Summary
Background
To retrospectively analyze the outcomes of interventional radiology treatment of patients with hepatic artery stenosis (HAS) after liver transplantation at our Institution.
Material/Methods
Hepatic artery stenosis was diagnosed and treated by endovascular technique in 8 (2.8%) patients, who underwent liver transplantation between July 2007 and July 2011. Patients entered the follow-up period, during which we analyzed hepatic artery patency with Doppler ultrasound at 1, 3, 6, and 12 months after percutaneous endovascular treatment (PTA), and every six months thereafter.
Results
During the 12-month follow-up period, 6 out of 8 patients (75%) were asymptomatic with patent hepatic artery, which was confirmed by multislice computed tomography (MSCT) angiography, or color Doppler (CD) ultrasound. One patient had a fatal outcome of unknown cause, and one patient underwent orthotopic liver retransplantation (re-OLT) procedure due to graft failure.
Conclusions
Our results suggest that HAS angioplasty and stenting are minimally invasive and safe endovascular procedures that represent a good alternative to open surgery, with good 12-month follow-up patency results comparable to surgery.
MeSH Keywords: Angioplasty, Hepatic Artery, Liver Transplantation
Background
Liver transplantation (LT) is nowadays a well-accepted treatment option for end-stage liver disease and acute liver failure. One of the main vascular complications after liver transplantation is hepatic artery stenosis (HAS) which can lead to deterioration of liver function, billiary damage, sepsis due to graft ischaemia, and possible progression to hepatic artery thrombosis [1]. Therefore, hepatic artery stenosis is associated with a high incidence of morbidity and mortality [2].
Hepatic artery stenoses are classified according to their location and multiplicity. Since this is a surgical complication, the classification according to the location is based on relative setting from the surgical anastomosis. There are different methods for treatment of HAS. Traditionally, the treatment of HAS included anticoagulation, surgical revascularisation, and even retransplantation [3]. However, retransplantation is limited, both by organ availability and by patient’s condition, and surgical revascularisation has a high risk of serious complications because of condensed adhesions around the liver and vessels [2]. With advancement of interventional radiological techniques, advancement in materials used (stents, balloons, wires), percutaneous interventional radiology procedures (IRP) have a promising role in the management of post-transplantation hepatic artery stenosis. To date, numerous case reports and case series describing interventional radiology treatment of HAS have been reported [4–10]. The purpose of our study was to retrospectively review and analyze the outcomes and technical successes of interventional radiology treatment in vascular complications after liver transplantation.
Material and Methods
A total of 292 liver transplantations were performed in our Institution on 281 patients between July 2007 and July 2011, including 7 simultaneous liver and kidney transplantations. The re-transplantation rate was 3.9% due to early post-operative complications including hepatic artery thrombosis, graft dysfunction etc. Hepatic artery stenosis was diagnosed and treated by endovascular technique in 8 (2.8%) patients. All patients gave their informed consent and underwent the procedure according to a protocol approved by the institutional review board. In Table 1 patients’ characteristics are summarized. There were 4 male and 4 female patients, with a mean age of 50.1 years (range 16 to 66 years). Indications for liver transplantation included alcoholic liver disease (ALD) (n=3), hepatocellular carcinoma (n=2), Wilson’s disease (n=1), autoimmune liver disease (n=1), and acute liver failure as a result of paracetamol overdose (n=1). Five of our patients underwent orthotopic liver transplantation (OLT), two living donor liver transplantation (LDLT) and one patient split transplantation of the right lobe. Immunosuppressive protocol consisted of calcineurin inhibitors (CNIs) namely tacrolimus (Prograf, Janssen-Cilag, Australia) or cyclosporine (Neoral, Novartis, Basel, Switzerland), mycophenolate mofetil (CellCept, Roche, Basel, Switzerland) and corticosteroids (Decortin, Merck KGaA, Darmstadt, Germany). Corticosteroids were administered for up to three months after LT. All patients received valganciclovir (Valcyte, Roche, Basel, Switzerland) sulfamethoxazole and trimethoprim (Sinersul, Pliva, Zagreb, Croatia) for prophylaxis of cytomegalovirus and Pneumocistis jiroveci infection for up to three months or up to 6–9 months, respectively. Immunosuppressive and prophylactic regimen was recorded at the time of stenting. Mean time between LT and IRP was 16 days (range from 2 to 61 days). Seven patients had a stent placed at the site of stenosis, one patient had PTA without stenting.
Table 1.
Characteristics of transplanted patients with hepatic artery stenosis.
| Case | Gende/age | Primary disease | Type of Tx | Tx-PTA interval (days) | Stent | Follow-up (months) | Clinical outcome* |
|---|---|---|---|---|---|---|---|
| 1 | F/21 | ALF (paracetamol) | OLT ×2 | 61 | No | 5 days | Death |
| 2 | F/16 | Wilson’s disease | LDLT | 16 | Yes | 48 | Asymptomatic |
| 3 | M/66 | ALD | OLT | 2 | Yes | 36 | Asymptomatic |
| 4 | F/60 | HCC | LDLT | 22 | Yes | 16 | Death |
| 5 | M/64 | HCC | OLT | 13 | Yes | 17 | Asymptomatic |
| 6 | M/50 | ALD | OLT | 3 | Yes | 13 | Asymptomatic |
| 7 | F/60 | Autoimmune liver disease | Split (right lobe) | 6 | Yes | 12 | Retransplantation |
| 8 | M/64 | ALD | OLT | 5 | Yes | 12 | Asymptomatic |
Clinical outcome at the time of writing this article.
Tx – transplantation; Tx-PTA – transplantation-percutaneous transluminal angioplasty; ALF – acute liver failure; OLT – orthotopic liver transplantation; LDLT – living donor liver transplantation; ALD – alcoholic liver disease; HCC – hepatocellular carcinoma.
None of the 8 patients received prophylactic anticoagulation therapy after LT. Four patients were receiving antiplatelet therapy (clopidogrel 75 mg/day) before LT (due to preexisting comorbidities) and remained on it after the procedure (Table 2). After IRP, dual antiplatelet therapy was given to all patients, clopidogrel (75 mg/day) and acetylsalicylic acid (100 mg/day) for up to three months after the procedure. In Table 2 patients’ preexisting comorbidities are summarized: cerebrovascular insult (n=1), coronary disease (n=1), kidney failure (n=1), hypertension (n=4) and diabetes mellitus (n=2).
Table 2.
Patients’ comorbidities and use of antiplatelet therapy before LT.
| Case | Antiplatelet therapy | CVI | Coronary disease | Kidney failure | Hypertension | Diabetes |
|---|---|---|---|---|---|---|
| 1 | No | No | No | No | No | No |
| 2 | No | No | No | No | No | No |
| 3 | No | No | No | Yes (chronic) | Yes | Yes |
| 4 | Yes | No | Yes | No | Yes | Yes |
| 5 | Yes | No | No | No | No | No |
| 6 | Yes | Yes | No | No | No | No |
| 7 | No | No | No | No | Yes | No |
| 8 | Yes | No | No | No | Yes | No |
LT – liver transplantation; CVI – cerebrovascular insult.
CD follow-ups were performed daily in the first week after LT, monthly after hospital discharge within the first 6 months, and in case of any blood liver test abnormalities after that period. Right and left hepatic arteries were evaluated and resistance index (RI) was calculated. Patients with abnormal CD finding were scheduled for multislice computed tomography (MSCT) angiography or digital subtraction angiography (DSA) (Figure 1). Hepatic artery stenosis was suspected when an intrahepatic Doppler waveform showed a prolonged systolic acceleration time (≥0.08 sec.) and a low RI (<0.5), and hepatic artery stenosis was confirmed by the detection of a focal peak velocity greater than 2 m/s. Early stenosis was defined as a stenosis that occurred within first 30 days after LT, and late stenosis after that period.
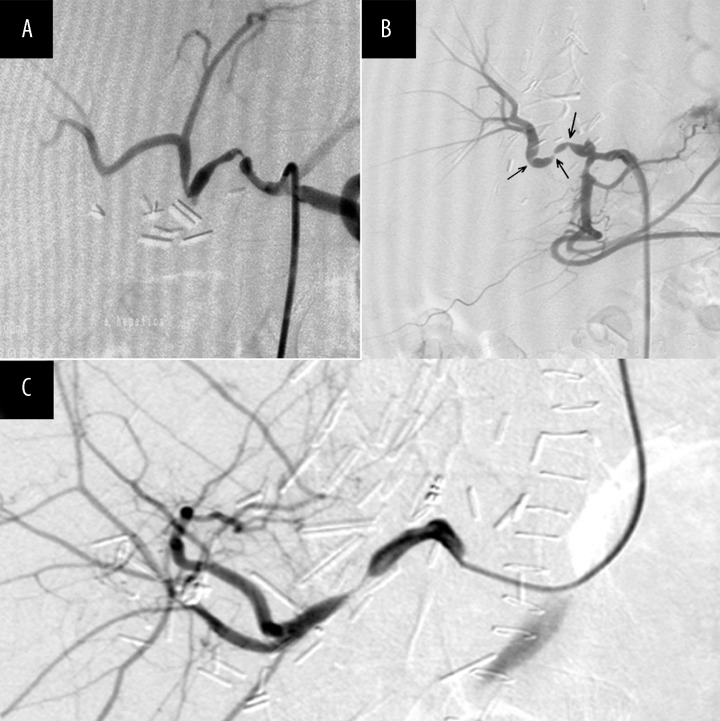
Figure 1.
Selective DSA of hepatic artery with two significant stenoses of the hepatic artery in the transplanted liver (A), selective DSA of hepatic artery using transfemoral approach showing three significant stenoses of the hepatic artery in the transplanted liver (B) and selective DSA of hepatic artery using a transbrachial approach, showing significant stenosis of the hepatic artery in the transplanted liver (C).
We used transfemoral or transbrachial approach in our patients, depending on the vascular anatomy of the celiac trunk, location of arterial anastomosis, and angulations of the anastomotic hepatic artery. A 4F sheath and a “pig tail” flush catheter were used to perform diagnostic angiography of the aorta and visceral arteries. Interventional procedures were carried out in full heparinization (5000 IU i.a.) with the use of 8F guiding catheters or 6F sheaths parked in the celiac trunk in order to obtain a stable position of wires and balloon catheters during maneuvering through tortuous vessels. Stenoses were crossed or selected with various types of 0.14″ wires (Nitrex, EV3, Plymouth, [MN] USA; Spartacore, Abbott Vascular, Beringen, CH; PT2, Boston Scientific, Miami, [FL] USA), and then PTA or PTA + stenting was performed. In case of a suboptimal PTA result, the stent was placed over the stenosed vessel segment. PTA without stenting was performed in one patient. We used both balloon-expandable and self-expandable stents, the choice of which was determined primarily on available dimensions in our IR operating theatre. Balloon-expandable stents were placed in five patients (Nexus II, OCCAM International, Eindhoven, NL; Palmaz BLUE, Cordis, Miami Lakes, [FL] USA; Tsunami GOLD, Terumo, Leuven, B; Palmaz GENESIS, Cordis, Miami Lakes (FL) USA). One of the implanted balloon-expandable stents was a coronary drug-eluting stent (DES) (Xience, Abbott Vascular, Beringen, CH). Self-expandable stents used included X-ACT (Abbott Vascular, Beringen, CH) and X-PERT (Abbott Vascular, Beringen, CH). Haemostasis was achieved with Exoseal (Cordis, Miami Lakes [FL] USA) vascular closure system.
After 24 hours following the procedure, CD was performed to evaluate hepatic artery flow, together with liver function tests. Also, for success analysis, during the follow-up period we evaluated the development of restenosis, pseudoaneurism, as well as development of dissection or rupture of artery, stent dislocation or distal embolism, during the procedure.
Results
Technical success was achieved in all 8 (100%) patients (Figure 2). We had no intraprocedural complications such as arterial rupture, dissection, acute hepatic artery thrombosis or stent misplacement. Patient no. 1 had 2 OLT procedures; the first one due to acute liver failure, and the second OLT due to primary graft dysfunction. Sixty days after the second OLT procedure, hepatic artery stenosis was detected and the patient was treated with PTA. Hepatic artery stenosis was treated with PTA at 60 days after the second LT, with satisfying results. Patency of the hepatic artery was achieved, but 5 days later the patient died of multi-organ failure.
Figure 2.
Selective DSA of hepatic artery post-balloon angioplasty showing a good vessel patency (A), selective DSA of common hepatic artery using a transfemoral approach after balloon-expandable stent placement (thick arrow) in the hepatic artery of the transplanted liver with visible artery spasms (thin arrow) (B), balloon-expandable stent dilation in subtotal hepatic artery stenosis in the transplanted liver (C) and selective DSA of hepatic artery using a transbrachial approach, showing the hepatic artery of the transplanted liver after balloon-expandable stent placement (arrow) (D).
Patient no. 4 had a successful procedure, with a patent artery after 12 months of follow-up, but died 16 months later of unknown cause.
One month after PTA, patient no. 7 showed a patent hepatic artery, but 3 months later graft failure developed and led to successful re-OLT, after which the patient was excluded from the follow-up.
Patient no. 8 developed splenic artery steal syndrome (Figure 3) three months after LT, but remained asymptomatic and with a patent hepatic artery during the follow-up.
Figure 3.
Selective DSA of coeliac trunk (catheter tip at the offspring of the common hepatic artery in the transplanted liver) with significant splenic artery steal syndrome (A), selective DSA of common hepatic artery in the transplanted liver with significant subtotal stenosis of the hepatic artery (arrow) (B), balloon-expandable stent dilatation in subtotal hepatic artery stenosis (C) and selective DSA of hepatic artery in the transplanted liver after placing a balloon-expandable coronary stent (D).
To summarize our results, during the 12-month follow-up period, 6 out of 8 patients (75%) were asymptomatic, with a patent hepatic artery, which was confirmed by MSCT angiography or CD ultrasound (Table 3). One patient had a fatal outcome of unknown cause, and one patient underwent a re-OLT procedure due to a graft failure. During the procedure, no pseudoaneurism occurred, there was no dissection or rupture of the artery, stent dislocation or distal embolism. In the 12-month follow-up we did not record restenosis in any of our patients.
Table 3.
Results of HA patency (immediately and 1–48 months after IR procedure).
| N | HA 0 | HA 1 | HA 3 | HA 6 | HA 12 | HA 18 | HA 24 | HA 36 | HA 48 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Patent | Death | |||||||
| 2 | Patent | Patent | Patent | Patent | Patent | Patent | Patent | Patent | Patent |
| 3 | Patent | Patent | Patent | Patent | Patent | Patent | Patent | Patent | |
| 4 | Patent | Patent | Patent | Patent | Patent | Death | |||
| 5 | Patent | Patent | Patent | Patent | Patent | Patent | |||
| 6 | Patent | Patent | Patent | Patent | Patent | ||||
| 7 | Patent | Patent | Re-OLT | ||||||
| 8 | Patent | Patent | Patent (SASS) | Patent (SASS) | Patent (SASS) |
HA – hepatic artery; IR – interventional radiology; re-OLT – retransplantation; SASS – splenic artery steal syndrome.
Discussion
Hepatic artery stenosis after liver transplantation usually occurs at the anastomotic site of the donor’s and recepient’s arteries within 3 months after surgery, with the incidence falling between 3 and 11% [2,11]. Out of 281 liver recipients subjected to transplantation between July 2007 and July 2011 at our Liver Transplant Center, 2.8% showed elevated liver chemistry tests. Stenosis of the hepatic artery was the proposed diagnosis, as it may present with unexplained elevated liver chemistry tests [12]. Color Doppler analysis confirmed symptomatic HAS.
Generally, HAS may be classified into: anastomotic (A-HAS) occurring at the surgical anastomosis, proximal (P-HAS) or distal (D-HAS) occurring at the recipient’s or donor’s artery, respectively [3]. All the treated patients with hepatic artery stenosis had anastomotic HAS. Risk factors of hepatic artery stenosis are numerous and include operative technique, vascular clamp injury, allograft rejection, preservation injury, anastomosis of the small arteries and procoagulant disorders [13,14]. Clinical signs of hepatic artery obstruction are: increased serum transaminase levels, cholestasis, liver abscess, ischemic biliary lesions, cholangitis, bile duct stenosis or necrosis. In addition, initial non-function and allograft dysfunction can occur [15]. The causes of hepatic dysfunction in transplant recipients are frequently multifactorial, and the pattern or severity of liver enzyme abnormalities is of little value in identifying the definitive cause of hepatic dysfunction [16]. It is important to note that hepatic artery stenosis may be asymptomatic, presenting only with moderate abnormalities in liver function tests. However, over time, asymptomatic HAS may lead to persistent ischemia and graft failure [14]. About 20% of patients have asymptomatic courses [17]. Therefore, early detection is of paramount importance for graft and patient survival. A mild degree of hepatic artery narrowing may be present even without Doppler abnormalities. Therefore, if the clinical suspicion is high, normal Doppler results should not prevent angiography follow-up [18]. In contrast to hepatic artery thrombosis, the clinical presentation of hepatic artery stenosis is variable and nonspecific [19]. Successful liver transplantation depends on uncompromised hepatic arterial inflow [19]. If left untreated, it can lead to hepatic artery thrombosis due to a slow flow or progress to liver ischemia with hepatic insufficiency, biliary strictures, sepsis, and graft loss [6]. The risk of thrombosis in non-treated hepatic artery stenosis is reported as up to 65%, which decreases to 19% after a successful treatment [3]. Thus, it is important to consider an interventional radiology procedure if asymptomatic stenosis is detected. Recently, the arterial steal syndromes have been recognized as one of the causes of hepatic hypoperfusion after LT [20]. These syndromes are characterized by a low arterial flow toward the graft, caused by a shift of flow into the splenic artery, called splenic artery steal syndrome (the most common), or into the gastroduodenal artery (gastroduodenal artery steal syndrome) [20]. In our study, only one patient was diagnosed with splenic artery steal syndrome, 3 months after the stenting procedure, although the hepatic artery remained patent and the patient was asyptomatic for 12 months of the follow-up period (Figure 3).
Recently, stenotic vessels may be managed by two new modalities: drug-eluting stents (DES) or drug-eluting balloons (DEB). Development of DEB derives mainly from the limitations of DES.
DES carries the risk of late or very late stent thrombosis, with a hazard ratio of up to 0.6 per year. This is a result of delayed healing, local inflammation, and impaired endothelial function, which lead to prolonged dual antiplatelet therapy. Restenosis is also reported with DES, especially in complex subsets of patients and lesions [21]. Nonstent-based local drug delivery using DEB maintains the antiproliferative properties of DES, but without the limitations of DES [21]. Another benefit of the DEB-based technology is that it is potentially cheaper, as balloon catheters are invariably cheaper than stents [22]. Moreover, DEB may be used in subsets of lesions where DES cannot be delivered or where DES does not perform well, such as in torturous vessels, small vessels, or long diffuse calcified lesions, which can result in stent fractures [21]. However, DEB cannot overcome the mechanical limitation of acute recoil seen after balloon angioplasty. Furthermore, it is not clear whether DEB can eliminate the late negative remodeling seen with noncoated balloons. Other potential limitations of DEB include the variability of pharmacokinetics and control [21].
We used a balloon-expandable stent coated with drug (Terumo Tsunami GOLD) in patient no. 4, who had a successful procedure, with a patent artery for 12 months of the follow-up period, and who died 16 months later of unknown cause.
The occurrence of restenosis in patients with PTA but without stenting reaches up to 33.3%, as reported by Kodama et al. [23]. However, Ueno et al. noted that restenosis is also common (25%) after stent placement [24]. A more recent large meta-analysis of case series from Rostambeigi et al. [25], which aimed to compare percutaneous balloon angioplasty (PBA) with stent placement, showed that PBA and stent placement are both efficacious, with similar complication rates. In our study, except for one death outcome, there was no restenosis (with or without stent) recorded during the follow-up, in accordance with the latter article. This leads us to a conclusion that individual approach should be considered, with both equal choices, taking into account the individual patient’s characteristics and lesion morphology. All patients had a patent HA immediately after the procedure, with normal follow-up results (HA patency) at 1, 3, 6, and 12 months. Some reports [24] suggest that percutaneous interventional procedures should not be performed earlier than 3 weeks after LT because of a high risk of anastomosis rupture, while others [23] suggest that such procedures can be performed as soon as one week after LT. In our case, the mean time between LT and the procedure was 16 days (range from 2 to 61 days), with no complications whatsoever. Taking into account a small number of treated patients, our results support a successful mid-term outcome of HAS after LT. PTA of HAS may help prevent biliary strictures and allow good long-term graft function in the majority of patients [6]. Therefore, based on currently growing data in the literature compatible with our results, the first-choice treatment for HAS should be evaluated in clinical trials, challenging the surgical procedure against alternative treatment modalities.
The two largest studies of HAS recommended a multidisciplinary approach, interventional radiology and open surgical management, as the best treatment option [5,6]. Because of the rapid development of new techniques in interventional radiology, interventional vascular procedures are used increasingly often as an alternative for the treatment of hepatic artery stenosis, and our results support the notion that percutaneous endovascular treatment is an important therapeutic alternative to surgical treatment (surgical revascularization and/or liver retransplantation). Since interventional procedures carry less morbidity and mortality than open surgery for arterial revascularization, some authors suggest that endovascular first strategy should be the appropriate approach to these types of lesions, followed by surgery in case of technical failures or complications [3,5].
Conclusions
Our results suggest that HAS angioplasty and stenting are minimally invasive and safe endovascular procedures that represent a good alternative to open surgery, with good 12-month follow-up patency results comparable to surgery [26].
Footnotes
Conflict of interest
None.
Satement
The authors received no financial support for the research and authorship.
References
- 1.Maruzzelli L, Miraglia R, Caruso S, et al. Percutaneous endovascular treatment of hepatic artery stenosis in adult and pediatric patients after liver transplantation. Cardiovasc Intervent Radiol. 2010;33:1111–19. doi: 10.1007/s00270-010-9848-4. [DOI] [PubMed] [Google Scholar]
- 2.Huang M, Shan H, Jiang Z, et al. The use of coronary stent in hepatic artery stenosis after orthotopic liver transplantation. Eur J Radiol. 2006;60:425–30. doi: 10.1016/j.ejrad.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 3.Saad WEA. Management of hepatic artery steno-occlusive complications after liver transplantation. Tech Vasc Interv Radiol. 2007;10:207–20. doi: 10.1053/j.tvir.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Orons PD, Zajko AB, Bron KM, et al. Hepatic artery angioplasty after liver transplantation: experience in 21 allografts. J Vasc Interv Radiol. 1995;6:523–29. doi: 10.1016/s1051-0443(95)71128-9. [DOI] [PubMed] [Google Scholar]
- 5.Saad WEA, Davies MG, Sahler LG, et al. Hepatic artery stenosis in liver transplant recipients: primary treatment with percutaneous transluminal angioplasty. J Vasc Interv Radiol. 2005;16:795–805. doi: 10.1097/01.RVI.0000156441.12230.13. [DOI] [PubMed] [Google Scholar]
- 6.Abassoglu O, Levy MF, Vodapally MS, et al. Hepatic artery stenosis after liver transplantation – incidence, presentation, treatment and long term outcome. Transplantation. 1997;63:250–55. doi: 10.1097/00007890-199701270-00013. [DOI] [PubMed] [Google Scholar]
- 7.Karatzas T, Lykaki Karatzas M, Webb JN, et al. Vascular complications, treatment and outcome following orthotopic liver transplantation. Transplant Proc. 1997;29:2853–55. doi: 10.1016/s0041-1345(97)00706-9. [DOI] [PubMed] [Google Scholar]
- 8.Denys AL, Qonadli SD, Durand F, et al. Feasibility and effectiveness of using coronary stents in the treatment of hepatic artery stenosis after orthotopic liver transplantation: preliminary report. Am J Roentgenol. 2002;178:1175–79. doi: 10.2214/ajr.178.5.1781175. [DOI] [PubMed] [Google Scholar]
- 9.Cotroneo AR, DiStasi C, Cina A, et al. Stent placement in four patients with hepatic artery stenosis or thrombosis after liver transplantation. J Vasc Interv Radiol. 2002;13:619–23. doi: 10.1016/s1051-0443(07)61657-1. [DOI] [PubMed] [Google Scholar]
- 10.Laštovičkova J, Peregrin J. Percutaneous transluminal angioplasty of hepatic artery stenosis in patients after orthotopic liver transplantation: mid-term results. Cardiovasc Intervent Radiol. 2011;34:1165–71. doi: 10.1007/s00270-010-0082-x. [DOI] [PubMed] [Google Scholar]
- 11.Quiroga S, Sebastia MC, Margarit C, et al. Complications of orthotopic liver transplantation: spectrum of findings with helical CT. Radiographics. 2001;21:1085–102. doi: 10.1148/radiographics.21.5.g01se061085. [DOI] [PubMed] [Google Scholar]
- 12.Haydon GH. Graft dysfunction. Graft. 2003;6:120–28. [Google Scholar]
- 13.Karani JB, Yu DF, Kane PA. Interventional radiology in liver transplantation. Cardiovasc Intervent Radiol. 2005;28:271–83. doi: 10.1007/s00270-004-0074-9. [DOI] [PubMed] [Google Scholar]
- 14.Boyvat F, Aytekin C, Harman A, et al. Endovascular Stent Placement In Patients With Hepatic Artery Stenoses Or Thromboses After Liver Transplant. Transplant Proc. 2008;40:22–26. doi: 10.1016/j.transproceed.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 15.Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl. 2003;9:612–20. doi: 10.1053/jlts.2003.50098. [DOI] [PubMed] [Google Scholar]
- 16.Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis in liver transplant recipients: prevalence and cholangiographic appearance of associated biliary complications. Am J Roentgenol. 1995;165:1145–49. doi: 10.2214/ajr.165.5.7572493. [DOI] [PubMed] [Google Scholar]
- 17.Silva RF, Raphe R, Felıcio HC, et al. Prevalence, treatment, and outcomes of the hepatic artery stenosis after liver transplantation. Transplant Proc. 2008;40:805–7. doi: 10.1016/j.transproceed.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 18.Crossin JD, Muradali D, Wilson SR. US of liver transplants: normal and abnormal. Radiographics. 2003;23:1093–114. doi: 10.1148/rg.235035031. [DOI] [PubMed] [Google Scholar]
- 19.Mueller AR, Platz KP, Kremer B. Early postoperative complications following liver transplantation. Best Pract Res. 2004;18:881–900. doi: 10.1016/j.bpg.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Criado A, Gilabert R, Berzigotti A, Bru C. Doppler ultrasound findings in the hepatic artery shortly after liver transplantation. Am J Roentgenol. 2009;193:128–35. doi: 10.2214/AJR.07.3919. [DOI] [PubMed] [Google Scholar]
- 21.Waksman R, Pakala R. Drug-eluting balloon: the comeback kid? Circ Cardiovasc Interv. 2009;2:352–58. doi: 10.1161/CIRCINTERVENTIONS.109.873703. [DOI] [PubMed] [Google Scholar]
- 22.Park K, Kim TE, Park KW. Analysis of potential cost-savings after introduction of drug-eluting balloon angioplasty for in-stent restenosis or small vessel disease. Korean Circ J. 2011;41:705–11. doi: 10.4070/kcj.2011.41.12.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kodama Y, Sakuhara Y, Abo D, et al. Percutaneous transluminal angioplasty for hepatic artery stenosis after living donor liver transplantation. Liver Transpl. 2006;12:465–69. doi: 10.1002/lt.20724. [DOI] [PubMed] [Google Scholar]
- 24.Ueno T, Jones G. Clinical outcomes from hepatic artery stenting in liver transplantation. Liver Transpl. 2006;12:422–27. doi: 10.1002/lt.20628. [DOI] [PubMed] [Google Scholar]
- 25.Rostambeigi N, Hunter D, Duval S, et al. Stent placement versus angioplasty for hepatic artery stenosis after liver transplant: a meta-analysis of case series. Eur Radiol. 2013;23(5):1323–34. doi: 10.1007/s00330-012-2730-9. [DOI] [PubMed] [Google Scholar]
- 26.Abbasoglu O, Levy MF, Vodapally MS, et al. Hepatic artery stenosis after liver transplantation-incidence, presentation, treatment, and long term outcome. Transplantation. 1997;63:250–55. doi: 10.1097/00007890-199701270-00013. [DOI] [PubMed] [Google Scholar]