Summary
Protein N-glycosylation can influence the nervous system in a variety of ways by affecting functions of glycoproteins involved in nervous system development and physiology. The importance of N-glycans for different aspects of neural development has been well documented. For example, some N-linked carbohydrate structures were found to play key roles in neural cell adhesion and axonal targeting during development. At the same time, the involvement of glycosylation in the regulation of neural physiology remains less understood. Recent studies have implicated N-glycosylation in the regulation of neural transmission, revealing novel roles of glycans in synaptic processes and the control of neural excitability. N-Glycans were found to markedly affect the function of several types of synaptic proteins involved in key steps of synaptic transmission, including neurotransmitter release, reception and uptake. Glycosylation also regulates a number of channel proteins, such as TRP channels that control responses to environmental stimuli and voltage-gated ion channels, the principal determinants of neuronal excitability. Sialylated carbohydrate structures play a particularly prominent part in the modulation of voltage gated ion channels. Sialic acids appear to affect channel functions via several mechanisms, including charge interactions, as well as other interactions that probably engage steric effects and interactions with other molecules. Experiments also indicated that some structural features of glycans can be particularly important for their function. Since glycan structures can vary significantly between different cell types and depends on the metabolic state of the cell, it is important to analyze glycan functions using in vivo approaches. While the complexity of the nervous system and intricacies of glycosylation pathways can create serious obstacles for in vivo experiments in vertebrates, recent studies have indicated that more simple and experimentally tractable model organisms like Drosophila should provide important advantages for elucidating evolutionarily conserved functions of N-glycosylation in the nervous system.
Keywords: glycosylation, sialylation, N-glycan, neural transmission, neural excitability, ion channel, Drosophila
Introduction
N-Linked glycan modifications of proteins exist in all three domains of life, Eukarya, Bacteria, and Archaea (Abu-Qarn et al. 2008). N-Glycosylation is especially abundant in eukaryotic cells, where it represents one of the most frequent and ubiquitous posttranslational protein modifications (Stanley et al. 2009, Moremen et al. 2012). In human cells, the majority of N-glycosylation sequon-containing proteins likely acquire N-glycans in the secretory pathway (Apweiler et al. 1999). Although N-glycosylation is not a prerequisite for the viability of mammalian cells in cultured conditions (Gottlieb et al. 1975, Stanley et al. 1975), the biosynthesis of N-glycans appears to be essential for cell communication as defects in N-glycosylation result in embryonic lethality (Ioffe and Stanley 1994, Metzler et al. 1994). The best known functions of N-glycosylation concentrate on promoting protein folding and mediating quality control within the secretory pathway inside the cell (Helenius and Aebi 2001). While biological functions of N-glycans outside of the cell are significantly less understood, they are involved in many essential processes, including cell communication and adhesion. It is more challenging to study these functions because they are less amenable to cell culture approaches and require in vivo analyses that are commonly complicated by pleiotropic effects and complex regulation of glycosylation pathways. The repertoire of N-glycan structures present on a protein can be very heterogeneous at the tissue and cellular level. Their biosynthesis is intimately linked to cell metabolism, reflecting a dynamic read-out of a physiological state of the cell (Dennis et al. 2009).
Many extracellular functions of N-glycans depend on interactions with specific lectins, proteins that bind particular carbohydrate structures (Varki et al. 2009). Glycoprotein-lectin interactions are known to affect a multitude of cell adhesion and signaling processes. These interactions are also involved in building a functional molecular landscape of cell surfaces (Sharon 2007, Dennis et al. 2009). Moreover, N-glycosylation can promote glycoprotein functions via stabilizing steric interactions that protect from proteolysis ((Wittwer and Howard 1990), reviewed in (Wormald and Dwek 1999)). All these functional outcomes of N-glycosylation are pertinent to the development and physiology of different organs and tissues, including the nervous system. In this review, we will focus on several novel paradigms of neural functions of N-glycans. Our goal is not to provide an extensive review of experimental data in this field. Instead, we will concentrate on the discussion of a number of recent studies that unraveled some interesting functional mechanisms underlying these paradigms.
1. N-Glycosylation in neural development
The critical involvement of N-glycosylation in development of the nervous system is evident from the studies of human congenital disorders of glycosylation (CDGs). They revealed that genetic defects in the N-glycosylation pathway are almost always associated with severe neurological abnormalities (reviewed in (Freeze et al. 2012)). Gene inactivation experiments in mice have shed light on the in vivo functions of several key glycosyltransferase genes and their glycan products in the nervous system (Lowe and Marth 2003). For example, brain-specific inactivation of GlcNAcT-I, a glycosyltransferase that mediates the biosynthesis of hybrid and complex N-linked carbohydrates, was found to result in severe neurological defects, including abnormal locomotion, tremors and paralysis (Ye and Marth 2004). However, pleiotropic effects of glycosylation on the development and physiology commonly obstruct conclusive analyses and interpretation of phenotypes produced by knockouts that affect core structures. On the other hand, mutations affecting more specialized and some terminal structures of glycans have proven to be more amenable to study. Phenotypes of such mutations demonstrated the involvement of certain N-glycan structures in specific regulatory events. Thus, genetic inactivation of ST8Sia II and ST8Sia IV polysialyltransferases that modify N-glycans of the neural cell adhesion molecule (NCAM) with polysialic acid (PSA) unveiled the prominent role of PSA in the nervous system (Weinhold et al. 2005, Angata et al. 2007, Hildebrandt et al. 2009). PSA is a long polymer of α2,8-linked sialic acid residues that can be attached to the termini of glycans on some glycoproteins, including N-glycans of NCAM. The PSA structure was shown to regulate brain development, neurite outgrowth and targeting, and to affect synaptic plasticity, learning and memory (for reviews on the structure and functions of NCAM-PSA see (Muhlenhoff et al. 1998, Rutishauser 2008, Muhlenhoff et al. 2009, Colley 2010)). Remarkably, the most severe phenotypes associated with PSA deficiency, including early postnatal lethality, defects in major axonal tracts and progressive hydrocephalus, result from the gain-of-function effect of NCAM that lacks proper PSA modification, and the genetic inactivation of NCAM rescues all gross morphological defects in the brain of PSA-deficient mice (Weinhold et al. 2005). Another notable example of a N-glycan structure that plays a specialized role in neural development is represented by poly-N-acetyllactosamine oligosaccharides (PLN). The synthesis of PLN in the developing olfactory system depends on the activity of β1,3-N-acetylglucosaminyltransferase (β3GnT2) that initiates and participates in the elongation of PLN on the terminal β1-linked galactose residues of N-glycans (Zhou et al. 1999). Targeted genetic inactivation of the β3GnT2 glycosyltransferase results in numerous abnormalities in the olfactory system in mice, including defects of axonal guidance and failure of glomeruli formation. The primary cause of this phenotype appears to be the hypoglycosylation of adenylyl cyclase 3. This enzyme generates cAMP, a key signaling molecule that functions in olfactory axon targeting, and the loss of PLN dramatically downregulates the activity of adenylyl cyclase and the production of cAMP (Henion et al. 2011).
These examples likely correspond to just the tip of the iceberg of numerous yet unknown important roles of N-glycosylation in the development of the nervous system. The nervous system is regulated by a broad spectrum of N-glycosylated proteins, including cell surface and extracellular matrix (ECM) glycoproteins participating in cell adhesion and signaling (Kleene and Schachner 2004, Dityatev et al. 2010). Intriguingly, the functions of a number of these glycoproteins are affected by N-glycosylation outside of the nervous system. Thus, β1,6-branching GlcNAc modifications were found to modulate cell adhesion and cell motility by affecting the functions of laminin 332 and α3β1 integrins (Zhao et al. 2006, Kariya et al. 2008). Another example of a carbohydrate structure that can markedly affect molecular interactions is α2,6-sialylation. It was found to regulate the functions of α4β1 integrins and receptor protein tyrosine phosphatase CD45 by modifying their conformation or interactions with functionally important partners in immune system cells (Amano et al. 2003, Woodard-Grice et al. 2008). Laminins, integrins and receptor protein tyrosine phosphatases also function in the nervous system, affecting neuronal migration, axonal growth and myelination, neuromuscular junction development, neuronal survival, etc. (Wang et al. 2009, Barros et al. 2010, Tan et al. 2011), and glycosylation of these proteins may be implicated in these neural functions. Several studies demonstrated that α2,6-linked sialic acids play an important role as negative regulators of galectin binding, which revealed a paradigm that is expected to be pertinent in many cellular and molecular contexts (reviewed in (Zhuo and Bellis 2011)). These and other examples suggest that similar glycan-dependent regulatory mechanisms may operate in the nervous system, and they need to be explored.
Several novel mechanisms implicating N-glycosylation in the modulation of neural transmission have been recently elucidated, and are discussed in more detail below.
2. N-Glycans in neural physiology
2.1. Glycans in synaptic transmission
Recent research revealed a connection between mutations in the gene encoding glutamine-fructose-6-phosphate transaminase 1 (GFPT1) and a group of congenital myasthenic syndromes (CMS, e.g. OMIM 608931) characterized by hereditary defects in synaptic transmission at neuromuscular junctions (Engel 2012). GFPT1 mediates the first, rate-limiting step in the synthesis of hexosamine needed for glycan biosynthesis (Senderek et al. 2011). The importance of protein glycosylation for different aspects of synaptic transmission was unveiled by a number of studies (see reviews (Kleene and Schachner 2004, Dityatev et al. 2010, Dani and Broadie 2012)). However, the molecular and cellular bases for the effects seen on synaptic transmission are complex and not well understood. Combined, these observations indicate that glycosylation in general is required for normal synaptic functions.
N-Glycosylation controls the function of many key players in synaptic processes and its effect on synaptic physiology is multifaceted. For instance, the function of synaptic vesicle protein 2 (SV2), ubiquitously present at vertebrate synapses, was found to depend on its N-glycosylation. Targeted gene inactivation in mice demonstrated the importance of SV2 for neural transmission as the deletion of two out of three existing SV2 isoforms resulted in postnatal lethality due to severe seizures. Notably, no developmental defects were found in the brains of these mutants, which indicated that SV2 proteins function mainly in synaptic physiology (Janz et al. 1999). It was suggested that they mediate a novel maturation step of primed synaptic vesicles, which potentiates responsiveness of synaptic vesicles to Ca2+ regulation. All SV2 isoforms have multiple N-linked glycan chains attached to their intravesicular loop. The most ubiquitous SV2 isoform, SV2a, has three glycosylation sites, and the removal of all of them inhibits the synaptic targeting of SV2a along with its function (Chang and Sudhof 2009). These results suggest that N-glycans are required for proper folding and trafficking of SV2 within the neuron. More recent analyses of SV2 mutants lacking individual glycosylation sites indicated that single N-glycans are partially dispensable and their function is redundant for the proper sorting of SV2a to synaptic vesicles (Kwon and Chapman 2012). Similar approaches were used to examine the role of N-glycosylation in the regulation of two other major glycoproteins of synaptic vesicles, synaptotagmin 1 and synaptophysin. It was found that the role of glycosylation in glycoprotein sorting to synaptic vesicles can range from dispensable (synaptotagmin 1) to essential (synaptophysin) (Kwon and Chapman 2012). These results illustrated that glycans can play highly individualized regulatory roles that are tailored for a particular glycoprotein and its specific function in the nervous system.
Another type of prominent players in synaptic transmission is represented by neurotransmitter receptors that function as ligand-gated channel proteins and mediate communication among neurons within the nervous system, or between neurons and muscles at neuromuscular junctions. Neurotransmitter receptors are commonly glycosylated, having several N-glycans attached to their extracellular domains. Substantial evidence indicating the functional importance of these carbohydrate modifications has started to emerge. Thus, a number of studies have demonstrated that N-linked carbohydrate chains are involved in the function of nicotinic acetylcholine receptors (nAChRs). nAChR proteins correspond to founding members of the pentameric ligand-gated super family of ion channels, that also includes serotonin, γ-aminobutyric acid (GABA) and glycine receptors (Chen 2010). nAChRs regulate postsynaptic responses at neuromuscular junctions and a variety of synaptic connections in the brain. These receptors are implicated in diverse neural functions, including the processing of sensory information and learning and memory (Miwa et al. 2011). Results of studies on the involvement of N-glycosylation in the function of nAChRs indicate that glycosylation affects functional properties of the receptors. It was proposed that N-glycans can promote the local folding of some functional protein domains, without influencing interactions between receptor subunits or their cell surface expression (Gehle et al. 1997, Chen et al. 1998). Using Torpedo nAChRs as a model system, experiments revealed that N-glycosylation is implicated in receptor modulation, as receptors with mutated glycosylation sites have abnormal conductance and desensitization (the rate of current decay) (Nishizaki 2003). Interestingly, the pharmacological application of concanavalin A (ConA) to in vitro assays of wild type receptors mimicked the effect of mutations affecting N-glycosylation. ConA is a lectin that binds N-linked glycans, and thus its effect on nAChRs was interpreted as evidence that the glycans may function as a modulating “lid” at the channel pore, and the lack of sugar chains or the inhibition of its movement by lectin binding caused the decreased rate of desensitization (Nishizaki 2003). More recent experiments indicated that carbohydrate modifications of nAChRs can influence their surface expression and cholinergic agonist-dependent gating. However, glycosylation does not change their binding affinity for the agonists or the stability of folded receptors (Dacosta et al. 2005, Dellisanti et al. 2007).
The role of N-glycosylation of ionotropic glutamate receptors (iGluRs) was also analyzed. iGluRs mediate fast transmission at the majority of excitatory synapses within the mammalian nervous system, and they play essential roles in synaptic plasticity and extrasynaptic modulation of neurons (reviewed in (Traynelis et al. 2010)). These receptors form tetrameric complexes that function as ligand-gated ion channels. iGluRs encompass large subfamilies of AMPA, kainate, and NMDA receptors (Traynelis et al. 2010). The majority of these receptors appear to be N-glycosylated, with consensus glycosylation sites in their amino-terminal domains involved in receptor assembly and modulation, as well as in their ligand-binding domains (Partin et al. 1993, Everts et al. 1997, Everts et al. 1999, Mah et al. 2005). While the presence of glycans at these sites has not been well characterized, N-glycans were found to affect desensitization of AMPA and kainite receptors (Hollmann et al. 1994, Everts et al. 1997). At the same time, N-glycosylation appears to not be generally required for iGluR function since the synthesis, transport and subunit assembly of functional receptors on the plasma membrane are not significantly affected by the lack of glycosylation (Sumikawa et al. 1988, Everts et al. 1997, Gill et al. 2009). In agreement with these data, crystallization studies of kainate receptors showed that N-linked sugar chains are not directly involved in ligand binding and subunit association of iGluRs (Armstrong et al. 1998, Nanao et al. 2005). In contrast to the AMPA and kainite receptors, functional expression of NMDA-type receptors was found to be dramatically downregulated by inhibition of N-glycosylation. This effect was shown to be associated with a specific reduction in expression of the NR1 subunit, suggesting that glycans are required for its folding and/or trafficking (Everts et al. 1997).
Some specialized carbohydrate structures that can be present on N-linked glycans were found to be involved in the functional modulation of glycoproteins participating in neural transmission. These structures include HNK-1 and sialic acid (discussed below). The HNK-1 glycoepitope (initially discovered on human natural killer cells (Abo and Balch 1981)) was shown to be involved in regulation of the AMPA-type receptor subunit GluR2. The HNK-1 epitope can be also present on some glycolipids. Using these glycolipids, two research groups independently demonstrated that the HNK-1 epitope represents a sulfated glucuronic acid linked to N-acetyllactosamine on the nonreducing termini of oligosaccharides (HSO3–3GlcAβ1–3Galβ1– 4GlcNAc) (Chou et al. 1986, Ariga et al. 1987). The expression of HNK-1 is highly enriched in the nervous system. Genetic inactivation of enzymes responsible for the biosynthesis of this epitope (glucuronyltransferase GlcAT-P, sulfotransferase HNK-1 ST, and β4-galactosyltransferase-2) lead to neurological phenotypes in mice, including reduced long term potentiation in hippocampal CA1 synapses, electrophysiological abnormalities of hippocampal interneurons, defects in neural plasticity, and learning and memory, which suggest that HNK-1 is important for synaptic functions (Senn et al. 2002, Yamamoto et al. 2002, Gurevicius et al. 2007, Yoshihara et al. 2009). It was found that the HNK-1 epitope downregulates endocytosis of the AMPA glutamate receptor subunit GluR2 and stabilizes its expression on neuronal plasma membranes. Moreover, the presence of HNK-1 promotes the interaction between GluR2 and N-cadherin, which probably regulates the stability of GluR2 on the cell surface at synaptic connections (Morita et al. 2009). The HNK-1 epitope is present on a number of glycoproteins involved in intracellular adhesion, cell migration and synaptic plasticity (reviewed in (Kleene and Schachner 2004, Yanagisawa and Yu 2007)). More recently this epitope was found to be expressed on a tenascin-C spliced variant and involved in the regulation of mouse neural stem cells (Yagi et al. 2010).
Synaptic transmission can be significantly influenced by the function of neurotransmitter transporters, synaptic proteins essential for control of the concentration of neurotransmitters in the synaptic cleft. The SLC6 (solute carrier) family of membrane proteins includes a subfamily of transporters that mediate the translocation of neurotransmitters across the plasma membrane by coupling it to the cotransport of Na+ and Cl− (reviewed in (Kristensen et al. 2011)). The members of this subfamily include the transporters for serotonin (5-hydroxytryptamine, or 5-HT), dopamine, norepinephrine, GABA and glycine. All these transport proteins appear to be N-glycosylated at the large extracellular loop 2 region, suggesting that this modification is functionally important. While removal of this glycosylation by mutagenesis or glycosidase treatment reduces the number of transporters at the cell surface it usually does not have a strong effect on ligand binding and transporter activity. The reduction in transporter number was attributed to a decrease in protein stability or a disruption in trafficking of nonglycosylated transporters to the plasma membrane ((Tate and Blakely 1994, Olivares et al. 1995, Melikian et al. 1996, Nguyen and Amara 1996, Martinez-Maza et al. 2001, Li et al. 2004, Kristensen et al. 2011)). N-Glycosylation of GAT1, the predominant GABA transporter in the brain, was found to promote both, the stability of the protein and its trafficking to the cell surface. Moreover, N-glycans were found to be important for GABA-uptake activity of the transporter, with sialic acids appearing to play an essential part in this regulation as the absence of sialylation slowed down the kinetics of the GABA transport cycle and reduced the apparent affinity of GAT1 for extracellular Na+ (Cai et al. 2005, Hu et al. 2011). Interestingly, a non-synonymous single nucleotide polymorphism (SNP) in the human SLC6A4 gene encoding the serotonin transporter (hSERT) creates an ectopic glycosylation site (K201N) that was found to enhance glycosylation of hSERT with a concomitant increase in the level of transporter expression and activity. Although it is not yet known whether this SNP is associated with a neurological phenotype, by analogy to a well-studied polymorphism that also changes the expression level of hSERT, it was suggested that the K201N allele may affect personality traits and psychiatric disease susceptibility (Rasmussen et al. 2009).
2.2 N-Glycosylation regulates ion channels in vertebrate neurons
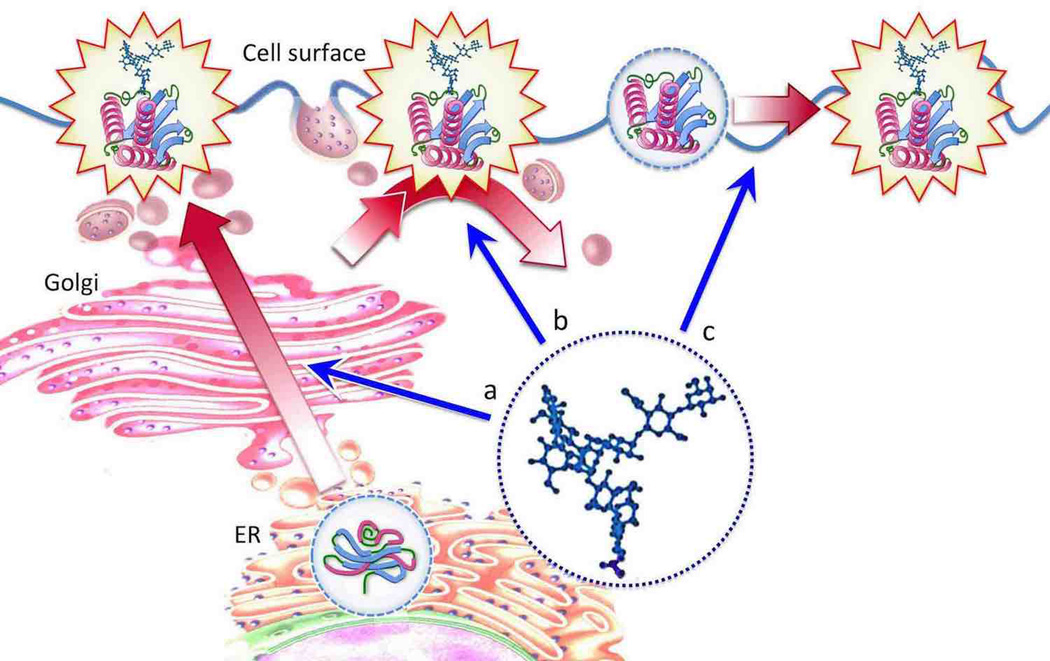
N-Glycosylation can be an important modulator of ion channels in the nervous system. In general, glycans can regulate channels via at least three different mechanisms: (i) by promoting their folding and trafficking to the cell surface, (ii) by affecting their stability and distribution on the cell surface (e.g., via regulating protein endocytosis and/or recycling at the plasma membrane), and (iii) by changing their molecular properties and thus potentiating channel functions (e.g., affecting biophysical characteristics and/or functional interactions with other molecules) (Fig. 1). Outcomes of the first two mechanisms impinge on the control of the number of channels on the cell surface. The effect of N-glycosylation on channel cell surface expression was demonstrated for several types of neuronal channels, including acid-sensing channels (e.g., ASIC1a & 1b (Kadurin et al. 2008, Jing et al. 2012)) and voltage gated ion channels (e.g., potassium channels Kv1.3, Kv1.4 and HERG (Gong et al. 2002, Watanabe et al. 2004, Zhu et al. 2012), and calcium channels Cav3.2 (Weiss et al. 2013)). The effect on biophysical properties is frequently mediated by glycans attached to channel pore loops that can influence channel gating. Channel pore N-glycan modifications can effectively modulate the function of the TRPM8 channel, a member of a large family of transient receptor potential (TRP) ion channels playing essential roles in sensory physiology. TRPM8 glycosylation was found to cause a marked shift in the voltage dependence of channel activation (Pertusa et al. 2012). TRPM8 is expressed in sensory neurons that respond to cold (Mckemy et al. 2002, Peier et al. 2002). The N-linked glycans affect the temperature threshold of TRPM8 activation, and therefore they can function as critical molecular determinants that establish cold sensitivity in primary sensory neurons (Pertusa et al. 2012). Notably, the membrane localization of channels in this case appears to be unaffected by glycosylation and therefore the effect of glycans is concentrated on the regulation of channel biophysical properties (Pertusa et al. 2012). Similarly, glycosylation was found to be an essential factor for agonist-mediated regulation of TRPV1 (TRP Vanilloid Type 1), a nonspecific cation channel that functions as a key sensor of pain-sensing nerve fibers. A non-glycosylated mutant TRPV1 (N604T) was shown to be properly expressed on the plasma membrane, however it did not undergo sustained regulation by capsaicin and had substantially altered desensitization properties (Veldhuis et al. 2012). While glycans affect the biophysical properties of several TRP channels (e.g., TRPC3 and TRPC6 (Dietrich et al. 2003, Wirkner et al. 2005)), glycosylation can also promote the function of TRP channels by regulating their expression and subcellular localization and thus influencing the number of available functional channels (TRPV4 and TRPV5 (Chang et al. 2005, Xu et al. 2006)). These different mechanisms mediated by N-glycosylation do not appear to be mutually exclusive. They could operate at the same time, while one of them could become more prominent, depending on particular molecular and cellular contexts.
Figure 1.
Main effects of protein N-glycosylation. N-glycans can potentiate glycoprotein functions by facilitating protein folding and trafficking to the cell surface (a), promoting protein stability on the cell surface via regulation of protein uptake and recycling to the plasma membrane (b), and by enhancing protein activity via changing protein biophysical properties (c). N-Glycosylation is sketched as a single generic N-glycan (not to scale). The number of glycans can be different for distinct proteins, while glycan structures can vary and have different effects on protein functions.
2.3 N-Glycans and interactions with lectins
Exogenous lectins that interact with N-linked glycan structures were found to have a strong modulatory effect on some neurotransmitter receptors in pharmacological assays. As mentioned above, ConA can bind to N-glycans of nAChRs and influence desensitization of wild-type receptors in a way that mimics the effect of mutations that eliminate N-glycosylation sites (Nishizaki 2003). The modulatory effect of ConA was also demonstrated for iGluR subfamilies of AMPA, kainate, and NMDA receptors (Traynelis et al. 2010). ConA exerts a pronounced effect on kainate receptors by inhibiting their desensitization (Partin et al. 1993, Everts et al. 1997, Everts et al. 1999). Experiments indicate that ConA can interact with N-glycans attached to the amino-terminal domain of iGluRs and affect receptor conformational changes. This action appears to depend on the conformational state of the channel, since agonist-induced desensitization prior to ConA application eliminates the effect (Everts et al. 1997, Everts et al. 1999, Fay and Bowie 2006). Some other lectins, such as wheat germ agglutinin, soybean agglutinin, and succinyl-ConA, were also shown to potentiate kainate receptors (Thio et al. 1993, Yue et al. 1995). The in vitro effects of these lectins suggested that glycans may play a specialized role in the modulation of receptors in vivo (Everts et al. 1997, Nanao et al. 2005), while some endogenous, yet unknown lectins can potentially bind to these glycans and regulate the function of neurotransmitter receptors. A related mechanism of lectin-dependent regulation has recently been described for the Ca2+ TRPV5 channel in renal epithelial cells. Reetention of TRPV5 on the cell surface is an essential regulatory process in the control of channel function. This regulation is mediated by Klotho, a humoral factor with glycosidase activity that appears to directly modify channel glycans, which in turn potentiates interactions with galectin and facilitates cell surface retention of the channel. (Chang et al. 2005, Cha et al. 2008, Leunissen et al. 2013). However, the regulation of TRPV5 is not fully understood, and it is likely mediated by the converging effects of several mechanisms, also including sialylation that appears to work in parallel to promote lipid-raft–mediated internalization of the channel (Leunissen et al. 2013). It will be important to investigate whether lectin-mediated regulation can also operate in the nervous system to regulate channels involved in neural transmission.
2.4 N-Glycans in regulation of voltage-gated ion channels and membrane excitability
A large group of voltage-gated ion channels represents principal regulators of cell excitability. Glycosylation can affect cell excitability of neurons by modulating the function of various members of this channel superfamily, including channels that regulate membrane permeability for Na+, K+, and Ca2+ ions (e.g., (Recio-Pinto et al. 1990, Zhang et al. 1999, Bennett 2002, Gong et al. 2002, Watanabe et al. 2003, Johnson et al. 2004, Watanabe et al. 2007, Schwetz et al. 2010, Weiss et al. 2013)). In mammals, N-glycosylation of voltage-gated Na+ and K+ channels was found to be regulated developmentally and in a cell-specific manner in the heart and the nervous system, suggesting that glycans participate in setting the distinct levels of excitability required in different cells and at various developmental stages (Castillo et al. 1997, Tyrrell et al. 2001, Schwalbe et al. 2008, Montpetit et al. 2009).
In addition to the direct effects of channel glycans, N-glycosylation can influence ion channels indirectly, in a molecule nonautonomous manner, by regulating other glycoproteins that control channel functions. For example, glycosylation of auxiliary subunits that interact with channels can promote cell surface localization and modify channel biophysical properties (Johnson et al. 2004, Cotella et al. 2010). The nonautonomous effect of N-glycans can be potentially pertinent for regulation of many channels, however this possibility remains largely unexplored.
Numerous studies of channel glycosylation have concentrated on sialylated glycans (reviewed in (Ednie and Bennett 2012)). Sialylated carbohydrate chains are negatively charged and can participate in electrostatic interactions with ions and other charged groups located on the cell surface, thus potentially affecting channel functions. Vertebrate voltage-gated Na+ channels are heavily decorated with sialylated structures. It was estimated that up to 30% of Na+ channel molecular mass is represented by carbohydrate chains, with sialic acids (Sia) comprising nearly 50% (Miller et al. 1983, Elmer et al. 1985, Messner and Catterall 1985, James and Agnew 1987, Roberts and Barchi 1987). Electrophysiological assays indicated that sialylated glycans can markedly affect the gating properties of Na+ channels (Recio-Pinto et al. 1990, Bennett et al. 1997, Zhang et al. 1999, Cronin et al. 2005). This effect varies significantly for different channels, and it can also be isoform- and subunit-specific (Bennett 2002, Johnson et al. 2004, Johnson and Bennett 2006).
The role of sialylation in the modulation of vertebrate voltage-gated Na+ channels has been generally explained by the electrostatic effect of the large negative charge provided by the numerous Sia residues present in the vicinity of the channel pore. Remarkably, more than 100 Sia residues can be attached to a channel protein, with the majority of them being incorporated as PSA structures (Miller et al. 1983, James and Agnew 1987, Zuber et al. 1992). The specific role of PSA in the regulation of voltage-gated Na+ channels was uncovered by analyses of mouse mutant cardiomyocytes that had genetically inactivated ST8Sia II polysialyltransferase, an enzyme involved in PSA biosynthesis. The ST8Sia II deficiency was found to cause defects in cell excitability and channel gating, including abnormal action potentials with a significantly broader waveform and a delayed peak, considerable depolarizing shifts of gating curves, and compromised fast inactivation of channels (Montpetit et al. 2009).
While the effect of PSA was confirmed by several studies, a line of evidence suggested that sialylation can also affect voltage-gated channels via mechanisms that cannot be attributed to PSA or the significant charge of numerous Sia residues attached to channel glycans. These data indicated that Sia can play a more specific role in the modulation of channel functions. Thus, electrophysiological analyses of the cardiac sodium channel in cell culture revealed that its function can be affected by some ‘functional’ Sia residues rather than by the total charge of channel sialylation (Stocker and Bennett 2006). Furthermore, experiments with rat hippocampal organotypic slice cultures suggested that PSA does not always influence the function of voltage-gated Na+ channels, since treatment with Endo-N sialidase, a glycosidase that specifically removes PSA, was found to have no apparent impact on intracellularly recorded action potentials and evoked synaptic transmission (Muller et al. 1996). Additionally, the effects of PSA and non-PSA Sia residues on the function of α-NaV1.4 channels were found to be distinct when they were analyzed using mutant Chinese hamster ovary (CHO) cell lines with defects in sialylation or polysialylation pathways. The loss of Sia and PSA in these mutant cells results in opposite shifts of voltage-dependent activation and steady-state inactivation of α-NaV1.4, while only the loss of Sia has a significant effect on recovery from fast inactivation (Ahrens et al. 2011). Finally, unnatural Sia residues with N-acetyl groups changed to N-pentanoyl or N-propanoyl structures, when introduced metabolically, were found to have a notable effect on conductance properties of the Kv3.1 voltage-gated K+ channel. Collectively, these data suggest that sialylation can modulate channels through specific steric effects, in addition to simply electrostatic interactions (Hall et al. 2011).
It is worth noting that most studies on the role of glycosylation in the regulation of ion channels and synaptic glycoproteins have been performed in vitro or in cell culture using transgenic approaches in various types of heterologous cells (for example, using frog oocytes (Everts et al. 1997, Gehle et al. 1997, Nishizaki 2003), different mammalian cell cultures (Bennett 2002, Dellisanti et al. 2007, Watanabe et al. 2007, Hu et al. 2011, Gurba et al. 2012), or in vitro reconstituted lipid membranes (Recio-Pinto et al. 1990, Castillo et al. 2003, Cronin et al. 2005)). It is important to keep in mind that the structure of glycosylation and its functional implications can vary significantly between different cell types, and between cultured cells and neural cells in vivo. Furthermore, the glycosylation of multisubunit protein complexes could also depend on a particular combination of subunits expressed by the cell (e.g., the glycosylation of GABAA β3 subunits can be affected by the co-expression of other receptor subunits (Gurba et al. 2012)). Therefore, it is important to exercise caution when interpreting data from in vitro and cell culture experiments in terms of mechanisms that operate in vivo. Nevertheless, taken together, experimental data clearly indicate that glycosylation can substantially influence the function of glycoproteins playing key roles in neural transmission (Fig. 2). This influence can be dissimilar for distinct types of factors regulating the nervous system. Moreover, even within the same family of related proteins (e.g., iGluRs) glycosylation can underlie distinct modulatory mechanisms that can also depend on the structure and location of carbohydrate chains. These effects of N-glycosylation potentially create an extra layer of regulatory processes that control neural physiology.
Figure 2.
N-Glycosylation can affect neural transmission by modulating voltage-gated ion channels that generate action potentials and determine neuronal excitability, and by influencing synaptic transmission via impact on the function of synaptic proteins, such as synaptic vesicle proteins and neurotransmitter receptors. N-Glycosylation is sketched as a generic N-glycan. Glycans can also include some specific modifications, such as polysialylation and the HNK-1 epitopes (not shown). Modified from (Scott and Panin 2014).
2.5. In vivo functions of sialylated N-glycans
Studies that investigate the function of sialic acids in vivo remain relatively scarce. The biological importance of sialylation of voltage-gated Na+ channels was most unambiguously demonstrated in the context of cardiac functions. Analyses of cardiomyocytes with defective channel sialylation (using mouse genetic models with diminished sialylation or glycosidase-treated rat cardiomyocytes) suggested that abnormal channel sialylation can result in cardiac excitability phenotypes and heart failure (Ufret-Vincenty et al. 2001, Stocker and Bennett 2006, Montpetit et al. 2009). Murine models were also used to examine the role of channel sialylation in the nervous system. These experiments analyzed neural excitability after treatment with glycosidases to remove sialylated glycans, as well as upon inhibition of endogenous neuraminidases that trim sialic acids from carbohydrate chains in vivo. It was found that glycoprotein sialylation can significantly modulate the excitability of neural networks and influence seizure threshold in kindling epilepsy models. These studies suggested that sialic acids can effectively modulate voltage-gated Na+ channels in brain neurons (Tyrrell et al. 2001, Isaev et al. 2007, Isaeva et al. 2010, Isaev et al. 2011).
Recent analyses of the mouse model of Angelman syndrome revealed the possibility that an abnormal sialylation of cell surface proteins plays a key role in the etiology of the syndrome (Condon et al. 2013). This neurological genetic disorder is caused by the maternal loss of the E3 ubiquitin ligase Ube3a and is associated with motor dysfunction, mental retardation, speech impairment, seizures, and a high prevalence of autism (Williams et al. 2006). Loss of Ube3a causes defects in synaptic development and function, including a deficit in experience-dependent synaptic plasticity and decreased plasma membrane localization of AMPA receptors at excitatory synapses (Jiang et al. 1998, Dindot et al. 2008, Yashiro et al. 2009, Greer et al. 2010). Intriguingly, the Ube3a defect also causes a dramatic reduction of glycoprotein sialylation due to the structural and homeostatic disruption of the Golgi apparatus, which indicated that the deficiency of glycoprotein sialylation likely underlies the pathobiological mechanism of Angelman syndrome (Condon et al. 2013).
Although a number of in vivo experiments indicate that glycoprotein sialylation can significantly influence the excitability of neural networks, it remains unknown whether this effect is primarily due to the sialylation of voltage-gated channels or some other glycoproteins. It is challenging to address this question in vertebrates because of the complexity of the nervous system, intricacies of glycosylation pathways, potential functional redundancy of glycosylation genes, as well as the ubiquity of sialylation that affects a panoply of glycoconjugates in the majority of vertebrate cells. With its power of genetic approaches, a spectrum of well-established neurobiological approaches, and simplified glycosylation pathways, Drosophila has recently emerged as a promising model for elucidating conserved genetic and molecular mechanisms of neural glycosylation.
3. N-Glycosylation regulates the nervous system of Drosophila
3.1 Drosophila mutations affecting N-glycosylation
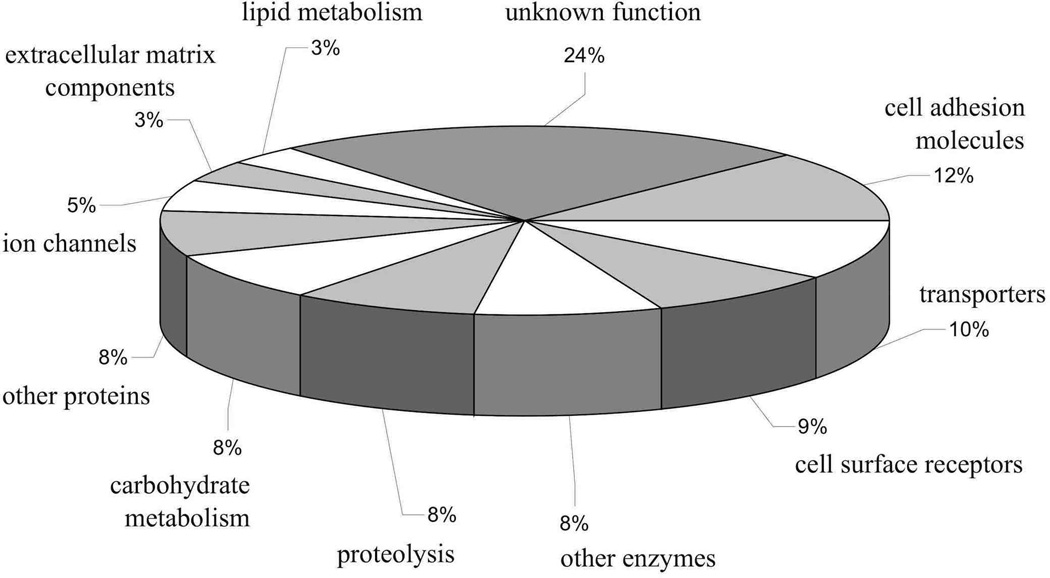
Recent glycoproteomic approaches characterized in detail the totality of Drosophila N-glycosylated proteins, identifying more than 450 glycoproteins expressed in the head (Koles et al. 2007, Vandenborre et al. 2010, Baycin-Hizal et al. 2011). These proteins comprise ion channels, transporters, cell surface receptors, cell adhesion molecules, molecules involved in proteolysis and carbohydrate metabolism, and some other protein families, including a large proportion of proteins with unknown functions (Fig. 3) (Koles et al. 2007, Baycin-Hizal et al. 2011). The repertoire of N-glycan structures in Drosophila is different from that in mammalian organisms. Detailed mass spectrometry analyses of the Drosophila N-glycome revealed that paucimannose and high mannose structures dominate the spectrum of N-glycosylation (Aoki et al. 2007, Koles et al. 2007). In contrast to mammalian N-glycans that are represented by abundant complex structures (Antonopoulos et al. 2011), complex and hybrid-type oligosaccharides that correspond to more processed mature structures represent only 12% of the total Drosophila N-glycan profile (Aoki et al. 2007). Nevertheless, these minor glycan species play prominent roles in the nervous system, suggesting that their functions are evolutionarily conserved (Schachter 2010). The importance of these glycans for the nervous system was revealed in a number of studies that analyzed mutants with defects in the N-glycosylation pathway. Thus, genetic inactivation of the MGAT1 gene that encodes GlcNAcT I, a key enzyme in the production of processed N-glycan structures, was found to result in severe neurological phenotypes, including locomotor abnormalities, significantly decreased life span, and the “fused lobes” phenotype, a developmental defect affecting a specialized brain structure involved in memory formation, the mushroom bodies (Sarkar et al. 2006, Sarkar et al. 2010). MGAT1 mutants have prominent synaptic defects, including overgrowth of neuromuscular junctions and abnormal synaptic vesicle cycling. MGAT1 mutant synapses have disrupted extracellular synaptomatrix and the accumulation of Mind the gap, a lectin-like extracellular matrix protein of the synaptic cleft. They also show a decreased expression of several key markers of functional synaptic morphology, such as Bruchpilot, a presynaptic active zone protein, and GLURIIB, a postsynaptic iGluR subunit B (Parkinson et al. 2013). Mutations of fused lobes cause cell-lethal phenotype in mosaic clones of olfactory projection neurons and result in mushroom body defects similar to those found in MGAT1 mutants (the mushroom body lobes become fused) (Boquet et al. 2000, Sekine et al. 2013). Fused lobes encodes Golgi β-N-acetylglucosaminidase that inhibits the biosynthesis of hybrid and complex N-glycans and concomitantly promotes the production of paucimannose structures (Leonard et al. 2006). Downregulation of sugar-free frosting, a gene encoding a Drosophila homolog of SAD kinase that regulates secretory flux through the Golgi, inhibits synthesis of the HRP glycoepitope (α3-linked core fucose) and increases the amount of hybrid and complex N-glycan structures. Sugar-free frosting mutations lead to neuromuscular junction defects in larvae and locomotor abnormalities in adult flies (Baas et al. 2011). Meigo, a putative nucleotide sugar transporter, appears to specifically regulate the targeting of neurite projections in the olfactory system by affecting N-glycosylation of ephrin (Sekine et al. 2013). Deficiency of β1,4-N-acetylgalactosaminyltransferase A (β4GalNAcTA), a glycosyltransferase potentially involved in the biosynthesis of complex and hybrid N-glycans, results in prominent neurological phenotypes, including defects of locomotion, reduction in the number of synaptic boutons at neuromuscular junctions and decreased frequency of spontaneous release of neurotransmitters (Haines and Irvine 2005, Haines and Stewart 2007, Nakamura et al. 2012). Taken together, these examples highlight the notion that protein N-glycosylation plays important and specific functions in the Drosophila nervous system, and that these functions require the structural diversity of N-glycans. These data also indicate an intriguing possibility that many genes involved in the N-glycosylation pathway could be associated with evolutionarily conserved mechanisms that regulate the nervous system in a wide range of animals, from arthropods to mammals.
Figure 3.
Distribution of the different protein classes among N-glycosylated proteins identified in Drosophila head by glycoproteomics approaches. Figure adapted with permission from (Koles et al. 2007).
3.2 Sialylated N-glycans control neural excitability in Drosophila
Sialylated glycans represent less than 0.1% of the total glycan profile of the Drosophila N-glycome. As a result, they can only be unambiguously detected and analyzed by the most sensitive glycomic approaches, such as multidimensional mass spectrometry (Aoki et al. 2007, Koles et al. 2007). Despite the fact that sialylation is so scarce, it has a prominent function in the nervous system of Drosophila, which was revealed by analysis of mutant phenotypes of the Drosophila sialyltransferase (DSiaT) and CMP-sialic acid synthetase (CSAS) genes that play key roles in the sialylation pathway (Koles et al. 2009). Unlike mammalian organisms that have twenty different sialyltransferases, Drosophila possesses only one sialyltransferase, DSiaT, which significantly simplifies the in vivo analysis of sialylation functions (Koles et al. 2009). DSiaT shows a close evolutionary relationship to the ST6Gal family of mammalian sialyltransferases; it modifies glycoproteins by attaching α2,6-linked sialic acids to LacNAc termini of N-glycans (Koles et al. 2004, Repnikova et al. 2010). The expression of DSiaT is dynamic and largely restricted to subsets of fully differentiated CNS neurons during development and in adult flies, which indicates that the pattern of sialylation is tightly controlled in a cell-specific and developmentally regulated manner (Koles et al. 2009, Repnikova et al. 2010, Islam et al. 2013). The expression of CSAS, an enzyme generating the CMP-sialic acid sugar donor for sialylation, is similarly restricted, which can partially explain the low overall amount of sialylated glycans present in Drosophila (Koles et al. 2007, Repnikova et al. 2010, Islam et al. 2013), even when DSiaT was ectopically expressed throughout the CNS (North et al. 2006).
Genetic inactivation of the sialylation pathway in vivo revealed that sialylated N-glycans play a prominent and specific role in the regulation of the nervous system. Targeted deletion of DSiaT results in a significantly shortened life span, locomotion abnormalities and temperature-sensitive paralysis phenotype. DSiaT mutant larvae have structural and physiological defects in their neuromuscular junction synaptic connections. Electrophysiological assays of DSiaT mutants indicated that DSiaT activity is required for normal neuronal excitability and specifically affects the function of Para, the main voltage-gated Na+ channel in Drosophila (Repnikova et al. 2010). Similar phenotypes result from CSAS mutations that are predicted to also block the sialylation pathway (Islam et al. 2013). Interestingly, the paralysis phenotype of CSAS mutants can be significantly ameliorated by an extra gene copy of para, which suggests that sialylation potentially controls the number of functional voltage-gated channels on the cell surface (Islam et al. 2013). Moreover, the genetic interactions between DSiaT and β4GalNAcTA indicated that sialic acids may function as masking residues hindering the recognition of LacNAc termini of glycans by some endogenous lectins (Nakamura et al. 2012). While further experiments are required to test these intriguing hypotheses, taken together, these results reveal an important novel, nervous system-specific function for α2,6-sialylated N-glycans in the regulation of neural transmission. It is tempting to speculate that this regulatory role corresponds to one of the most ancient evolutionarily conserved functions of sialylation in metazoan organisms. This intriguing hypothesis requires further investigation.
Conclusions
N-Glycosylation can affect glycoproteins by a number of mechanisms, e.g., by facilitating protein folding and stability, supporting trafficking, participating in interactions with other molecules, including lectins, as well as by mediating electrostatic and steric effects on protein dynamics and conformation. In the nervous system, many key players of neural transmission bear N-linked carbohydrate modifications. The roles of these modifications usually depend on molecular and cellular contexts and can vary from non-essential effects to obligatory requirements for protein functions (Table 1). This broad range of putative effects is expected to create a full gamut of states of neural transmission that can be controlled by glycosylation pathways (Fig. 2). Collectively, these data suggest that N-glycans can function in vivo as potent regulators of synaptic transmission and excitability of neural circuits, while also providing an important link between neural transmission and metabolism. These data also pose a number of outstanding questions about molecular, cellular and genetic mechanisms that can underlie the glycan-mediated neural regulation in vivo, as well as a potential involvement of neural N-glycosylation in the pathobiology of neurological disorders. Obtaining answers to these challenging but fundamentally important questions is expected to require a combination of in vitro and in vivo approaches, and should be facilitated by studies using genetically tractable model organisms with simplified glycosylation pathways and a decreased complexity of the nervous system.
Table 1.
Examples of effects of N-glycosylation on glycoproteins involved in neural physiology.
| Glycoprotein | Function in the nervous system |
Role of N-glycosylation | References |
|---|---|---|---|
| SV2 (synaptic vesicle protein 2) | Major synaptic vesicle protein, controls maturation step of primed synaptic vesicles | Required for proper folding and trafficking to synapses | (Chang and Sudhof 2009; Kwon and Chapman 2012) |
| Synaptophysin | Major synaptic vesicle protein, regulates the kinetics of synaptic vesicle endocytosis | Required for synaptic localization | (Kwon and Chapman 2012) |
| Nicotinic acetylcholine receptors (nAChRs) | Ligand-gated cation channels, regulate postsynaptic responses to neurotransmitter acetylcholine, regulates diverse brain functions | Regulates desensitization and channel gating | (Chen et al. 1998; Gehle et al. 1997; Nishizaki 2003) |
| Ionotropic glutamate receptors (iGluRs) | Ligand-gated ion channels, regulate fast transmission at the majority of excitatory synapses | Affects maximal currents and desensitization of AMPA and kainite receptors. Required for folding or trafficking of NMDA receptors. HNK-1 structure downregulates endocytosis of AMPA GluR2 subunit and promotes receptors’ stability on neuronal membranes |
(Everts et al. 1999; Everts et al. 1997; Partin et al. 1993; Thio at al. 1993; Yue et al. 1995) (Morita at al. 2009; Senn et al. 2002; Yamamoto et al. 2002; Yoshihara et al. 2009) |
| Neurotransmitter transporters | Major determinants of synaptic signaling, mediate uptake of neurotransmitters and regulate synaptic concentration of neurotransmitters | Promotes protein stability and trafficking, increases the number of transporters at the cell surface. Sialylated glycans can affect the kinetics of GABA transporter activity and it’s affinity for Na−. |
(Hu et al. 2011; Kristensen et al. 2011; Li at al. 2004; Martinez-Maze et al. 2001; Melikian et al. 1996; Nguyen and Amara 1996; Olivares et al. 1995; Tate and Blakely 1994) |
| Acid-sensing channels (ASICs) | Acidosis-activated cation channels. Play roles in pain, neurological and psychiatric diseases, potential mechanosensory function in sensory neurons | Effect on cell surface expression of ASIC1a and ASIC 1b | (Jing et al. 2012; Kadurin et al. 2008) |
| TRP channels (transient receptor potential ion channels) | Playing essential roles in sensory physiology | Affects the temperature threshold of TRPM8 activation in response to cold. Affect biophysical properties of TRPC3, TRPC6 and TRPV1. Affect expression and subcellular localization of TRPV4 and TRPV5. |
(Chang et al. 2005; Dietrich et al. 2003; Pertusa et al. 2012; Wirkner et al. 2005; Xu et al. 2006) |
|
Voltage-gated ion channels Potassium channels Calcium channels Sodium channels |
Control cell excitability, generate action potentials, affect neural transmission |
Affects cell surface expression and stability of Kv11.1, Kv1.3, Kv12.2 and Kv1.4. Affects gating of Kv1.1, Kv1.5, Kv12.2, IsK Affects trafficking and gating of Kv1.2 Affects simulated action potentials for Kv1.1 and Kv1.2 Sialylation: Affects gating of Kv1.1, Kv1.5, Kv3.1 Gating of Drosophila Shaker channel expressed heterologously in mammalian cells is affected by N-glycans and sialylation Controls activity and cell surface expression of Cav3.2, affects glucose-dependent potentiation Affects gating of Nav1.4 and Nav1.5, Alters steady-state inactivation of Nav1.9 Sialylation: Affects gating of Nav1.4, and Nav1.5, electroplax channel. Sialylation of Nav beta(2) subunit affects gating of Nav1.5. Affects functional properties of Drosophila Nav Para (unknown if the effect is direct) |
(Freeman et al. 2000; Gong et al. 2002; Hall et al. 2011; Johnson and Bennett 2008; Noma et al. 2009; Schwetz et al. 2010; Sutachan et al. 2005; Thornhill et al. 1996; Watanabe et al. 2003; Watanabe et al. 2004; Watanabe et al. 2007; Zhu et al. 2009; Zhu et al. 2012) (Weiss et al. 2013) (Bennett et al. 1997; Cronin at al. 2005; Johnson at al. 2004; Recio-Pinto et al. 1990; Repnikova et al. 2010; Stocker and Bennett 2006; Zhang et al. 1999) |
ACKNOWLEDGEMENTS
We are grateful to Dr. Mark Zoran for stimulating discussions, Dr. Linda Baum and Dr. Mark Lehrman for their inspiration to review the topics discussed in the paper; Dr. Daria Panina for comments on the manuscript. We thank all members of the Panin laboratory for helpful discussions. This work was supported in part by NIH grant NS075534 to V.M.P.
Ethical and Biosafety Standards Policy: Research experiments in the Panin laboratory have been approved by the Institutional Biosafety Committee of Texas A&M University (Permit IBC2013-053).
Abbreviations
- CDGs
congenital disorders of glycosylation
- NCAM
neural cell adhesion molecule
- PSA
polysialic acid
- SV2
synaptic vesicle protein 2
- nAChR
nicotinic acetylcholine receptor
- iGluR
ionotropic glutamate receptor
- GABA
γ-aminobutyric acid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- NMDA
N-methyl-D-aspartate
- ASIC
acid-sensing ion channel
- TRP
transient receptor potential
- β4GalNAcTA
β1,4-N-acetylgalactosaminyltransferase A
- GnTI
N-acetylglucosaminyltransferase I
- NMJ
neuromuscular junction
- CSAS
CMP-sialic acid synthetase
- Para
paralytic
- DSiaT
Drosophila sialyltransferase
- LacNAc
N-Acetyllactosamine
- GalNAc
N-Acetylgalactosamine
- Sia
sialic acid(s)
- ConA
concanavalin A
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J Immunol. 1981;127(3):1024–1029. [PubMed] [Google Scholar]
- Abu-Qarn M, Eichler J, Sharon N. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr Opin Struct Biol. 2008;18(5):544–550. doi: 10.1016/j.sbi.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Ahrens J, Foadi N, Eberhardt A, Haeseler G, Dengler R, Leffler A, Muhlenhoff M, Gerardy-Schahn R, Leuwer M. Defective polysialylation and sialylation induce opposite effects on gating of the skeletal Na+ channel NaV1.4 in Chinese hamster ovary cells. Pharmacology. 2011;87(5–6):311–317. doi: 10.1159/000327389. [DOI] [PubMed] [Google Scholar]
- Amano M, Galvan M, He J, Baum LG. The ST6Gal I sialyltransferase selectively modifies N-glycans on CD45 to negatively regulate galectin-1-induced CD45 clustering, phosphatase modulation, and T cell death. J Biol Chem. 2003;278(9):7469–7475. doi: 10.1074/jbc.M209595200. [DOI] [PubMed] [Google Scholar]
- Angata K, Huckaby V, Ranscht B, Terskikh A, Marth JD, Fukuda M. Polysialic acid-directed migration and differentiation of neural precursors are essential for mouse brain development. Mol Cell Biol. 2007;27(19):6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonopoulos A, North SJ, Haslam SM, Dell A. Glycosylation of mouse and human immune cells: insights emerging from N-glycomics analyses. Biochem Soc Trans. 2011;39(5):1334–1340. doi: 10.1042/BST0391334. [DOI] [PubMed] [Google Scholar]
- Aoki K, Perlman M, Lim JM, Cantu R, Wells L, Tiemeyer M. Dynamic developmental elaboration of N-linked glycan complexity in the Drosophila melanogaster embryo. J Biol Chem. 2007;282(12):9127–9142. doi: 10.1074/jbc.M606711200. [DOI] [PubMed] [Google Scholar]
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- Ariga T, Kohriyama T, Freddo L, Latov N, Saito M, Kon K, Ando S, Suzuki M, Hemling ME, Rinehart KL, Jr, et al. Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J Biol Chem. 1987;262(2):848–853. [PubMed] [Google Scholar]
- Armstrong N, Sun Y, Chen GQ, Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature. 1998;395(6705):913–917. doi: 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Baas S, Sharrow M, Kotu V, Middleton M, Nguyen K, Flanagan-Steet H, Aoki K, Tiemeyer M. Sugar-free frosting, a homolog of SAD kinase, drives neural-specific glycan expression in the Drosophila embryo. Development. 2011;138(3):553–563. doi: 10.1242/dev.055376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CS, Franco SJ, Muller U. Extracellular matrix: functions in the nervous system. Cold Spring Harb Perspect Biol. 2010;3(1):a005108. doi: 10.1101/cshperspect.a005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baycin-Hizal D, Tian Y, Akan I, Jacobson E, Clark D, Chu J, Palter K, Zhang H, Betenbaugh MJ. GlycoFly: a database of Drosophila N-linked glycoproteins identified using SPEG--MS techniques. J Proteome Res. 2011;10(6):2777–2784. doi: 10.1021/pr200004t. [DOI] [PubMed] [Google Scholar]
- Bennett E, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating: A possible electrostatic mechanism. Journal of General Physiology. 1997;109(3):327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ES. Isoform-specific effects of sialic acid on voltage-dependent Na+ channel gating: Functional sialic acids are localized to the S5-S6 loop of domain I. Journal of Physiology. 2002;538(3):675–690. doi: 10.1113/jphysiol.2001.013285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boquet I, Hitier R, Dumas M, Chaminade M, Preat T. Central brain postembryonic development in Drosophila: implication of genes expressed at the interhemispheric junction. J Neurobiol. 2000;42(1):33–48. [PubMed] [Google Scholar]
- Cai G, Salonikidis PS, Fei J, Schwarz W, Schulein R, Reutter W, Fan H. The role of N-glycosylation in the stability, trafficking and GABA-uptake of GABA-transporter 1. Terminal N-glycans facilitate efficient GABA-uptake activity of the GABA transporter. FEBS J. 2005;272(7):1625–1638. doi: 10.1111/j.1742-4658.2005.04595.x. [DOI] [PubMed] [Google Scholar]
- Castillo C, Diaz ME, Balbi D, Thornhill WB, Recio-Pinto E. Changes in sodium channel function during postnatal brain development reflect increases in the level of channel sialidation. Brain Res Dev Brain Res. 1997;104(1–2):119–130. doi: 10.1016/s0165-3806(97)00159-4. [DOI] [PubMed] [Google Scholar]
- Castillo C, Thornhill WB, Zhu J, Recio-Pinto E. The permeation and activation properties of brain sodium channels change during development. Developmental Brain Research. 2003;144(1):99–106. doi: 10.1016/s0165-3806(03)00164-0. [DOI] [PubMed] [Google Scholar]
- Cha SK, Ortega B, Kurosu H, Rosenblatt KP, Kuro OM, Huang CL. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105(28):9805–9810. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Hoefs S, Van Der Kemp AW, Topala CN, Bindels RJ, Hoenderop JG. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310(5747):490–493. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- Chang WP, Sudhof TC. SV2 renders primed synaptic vesicles competent for Ca2+-induced exocytosis. J Neurosci. 2009;29(4):883–897. doi: 10.1523/JNEUROSCI.4521-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Dang H, Patrick JW. Contributions of N-linked glycosylation to the expression of a functional alpha7-nicotinic receptor in Xenopus oocytes. J Neurochem. 1998;70(1):349–357. doi: 10.1046/j.1471-4159.1998.70010349.x. [DOI] [PubMed] [Google Scholar]
- Chen L. In pursuit of the high-resolution structure of nicotinic acetylcholine receptors. J Physiol. 2010;588(Pt 4):557–564. doi: 10.1113/jphysiol.2009.184085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou DK, Ilyas AA, Evans JE, Costello C, Quarles RH, Jungalwala FB. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J Biol Chem. 1986;261(25):11717–11725. [PubMed] [Google Scholar]
- Colley KJ. Structural basis for the polysialylation of the neural cell adhesion molecule. Adv Exp Med Biol. 2010;663:111–126. doi: 10.1007/978-1-4419-1170-4_7. [DOI] [PubMed] [Google Scholar]
- Condon KH, Ho J, Robinson CG, Hanus C, Ehlers MD. The Angelman syndrome protein Ube3a/E6AP is required for Golgi acidification and surface protein sialylation. J Neurosci. 2013;33(9):3799–3814. doi: 10.1523/JNEUROSCI.1930-11.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotella D, Radicke S, Bortoluzzi A, Ravens U, Wettwer E, Santoro C, Sblattero D. Impaired glycosylation blocks DPP10 cell surface expression and alters the electrophysiology of Ito channel complex. Pflugers Arch. 2010;460(1):87–97. doi: 10.1007/s00424-010-0824-2. [DOI] [PubMed] [Google Scholar]
- Cronin NB, O'reilly A, Duclohier H, Wallace BA. Effects of deglycosylation of sodium channels on their structure and function. Biochemistry. 2005;44(2):441–449. doi: 10.1021/bi048741q. [DOI] [PubMed] [Google Scholar]
- Dacosta CJ, Kaiser DE, Baenziger JE. Role of glycosylation and membrane environment in nicotinic acetylcholine receptor stability. Biophys J. 2005;88(3):1755–1764. doi: 10.1529/biophysj.104.052944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani N, Broadie K. Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev Neurobiol. 2012;72(1):2–21. doi: 10.1002/dneu.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellisanti CD, Yao Y, Stroud JC, Wang ZZ, Chen L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat Neurosci. 2007;10(8):953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- Dennis JW, Nabi IR, Demetriou M. Metabolism, cell surface organization, and disease. Cell. 2009;139(7):1229–1241. doi: 10.1016/j.cell.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich A, Mederos Y, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem. 2003;278(48):47842–47852. doi: 10.1074/jbc.M302983200. [DOI] [PubMed] [Google Scholar]
- Dindot SV, Antalffy BA, Bhattacharjee MB, Beaudet AL. The Angelman syndrome ubiquitin ligase localizes to the synapse and nucleus, and maternal deficiency results in abnormal dendritic spine morphology. Hum Mol Genet. 2008;17(1):111–118. doi: 10.1093/hmg/ddm288. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Schachner M, Sonderegger P. The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci. 2010;11(11):735–746. doi: 10.1038/nrn2898. [DOI] [PubMed] [Google Scholar]
- Ednie AR, Bennett ES. Modulation of voltage-gated ion channels by sialylation. Compr Physiol. 2012;2(2):1269–1301. doi: 10.1002/cphy.c110044. [DOI] [PubMed] [Google Scholar]
- Elmer LW, O'brien BJ, Nutter TJ, Angelides KJ. Physicochemical characterization of the alpha-peptide of the sodium channel from rat brain. Biochemistry. 1985;24(27):8128–8137. doi: 10.1021/bi00348a044. [DOI] [PubMed] [Google Scholar]
- Engel AG. Current status of the congenital myasthenic syndromes. Neuromuscul Disord. 2012;22(2):99–111. doi: 10.1016/j.nmd.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Petroski R, Kizelsztein P, Teichberg VI, Heinemann SF, Hollmann M. Lectin-induced inhibition of desensitization of the kainate receptor GluR6 depends on the activation state and can be mediated by a single native or ectopic N-linked carbohydrate side chain. J Neurosci. 1999;19(3):916–927. doi: 10.1523/JNEUROSCI.19-03-00916.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts I, Villmann C, Hollmann M. N-Glycosylation is not a prerequisite for glutamate receptor function but Is essential for lectin modulation. Mol Pharmacol. 1997;52(5):861–873. doi: 10.1124/mol.52.5.861. [DOI] [PubMed] [Google Scholar]
- Fay AM, Bowie D. Concanavalin-A reports agonist-induced conformational changes in the intact GluR6 kainate receptor. J Physiol. 2006;572(Pt 1):201–213. doi: 10.1113/jphysiol.2005.103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze HH, Eklund EA, Ng BG, Patterson MC. Neurology of inherited glycosylation disorders. Lancet Neurol. 2012;11(5):453–466. doi: 10.1016/S1474-4422(12)70040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehle VM, Walcott EC, Nishizaki T, Sumikawa K. N-glycosylation at the conserved sites ensures the expression of properly folded functional ACh receptors. Brain Res Mol Brain Res. 1997;45(2):219–229. doi: 10.1016/s0169-328x(96)00256-2. [DOI] [PubMed] [Google Scholar]
- Gill MB, Vivithanaporn P, Swanson GT. Glutamate binding and conformational flexibility of ligand-binding domains are critical early determinants of efficient kainate receptor biogenesis. J Biol Chem. 2009;284(21):14503–14512. doi: 10.1074/jbc.M900510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Anderson CL, January CT, Zhou Z. Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am J Physiol Heart Circ Physiol. 2002;283(1):H77–H84. doi: 10.1152/ajpheart.00008.2002. [DOI] [PubMed] [Google Scholar]
- Gottlieb C, Baenziger J, Kornfeld S. Deficient uridine diphosphate-N-acetylglucosamine: glycoprotein N-acetylglucosaminyltransferase activity in a clone of Chinese hamster ovary cells with altered surface glycoproteins. J Biol Chem. 1975;250(9):3303–3309. [PubMed] [Google Scholar]
- Greer PL, Hanayama R, Bloodgood BL, Mardinly AR, Lipton DM, Flavell SW, Kim TK, Griffith EC, Waldon Z, Maehr R, Ploegh HL, Chowdhury S, Worley PF, Steen J, Greenberg ME. The Angelman Syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurba KN, Hernandez CC, Hu N, Macdonald RL. GABRB3 mutation, G32R, associated with childhood absence epilepsy alters alpha1beta3gamma2L gamma-aminobutyric acid type A (GABAA) receptor expression and channel gating. J Biol Chem. 2012;287(15):12083–12097. doi: 10.1074/jbc.M111.332528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevicius K, Gureviciene I, Sivukhina E, Irintchev A, Schachner M, Tanila H. Increased hippocampal and cortical beta oscillations in mice deficient for the HNK-1 sulfotransferase. Mol Cell Neurosci. 2007;34(2):189–198. doi: 10.1016/j.mcn.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Haines N, Irvine KD. Functional analysis of Drosophila beta1,4-N-acetlygalactosaminyltransferases. Glycobiology. 2005;15(4):335–346. doi: 10.1093/glycob/cwi017. [DOI] [PubMed] [Google Scholar]
- Haines N, Stewart BA. Functional roles for beta1,4-N-acetlygalactosaminyltransferase- A in Drosophila larval neurons and muscles. Genetics. 2007;175(2):671–679. doi: 10.1534/genetics.106.065565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MK, Reutter W, Lindhorst T, Schwalbe RA. Biochemical engineering of the N-acyl side chain of sialic acids alters the kinetics of a glycosylated potassium channel Kv3.1. FEBS Lett. 2011;585(20):3322–3327. doi: 10.1016/j.febslet.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Helenius A, Aebi M. Intracellular functions of N-linked glycans. Science. 2001;291(5512):2364–2369. doi: 10.1126/science.291.5512.2364. [DOI] [PubMed] [Google Scholar]
- Henion TR, Faden AA, Knott TK, Schwarting GA. beta3GnT2 maintains adenylyl cyclase-3 signaling and axon guidance molecule expression in the olfactory epithelium. J Neurosci. 2011;31(17):6576–6586. doi: 10.1523/JNEUROSCI.0224-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt H, Muhlenhoff M, Oltmann-Norden I, Rockle I, Burkhardt H, Weinhold B, Gerardy-Schahn R. Imbalance of neural cell adhesion molecule and polysialyltransferase alleles causes defective brain connectivity. Brain. 2009;132(Pt 10):2831–2838. doi: 10.1093/brain/awp117. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Maron C, Heinemann S. N-glycosylation site tagging suggests a three transmembrane domain topology for the glutamate receptor GluR1. Neuron. 1994;13(6):1331–1343. doi: 10.1016/0896-6273(94)90419-7. [DOI] [PubMed] [Google Scholar]
- Hu J, Fei J, Reutter W, Fan H. Involvement of sialic acid in the regulation of gamma--aminobutyric acid uptake activity of gamma-aminobutyric acid transporter 1. Glycobiology. 2011;21(3):329–339. doi: 10.1093/glycob/cwq166. [DOI] [PubMed] [Google Scholar]
- Ioffe E, Stanley P. Mice lacking N-acetylglucosaminyltransferase I activity die at mid-gestation, revealing an essential role for complex or hybrid N-linked carbohydrates. Proc Natl Acad Sci U S A. 1994;91(2):728–732. doi: 10.1073/pnas.91.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Isaeva E, Shatskih T, Zhao Q, Smits NC, Shworak NW, Khazipov R, Holmes GL. Role of extracellular sialic acid in regulation of neuronal and network excitability in the rat hippocampus. J Neurosci. 2007;27(43):11587–11594. doi: 10.1523/JNEUROSCI.2033-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Zhao Q, Kleen JK, Lenck-Santini PP, Adstamongkonkul D, Isaeva E, Holmes GL. Neuroaminidase reduces interictal spikes in a rat temporal lobe epilepsy model. Epilepsia. 2011;52(3):e12–e15. doi: 10.1111/j.1528-1167.2011.02988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva E, Lushnikova I, Savrasova A, Skibo G, Holmes GL, Isaev D. Blockade of endogenous neuraminidase leads to an increase of neuronal excitability and activity-dependent synaptogenesis in the rat hippocampus. Eur J Neurosci. 2010;32(11):1889–1896. doi: 10.1111/j.1460-9568.2010.07468.x. [DOI] [PubMed] [Google Scholar]
- Islam R, Nakamura M, Scott H, Repnikova E, Carnahan M, Pandey D, Caster C, Khan S, Zimmermann T, Zoran MJ, Panin VM. The role of Drosophila cytidine monophosphate-sialic Acid synthetase in the nervous system. J Neurosci. 2013;33(30):12306–12315. doi: 10.1523/JNEUROSCI.5220-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James WM, Agnew WS. Multiple oligosaccharide chains in the voltage-sensitive Na channel from electrophorus electricus: evidence for alpha-2,8-linked polysialic acid. Biochem Biophys Res Commun. 1987;148(2):817–826. doi: 10.1016/0006-291x(87)90949-1. [DOI] [PubMed] [Google Scholar]
- Janz R, Goda Y, Geppert M, Missler M, Sudhof TC. SV2A and SV2B function as redundant Ca2+ regulators in neurotransmitter release. Neuron. 1999;24(4):1003–1016. doi: 10.1016/s0896-6273(00)81046-6. [DOI] [PubMed] [Google Scholar]
- Jiang YH, Armstrong D, Albrecht U, Atkins CM, Noebels JL, Eichele G, Sweatt JD, Beaudet AL. Mutation of the Angelman ubiquitin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21(4):799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- Jing L, Chu XP, Jiang YQ, Collier DM, Wang B, Jiang Q, Snyder PM, Zha XM. N-glycosylation of acid-sensing ion channel 1a regulates its trafficking and acidosis-induced spine remodeling. J Neurosci. 2012;32(12):4080–4091. doi: 10.1523/JNEUROSCI.5021-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D, Bennett ES. Isoform-specific effects of the beta(2) subunit on voltage-gated sodium channel gating. Journal of Biological Chemistry. 2006;281(36):25875–25881. doi: 10.1074/jbc.M605060200. [DOI] [PubMed] [Google Scholar]
- Johnson D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the beta(1) subunit modulates voltage-gated sodium channel function. Journal of Biological Chemistry. 2004;279(43):44303–44310. doi: 10.1074/jbc.M408900200. [DOI] [PubMed] [Google Scholar]
- Kadurin I, Golubovic A, Leisle L, Schindelin H, Grunder S. Differential effects of N-glycans on surface expression suggest structural differences between the acid-sensing ion channel (ASIC) 1a and ASIC1b. Biochem J. 2008;412(3):469–475. doi: 10.1042/BJ20071614. [DOI] [PubMed] [Google Scholar]
- Kariya Y, Kato R, Itoh S, Fukuda T, Shibukawa Y, Sanzen N, Sekiguchi K, Wada Y, Kawasaki N, Gu J. N-Glycosylation of laminin-332 regulates its biological functions. A novel function of the bisecting GlcNAc. J Biol Chem. 2008;283(48):33036–33045. doi: 10.1074/jbc.M804526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R, Schachner M. Glycans and neural cell interactions. Nat Rev Neurosci. 2004;5(3):195–208. doi: 10.1038/nrn1349. [DOI] [PubMed] [Google Scholar]
- Koles K, Irvine KD, Panin VM. Functional characterization of Drosophila sialyltransferase. J Biol Chem. 2004;279(6):4346–4357. doi: 10.1074/jbc.M309912200. [DOI] [PubMed] [Google Scholar]
- Koles K, Lim JM, Aoki K, Porterfield M, Tiemeyer M, Wells L, Panin V. Identification of N-glycosylated proteins from the central nervous system of Drosophila melanogaster. Glycobiology. 2007;17(12):1388–1403. doi: 10.1093/glycob/cwm097. [DOI] [PubMed] [Google Scholar]
- Koles K, Repnikova E, Pavlova G, Korochkin LI, Panin VM. Sialylation in protostomes: a perspective from Drosophila genetics and biochemistry. Glycoconj J. 2009;26(3):313–324. doi: 10.1007/s10719-008-9154-4. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63(3):585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Kwon SE, Chapman ER. Glycosylation is dispensable for sorting of synaptotagmin 1 but is critical for targeting of SV2 and synaptophysin to recycling synaptic vesicles. J Biol Chem. 2012;287(42):35658–35668. doi: 10.1074/jbc.M112.398883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R, Rendic D, Rabouille C, Wilson IB, Preat T, Altmann F. The Drosophila fused lobes gene encodes an N-acetylglucosaminidase involved in N-glycan processing. J Biol Chem. 2006;281(8):4867–4875. doi: 10.1074/jbc.M511023200. [DOI] [PubMed] [Google Scholar]
- Leunissen EH, Nair AV, Bull C, Lefeber DJ, Van Delft FL, Bindels RJ, Hoenderop JG. The epithelial calcium channel TRPV5 is regulated differentially by klotho and sialidase. J Biol Chem. 2013 doi: 10.1074/jbc.M113.473520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LB, Chen N, Ramamoorthy S, Chi L, Cui XN, Wang LC, Reith ME. The role of N-glycosylation in function and surface trafficking of the human dopamine transporter. J Biol Chem. 2004;279(20):21012–21020. doi: 10.1074/jbc.M311972200. [DOI] [PubMed] [Google Scholar]
- Lowe JB, Marth JD. A genetic approach to Mammalian glycan function. Annu Rev Biochem. 2003;72:643–691. doi: 10.1146/annurev.biochem.72.121801.161809. [DOI] [PubMed] [Google Scholar]
- Mah SJ, Cornell E, Mitchell NA, Fleck MW. Glutamate receptor trafficking: endoplasmic reticulum quality control involves ligand binding and receptor function. J Neurosci. 2005;25(9):2215–2225. doi: 10.1523/JNEUROSCI.4573-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Maza R, Poyatos I, Lopez-Corcuera BENU, Gimenez C, Zafra F, Aragon C. The role of N-glycosylation in transport to the plasma membrane and sorting of the neuronal glycine transporter GLYT2. J Biol Chem. 2001;276(3):2168–2173. doi: 10.1074/jbc.M006774200. [DOI] [PubMed] [Google Scholar]
- Mckemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Melikian HE, Ramamoorthy S, Tate CG, Blakely RD. Inability to N-glycosylate the human norepinephrine transporter reduces protein stability, surface trafficking, and transport activity but not ligand recognition. Mol Pharmacol. 1996;50(2):266–276. [PubMed] [Google Scholar]
- Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985;260(19):10597–10604. [PubMed] [Google Scholar]
- Metzler M, Gertz A, Sarkar M, Schachter H, Schrader JW, Marth JD. Complex asparagine-linked oligosaccharides are required for morphogenic events during post-implantation development. EMBO J. 1994;13(9):2056–2065. doi: 10.1002/j.1460-2075.1994.tb06480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Agnew WS, Levinson SR. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Miwa JM, Freedman R, Lester HA. Neural systems governed by nicotinic acetylcholine receptors: emerging hypotheses. Neuron. 2011;70(1):20–33. doi: 10.1016/j.neuron.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montpetit ML, Stocker PJ, Schwetz TA, Harper JM, Norring SA, Schaffer L, North SJ, Jang-Lee J, Gilmartin T, Head SR, Haslam SM, Dell A, Marth JD, Bennett ES. Regulated and aberrant glycosylation modulate cardiac electrical signaling. Proc Natl Acad Sci U S A. 2009;106(38):16517–16522. doi: 10.1073/pnas.0905414106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moremen KW, Tiemeyer M, Nairn AV. Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol. 2012;13(7):448–462. doi: 10.1038/nrm3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita I, Kakuda S, Takeuchi Y, Itoh S, Kawasaki N, Kizuka Y, Kawasaki T, Oka S. HNK-1 glyco-epitope regulates the stability of the glutamate receptor subunit GluR2 on the neuronal cell surface. J Biol Chem. 2009;284(44):30209–30217. doi: 10.1074/jbc.M109.024208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlenhoff M, Eckhardt M, Gerardy-Schahn R. Polysialic acid: three-dimensional structure, biosynthesis and function. Curr Opin Struct Biol. 1998;8(5):558–564. doi: 10.1016/s0959-440x(98)80144-9. [DOI] [PubMed] [Google Scholar]
- Muhlenhoff M, Oltmann-Norden I, Weinhold B, Hildebrandt H, Gerardy-Schahn R. Brain development needs sugar: the role of polysialic acid in controlling NCAM functions. Biol Chem. 2009;390(7):567–574. doi: 10.1515/BC.2009.078. [DOI] [PubMed] [Google Scholar]
- Muller D, Wang C, Skibo G, Toni N, Cremer H, Calaora V, Rougon G, Kiss JZ. PSA-NCAM is required for activity-induced synaptic plasticity. Neuron. 1996;17(3):413–422. doi: 10.1016/s0896-6273(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Pandey D, Panin VM. Genetic Interactions Between Drosophila sialyltransferase and beta1,4-N-acetylgalactosaminyltransferase-A Genes Indicate Their Involvement in the Same Pathway. G3 (Bethesda) 2012;2(6):653–656. doi: 10.1534/g3.112.001974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanao MH, Green T, Stern-Bach Y, Heinemann SF, Choe S. Structure of the kainate receptor subunit GluR6 agonist-binding domain complexed with domoic acid. Proc Natl Acad Sci U S A. 2005;102(5):1708–1713. doi: 10.1073/pnas.0409573102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Amara SG. N-linked oligosaccharides are required for cell surface expression of the norepinephrine transporter but do not influence substrate or inhibitor recognition. J Neurochem. 1996;67(2):645–655. doi: 10.1046/j.1471-4159.1996.67020645.x. [DOI] [PubMed] [Google Scholar]
- Nishizaki T. N-glycosylation sites on the nicotinic ACh receptor subunits regulate receptor channel desensitization and conductance. Brain Res Mol Brain Res. 2003;114(2):172–176. doi: 10.1016/s0169-328x(03)00171-2. [DOI] [PubMed] [Google Scholar]
- North SJ, Koles K, Hembd C, Morris HR, Dell A, Panin VM, Haslam SM. Glycomic studies of Drosophila melanogaster embryos. Glycoconj J. 2006;23(5–6):345–354. doi: 10.1007/s10719-006-6693-4. [DOI] [PubMed] [Google Scholar]
- Olivares L, Aragon C, Gimenez C, Zafra F. The role of N-glycosylation in the targeting and activity of the GLYT1 glycine transporter. J Biol Chem. 1995;270(16):9437–9442. doi: 10.1074/jbc.270.16.9437. [DOI] [PubMed] [Google Scholar]
- Parkinson W, Dear ML, Rushton E, Broadie K. N-glycosylation requirements in neuromuscular synaptogenesis. Development. 2013;140(24):4970–4981. doi: 10.1242/dev.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, Mcintyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Pertusa M, Madrid R, Morenilla-Palao C, Belmonte C, Viana F. N-glycosylation of TRPM8 ion channels modulates temperature sensitivity of cold thermoreceptor neurons. J Biol Chem. 2012;287(22):18218–18229. doi: 10.1074/jbc.M111.312645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen TN, Plenge P, Bay T, Egebjerg J, Gether U. A single nucleotide polymorphism in the human serotonin transporter introduces a new site for N-linked glycosylation. Neuropharmacology. 2009;57(3):287–294. doi: 10.1016/j.neuropharm.2009.05.009. [DOI] [PubMed] [Google Scholar]
- Recio-Pinto E, Thornhill WB, Duch DS, Levinson SR, Urban BW. Neuraminidase treatment modifies the function of electroplax sodium channels in planar lipid bilayers. Neuron. 1990;5(5):675–684. doi: 10.1016/0896-6273(90)90221-z. [DOI] [PubMed] [Google Scholar]
- Repnikova E, Koles K, Nakamura M, Pitts J, Li H, Ambavane A, Zoran MJ, Panin VM. Sialyltransferase regulates nervous system function in Drosophila. J Neurosci. 2010;30(18):6466–6476. doi: 10.1523/JNEUROSCI.5253-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RH, Barchi RL. The voltage-sensitive sodium channel from rabbit skeletal muscle. Chemical characterization of subunits. J Biol Chem. 1987;262(5):2298–2303. [PubMed] [Google Scholar]
- Rutishauser U. Polysialic acid in the plasticity of the developing and adult vertebrate nervous system. Nat Rev Neurosci. 2008;9(1):26–35. doi: 10.1038/nrn2285. [DOI] [PubMed] [Google Scholar]
- Sarkar M, Iliadi KG, Leventis PA, Schachter H, Boulianne GL. Neuronal expression of Mgat1 rescues the shortened life span of Drosophila Mgat11 null mutants and increases life span. Proc Natl Acad Sci U S A. 2010;107(21):9677–9682. doi: 10.1073/pnas.1004431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M, Leventis PA, Silvescu CI, Reinhold VN, Schachter H, Boulianne GL. Null mutations in Drosophila N-acetylglucosaminyltransferase I produce defects in locomotion and a reduced life span. J Biol Chem. 2006;281(18):12776–12785. doi: 10.1074/jbc.M512769200. [DOI] [PubMed] [Google Scholar]
- Schachter H. Mgat1-dependent N-glycans are essential for the normal development of both vertebrate and invertebrate metazoans. Semin Cell Dev Biol. 2010;21(6):609–615. doi: 10.1016/j.semcdb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Schwalbe RA, Corey MJ, Cartwright TA. Novel Kv3 glycoforms differentially expressed in adult mammalian brain contain sialylated N-glycans. Biochem Cell Biol. 2008;86(1):21–30. doi: 10.1139/o07-152. [DOI] [PubMed] [Google Scholar]
- Schwetz TA, Norring SA, Bennett ES. N-glycans modulate K(v)1.5 gating but have no effect on K(v)1.4 gating. Biochim Biophys Acta. 2010;1798(3):367–375. doi: 10.1016/j.bbamem.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Sekine SU, Haraguchi S, Chao K, Kato T, Luo L, Miura M, Chihara T. Meigo governs dendrite targeting specificity by modulating ephrin level and N-glycosylation. Nat Neurosci. 2013;16(6):683–691. doi: 10.1038/nn.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senderek J, Muller JS, Dusl M, Strom TM, Guergueltcheva V, Diepolder I, Laval SH, Maxwell S, Cossins J, Krause S, Muelas N, Vilchez JJ, Colomer J, Mallebrera CJ, Nascimento A, Nafissi S, Kariminejad A, Nilipour Y, Bozorgmehr B, Najmabadi H, Rodolico C, Sieb JP, Steinlein OK, Schlotter B, Schoser B, Kirschner J, Herrmann R, Voit T, Oldfors A, Lindbergh C, Urtizberea A, Von Der Hagen M, Hubner A, Palace J, Bushby K, Straub V, Beeson D, Abicht A, Lochmuller H. Hexosamine biosynthetic pathway mutations cause neuromuscular transmission defect. Am J Hum Genet. 2011;88(2):162–172. doi: 10.1016/j.ajhg.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn C, Kutsche M, Saghatelyan A, Bosl MR, Lohler J, Bartsch U, Morellini F, Schachner M. Mice deficient for the HNK-1 sulfotransferase show alterations in synaptic efficacy and spatial learning and memory. Mol Cell Neurosci. 2002;20(4):712–729. doi: 10.1006/mcne.2002.1142. [DOI] [PubMed] [Google Scholar]
- Sharon N. Lectins: carbohydrate-specific reagents and biological recognition molecules. J Biol Chem. 2007;282(5):2753–2764. doi: 10.1074/jbc.X600004200. [DOI] [PubMed] [Google Scholar]
- Stanley P, Narasimhan S, Siminovitch L, Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P, Schachter H, Taniguchi N. N-Glycans. In: Varki A, Cummings RD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- Stocker PJ, Bennett ES. Differential sialylation modulates voltage-gated Na+ channel gating throughout the developing myocardium. Journal of General Physiology. 2006;127(3):253–265. doi: 10.1085/jgp.200509423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K, Parker I, Miledi R. Effect of tunicamycin on the expression of functional brain neurotransmitter receptors and voltage-operated channels in Xenopus oocytes. Brain Res. 1988;464(3):191–199. doi: 10.1016/0169-328x(88)90025-3. [DOI] [PubMed] [Google Scholar]
- Tan CL, Kwok JC, Patani R, Ffrench-Constant C, Chandran S, Fawcett JW. Integrin activation promotes axon growth on inhibitory chondroitin sulfate proteoglycans by enhancing integrin signaling. J Neurosci. 2011;31(17):6289–6295. doi: 10.1523/JNEUROSCI.0008-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate CG, Blakely RD. The effect of N-linked glycosylation on activity of the Na(+)- and Cl(−)-dependent serotonin transporter expressed using recombinant baculovirus in insect cells. J Biol Chem. 1994;269(42):26303–26310. [PubMed] [Google Scholar]
- Thio LL, Clifford DB, Zorumski CF. Concanavalin A enhances excitatory synaptic transmission in cultured rat hippocampal neurons. Synapse. 1993;13(1):94–97. doi: 10.1002/syn.890130111. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, Mcbain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell L, Renganathan M, Dib-Hajj SD, Waxman SG. Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J Neurosci. 2001;21(24):9629–9637. doi: 10.1523/JNEUROSCI.21-24-09629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ufret-Vincenty CA, Baro DJ, Lederer WJ, Rockman HA, Quinones LE, Santana LF. Role of sodium channel deglycosylation in the genesis of cardiac arrhythmias in heart failure. J Biol Chem. 2001;276(30):28197–28203. doi: 10.1074/jbc.M102548200. [DOI] [PubMed] [Google Scholar]
- Vandenborre G, Van Damme EJ, Ghesquiere B, Menschaert G, Hamshou M, Rao RN, Gevaert K, Smagghe G. Glycosylation signatures in Drosophila: fishing with lectins. J Proteome Res. 2010;9(6):3235–3242. doi: 10.1021/pr1001753. [DOI] [PubMed] [Google Scholar]
- Varki A, Etzler ME, Cummings RD, Esko JD. Discovery and Classification of Glycan-Binding Proteins. In: Varki A, Cummings RD, et al., editors. Essentials of Glycobiology. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- Veldhuis NA, Lew MJ, Abogadie FC, Poole DP, Jennings EA, Ivanusic JJ, Eilers H, Bunnett NW, Mcintyre P. N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J Biol Chem. 2012;287(26):21765–21772. doi: 10.1074/jbc.M112.342022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PS, Wang J, Xiao ZC, Pallen CJ. Protein-tyrosine phosphatase alpha acts as an upstream regulator of Fyn signaling to promote oligodendrocyte differentiation and myelination. J Biol Chem. 2009;284(48):33692–33702. doi: 10.1074/jbc.M109.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Wang HG, Sutachan JJ, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects rat Kv1.1 potassium channel gating by a combined surface potential and cooperative subunit interaction mechanism. Journal of Physiology. 2003;550(1):51–66. doi: 10.1113/jphysiol.2003.040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe I, Zhu J, Recio-Pinto E, Thornhill WB. Glycosylation affects the protein stability and cell surface expression of Kv1.4 but Not Kv1.1 potassium channels. A pore region determinant dictates the effect of glycosylation on trafficking. J Biol Chem. 2004;279(10):8879–8885. doi: 10.1074/jbc.M309802200. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Zhu J, Sutachan JJ, Gottschalk A, Recio-Pinto E, Thornhill WB. The glycosylation state of Kv1.2 potassium channels affects trafficking, gating, and simulated action potentials. Brain Res. 2007;1144:1–18. doi: 10.1016/j.brainres.2007.01.092. [DOI] [PubMed] [Google Scholar]
- Weinhold B, Seidenfaden R, Rockle I, Muhlenhoff M, Schertzinger F, Conzelmann S, Marth JD, Gerardy-Schahn R, Hildebrandt H. Genetic ablation of polysialic acid causes severe neurodevelopmental defects rescued by deletion of the neural cell adhesion molecule. J Biol Chem. 2005;280(52):42971–42977. doi: 10.1074/jbc.M511097200. [DOI] [PubMed] [Google Scholar]
- Weiss N, Black SA, Bladen C, Chen L, Zamponi GW. Surface expression and function of Cav3.2 T-type calcium channels are controlled by asparagine-linked glycosylation. Pflugers Arch. 2013;465(8):1159–1170. doi: 10.1007/s00424-013-1259-3. [DOI] [PubMed] [Google Scholar]
- Williams CA, Beaudet AL, Clayton-Smith J, Knoll JH, Kyllerman M, Laan LA, Magenis RE, Moncla A, Schinzel AA, Summers JA, Wagstaff J. Angelman syndrome 2005: updated consensus for diagnostic criteria. Am J Med Genet A. 2006;140(5):413–418. doi: 10.1002/ajmg.a.31074. [DOI] [PubMed] [Google Scholar]
- Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport. 2005;16(9):997–1001. doi: 10.1097/00001756-200506210-00023. [DOI] [PubMed] [Google Scholar]
- Wittwer AJ, Howard SC. Glycosylation at Asn-184 inhibits the conversion of single-chain to two-chain tissue-type plasminogen activator by plasmin. Biochemistry. 1990;29(17):4175–4180. doi: 10.1021/bi00469a021. [DOI] [PubMed] [Google Scholar]
- Woodard-Grice AV, Mcbrayer AC, Wakefield JK, Zhuo Y, Bellis SL. Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of alpha4beta1 integrins. J Biol Chem. 2008;283(39):26364–26373. doi: 10.1074/jbc.M800836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormald MR, Dwek RA. Glycoproteins: glycan presentation and protein-fold stability. Structure. 1999;7(7):R155–R160. doi: 10.1016/s0969-2126(99)80095-1. [DOI] [PubMed] [Google Scholar]
- Xu H, Fu Y, Tian W, Cohen DM. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am J Physiol Renal Physiol. 2006;290(5):F1103–F1109. doi: 10.1152/ajprenal.00245.2005. [DOI] [PubMed] [Google Scholar]
- Yagi H, Yanagisawa M, Suzuki Y, Nakatani Y, Ariga T, Kato K, Yu RK. HNK-1 epitope-carrying tenascin-C spliced variant regulates the proliferation of mouse embryonic neural stem cells. J Biol Chem. 2010;285(48):37293–37301. doi: 10.1074/jbc.M110.157081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Oka S, Inoue M, Shimuta M, Manabe T, Takahashi H, Miyamoto M, Asano M, Sakagami J, Sudo K, Iwakura Y, Ono K, Kawasaki T. Mice deficient in nervous system-specific carbohydrate epitope HNK-1 exhibit impaired synaptic plasticity and spatial learning. J Biol Chem. 2002;277(30):27227–27231. doi: 10.1074/jbc.C200296200. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, Weinberg RJ, Ehlers MD, Philpot BD. Ube3a is required for experience-dependent maturation of the neocortex. Nat Neurosci. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z, Marth JD. N-glycan branching requirement in neuronal and postnatal viability. Glycobiology. 2004;14(6):547–558. doi: 10.1093/glycob/cwh069. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Sugihara K, Kizuka Y, Oka S, Asano M. Learning/memory impairment and reduced expression of the HNK-1 carbohydrate in beta4-galactosyltransferase-II-deficient mice. J Biol Chem. 2009;284(18):12550–12561. doi: 10.1074/jbc.M809188200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue KT, Macdonald JF, Pekhletski R, Hampson DR. Differential effects of lectins on recombinant glutamate receptors. Eur J Pharmacol. 1995;291(3):229–235. doi: 10.1016/0922-4106(95)90062-4. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Hartmann HA, Satin J. Glycosylation influences voltage-dependent gating of cardiac and skeletal muscle sodium channels. Journal of Membrane Biology. 1999;171(3):195–207. doi: 10.1007/s002329900571. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Nakagawa T, Itoh S, Inamori K, Isaji T, Kariya Y, Kondo A, Miyoshi E, Miyazaki K, Kawasaki N, Taniguchi N, Gu J. N-acetylglucosaminyltransferase III antagonizes the effect of N-acetylglucosaminyltransferase V on alpha3beta1 integrin-mediated cell migration. J Biol Chem. 2006;281(43):32122–32130. doi: 10.1074/jbc.M607274200. [DOI] [PubMed] [Google Scholar]
- Zhou D, Dinter A, Gutierrez Gallego R, Kamerling JP, Vliegenthart JF, Berger EG, Hennet T. A beta-1,3-N-acetylglucosaminyltransferase with poly-N-acetyllactosamine synthase activity is structurally related to beta-1,3- galactosyltransferases. Proc Natl Acad Sci U S A. 1999;96(2):406–411. doi: 10.1073/pnas.96.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yan J, Thornhill WB. N-glycosylation promotes the cell surface expression of Kv1.3 potassium channels. FEBS J. 2012;279(15):2632–2644. doi: 10.1111/j.1742-4658.2012.08642.x. [DOI] [PubMed] [Google Scholar]
- Zhuo Y, Bellis SL. Emerging role of alpha2,6-sialic acid as a negative regulator of galectin binding and function. J Biol Chem. 2011;286(8):5935–5941. doi: 10.1074/jbc.R110.191429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber C, Lackie PM, Catterall WA, Roth J. Polysialic acid is associated with sodium channels and the neural cell adhesion molecule N-CAM in adult rat brain. J Biol Chem. 1992;267(14):9965–9971. [PubMed] [Google Scholar]