabello et al reported minimum variability in osteosarcoma incidence rates between countries in individuals ≤24 years. The country data were from the Cancer Incidence in Five Continents from the International Agency for Cancer Research (IARC) database. Overall, they reported that worldwide osteosarcoma incidence rates varied mostly among the elderly with little geographic variation among the younger age group.17
Our findings should be considered in light of several limitations. In registry data, there is a possibility of misclassification or miscoding especially with rare tumors. In addition, data were not available for 7 CCR in the 1999-2008 analytic dataset because they did not meet case ascertainment and quality criteria.9 The exclusion of these CCRs may have influenced the observed osteosarcoma incidence rates. Although we used data that had been linked to the IHS database to increase the accuracy of the AI/AN designation, race misclassification of these cases and other cases may still remain. However, a study by Clegg et al indicated that this may not be a strong limitation for cancer incidence any longer.24 Finally, even though the NHIA algorithm was used to assign ethnicity for Hispanics, ethnicity misclassification may also occur,24 Despite these limitations, our study is one of the first to report on malignant osteosarcoma incidence by primary site which covers over 90% of the US population. In addition, we were among the first studies to categorize osteosarcoma by appendicular and axial sites. Differences observed in these analyses between these sites provide further insight into the incidence of these tumors in these sites.
These analyses provide a better understanding of the incidence of malignant osteosarcoma using population-based data covering approximately 90.1% of the US population during 1999-2008. In addition, these analyses provide a more detailed understanding of age, gender, race, and ethnicity by primary site for malignant osteosarcoma incidence on a national level in the United States. More importantly, differences between appendicular and axial sites were observed overall by selected demographic characteristics, in particular regional variations. These analyses by subsites are a strength of this study.
Acknowledgements
The authors would like to acknowledge the contribution of hospital and central cancer registry staff who collected and processed the data used in this study.
These data were provided by the statewide central cancer registries participating in either the National Program of Cancer Registries (NPCR) and were submitted to CDC in the November 2010 data submission or to the Surveillance, Epidemiology, and End Results (SEER) Program in the November 2010 submission. The dataset includes data for diagnosis years 1999-2008.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- 1.Bleyer A, O’Leary M, Barr R, Ries LAG. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. National Cancer Institute; Bethesda, MD: 2000. NIH publication 06-5767. [Google Scholar]
- 2.Harting MT, Lally KP, Andrassy RJ, et al. Age as a prognostic factor for patients with osteosarcoma: an analysis of 438 patients. J Cancer Res Clin Oncol. 2010;136(4):561–570. doi: 10.1007/s00432-009-0690-5. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi: 10.1007/978-1-4419-0284-9_1. [DOI] [PubMed] [Google Scholar]
- 5.Savage SA, Mirabello L. Using epidemiology and genomics to understand osteosarcoma etiology. Sarcoma. 2011 doi: 10.1155/2011/548151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anfinsen KP, Devesa SS, Gray F, et al. Age-period-cohort analysis of primary bone cancer incidence rates in the United States (1976-2005) Cancer Epidemiol Biomarkers Prevent. 2011;20(8):1770–1777. doi: 10.1158/1055-9965.EPI-11-0136. [DOI] [PubMed] [Google Scholar]
- 7.Hayden JB, Hoang BH. Osteosarcoma: basic science and clinical implications. Orthop Clin North Am. 2006;37(1):1–7. doi: 10.1016/j.ocl.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention [Accessed March 8, 2012];National Program of Cancer Registries: About the Program. Available at: http://www.cdc.gov/cancer/npcr/about.htm.
- 9.Centers for Disease Control and Prevention [Accessed March 8, 2012];National Program of Cancer Registries: United States Cancer Statistics (USCS) Technical Notes: USCS Publication Criteria. Available at: http://www.cdc.gov/cancer/npcr/uscs/2005/technical_notes/criteria.htm.
- 10.World Health Organization . International Classification of Diseases for Oncology. 3rd ed. World Health Organization; Geneva: 2000. [Google Scholar]
- 11.American Joint Committee on Cancer . AJCC Cancer Staging Handbook. 6th ed. Springer; New York: 2002. [Google Scholar]
- 12.World Health Organization . International Classification of Diseases for Oncology. 2nd ed. World Health Organization; Geneva: 1990. [Google Scholar]
- 13.Young JL, Roffers S, Ries L, Fritz A, Hurlbut AA. SEER Summary Staging Manual - 2000. National Cancer Institute; Bethesda, MD: 2000. [Google Scholar]
- 14.North American Association of Central Cancer Registries [Accessed July 10, 2012];NAACCR Guideline for Enhancing Hispanic-Latino Identification; Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2] Available at: http://www.naaccr.org/LinkClick.aspx?fileticket=i6MN9F8c1eU%3D&tabid=92.
- 15.Centers for Disease Control and Prevention [Accessed July 10, 2012];Interpreting the Data: Race and Ethnicity in Cancer Data. Available at: http://www.cdc.gov/cancer/npcr/uscs/2007/technica_notes/interpreting/race.htm.
- 16.SEER*Stat [computer program]. Version 7.0.5. Surveillance Research Program, National Cancer Institute; Bethesda, Maryland: www.seer.cancer.gov/seerstat. [Google Scholar]
- 17.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boden SD, Joyce ME, Oliver BV, Heydemann A, Bolander ME. Estrogen receptor mRNA expression in callus during fracture healing in the rat. Calcif Tissue Int. 1989;45(5):324–325. doi: 10.1007/BF02556027. [DOI] [PubMed] [Google Scholar]
- 19.Colvard DS, Eriksen EE, Keeting PE, et al. Identification of androgen receptors in normal human osteoblast-like cells. Proc Natl Acad Sci USA. 1989;45:854–357. doi: 10.1073/pnas.86.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garnero P, Sornay-Rendu E, Chapuy MC, Delmas PD. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res. 1996;11(3):337–349. doi: 10.1002/jbmr.5650110307. [DOI] [PubMed] [Google Scholar]
- 21.Cooley DM, Beranek BC, Schlittler DL, Glickman NW, Glickman LT, Waters DJ. Endogenous gonadal hormone exposure and bone sarcoma risk. Cancer Epidemiol Biomarkers Prevent. 2002;11(11):1434–1440. [PubMed] [Google Scholar]
- 22.Dohi O, Hatori M, Suzuki T, et al. Sex steroid receptors expression and hormone-induced cell proliferation in human osteosarcoma. Cancer Sci. 2008;99(3):518–523. doi: 10.1111/j.1349-7006.2007.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora RS, Alston RD, Eden TO, Geraci M, Birch JM. The contrasting age-incidence patterns of bone tumours in teenagers and young adults: Implications for aetiology. Int J Cancer. 2012;131(7):1678–1685. doi: 10.1002/ijc.27402. [DOI] [PubMed] [Google Scholar]
- 24.Clegg LX, Reichman ME, Hankey BF, et al. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18(2):177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]