Abstract
The underlying mechanisms by which n-3 polyunsaturated fatty acids (PUFA) exert a chemopreventive effect in the colon have not been elucidated. Retinoid X receptors (RXR) are a family of nuclear receptors implicated in cancer chemoprevention. Since docosahexaenoic acid (DHA), an n-3 PUFA enriched in fish oil, reduces colonocyte proliferation and enhances apoptosis relative to n-6 PUFA-treated cells, we determined whether DHA can serve as a specific ligand for RXRα activation relative to n-6 PUFA in colonocytes. In a mammalian one-hybrid assay, immortalized young adult mouse colonic (YAMC) cells were co-transfected with a yeast galactose upstream activating sequence (UAS)4-tk-Luciferase (Luc) reporter plasmid, plus either GAL4 DNA-binding domain fused to RXRα, retinoic acid receptor α or GAL4 alone, followed by an n-3, n-6 or n-9 fatty acid incubation. Luc activity levels were dose-dependently elevated only in n-3 PUFA (DHA)-treated RXRα. Since RXR homodimers and RXR/peroxisome proliferator-activated receptor (PPAR) heterodimers bind consensus direct repeat (DR1) motifs, YAMC and NCM460 (a normal human colonic cell line), were respectively, co-transfected with RXRα and DR1-Luc, followed by different PUFA treatment. Luc activity levels were increased (P < 0.05) only in DHA groups. The DHA-dependent induction of DR-1-Luc was reduced to basal levels upon RXRα antagonist-treatment, with no effect on PPARγ antagonist-treatment. A role for select RXR isoforms in colonocyte biology was also determined by examining nuclear receptor mRNA levels in rat colon following dietary lipid and carcinogen exposure over time. RXRα, RXRβ and RXRγ were detected in rat colonic mucosa, and the levels of RXRα and RXRγ were elevated in fish oil (n-3 PUFA) versus corn oil (n-6 PUFA) fed animals after 16 weeks. These data indicate that, RXRα, an obligatory component of various nuclear receptors, preferentially binds n-3 PUFA in colonocytes, and that the nuclear receptor targets for PUFA in the colon are modulated by dietary lipid exposure.
Introduction
Many epidemiological, clinical and experimental studies have demonstrated that n-3 polyunsaturated fatty acids (PUFA) reduce colon cancer risk (1–8). In contrast, n-6 PUFA enhance the development of colonic tumors (3,5,7). This is noteworthy because the typical Western diet contains ~10 times more n-6 than n-3 PUFA (9). Despite the overwhelming scientific evidence linking dietary fat intake to colon cancer, the molecular mechanisms by which the dietary n-3 versus n-6 PUFA classes differentially modulate colon cancer development have not been fully elucidated.
Much of our work to date has focused on the prevailing hypothesis that dietary n-3 PUFA alter membrane composition and therefore the organization of signaling complexes capable of regulating epithelial cell cytokinetics (4,10–12). Alternatively, recent data indicate that dietary PUFA are also ligands for nuclear receptors (13–15). Nuclear receptors function as ligand-activated transcription factors that regulate the expression of target genes to affect almost all biologic processes, as diverse as reproduction, development and general metabolism (13,16).
Among the different nuclear receptors, peroxisome proliferator-activated receptors (PPARs) have been shown to be one of the major targets for fatty acids (13,17). However, this class of nuclear receptor binds n-3 and n-6 PUFA with equal affinity and appears to lack fatty acid class specificity (18–20). Therefore, the unique protective effects of n-3 PUFA are likely not directly mediated through activation of PPARs.
The lack of experimental data on the mechanism by which docosahexaenoic acid (DHA, 22:6Δ4,7,10,13,16,19), a major n-3 PUFA found in fish oil, reduces colonocyte proliferation and enhances apoptosis relative to n-6 PUFA (11,21) prompted us to identify ‘non-membrane’, non-PPAR molecular targets, which selectively respond to n-3 PUFA. Here we report for the first time that retinoid X receptor (RXR) is preferentially activated by n-3 PUFA in mouse and human colonocytes. Moreover, we found that colonocyte expression of RXRs and PPARγ mRNA is modulated by dietary PUFA content in the presence and absence of carcinogen exposure.
Materials and methods
Materials
RPMI 1640 was purchased from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was obtained from Hyclone (Logan, UT). Insulin/transferrin/selenium (ITS) was purchased from Collaborative Biomedical Products (Bedford, MA). GlutaMAX-1 and recombinant mouse interferon-γ (IFN-γ) were from Gibco BRL (Grand Island, NY). M3:10 medium was obtained from INCELL Corporation (San Antonio, TX). Fatty acid-free bovine serum albumin (BSA) was from Roche Diagnostics (Indianapolis, IN). Fatty acids were purchased from NuChek Prep (Elysian, MN). Pre-cast 4–20% Tris– glycine gels were obtained from Invitrogen (Carlsbad, CA). Electroblotting polyvinylidene difluoride (PVDF) membranes were obtained from Millipore (Burlington, MA). Rabbit polyclonal anti-RXRα and anti-PPARγ were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Peroxidase labeled goat anti-rabbit IgG was purchased from Kirkegaard & Perry Laboratories (Gaithesburg, MD). RXR agonist (AGN 194204) and RXR antagonist (AGN 195393) were generous gifts from Dr Richard Beard (Allergan, Irvine, CA). PPARγ agonist 15-deoxy-Δ12,14-prostaglandin J2 was purchased from Cayman Chemical (Ann Arbor, MI). PPARγ antagonist (GW9662) was a gift from Dr Timothy Willson (GlaxoSmithKline, Research Triangle Park, NC). Effectene Transfection Reagent was purchased from Qiagen (Valencia, CA). Luciferase assay system and Dual-Luciferase reporter assay system were purchased from Promega (Madison, WI). BCA protein assay system was obtained from Pierce (Rockford, IL). All other reagents and tissue culture wares were from Fisher Scientific (Fair Lawn, NJ).
Cell lines and plasmids
Conditionally immortalized young adult mouse colon (YAMC) cells originally obtained from Dr Robert Whitehead (Ludwig Institute for Cancer Research, Melbourne, Australia) (22) (passages 16–20), were cultured in RPMI 1640 media supplemented with 5% FBS, 1% ITS, 1% 200 mM GlutaMAX and 5000 U/l of recombinant IFN-γ (23). Cells were cultured under permissive (33°C) conditions as described previously (24). NCM460, a non-tumorigenic epithelial cell line derived from the normal human colon mucosa (25) were maintained in M3:10 medium at 37°C. Human GAL4-fused expression plasmids (pCMV-RXRα-GAL4, pCMV-RARα-GAL4, pCMV-GAL4 and UASx4-tk-luciferase reporter) were generous gifts from Dr Ronald Evans (Salk Institute for Biological Studies, La Jolla, CA). Full-length mouse RXRα cDNA (pSG5RXRα) was from Dr Pierre Chambon (Institut de Chimie Biologique, Strasbourg, France) (26). Mouse PPARγ cDNA (pSG5mPPARγ) was kindly provided by Dr Vanden Heuvel (Pennsylvania State University, University Park, PA). Wild-type PPRE3 Luciferase reporter plasmid (DR1-Luc) was generously provided by Dr Steve Safe (Texas A&M University, College Station, TX), and pRL-CMV Renilla Luciferase reporter plasmid (Renilla luciferase) was purchased (Promega).
Transfections and luciferase assays
A series of transient transfections were conducted. YAMC or NCM 460 cells were transiently co-transfected with the appropriate expression and reporter plasmids as indicated using Effectene Transfection Reagent (Qiagen). Following an overnight incubation, transfected cells were provided fresh media containing fatty acid–BSA complexes and RXRα/PPARγ agonists or antagonists at various concentrations for 24 h. Cells were subsequently harvested in luciferase assay buffer. Luciferase activity in cell lysates was measured using a LumiCount luminometer (Packard, Meriden, CT) and the Luciferase Assay System (Promega). Values were normalized by protein content measured using the BCA protein assay. For those cultures containing Firefly and Renilla Luc reporters, relative light units from firefly luciferase activity were normalized to the relative light units from Renilla luciferase using a Dual Luciferase Assay System (Promega) (27).
Western blotting
Cell lysates from RXRα or PPARγ-transfected and Mock-transfected cells were immunoblotted with RXRα or PPARγ antibody using the method of Davidson et al. (12) to evaluate the protein expression level in basal versus transfected cells. Briefly, samples were treated with SDS sample buffer and subjected to electrophoresis in a 4–20% pre-cast Tris–glycine gel. After electrophoresis, proteins were electroblotted onto PVDF membranes using a Hoefer Mighty Small Transphor Unit (Pharmacia, Piscataway, NJ) at 400 mA for 100 min. After transfer, the membrane was incubated with rabbit anti-RXRα or anti-PPARγ antibody overnight at 4°C, followed by peroxidase labeled goat anti-rabbit IgG incubation for 1 h at room temperature. Bands were developed using Super Signal West Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL), and the blots were scanned using a Bio-Rad Fluor-S Max MultiImager System (Hercules, CA).
Animal experiments
The animal use protocol was approved by the University Animal Care Committee of Texas A&M University and conformed to National Institutes of Health guidelines. Male weanling (21-day-old) Sprague–Dawley rats (Harlan Sprague-Dawley, Houston, TX) were housed individually and assigned to one of two different experimental diets differing only in their lipid composition (28,29). Specifically, animals were fed either an n-6 PUFA-enriched corn oil based diet (devoid of long chain n-3 PUFA) or a DHA-enriched fish oil-corn oil mixture at 15 g/100 g as described previously (30). The n-6 PUFA-corn oil contained linoleic acid (18:2n-6) at 54% by weight, as the major fatty acid constituent. Less than 1% n-3 PUFA was detectable by gas chromatography. In contrast, the fish oil diet contained ~15% 18:2n-6, 12% 20:5n-3 and 8% DHA (22:6n-3). Each diet contained identical levels of tert-butyl-hydroquinone (2 mg), alpha-tocopherol (26 mg) and gamma-tocopherol (14 mg)/100 g diet. Rats were injected with saline (control) or azoxymethane (AOM) at weeks 2 and 3 as described previously (2). Animals were killed by CO2 asphyxiation at 12 h, 10, 16 or 36 weeks after the first injection. Colonic mucosa was subsequently scraped and immediately processed for RNA analysis described below. The time points represent distinct phases of the malignant transformation process, i.e. 12 h, initiation stage; 10 weeks, early promotion stage; 16 weeks, late promotion stage and 36 weeks, tumor end stage.
Reverse transcriptase–PCR
Colon mucosa total RNA was isolated using the Totally RNA isolation kit from Ambion (Austin, TX). The isolated total RNA was subsequently treated with DNase to remove contaminating DNA. RNA was reverse-transcribed to cDNA using SuperScript II (Gibco BRL). Real-time PCR was performed using the ABI 7700 (Applied Biosystems, Foster City, CA) and Taqman probes as described previously (31). Probes and primer pairs for rat RXRs and PPARγ genes are summarized in Table I.
Table I.
Real time PCR primer sets and Taqman probes for nuclear receptor genes
| Gene name | Oligo sequence (5′–3′) |
|---|---|
| RXRα | Forward primer: CCTGCCGTGACAACAAGGA |
| Reversed primer: CACTTCTGGTATCGGCAGTACTG | |
| Taqman probe: CCGGTTCCGCTGTCTCTTGTCGA | |
| RXRβ | Forward primer: AAGCTCAGGCAAGCACTATGG |
| Reversed primer: GTAGGTCAGGTCCTTCCGAATG | |
| Taqman probe: CCTTGCAGCCCTCGCAGCTGTAA | |
| RXRγ | Forward primer: AGGACATGCGGATGGATAAGTC |
| Reversed primer: CATCTGGATTGAACAGCACAATG | |
| Taqman probe: CGCGCAGGCACCCGAGCT | |
| PPARγ | Forward primer: GCTGAACCCAGAGTCTGCTGAT |
| Reversed primer: GTCAGCGGGAAGGACTTTATGT | |
| Taqman probe: TGCGAGCCCTGGCAAAGCATTT |
Statistics
For all transient transfection experiments, data were analyzed using one-way ANOVA and Duncan’s New Multiple Range procedure for comparing treatment means. For RT–PCR analyses, RNA expression was ranked across all diet/treatment groups in order to eliminate potential artifacts created by outlying observations. Tukey’s Studentized range test (32) was used to compare the effects of diet and treatment. Differences of P < 0.05 were considered statistically significant.
Results
DHA acts as an agonist of RXRα in an RXR-dependent GAL4-containing reporter construct in RXRα-transfected cultures
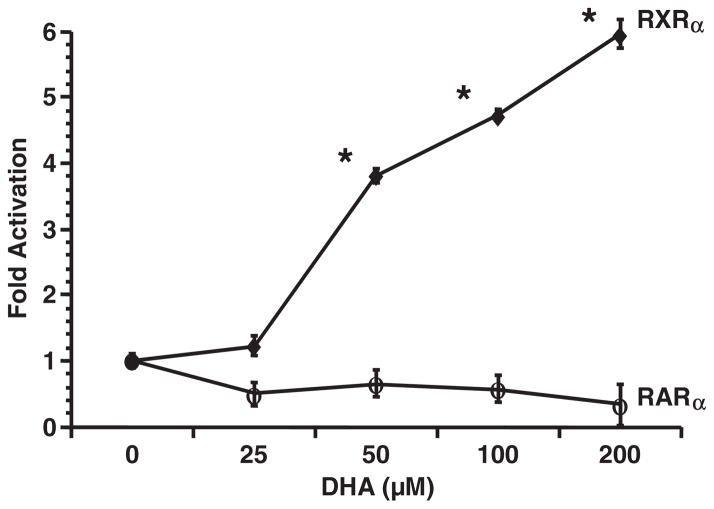
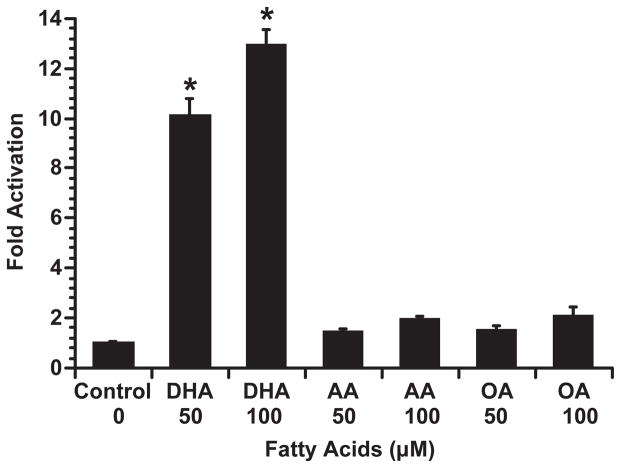
In order to test the specific activation potential of DHA on colonocyte RXRα or retinoic acid receptor (RAR)α, YAMC cells were co-transfected with UASx4-tk-luciferase reporter plasmid, and either pCMV-RXRα-GAL4, pCMV-RARα-GAL4 or pCMV-GAL4 expression plasmid, followed by DHA incubation at different concentrations. As shown in Figure 1, DHA dose-dependently activated RXRα, with no effect on RARα, indicating DHA is a specific ligand for RXRα. In addition, the transactivation of RXRα by DHA (an n-3 PUFA) in this culture system was specific relative to the other n-6 [arachidonic acid (AA), 20:4n-6, all cis-Δ5,8,11,14] and n-9 [oleic acid (OA), 18:1n-9, cis-Δ9] fatty acids (Figure 2).
Fig. 1.
DHA dose-dependently transactivates an RXR-dependent GAL4-containing reporter construct in RXRα-transfected colonocyte cultures. YAMC cells were transiently co-transfected with UASx4-tk-luciferase reporter plasmid, and either pCMV-RXRα-GAL4, pCMV-RARα-GAL4 or GAL4 expression plasmid, followed by DHA incubation (0–200 μM) for 24 h. Cell lysates were harvested, and luciferase activity and protein concentrations were determined. Data are expressed as fold activation over the control [GAL4 alone at each dosage (0–200 μM)]. Data (n = 7) are presented as mean ± SE. *Indicates a difference, P < 0.05.
Fig. 2.
DHA acts as an agonist of RXRα. YAMC cells were transiently co-transfected with UASx4-tk-luciferase reporter and pCMV-RXRα-GAL4 expression plasmid, followed by incubation with n-3, n-6 and n-9 fatty acids (DHA, AA, OA, respectively, at 0, 50 or 100 μM) for 24 h. Cell lysates were harvested, and luciferase activity and protein concentrations were determined. Data (n = 9–16) are expressed as fold activation over control (no FA added)
DR1 activity is induced by DHA in RXRα-transfected colonocytes
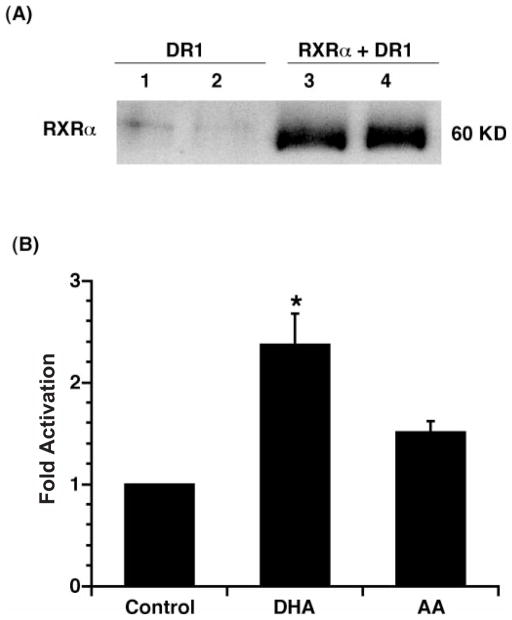
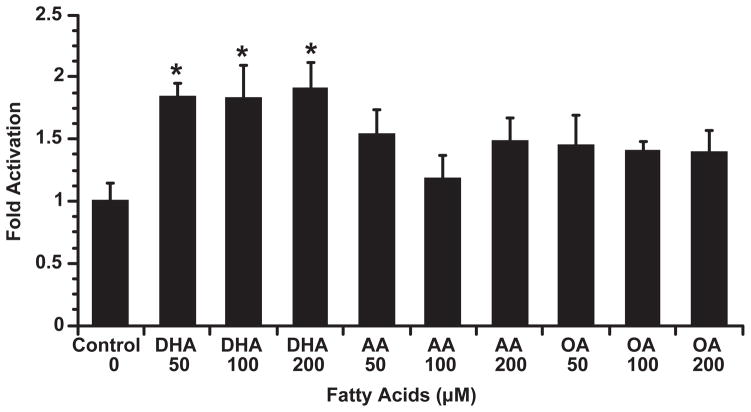
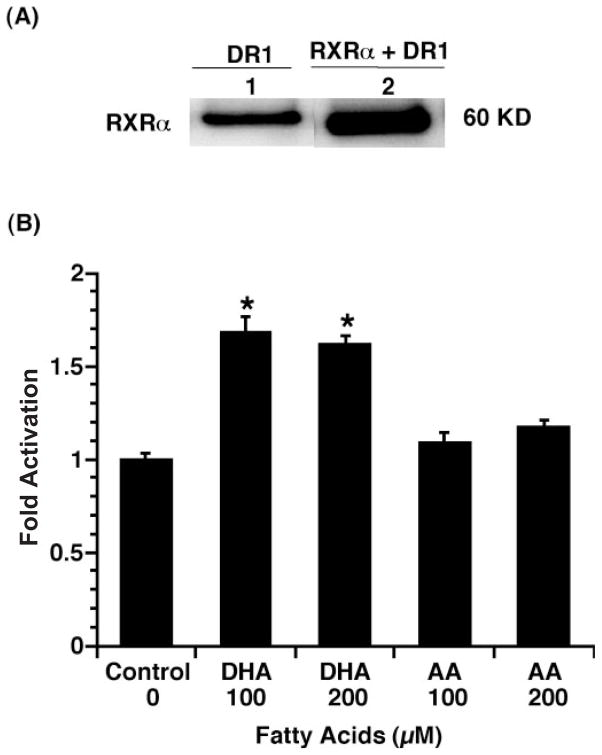
Since RXR-dependent gene transcription is largely mediated by a DR1 element (PPRE), a combined RXRα and DR1-Luc reporter transfection system was utilized to examine the effect of DHA on colonocyte DR1 activity. YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα, followed by DHA, AA (100 μM) or no fatty acid incubation for 24 h. Immunoblot analysis for RXRα expression was increased in culture transfected with RXRα and DR1-Luc as shown in Figure 3A. These data suggest that the DHA enhancement of DR1-Luc activity is mediated by RXRα. Similar to the GAL4 RXRα system, DHA, but not AA, activated (P < 0.05) DR1-dependent Luc activity (Figure 3B). Furthermore, as shown in Figure 4, transactivation of a DR1-containing reporter was limited to n-3 PUFA, with no effect (P < 0.05) of n-6 (AA) or n-9 (OA) fatty acids.
Fig. 3.
DR1 activity is induced by DHA but not AA in RXRα-transfected cultures. (A) Immunoblot analysis examining RXRα protein expression. Lanes 1 and 2, transfection with DR1-Luc reporter only; lanes 3 and 4, co-transfection with pSG5RXRα expression plasmid and the DR1-Luc reporter. (B) YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα expression plasmids, followed by DHA, AA (100 μM) or no fatty acid incubation for 24 h. Cell lysates were harvested, and dual luciferase activities were determined. DR1-Luc activity was normalized relative to Renilla luciferase activity. Data (n = 8) are expressed as fold activation over control (mean ± SE). *Indicates that DHA treatment is different (P < 0.05) from control and AA groups.
Fig. 4.
DHA dose-dependently transactivates a DR1 containing reporter construct in RXRα-transfected cultures. YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα expression plasmids, followed by incubation with n-3, n-6 and n-9 fatty acids (DHA, AA, OA), respectively, at 0, 50, 100 or 200 μM for 24 h. Cell lysates were harvested, and dual luciferase activities were measured. DR1-Luc activity was normalized relative to Renilla luciferase activity. Data (n = 4) are expressed as fold activation over no fatty acid control (mean ± SE). *Indicates that DHA treatment is different (P < 0.05) from control (no FA added) treatment.
DHA enhances DR1 activity in human colonocytes
In order to further evaluate the effect of DHA on RXRα activation, co-transfection experiments were conducted on the human colonocyte cell line, NCM460. NCM460 cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα, followed by incubation with DHA, AA (100 or 200 μM) or no fatty acid incubation for 24 h. Comparable with the results from YAMC cells (Figures 3 and 4), DHA but not AA, enhanced (P < 0.05) DR1 activity in NCM460 (Figure 5). These data suggest that the activation of DR1 by DHA is a general effect, as seen in both mouse and human colonocytes.
Fig. 5.
DHA enhances DR1 activity in human colonocytes. (A) Immunoblot analysis examining RXRα protein expression. Lane 1, transfection with DR1-Luc reporter only; lane 2, co-transfection with pSG5mRXRα expression plasmid and the DR1-Luc reporter. (B) NCM460 cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα expression plasmids, followed by incubation with DHA, AA (100 or 200 μM) or no fatty acid incubation for 24 h. Cell lysates were harvested, and dual luciferase activities were measured. DR1-Luc activity was normalized relative to Renilla luciferase activity. Data (n = 4) are expressed as fold activation over no fatty acid control (mean ± SE). *Indicates DHA different (P < 0.05) from other treatments.
PUFA (n-3 and n-6) non-specifically increase DR1 activity in PPARγ-transfected cultures
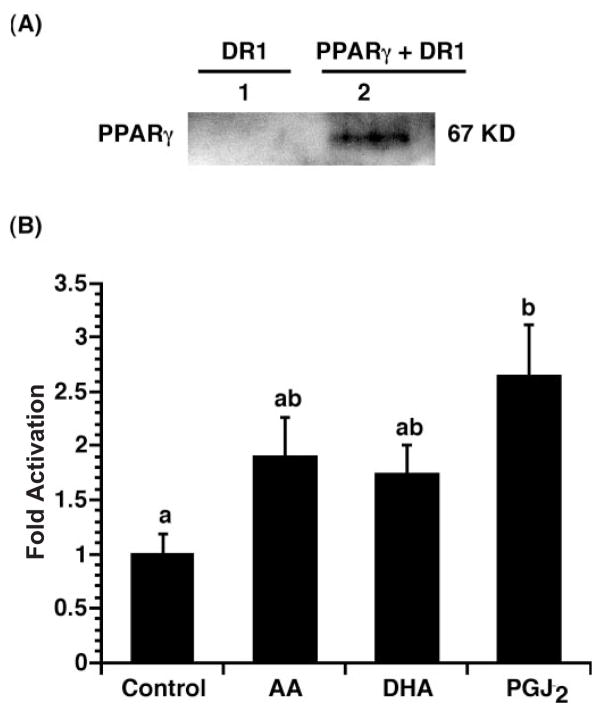
Since DR1 also mediates PPAR-dependent transactivation, we investigated the ability of PPARγ to mediate DHA-dependent DR1 activation. For this purpose, YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5mPPARγ, followed by incubation with 100 μM DHA, AA, 2 μM 15-deoxy-Δ12,14-PGJ2 (PPARγ agonist) or medium alone for 24 h. Figure 6A shows that PPARγ expression was increased in cultures transfected with PPARγ and DR1-Luc. As shown in Figure 6B, except for PGJ2 (a PPARγ agonist), DHA and AA only slightly enhanced DR1 activation (P < 0.05). The levels of activation with both fatty acids were similar, suggesting that activation of PPARγ is not fatty acid specific in colonocytes.
Fig. 6.
PUFA (n-3 and n-6) non-specifically increase DR1 activity in PPARγ-transfected cultures. (A) Immunoblot analysis examining PPARγ protein expression. Lane 1, transfection with DR1-Luc reporter only; lane 2, co-transfection with pSG5mPPARγ expression plasmid and the DR1-Luc reporter. (B) YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5mPPARγ expression plasmids, followed by incubation with 100 μM DHA, AA, 2 μM 15-deoxy-Δ12,14-PGJ2, or medium alone for 24 h. Cell lysates were harvested, and dual luciferase activities were measured. DR1-Luc activity was normalized relative to Renilla luciferase activity. Data (n = 4) are expressed as fold activation over no fatty acid control (mean ± SE). Values not sharing the same superscript are different (P < 0.05).
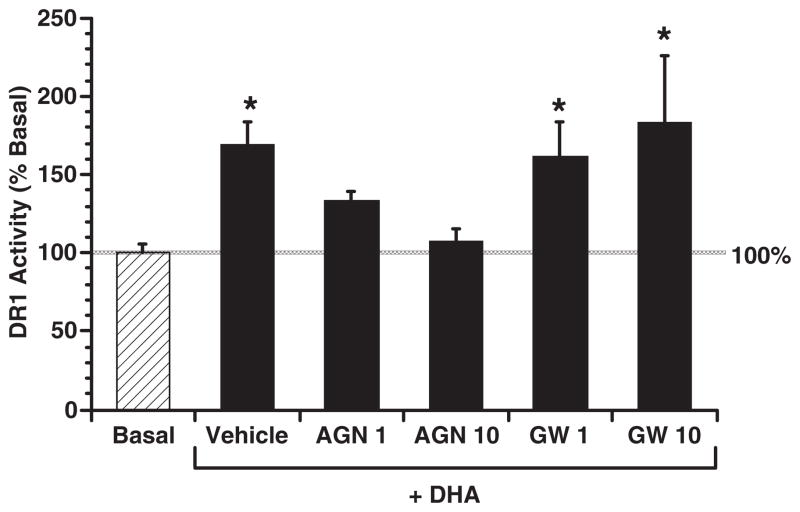
RXR antagonist blocks DHA-dependent activation of DR1 in YAMC cells
DR1 serves as a response element for both RXR and PPAR. In order to further determine whether the effect of DHA on DR1 activation is RXRα specific, antagonists for RXR or PPARγ were added to cultures. YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα, followed by incubation with DHA (100 μM) ± RXR antagonist (AGN195393) or PPARγ antagonist (GW259662X) (27) at 1 or 10 μM for 24 h (Figure 7). DHA-induced DR1 activity was dose-dependently reduced to the basal level upon RXRα antagonist-treatment. In contrast, PPARγ antagonist had no inhibitory effect, indicating that RXRα is a specific nuclear target for DHA.
Fig. 7.
RXR antagonist blocks DHA-dependent activation of DR1 in YAMC cells. YAMC cells were transiently co-transfected with DR1-Luc, Renilla luciferase and pSG5RXRα expression plasmids, followed by incubation with DHA (100 μM) ± RXR antagonist (AGN195393, abbreviated as AGN) or PPARγ antagonist (GW259662X, abbreviated as GW) at 1 or 10 μM for 24 h. Cell lysates were harvested, and dual luciferase activities were measured. DR1-Luc activity was normalized relative to Renilla luciferase activity. Data (n = 4) are expressed as percent activation over basal (no fatty acid, no antagonist), mean ± SE. *Indicates a difference (P<0.05) from the basal group.
Effect of dietary n-3 PUFA and carcinogen on RXR and PPARγ expression levels in rat colon
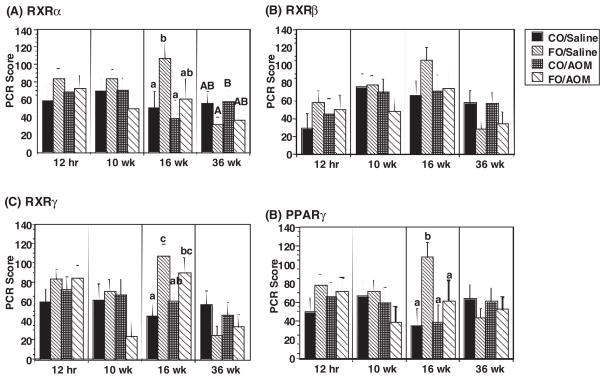
The regulation of target gene expression can be influenced in part by the cellular accessability of ligands and/or by cellular/nuclear levels of receptor (33). This is noteworthy, because RXR expression is frequently perturbed in certain forms of cancer (34,35). Therefore, in order to better understand how n-3 PUFA influence RXR nuclear receptor function in vivo, RNA expression levels of RXRs and PPARγ in rat colonic mucosa were measured. Animals were fed diets containing fish oil (containing n-3 PUFA) or corn oil (containing n-6 PUFA) and treated with a colon specific carcinogen, AOM or saline (control). RXRα, β and γ and PPARγ mRNA expression was subsequently measured by real time PCR at different stages of the malignant transformation process. As shown in Figure 8, RXRα, RXRβ, RXRγ and PPARγ were expressed in rat colonic mucosa, and the expression levels of RXRα, RXRγ and PPARγ were elevated in fish oil (n-3 PUFA) versus corn oil (n-6 PUFA) fed animals after 16 weeks. Interestingly, carcinogen exposure attenuated the fish oil effect with respect to PPARγ expression. These data indicate that the steady-state nuclear receptor targets for PUFA in the colon are modulated by dietary lipid and carcinogen exposure over time.
Fig. 8.
Elevation of colonic RXRs and PPARγ expression levels in fish oil-fed rats. Rats were fed semi-purified diets containing either corn oil (containing n-6 PUFA) or fish oil (containing n-3 PUFA) for 38 weeks. AOM or saline (vehicle-injected control) was injected at weeks 2 and 3. Animals were killed at 12 h, 10, 16 and 36 weeks after the first injection. Total RNA was isolated from colon mucosa. The RXRα, β and γ and PPARγ mRNA levels were measured using Taqman real-time PCR. Data were expressed as PCR score (n = 5–15, mean ± SE). Values not sharing the same superscript are different (P < 0.05). Saline, saline injected control mice; AOM, azoxymethane-injected; CO, rats fed corn oil containing n-6 PUFA; FO, rats fed fish oil containing n-3 PUFA.
Discussion
We have shown previously that n-3 PUFA selectively enhance apoptosis and suppress colonic cell proliferation compared with n-6 PUFA, the major dietary form of polyunsaturated fatty acid in the US diet (9,36,37). This is significant because the balance between colonic epithelial cell proliferation and apoptosis in part determines susceptibility to toxic carcinogenic agents and colon cancer risk (2,38–40). Unfortunately, to date, the underlying molecular mechanisms by which the n-3 class of fatty acids exerts its chemopreventive effects in the colon are not known. Therefore, the major goal of this study was to identify additional molecular targets through which n-3 PUFA modulate colonocyte signaling and function.
In addition to their ability to alter membrane function/dynamics, recent studies indicate that dietary fatty acids are also ligands for nuclear receptors and, therefore, could act as agonists inducing the sequence of events necessary for trans-activation of cognate target genes (14,41,42). An intriguing possibility is that DHA, 22:6n-3, all cis-Δ4,7,10,13,16,19, and perhaps other n-3 PUFA, modulate colonic cytokinetics and cancer risk by selectively activating the RXR subunit of nuclear receptor heterodimers. In order to investigate this possibility, we examined the ability of DHA, compared with n-6 and n-9 fatty acids, to transactivate RXRα in mouse and human colonic cell lines. In the present study, DHA (a major n-3 PUFA found in fish oil) was found to selectively activate RXR-responsive reporters in colonocytes (Figures 2, 4 and 5). In addition, in dose–response experiments, DHA was an efficient activator at a concentration considered physiologically relevant, because it lies within the range of blood levels in human subjects supplemented with DHA (43). In contrast, neither n-6 (20:4n-6, all cis-Δ5,8,11,14) nor n-9 (18:1n-9, cis-Δ9) fatty acids elicited a response. These data indicate for the first time that DHA can act as an RXR agonist in mouse and human colonocytes. With respect to ligand structure– function features, there are no structural similarities between DHA and 9-cis retinoic acid, a potent RXR activator. Both agonists contain a carboxyl function, which is an essential element for binding to amino acids in the ligand-binding pocket of RXR (44). Interestingly, 20:4n-6, which also contains this structural feature, does not activate the RXR signaling pathway (Figures 2–4). Additional characterization of the predominantly hydrophobic pocket of the RXRα active site is required in order to further elucidate the process of molecular recognition.
There are two subtypes of retinoid receptors, RARs and RXRs, which include three isotypes each, designated α, β and γ. RXRs are transcription factors that can act as ligand-dependent and -independent partners for RARs and other nuclear receptors. Regulation of gene transcription by RAR– RXR and RXR–RXR dimers is largely mediated by DR5 (an RAR response element) and DR1 (an RXR response element), respectively (45). RXRs are also promiscuous heterodimeric partners for thyroid hormone receptor (TR), vitamin D receptor, PPARs, and nuclear oxysterol receptors (45,46). Although little is known regarding the ligands that activate RXR in vivo, it seems likely that ligand-induced activation of RXR does occur in vivo (47). With respect to cancer chemoprevention, there is evidence that RAR and RXR selective ligands cooperatively induce apoptosis, in part by antagonizing activator protein-1 (AP-1) and the β-catenin LEF/TCF signaling pathways (48–51). It is possible, therefore, that n-3 PUFA enhance colonocyte apoptosis and suppress cell proliferation via RXR. Further investigation is needed in order to evaluate the biological properties of RXR agonists and antagonists with respect to colonocyte growth and differentiation.
To determine whether DHA modulates the heterodimerizing partners of RXR, we also performed a number of experiments using RAR and PPAR responsive reporters. Our data indicate that DHA fails to activate RAR (Figure 1). In addition, with respect to n-3 PUFA as agonists for PPARs, in this study, colonic PPAR-dependent transactivation was not specifically associated with DHA (Figure 6). These data indicate that although some PPAR (γ, δ) agonists are known to modulate colonic tumor growth (6,52), the PPARγ class of nuclear receptor binds n-3 and n-6 PUFA with equal affinity and lacks fatty acid class (n-3 versus n-6) specificity (18–20). Therefore, the unique effects of n-3 PUFA in the colon are likely not directly mediated via PPARγ. Interesting, Lee and Hwang (53) have recently demonstrated that DHA suppresses PPARδ transactivation in HCT116 colon tumor cells. These results indicate that DHA has the potential to differentially influence both RXR and PPAR heterodimeric partners in colonocytes.
We have documented for the first time that all three isoforms of RXR are expressed in normal rat colonic mucosa (Figure 8). This suggests a physiological function of RXRs on colonocyte biology. In addition, RXRα, RXRγ and PPARγ expression levels were elevated in fish oil-fed animals after 16 weeks. Interestingly, carcinogen exposure attenuated the fish oil effect with respect to PPARγ expression. Taken together, these data indicate that the steady-state receptor targets for PUFA in the colon are modulated by dietary lipid and carcinogen exposure over time. The increase in RXR and PPARγ mRNA during the late promotion stage of colon carcinogenesis in n-3 PUFA (fish oil) fed rats, is consistent with the ability of DHA to modulate colonic cytokinetics during discrete stages of malignant transformation (54,55). Recent studies have shown that RXR gene regulation and the steady-state levels of dietary DHA in cells are under homeostatic control (28,56–58). Specifically, with respect to RXRα gene transcription, it has been demonstrated in rat hepatic cells that fatty acids are capable of modulating RXR expression (59). Although we did not detect a direct effect of carcinogen on RXR expression in the colon, a number of studies have reported that malignant transformation is associated with alterations in the expression RXRs in gastric and skin tumors (34,35,60). Further investigation of the mechanism by which environmental agents, i.e. diet, modulate colonic RXR expression may provide additional insight into the establishment of new therapeutic/chemopreventive strategies.
In conclusion, we have demonstrated for the first time that RXR, an obligatory component of a large number of nuclear receptors, is preferentially activated by DHA, an n-3 PUFA, in mouse and human colonocytes. This raises the intriguing possibility that n-3 PUFA mediate growth inhibitory effects in the colon through the RXR subunit of nuclear receptor heterodimers. Additional studies are required in order to identify the genes that are immediate responders to this class of activated receptors.
Acknowledgments
This study was supported by NIH grants CA59034, CA74552, P30-ES09106 and TARP 010366-0194-2001. We also gratefully acknowledge the provision of reagents from Drs Timothy Willson (GlaxoSmithKline), Richard Beard (Allergan), Ronald Evans (Salk Institute), Pierre Chambon (Institut de Chimie Biologique), Jack Vanden Heuvel (Penn State University) and Steven Safe (Texas A&M University).
Abbreviations
- AA
arachidonic acid
- AOM
azoxymethane
- DHA
docosahexaenoic acid
- OA
oleic acid
- PPAR
peroxisome proliferator-activated receptor
- PUFA
polyunsaturated fatty acids
- RAR
retinoic acid receptor
- RXR
retinoid X receptor
- UAS
upstream activating sequence
- YAMC
young adult mouse colon
References
- 1.Anti M, Armelao F, Marra G, Percesepe A, Bartoli GM, Palozz P, Parrella P, Canetta C, Gentiloni N, De Vitis I, Gasbarrini G. Effects of different doses of fish oil on rectal cell proliferation in patients with sporadic colonic adenomas. Gastroenterology. 1994;107:1709–1718. doi: 10.1016/0016-5085(94)90811-7. [DOI] [PubMed] [Google Scholar]
- 2.Chang WC, Chapkin RS, Lupton JR. Predictive value of proliferation, differentiation and apoptosis as intermediate markers for colon tumorigenesis. Carcinogenesis. 1997;4:721–730. doi: 10.1093/carcin/18.4.721. [DOI] [PubMed] [Google Scholar]
- 3.Deschner EE, Lytle J, Wong G, Ruperto J, Newmark HL. The effect of dietary omega-3 fatty acids (fish oil) on azoxymethanol-induced focal areas of dysplasia and colon tumor incidence. Cancer. 1990;66:2350–2356. doi: 10.1002/1097-0142(19901201)66:11<2350::aid-cncr2820661117>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 4.Lee DY, Lupton JR, Aukema HM, Chapkin RS. Dietary fat and fiber alter rat colonic mucosal lipid mediators and cell proliferation. J Nutr. 1993;123:1808–1817. doi: 10.1093/jn/123.11.1808. [DOI] [PubMed] [Google Scholar]
- 5.Minoura T, Takata T, Sakaguchi M, Takada H, Yamamura M, Yamamoto M. Effect of dietary eicosapentaenoic acids on azoxymethane-induced colon carcinogenesis in rat. Cancer Res. 1988;48:4790–4794. [PubMed] [Google Scholar]
- 6.Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARdelta decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA. 2001;98:2598–2603. doi: 10.1073/pnas.051630998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy BS. Dietary fat, calories and fiber in colon cancer. Prev Med. 1993;22:738–749. doi: 10.1006/pmed.1993.1068. [DOI] [PubMed] [Google Scholar]
- 8.Willet WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relations of meat, fat and fiber intake to the risk of colon cancer in the prospective study among women. N Engl J Med. 1990;323:1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]
- 9.Spector AA. Essentiality of fatty acids. Lipids. 1999;34:S1–S3. doi: 10.1007/BF02562220. [DOI] [PubMed] [Google Scholar]
- 10.Chapkin RS, Hong MY, Fan YY, Davidson LA, Sanders LM, Henderson CE, Turner ND, Barhoumi R, Burghardt RC, Lupton JR. Dietary n-3 fatty acids alter colonocyte mitochondrial membrane composition and function. Lipids. 2002;37:193–199. doi: 10.1007/s11745-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 11.Collett ED, Davidson LA, Fan YY, Lupton JR, Chapkin RS. n-6 and n-3 polyunsaturated fatty acids differentially modulate oncogenic ras activation in colonocytes. Am J Physiol Cell Physiol. 2001;280:C1066–C1075. doi: 10.1152/ajpcell.2001.280.5.C1066. [DOI] [PubMed] [Google Scholar]
- 12.Davidson LA, Lupton JR, Jiang YH, Chapkin RS. Carcinogen and dietary lipid regulate ras expression and localization in rat colon without affecting farnesylation kinetics. Carcinogenesis. 1999;20:785–791. doi: 10.1093/carcin/20.5.785. [DOI] [PubMed] [Google Scholar]
- 13.Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the x-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- 14.de Urquiza AM, Liu S, Sjoberg M, Zetterstrom RH, Griffiths W, Sjovall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 15.Wolfrum C, Borrmann CM, Borchers T, Spener F. Fatty acids and hypolipidemic drugs regulate peroxisome proliferator-activated receptors α- and γ-mediated gene expression via liver fatty acid binding protein: a signaling path to the nucleus. Proc Natl Acad Sci. 2001;98:2323–2328. doi: 10.1073/pnas.051619898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olefsky JM. Nuclear receptor minireview series. J Biol Chem. 2001;276:36863–36864. doi: 10.1074/jbc.R100047200. [DOI] [PubMed] [Google Scholar]
- 17.Forman BM. The PPARs: it’s not over ‘til the fat lady sings. Trends Mol Med. 2001;7:331–332. doi: 10.1016/s1471-4914(01)02074-3. [DOI] [PubMed] [Google Scholar]
- 18.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kliewer SZ, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, Devchand P, Wahli W, Willson TM, Lenhard JM, Lehmann JM. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome-activated receptors alpha and gamma. Proc Natl Acad Sci USA. 1997;94:4318–4323. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu HE, Lambert HM, Montana VG, et al. Molecular recognition of fatty acids by peroxisome proliferator-activated receptors. Mol Cell. 1999;3:397–403. doi: 10.1016/s1097-2765(00)80467-0. [DOI] [PubMed] [Google Scholar]
- 21.Turner ND, Zhang J, Davidson LA, Lupton JR, Chapkin RS. Oncogenic ras alters sensitivity to mouse colonocytes to butyrate and fatty acid mediated growth arrest and apoptosis. Cancer Lett. 2002;186:29–35. doi: 10.1016/s0304-3835(02)00325-7. [DOI] [PubMed] [Google Scholar]
- 22.Whitehead RH, VanEeden PE, Noble MD, Ataliotis P, Jat PS. Establishment of conditionally immortalized epithelial cell lines from both colon and small intestine of adult H-2Kb-tsA58 transgenic mice. Proc Natl Acad Sci USA. 1993;90:587–591. doi: 10.1073/pnas.90.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fan YY, Zhang J, Barhoumi R, Burghardt RC, Turner N, Lupton JR, Chapkin RS. Antagonism of CD95 (APO-1/Fas) signaling blocks butyrate induction of apoptosis in young adult mouse colonic (YAMC) cells. Am J Physiol. 1999;277:C310–C319. doi: 10.1152/ajpcell.1999.277.2.C310. [DOI] [PubMed] [Google Scholar]
- 24.Aukema HM, Davidson LA, Pence BC, Jiang YH, Lupton JR, Chapkin RS. Butyrate alters activity of specific cAMP-receptor proteins in a transgenic mouse colonic cell line. J Nutr. 1997;127:18–24. doi: 10.1093/jn/127.1.18. [DOI] [PubMed] [Google Scholar]
- 25.Moyer MP, Manzano LA, Merriman RL, Stauffer JS, Tanzer LR. NCM460, a normal human colon epithelial cell line. In Vitro Cell Dev Bio Animal. 1996;32:315–317. doi: 10.1007/BF02722955. [DOI] [PubMed] [Google Scholar]
- 26.Leid M, Kastner P, Lyons R, Nakshatri H, Saunders M, Zacharewski T, Chen JY, Staub A, Garnier JM, Mader S. Purification, cloning and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequence efficiently. Cell. 1992;68:377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- 27.Gupta RA, Brockman JA, Sarraf P, Willson TM, DuBois RN. Target genes of peroxisome proliferator-activated receptor γ in colorectal cancer cells. J Biol Chem. 2001;276:29681–29687. doi: 10.1074/jbc.M103779200. [DOI] [PubMed] [Google Scholar]
- 28.Hong MY, Chapkin RS, Morris JS, Wang N, Carroll RJ, Turner ND, Chang WC, Davidson LA, Lupton JR. Anatomical site-specific response to DNA damage is related to later tumor development in the rat azoxymethane colon carcinogenesis model. Carcinogenesis. 2001;22:1831–1835. doi: 10.1093/carcin/22.11.1831. [DOI] [PubMed] [Google Scholar]
- 29.Jiang YH, Lupton JR, Chapkin RS. Dietary fat and fiber modulate the effect of carcinogen on colonic protein kinase C lambda expression in rats. J Nutr. 1997;127:1938–1943. doi: 10.1093/jn/127.10.1938. [DOI] [PubMed] [Google Scholar]
- 30.Pickering JS, Lupton JR, Chapkin RS. Dietary fat, fiber and carcinogen alter fecal diacylglycerol composition and mass. Cancer Res. 1995;55:2293–2298. [PubMed] [Google Scholar]
- 31.Davidson LA, Lupton JR, Miskovsky E, Fields AP, Chapkin RS. Quantification of human intestinal gene expression profiles using exfoliated colonocytes: a pilot study. Biomarkers. 2003;8:51–61. doi: 10.1080/1354750021000042268. [DOI] [PubMed] [Google Scholar]
- 32.Ott EL, Longnecker M. An Introduction to Statistical Methods and Data Analysis. 5. Duxbury, NY: 2001. [Google Scholar]
- 33.Akiyama TE, Baumann CT, Sakai S, Hager GL, Gonzalez FJ. Selective intranuclear redistribution of PPAR isoforms by RXRα. Mol Endocrinol. 2002;16:707–721. doi: 10.1210/mend.16.4.0797. [DOI] [PubMed] [Google Scholar]
- 34.Darwiche B, Celli G, Tennenbaum T, Glick AB, Yuspa SH, DeLuca LM. Mouse skin tumor progression results in differential expression of retinoic acid and retinoid X receptors. Cancer Res. 1995;55:2774–2782. [PubMed] [Google Scholar]
- 35.Jiang S-Y. Expression of nuclear retinoid receptors in normal, premalignant and malignant gastric tissues determined by in situ hydridization. Br J Cancer. 1999;80:206–214. doi: 10.1038/sj.bjc.6690340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong MY, Lupton JR, Morris JS, Wang N, Carroll RJ, Davidson LA, Elder RH, Chapkin RS. Dietary fish oil reduces DNA adduct levels in rat colon in part by increasing apoptosis during tumor initiation. Cancer Epidemiol Biomark Prev. 2000;9:819–826. [PubMed] [Google Scholar]
- 37.Hong MY, Chapkin RS, Barhoumi R, Burghardt RC, Turner ND, Henderson CE, Sanders LM, Fan YY, Davidson LA, Murphy M, Carroll RJ, Lupton JR. Fish oil feeding increases the unsaturation index in mitochondrial phospholipids, enhancing reactive oxygen species generation and initiating apoptosis in rat colonocytes. Carcinogenesis. 2002;23:1919–1925. doi: 10.1093/carcin/23.11.1919. [DOI] [PubMed] [Google Scholar]
- 38.Bedi A, Pasricha PJ, Alchtar AJ, Barber JP, Bedi GC, Giairdiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- 39.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 40.Siniscrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995;55:237–241. [PubMed] [Google Scholar]
- 41.Bourguet W, Vivat VA, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000;5:289–298. doi: 10.1016/s1097-2765(00)80424-4. [DOI] [PubMed] [Google Scholar]
- 42.Egea PF, Mitschler A, Moras D. Molecular recognition of agonist ligands by RXRs. Mol Endocrinol. 2002;16:987–997. doi: 10.1210/mend.16.5.0823. [DOI] [PubMed] [Google Scholar]
- 43.Conquer JA, Holub BJ. Effect of supplementation with different doses of DHA on the levels of circulating DHA as non-esterified fatty acid in subjects of Asian Indian background. J Lipid Res. 1998;39:286–292. [PubMed] [Google Scholar]
- 44.Radominska A, Chen G. Photoaffinity labeling of human retinoid X receptor β (RXRβ) with 9-cis retinoic acid: Identification of phytanic acid, docosahexaenoic acid and lithocholic acid as ligands for RXRβ. Biochemistry. 2002;41:4883–4890. doi: 10.1021/bi0121151. [DOI] [PubMed] [Google Scholar]
- 45.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 46.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 47.Solomin L, Johansson CB, Zetterstrom RH, Bissonnette RP, Heyman RA, Olson L, Lendahl U, Frisen J, Perlmann T. Retinoid-X receptor signalling in the developing spinal cord. Nature. 1998;395:398–402. doi: 10.1038/26515. [DOI] [PubMed] [Google Scholar]
- 48.Easwaran V, Pishvaian M, Salimuddin, Byers S. Cross-regulation of beta-catenin-LEF/TCF and retinoid signaling pathways. Curr Biol. 1999;9:1415–1418. doi: 10.1016/s0960-9822(00)80088-3. [DOI] [PubMed] [Google Scholar]
- 49.Horn V, Minucci S, Ogryzko V, Adamson ED, Howard BH, Levin A, Ozato K. RAR and RXR selective ligands cooperatively induce apoptosis and neuronal differentiation in P19 embryonal carcinoma cells. FASEB J. 1996;10:1071–1077. doi: 10.1096/fasebj.10.9.8801169. [DOI] [PubMed] [Google Scholar]
- 50.Lotan R. Retinoids in cancer chemoprevention. FASEB J. 1996;10:1031–1039. doi: 10.1096/fasebj.10.9.8801164. [DOI] [PubMed] [Google Scholar]
- 51.Nagy L, Thomazy VA, Shipley GL, Fesus L, Lamph W, Heyman RA, Chandraratna RAS, Davies PJA. Activation of retinoid X receptors induces apoptosis in HL-60 cell lines. Mol Cell Biol. 1995;15:3540–2551. doi: 10.1128/mcb.15.7.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- 53.Lee JY, Hwang DH. Docosahexaenoic acid suppresses the activity of peroxisome proliferators-activated receptors in a colon tumor cell line. Biochem Biophys Res Commun. 2002;298:667–674. doi: 10.1016/s0006-291x(02)02530-5. [DOI] [PubMed] [Google Scholar]
- 54.Chang WC, Chapkin RS, Lupton JR. Fish oil blocks azoxymethane-induced tumorigenesis by increased cell differentiation and apoptosis rather than decreased cell proliferation. J Nutr. 1998;128:491–497. doi: 10.1093/jn/128.3.491. [DOI] [PubMed] [Google Scholar]
- 55.Murray NR, Weems C, Chen L, Leon J, Yu W, Davidson LA, Jamieson L, Chapkin RS, Thompson A, Fields AP. Protein kinase CβII and TGFβRII in ω-3 fatty acid-mediated inhibition of colon carcinogenesis. J Cell Biol. 2002;157:915–920. doi: 10.1083/jcb.200201127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cao Y, Pearman T, Zimmerman GA, McIntyre TM, Prescott SM. Intracellular unesterified arachidonic acid signals apoptosis. Proc Natl Acad Sci USA. 2000;97:11280–11285. doi: 10.1073/pnas.200367597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan NS, Shaw NS, Vinckenbosch N, Liu P, Yasmin R, Desvergne B, Wahli W, Noy N. Selective cooperation between fatty acid binding proteins and peroxisome proliferator-activated receptors in regulating transcription. Mol Cell Biol. 2002;22:5114–5127. doi: 10.1128/MCB.22.14.5114-5127.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamashita A, Sugiura T, Waku K. Acyltransferases and transacylases involved in fatty acid remodeling of phospholipids and metabolism of bioactive lipids in mammalian cells. J Biochem. 1997;122:1–16. doi: 10.1093/oxfordjournals.jbchem.a021715. [DOI] [PubMed] [Google Scholar]
- 59.Steinegar HH, Arntsen B, Spydevold O, Sorensen HN. Gene transcription of the retinoid X receptor α (RXRα) is regulated by fatty acids and hormones in rat hepatic cells. J Lipid Res. 1998;39:744–754. [PubMed] [Google Scholar]
- 60.Xu XC, Wong WYL, Goldberg L, Baer SC, Wolf JE, Ramsdell WM, Alberts DS, Lippman SM, Lotan R. Progressive decreases in nuclear retinoid receptors during skin squamous carcinogenesis. Cancer Res. 2001;61:4306–4310. [PubMed] [Google Scholar]