Abstract
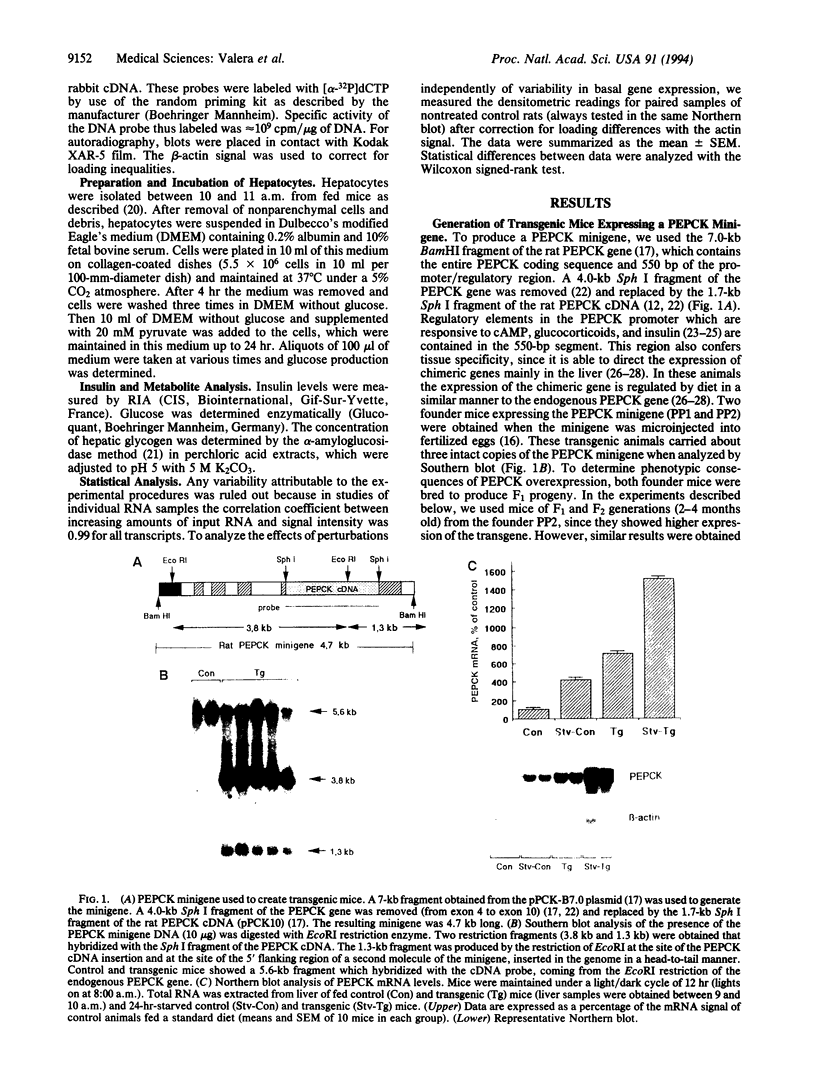
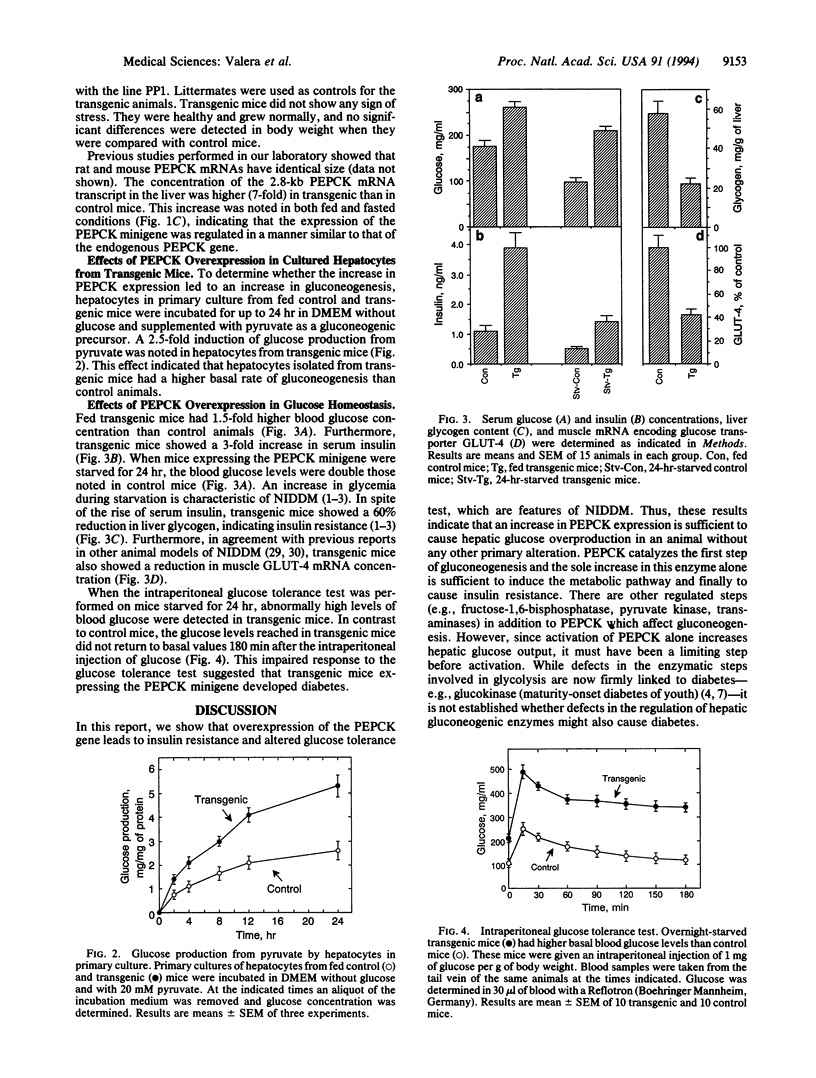
An increase in hepatic gluconeogenesis is believed to be an important factor responsible for the fasting hyperglycemia detected in patients with non-insulin-dependent diabetes mellitus (NIDDM). Phosphoenolpyruvate carboxykinase (GTP) (PEPCK; EC 4.1.1.32) is a regulatory enzyme of gluconeogenesis. To study the role of the expression of PEPCK gene in the development of NIDDM, we have produced lines of transgenic mice expressing a PEPCK minigene under control of its own promoter. Transgenic mice were hyperglycemic and had higher serum insulin concentrations. In addition, alterations in liver glycogen content and muscle glucose transporter GLUT-4 gene expression were detected. The overexpression of the PEPCK gene led to an increase in glucose production from pyruvate in hepatocytes in primary culture. When intraperitoneal glucose tolerance tests were performed, blood glucose levels were higher than those detected in normal mice. This animal model shows that primary alterations in the rate of liver glucose production may induce insulin resistance and NIDDM.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale E. G., Chrapkiewicz N. B., Scoble H. A., Metz R. J., Quick D. P., Noble R. L., Donelson J. E., Biemann K., Granner D. K. Rat hepatic cytosolic phosphoenolpyruvate carboxykinase (GTP). Structures of the protein, messenger RNA, and gene. J Biol Chem. 1985 Sep 5;260(19):10748–10760. [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Bonadonna R. C., Ferrannini E. Pathogenesis of NIDDM. A balanced overview. Diabetes Care. 1992 Mar;15(3):318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Simonson D. C. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989 Apr;38(4):387–395. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- Dimitrakoudis D., Ramlal T., Rastogi S., Vranic M., Klip A. Glycaemia regulates the glucose transporter number in the plasma membrane of rat skeletal muscle. Biochem J. 1992 Jun 1;284(Pt 2):341–348. doi: 10.1042/bj2840341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Flier J. S. Lilly Lecture: syndromes of insulin resistance. From patient to gene and back again. Diabetes. 1992 Sep;41(9):1207–1219. doi: 10.2337/diab.41.9.1207. [DOI] [PubMed] [Google Scholar]
- Friedman J. E., de Venté J. E., Peterson R. G., Dohm G. L. Altered expression of muscle glucose transporter GLUT-4 in diabetic fatty Zucker rats (ZDF/Drt-fa). Am J Physiol. 1991 Dec;261(6 Pt 1):E782–E788. doi: 10.1152/ajpendo.1991.261.6.E782. [DOI] [PubMed] [Google Scholar]
- Granner D., Andreone T., Sasaki K., Beale E. Inhibition of transcription of the phosphoenolpyruvate carboxykinase gene by insulin. Nature. 1983 Oct 6;305(5934):549–551. doi: 10.1038/305549a0. [DOI] [PubMed] [Google Scholar]
- Groop L. C., Kankuri M., Schalin-Jäntti C., Ekstrand A., Nikula-Ijäs P., Widén E., Kuismanen E., Eriksson J., Franssila-Kallunki A., Saloranta C. Association between polymorphism of the glycogen synthase gene and non-insulin-dependent diabetes mellitus. N Engl J Med. 1993 Jan 7;328(1):10–14. doi: 10.1056/NEJM199301073280102. [DOI] [PubMed] [Google Scholar]
- Imai E., Stromstedt P. E., Quinn P. G., Carlstedt-Duke J., Gustafsson J. A., Granner D. K. Characterization of a complex glucocorticoid response unit in the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1990 Sep;10(9):4712–4719. doi: 10.1128/mcb.10.9.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Cam A., Guillouzo A., Freychet P. Ultrastructual and biochemical studies of isolated adult rat hepatocytes prepared under hypoxic conditions. Cryopreservation of hepatocytes. Exp Cell Res. 1976 Mar 15;98(2):382–395. doi: 10.1016/0014-4827(76)90448-1. [DOI] [PubMed] [Google Scholar]
- Liu J. S., Park E. A., Gurney A. L., Roesler W. J., Hanson R. W. Cyclic AMP induction of phosphoenolpyruvate carboxykinase (GTP) gene transcription is mediated by multiple promoter elements. J Biol Chem. 1991 Oct 5;266(28):19095–19102. [PubMed] [Google Scholar]
- Magnuson M. A., Quinn P. G., Granner D. K. Multihormonal regulation of phosphoenolpyruvate carboxykinase-chloramphenicol acetyltransferase fusion genes. Insulin's effects oppose those of cAMP and dexamethasone. J Biol Chem. 1987 Nov 5;262(31):14917–14920. [PubMed] [Google Scholar]
- Magnusson I., Rothman D. L., Katz L. D., Shulman R. G., Shulman G. I. Increased rate of gluconeogenesis in type II diabetes mellitus. A 13C nuclear magnetic resonance study. J Clin Invest. 1992 Oct;90(4):1323–1327. doi: 10.1172/JCI115997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992 Oct 30;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- McGrane M. M., Yun J. S., Moorman A. F., Lamers W. H., Hendrick G. K., Arafah B. M., Park E. A., Wagner T. E., Hanson R. W. Metabolic effects of developmental, tissue-, and cell-specific expression of a chimeric phosphoenolpyruvate carboxykinase (GTP)/bovine growth hormone gene in transgenic mice. J Biol Chem. 1990 Dec 25;265(36):22371–22379. [PubMed] [Google Scholar]
- McGrane M. M., de Vente J., Yun J., Bloom J., Park E., Wynshaw-Boris A., Wagner T., Rottman F. M., Hanson R. W. Tissue-specific expression and dietary regulation of a chimeric phosphoenolpyruvate carboxykinase/bovine growth hormone gene in transgenic mice. J Biol Chem. 1988 Aug 15;263(23):11443–11451. [PubMed] [Google Scholar]
- O'Brien R. M., Lucas P. C., Forest C. D., Magnuson M. A., Granner D. K. Identification of a sequence in the PEPCK gene that mediates a negative effect of insulin on transcription. Science. 1990 Aug 3;249(4968):533–537. doi: 10.1126/science.2166335. [DOI] [PubMed] [Google Scholar]
- Permutt M. A., Chiu K. C., Tanizawa Y. Glucokinase and NIDDM. A candidate gene that paid off. Diabetes. 1992 Nov;41(11):1367–1372. doi: 10.2337/diab.41.11.1367. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Glucokinase and candidate genes for type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993 Apr;36(4):269–275. doi: 10.1007/BF00400227. [DOI] [PubMed] [Google Scholar]
- Short M. K., Clouthier D. E., Schaefer I. M., Hammer R. E., Magnuson M. A., Beale E. G. Tissue-specific, developmental, hormonal, and dietary regulation of rat phosphoenolpyruvate carboxykinase-human growth hormone fusion genes in transgenic mice. Mol Cell Biol. 1992 Mar;12(3):1007–1020. doi: 10.1128/mcb.12.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R., Agius L. The biochemistry of diabetes. Biochem J. 1988 Mar 15;250(3):625–640. doi: 10.1042/bj2500625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S. I. Lilly Lecture: molecular mechanisms of insulin resistance. Lessons from patients with mutations in the insulin-receptor gene. Diabetes. 1992 Nov;41(11):1473–1490. doi: 10.2337/diab.41.11.1473. [DOI] [PubMed] [Google Scholar]
- Yoo-Warren H., Monahan J. E., Short J., Short H., Bruzel A., Wynshaw-Boris A., Meisner H. M., Samols D., Hanson R. W. Isolation and characterization of the gene coding for cytosolic phosphoenolpyruvate carboxykinase (GTP) from the rat. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3656–3660. doi: 10.1073/pnas.80.12.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]




