Abstract
Objective
Previously studies have associated visuospatial tasks, particularly “clock-drawing”, with mortality. We sought to determine whether clock-drawing also mediates the association between depressive symptoms and mortality.
Participants
Non-institutionalized Hispanic and non-Hispanic White elderly volunteers.
Measurements
Survival curves were generated as a function of baseline depressive symptom ratings. Significant models were adjusted for CLOX performance. CLOX is divided into CLOX1, a measure of executive control, and CLOX2, a measure of visuospatial skills.
Design
Retrospective analysis of three longitudinal cohorts.
Results
CLOX2 and depressive symptoms were both associated with mortality in unadjusted models. CLOX2 predicted survival independently of CLOX1 in all three cohorts. CLOX2 also attenuated, and /or mediated the association between depressive symptoms and mortality. These results withstood adjustment for age and education in all three cohorts.
Conclusion
Regardless of the sample examined, or the measure of depressive symptoms applied, the association between depressive symptoms and mortality appears to be at least partially mediated by visuospatial skills. This finding supports our hypothesis that right hemisphere structural brain disease, particularly that involving the insula, may mediate depression's effects on mortality.
Keywords: Cognition, Old Age, Longitudinal, Survival Analysis
Introduction
Depression is a significant and independent predictor of mortality in elderly populations. It is also associated with autonomic dysfunction (Carney et al., 2005), syncope (Ventura et al., 2001), falls, and hip fractures (Kron et al., 2003). In the elderly, significant associations can be demonstrated regardless of whether “depression” is established by formal structured psychiatric interviews, or symptom rating scales (Adamson et al., 2005; Whooley and Browner, 1998;Mehta et al., 2003;Wulsin et al., 2005;Taketshita et al., 2002). Moreover, the effects of depressive symptoms on survival may extend to “subsyndromal” presentations, as “dose-dependent” risks can often be demonstrated below clinical thresholds (Whooley and Browner, 1998; Mehta et al., 2003).
The mechanism by which depressive symptoms increase mortality is not-well established. Although depressive symptoms are co-morbidly associated with medical problems which might also increase mortality, their effect on mortality, especially in the elderly, generally resists adjustment for medications or medical diagnoses (Adamson et al., 2005; Whooley and Browner, 1998; Mehta et al., 2003; Wulsin et al., 2005; Takeshita et al., 2002). This suggests that depression may exert direct central nervous system (CNS) effects on survival.
Specifically, depression's effects on survival may be mediated by neurogenic cardiac rhythm disturbances. It has recently been shown that diminished heart rate variability (HRV) partially mediates depression's effect on survival after myocardial infarction (Carney et al., 2005). Similarly, Whang et al. (2005) have found depression to be a predictor of ventricular arrhythmias in patients with implantable defibrillators. Although their patients all had conditions affecting cardiac conduction, the effect of depressive symptoms on ventricular arrhythmias survived adjustment for time from device implant, cardiac arrest as an indication, angina, congestive heart failure, left ventricular ejection fraction, smoking, alcohol use, serotonin selective reuptake inhibitor (SSRI) use, and the use of either angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers. Depression is also significantly associated with mortality in patients referred for unexplained syncope (Kennedy et al., 1987).
It is interesting to note that the mortality of older depressed patients is increased relative to younger depressed patients. In the most comprehensive study to date, Philibert et al., (1997) found that late onset depression doubled the risk of depression related mortality relative to young onset cases. This suggests that the association between depression and mortality is moderated by an age-related factor (e.g., structural brain disease). This may explain why studies that involve younger persons sometimes fail to confirm an independent effect of depressive symptoms on survival (Evanson-Rose et al., 2004), while studies in the elderly generally support an independent effect (Adamson et al., 2005; Whooley and Browner, 1998; Mehta et al., 2003; Wulsin et al., 2005; Takeshita et al., 2002).
Like depression, visuospatial cognitive measures, especially clock-drawing, are also robust correlates of mortality in otherwise well elderly samples (Lavery et al., 2005;Royall et al., 2007). The pathological substrates of impaired constructions are complex, and potentially include both left and right hemisphere regions of interest (ROIs) (Kirk & Kertesz, 1994). However, structural right hemisphere pathology and visuospatial cognitive measures have previously been associated with mortality in Alzheimer's disease (AD), head injury, stroke, epilepsy and Chronic Obstructive Pulmonary Disease (Antonelli-Incalzi et al., 2006). In this study, we examine whether the effects of depressive symptoms on mortality are also mediated by visuospatial cognitive impairments, and attempt to replicate that observation in three different longitudinal cohorts.
Methods
Subjects
We have collected longitudinal visuospatial performance data in the Air Force Villages’ Freedom House Study (FHS) and two additional community samples involving Mexican American Hispanics. These are the Hispanic Established Population for Epidemiological Studies in the Elderly (HEPESE), and the San Antonio Longitudinal Study of Aging (SALSA). These data were collected after complete description of the study to the subjects, written informed consent was obtained. The FHS and SALSA have been approved by the University of Texas Health Science Center's Institutional Review Board (IRB). HEPESE was approved by the IRB of the University of Texas Medical Branch in Galveston, TX.
Freedom House Study (FHS) (Royall et al., 2005)
The FHS cohort consists of 546 initially non-institutionalized elderly CCRC residents over the age of 70. At inception, the FHS cohort represented 57.3% of 954 potentially eligible participants and did not differ significantly from non-solicited residents with regard to age, gender, or baseline level of care. Our protocol was approved by the University of Texas’ Health Science Center at San Antonio's Institutional Review Board.
In the FHS, clock drawing, as measured by CLOX
An Executive Clock-drawing task (CLOX) has the strongest independent effect on survival in multivariate models (Royall et al., 2007). CLOX was introduced in the second FHS wave (approximately 1996) and collected on 242 residents. These form the basis of this analysis. This subgroup was slightly older than the larger FHS cohort (mean age at baseline = 78.5 years vs. 77.7 years respectively) but did not differ significantly with regard to gender, education, baseline level of care, or MMSE scores. Survival was dated from first CLOX administration.
The Hispanic Established Population for Epidemiological Studies in the Elderly (HEPESE) (Markides et al., 1996)
The HEPESE is a longitudinal study of a representative sample of Mexican Americans, aged 65 years, residing in the Southwestern United States. The sample was drawn using area probability sampling procedures to represent Mexican American elderly in five Southwestern states, Texas, New Mexico, Colorado, Arizona, and California. Subjects were interviewed in their own homes. The response rate for eligible respondents was 83%.
The San Antonio Longitudinal Study of Aging (SALSA) (Hazuda et al., 1998)
SALSA is a community-based study of the disablement process in older (65+ years) Mexican Americans and European Americans. SALSA is an extension of the San Antonio Heart Study (SAHS), a population-based study begun in 1979 to investigate ethnic and sociocultural differences in the etiology and incidence of diabetes mellitus and cardiovascular disease among Mexican Americans and European Americans.
Participants were randomly sampled from three types of neighborhoods
1) low-income, almost exclusively Mexican-American neighborhoods, where a highly traditional Mexican-American cultural orientation predominated (barrio); 2) middle income, ethnically balanced neighborhoods, where upwardly mobile Mexican-American families had gradually moved in and European-American families had moved out (transitional); and 3) high income, predominantly European-American neighborhoods, where Mexican Americans had largely adopted the cultural orientation of the broader society (suburbs).
Clinical Variables
Depressive symptoms
Depressive symptoms were assessed using different measures in each cohort. In SALSA and the FHS, depressive symptoms were assessed with the Geriatric Depression Scale (GDS). However, the full 30 item version was used in SALSA, while the 15 item short form (sGDS) was used in the FHS (Sheikh et al., 1986). In HEPESE, depressive symptoms were assessed using the Centers for Epidemiological Studies depression scale (CES-D) (Radloff and Teri, 1986). The psychometric characteristics of these measures are well known. Gerety et al., (Gerety et al., 1994) have found no differences among the GDS, sGDS and CES-D as screens for depression in elderly patients [sensitivity (range 0.74-0.89), specificity (range 0.62-0.77), or area under the receiver operating curve (ROC) (range 0.85-0.91) relative to blind expert structured clinical assessment].
General Cognition
The Mini-Mental State Examination (MMSE) (Folstein et al., 1975)
The MMSE is a well-known and widely used test for screening cognitive impairment. Scores range from 0-30. Scores below 24 reflect cognitive impairment.
CLOX: An Executive Clock-drawing Task (Royall et al., 1998)
CLOX is a two-part instrument designed to discriminate the executive control of clock-drawing (CLOX1) from drawing per-se (CLOX2). The patient is first instructed to draw a clock on a blank page (CLOX1). He or she is instructed only to “Draw a clock that says 1:45. Set the hands and numbers on the face so that a child could read them.” Once the subject begins to draw, no further assistance is allowed. Then the examiner draws a clock and invites the subject to copy it (CLOX2). Both drawings are rated on the same 15-point scale (lower scores indicate greater impairment). Cut-points of 10/15 (CLOX1) and 12/15 (CLOX2) represent the 5th percentile for young adult controls. Normative data have been published for elderly Hispanics and Non-Hispanic whites (Royall et al., 2003; Heller et al., 2006).
CLOX1 is the most “executive” of six published clock-drawing tests (Royall et al., 1999). CLOX1, but not CLOX2, is significantly associated with the level of care received by elderly retirees in both cross-sectional (Royall, Chiodo & Polk, 2000) and longitudinal (Lavery et al., 2005) models. In contrast, CLOX is also significant predictor of mortality in elderly retirees (Lavery et al., 2005; Royall et al., 2007).
Survival
Cox Proportional Hazards models of survival time were generated for each cognitive measure. Kaplan-Meier survival curves were generated for selected variables. All models were replicated independently in each of the three cohorts.
In each sample, survival time was calculated based on the date of each subject's first CLOX administration (i.e., wave 2 of the FHS, wave 3 of the HEPESE, and wave 3 of the SALSA). Survivor data was censored as of the last available clinical exam (SALSA & HEPESE).
In the FHS, survival data was censored based on internal CCRC records of participant survival status as of June 1, 2001, approximately 70 months after baseline CLOX testing. This date was used to censor survival times. Only n = 5 (0.9%) of the original 546 subjects had been lost to follow-up by leaving the facility since inception of the cohort. Subjects of the current study were divided into 1) Survivors (n = 189; mean survival = 68.4 months) and 2) Non-survivors (n = 53; mean survival = 38.3 months) based on their final survival status.
The total number of HEPESE subjects surveyed at baseline (Fall, 1993–Spring 1994) was 3,050. Of these, 1713 (56.2%) were still available at wave three (Fall–Spring 1998–99), when the CLOX was introduced. A valid CLOX (either CLOX1 or CLOX2) was obtained in 1089 subjects (64%). One thousand twenty-five had a valid CLOX1. One thousand eighty nine had a valid CLOX2.
The final survival status of each subject in the cohort was ascertained as of Dec 12, 2004 (approximately 4 years after wave three) and verified from death records. This date was used to censor survival times. In the current study there were 999 survivors; mean survival = 24.23 months) and 90 non-survivors (mean survival = 13.66 months).
SALSA data are based on 429 participants (216 Mexican Americans, 213 European Americans), assessed with the introduction of CLOX at the first SALSA follow-up. After two additional follow-ups, each separated by approximately 18 months, there were 392 survivors (mean survival = 44.83 months) and 90 non-survivors (mean survival = 22.2 months).
Results
Baseline Descriptors
Table 1 presents baseline subject characteristics for each cohort. In all three samples, there is a high prevalence of CLOX failure, notably CLOX1 failure (Table 2). CLOX2 failure rates range from 17% (FHS) to 36% (SALSA) consistent with previous studies.
Table 1.
Baseline Clinical Features of Cases, Stratified by Cohort
| Sample | SALSA | HEPESE | FHS |
|---|---|---|---|
| Mean (SD) follow-up in months | 42.3 (11.3) | 22.6 (4.9) | 62.0 (15.0) |
| N at CLOX baseline | 441 | 1,088 | 242 |
| % Hispanic | 50 | 100 | 0 |
| Education (years) | |||
| Age at CLOX baseline | 68.7 (3.1) | 77.5 (6.1) | 78.5 (5.3) |
| Mean MMSE | 25.8 (4.1) | 21.1 (7.3) | 27.2 (2.8) |
| Mean (SD) CLOX1 | 9.4 (3.1) | 8.4 (3.2) | 9.9 (3.3) |
| % failing CLOX1 @ ≤ 9/15 | 43.8% | 56.1% | 38% |
| Mean (SD) CLOX2 | 12.0 (2.3) | 12.3 (2.4) | 13.0 (2.1) |
| % failing CLOX2 @ ≤ 11/15 | 36.0% | 30.0% | 17% |
| Mean (SD) depression scales | 5.7 (4.8)⁎ | 8.1 (8.7)⁂ | 1.8 (2.1)† |
Geriatric Depression Scale -30 item
Centers for Epidemiological Studies – depression
Geriatric Depression Scale-15 item
Table 2.
Cox Proportional Hazards Models of Psychometric Predictors of Survival
| Sample | SALSA⁎ | HEPESE⁂ | FHS† |
|---|---|---|---|
| Model # | |||
|
1. CLOX2
estimate (standard error) |
−0.17 (0.05) χ2 = 11.50 (p =0.001) HR = 0.848 |
−0.11 (0.37) χ2 = 9.57 (p=0.002) HR = 0.892 |
−0.27 (0.05) χ2 = 28.17 (p <0.001) HR = 0.764 |
|
2. CLOX2
adjusted for CLOX1 estimate (standard error) |
−0.07 (0.05) χ2 = 1.63 (p = 0.20) HR = 0.93* |
−0.09 (.04) χ2 = 4.43 (p <0.035) HR = 0.910 |
−0.25 (.06) χ2 = 19.07 (p <0.001) HR = 0.780 |
|
3. Depressive symptoms
estimate (standard error) |
0.08 (0.03) χ2 = 8.60 (p =0.003) HR =1.079 |
0.03 (0.01) χ2 = 22.62 (p <0.0001) HR = 1.034 |
0.21 (0.05) χ2 = 17.61 (p <0.0001) HR = 1.24 |
HR = Hazard Ratio
CLOX2 tends to significance after adjustment in Hispanic SALSA subset (estimate −0.13 (0.07) χ2= 3.07 (p = 0.08); HR = 0.88).
Geriatric Depression Scale -30 item from Hispanic sample only
Centers for Epidemiological Studies – depression
Geriatric Depression Scale-15 item
Univariate Survival Models
We examined the association between survival and performance on CLOX2 in each cohort (Table 2). As previously reported, CLOX2 is a significant univariate predictor of survival in the FHS (Royall et al., 2007). Similarly, it is also associated with survival in HEPSE and SALSA. In FHS and HEPESE, the association between CLOX2 and survival withstands adjustment for CLOX1. SALSA shows a trend [estimate 0.13 (0.07), χ2= 3.07 (p = 0.08); HR = 0.88], but only among Hispanics. Anglo SALSA subjects had a very low rate of CLOX2 failure, suggesting a ceiling effect. In each sample, depressive symptom scales were also significantly associated with survival (Table 2).
Multivariate Models
Table 3 presents CLOX2's mediation effect on the association between depressive symptom burden and survival. In SALSA, the association between GDS scores and survival was no longer significant after adjustment for CLOX2 scores. The effect of depressive symptoms on survival in both HEPESE and FHS were attenuated after adjustment for CLOX2. The results in each cohort are not affected by further education.
Table 3.
Cox Proportional Hazards Model of Depressive Symptoms as predictors of Survival Time, adjusted for CLOX2
| Sample | SALSA⁎ | HEPESE⁂ | FHS† |
|---|---|---|---|
| Model | |||
|
Depressive symptoms
estimate (standard error) |
0.05 (0.03) χ2 =2.77 (p = 0.096) HR =1.049 |
0.02 (0.01) χ2 =5.42 (p = 0.020) HR =1.025 |
0.24 (0.06) χ2 =15.95 (p < 0.001) HR =1.272 |
|
CLOX2
estimate (standard error) |
−0.12 (0.05) χ2 = 4.27 (p = 0.034) HR=0.889 |
−0.10 (0.03) χ2 =5.95 (p = 0.015) HR =0.910 |
−0.27 (.07) χ2 =16.96 (p < 0.001) HR = 0.764 |
Geriatric Depression Scale -30 item from Hispanic sample only
Centers for Epidemiological Studies – depression
Geriatric Depression Scale-15 item
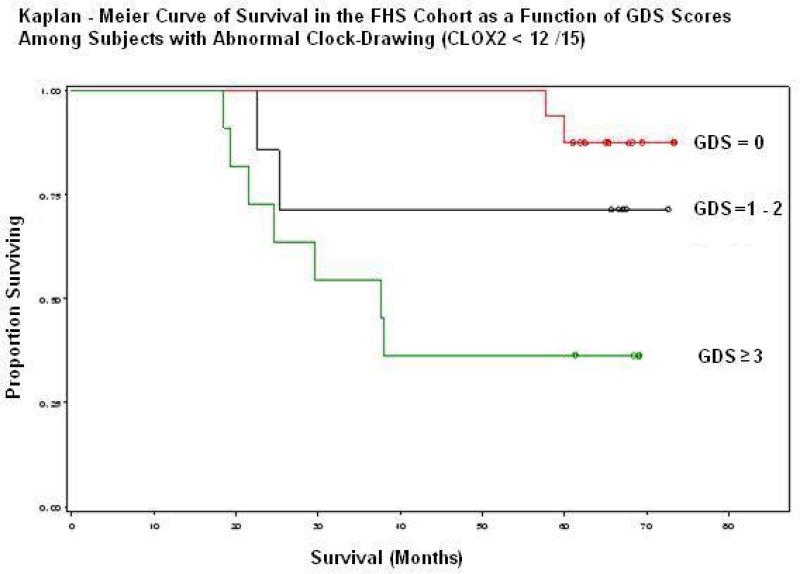
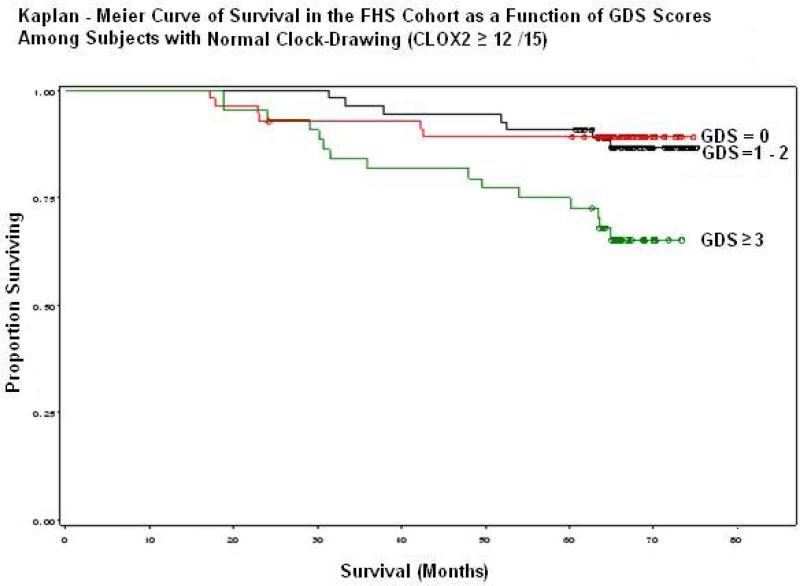
Figures 1& 2 present Cox proportional Hazards Models of survival in the FHS as a function of baseline sGDS scores, stratified by CLOX2 performance. sGDS scores were divided into terciles due to the paucity of subjects who failed both the sGDS (at >6 /15) and CLOX2 (at <12/15). Figure 1 shows a dose-dependent degradation in survival among subjects who also fail CLOX2 (log rank test: p = 0.007). This is not observed in the subset that passed CLOX2 (Figure 2). Mortality was increased even at subclinical levels of depressive symptoms relative to the absence of such symptoms (log rank test, p = 0.005).
Figure 1.
SGDS performance has a significant effect on survival in those who also fail CLOX2. Note near term mortality at sGDS > 0.
Figure 2.
The effect of sGDS scores on survival is attenuated by intact CLOX2 performance. Note long term mortality in sGDS ≥ 3.
However, in order to formally demonstrate a mediation effect, we must also show that depressive symptoms are associated with CLOX2 scores. In SALSA and HEPESE, CLOX2 was significantly associated with the GDS [β = −0.11 (0.02), p <0.001] and CES-D [β = −0.04 (0.008), p = 0.001] respectively. CLOX2 was not significantly associated with sGDS in FHS [β = −0.06 (0.07), p = ns].
Following the methods put forth by MacKinnon et al., (1994;2006), the mediation effect [i.e., the standard error (se) of the remaining effect of depression on mortality after adjusting for CLOX2] was calculated as the difference in the unadjusted direct effect of depressive symptoms on survival less that of the adjusted model; i.e, a - b = c for SALSA and a2 – b2 = c2 for HEPESE). To determine if this mediation effect is significant, Mackinnon et al., (1994;2006) give calculations for obtaining the se of this effect. We found that the mediation effect was significant for SALSA (z = 5.60) and marginal in HEPESE (z = 1.88). The percentage of effect can also be calculated. CLOX2 mediated 36% of the association between GDS and mortality in SALSA and 15% of the association between CES-D and mortality in HEPESE.
Discussion
We have observed that CLOX2 scores attenuate, and potentially mediate depression's well established association with mortality. This can be demonstrated in both Hispanic and Non-Hispanic whites, in a variety of settings, with varying lengths of follow-up, and regardless of the depression rating measures employed. This finding is remarkable given that previous studies have shown the association between depressive symptoms and survival to survive adjustment for a variety of other clinical covariates. Unfortunately, methodological differences across these three cohorts preclude a valid comparison of comorbidities across them. Future studies in selected cohorts will be needed to establish that the specific effect of CLOX2 on survival reported in these studies is independent of medical comorbidity, as is suggested by previous work in other cohorts.
In non-institutionalized community-dwelling elderly (Lavery et al., 2005;Royall et al., 2007) CLOX scores predict survival independently of medical comorbidities and healthcare utilization. In the FHS (Royall et al., 2007) CLOX2 scores predicted 5-year survival independently of age, self-reported health, the number of physician visits in the last 6 months, the number of hospitalizations in the last year, IADL's and ADL's. Only age and ADL's had significant effects on survival, independent of CLOX2 scores. In Lavery et al.'s study (Lavery et al., 2005) CLOX1 scores predicted 3-year survival independently of age, gender, MMSE, comorbid conditions, gait & balance, and comorbid medications.
The apparent discrepancy between our results and those of Lavery et al. (2005) can be explained by the fact that that earlier study retrospectively recoded CDT productions obtained by some other CDT method. We believe that CDT psychometric properties depend more upon the details of their administration than their scoring (Royall and Espino, 2002). CLOX1 and CLOX2 are scored identically by the CLOX scoring metric, yet they are administered differently, and can produce clearly different CDT productions. Thus, depending on how Lavery et al.'s (2005) CDT's were originally administered, they may have produced a hybrid task that is psychometrically closer to CLOX2 than CLOX1. This possibility is reflected by the fact that Lavery et al. (2005) were forced to use a threshold of 12/15 to define CDT impairment. This is our recommended threshold for impairment on CLOX2 (Royall et al., 1998). Similarly, in this study, CLOX2's association survives adjustment for CLOX1 (in FHS and HEPESE; SALSA shows a trend).
We recently proposed that CLOX's association with survival may be mediated through preclinical AD pathology affecting the right insula (Royall et al., 2006). The insula is a limbic structure that modulates autonomic responses to somatic and affective processes. In stroke and epilepsy, right insular pathology can be associated with autonomic dysfunctions, including reduced heart rate variability (which has previously been associated with mortality) and asystole (Aszalos et al., 2002; Howell et al., 1989). Thus, right hemisphere brain disease generally and insular lesions in particular are candidate mediators of the excess depression related mortality seen in older populations.
Moreover, the insular control of autonomic function is lateralized. Right insular lesions are associated with decreased sympathetic modulation and relatively increased parasympathetic modulation. If poor CLOX2 performance is associated with right insular brain disease, then it can be expected to be associated with bradyarrhythmias, orthostasis, syncope and /or falls. In contrast, left insular dysfunction should be associated with increased autonomic tone, accelerated heart rate, and shortened QT intervals. Such symptoms could easily explain depression's relatively strong association with mortality in cardiac patients, but are less likely to be relevant to the community based samples reflected in the current study, particularly the FHS, where verbal, verbal fluency, and verbal memory tests had no significant associations with survival, in contrast to Trail-Making, Digit-symbol Substitution, and CLOX (i.e., measures with strong visuo-spatial properties) (Royall et al., 2007). Although the current data do not address lateralized autonomic functions directly, future analyses, particularly of the SALSA cohort, may be able to test this hypothesis explicitly.
Finally, we do not need to claim that CLOX2 “preferentially affects” the right insula in order to explain the observed mediational effects. CLOX2 must only be associated with both depression and mortality, and attenuate depression's apparent association in a multivariate model. Depression might actually be more strongly associated with left insular function, but if that dysfunction is not in turn associated with mortality, then depression's association with survival is not mediated via that path. We expect that our results might be very different if replicated in a sample with recent myocardial infarction or congestive heart failure. However, because these samples are unselected community samples they may be more generalizable and the population at risk may be much greater. In summary, we have demonstrated that a visuospatial clock-drawing task attenuates and potentially mediates the otherwise robust association between depressive symptoms and mortality. This effect can be demonstrated in both Hispanic and Non-Hispanic whites, in a variety of settings, with varying lengths of follow-up, and regardless of the depression rating measures employed. The effect of clock-drawing on mortality withstands adjustment for the executive control of visuospatial task performance, and may reflect pathology outside the frontal lobe. We suspect a specific association with asymmetric insular dysfunction in the right hemisphere. Clock-drawing may represent a relatively easy means of identifying persons at risk.
Acknowledgments
This study has been supported by grants from the National Institute on Aging 5 R01 AG010939-12; 5 RO1 AG16518, the National Institute for Neurological Disorders and Stroke NS45121-01A1, and from the Alzheimer's Research Foundation.
Footnotes
Financial Disclosures:
Dr. Royall holds the copyright for CLOX: An Executive Clock-drawing task.
Abstracts of this work have been presented at the 59th Annual Scientific Meeting of the Gerontological Society of America and the 2007 Annual Meeting of the American Association for Geriatric Psychiatry.
References
- Adamson JA, Price GM, Breeze E, et al. Are older people dying of depression? Findings from the medical research council trial of the assessment and management of older people in the community. J Am Geriatr Soc. 2005;53:1128–1132. doi: 10.1111/j.1532-5415.2005.53355.x. [DOI] [PubMed] [Google Scholar]
- Antonelli-Incalzi R, Corsonello A, Pedone C, et al. Drawing impairment predicts mortality in severe COPD. Chest. 2006;130:1687–1694. doi: 10.1378/chest.130.6.1687. [DOI] [PubMed] [Google Scholar]
- Aszalos Z, Barsi P, Vitrai J, et al. Lateralization as a factor in the prognosis of middle cerebral artery territorial infarct. Eur Neurol. 2002;48:141–145. doi: 10.1159/000065515. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Freedland KE, et al. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165:1486–1491. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, House JS, Mero RP. Depressive symptoms and mortality risk in a national sample: confounding effects of health status. Psychosom Med. 2004;66:823–830. doi: 10.1097/01.psy.0000145903.75432.1f. [DOI] [PubMed] [Google Scholar]
- Folstein M, Folstein S, McHugh PR. ‘Mini-Mental State’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatry Res. 1975;12:169–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gerety MB, Williams JW, Jr, Mulrow CD, et al. Performance of case-finding tools for depression in the nursing home: influence of clinical and functional characteristics and selection of optimal threshold scores. J Am Geriatr Soc. 1994;42:1103–1109. doi: 10.1111/j.1532-5415.1994.tb06217.x. [DOI] [PubMed] [Google Scholar]
- Hazuda HP, Wood RC, Lichtenstein MJ, et al. Sociocultural status, psychosocial factors and cognitive functional limitation in elderly Mexican Americans: Findings from the San Antonio Longitudinal Study of Aging (SALSA). J Gerontol Soc Work. 1998;30:99–121. [Google Scholar]
- Heller PL, Briones DF, Schiffer RB, Guerrero M, Jr, et al. Mexican heritage and cognitive functioning: Findings from an elderly southwestern sample. J Neuropsychiatry Clin Neurosci. 2006;18:350–355. doi: 10.1176/jnp.2006.18.3.350. [DOI] [PubMed] [Google Scholar]
- Hilz MJ, Dutsch M, Perrine K, et al. Hemispheric influence on autonomic modulation and baroreflex sensitivity. Ann Neurol. 2001;49:575–584. [PubMed] [Google Scholar]
- Howell SJ, Blumhardt LD. Cardiac asystole associated with epileptic seizures: a case report with simultaneous EEG and ECG. J Neurol Neurosurg Psychiatry. 1989;52:795–798. doi: 10.1136/jnnp.52.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GJ, Hofer MA, Cohen D, et al. Significance of depression and cognitive impairment in patients undergoing programmed stimulation of cardiac arrhythmias. Psychosom Med. 1987;49:410–421. doi: 10.1097/00006842-198707000-00010. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Harlock W, Coates R. Computer tomographic localization, lesion size, and prognosis in aphasia and nonverbal impairment. Brain Lang. 1979;8:34–50. doi: 10.1016/0093-934x(79)90038-5. [DOI] [PubMed] [Google Scholar]
- Kirk A, Kertesz A. Localization of lesions in constructional impairment. In: Kertesz Andrew., editor. Localization and neuroimaging in Neuropsychology. Academic Press; New York: 1994. pp. 525–540. Chapter 17. [Google Scholar]
- Kron M, Loy S, Sturm E, Nikolaus T, et al. Risk indicators for falls in institutionalized frail elderly. Am J Epidemiol. 2003;158:645–53. doi: 10.1093/aje/kwg203. [DOI] [PubMed] [Google Scholar]
- Lavery LL, Starenchak SM, Flynn WB, et al. The Clock Drawing Test is an independent predictor of incident use of 24-hour care in a retirement community. J Gerontol A Biol Sci Med Sci. 2005;60:928–932. doi: 10.1093/gerona/60.7.928. [DOI] [PubMed] [Google Scholar]
- MacKinnon D. Czarees A, Beatty L, editors. Analysis of mediating variables in prevention and intervention research. Scientific Methods for Prevention Intervention Research. NIDA Research Monograph. 1994;139:137–153. [PubMed] [Google Scholar]
- MacKinnon DP. Introduction to Statistical Mediation Analysis. Erlbaum; Mahwah, NJ.: 2006. [Google Scholar]
- Markides KS, Stroup-Benham CA, Goodwin JS, et al. The effect of medical conditions on the functional limitations of Mexican-American elderly. Ann Epidemiol. 1996;6:386–91. doi: 10.1016/s1047-2797(96)00061-0. [DOI] [PubMed] [Google Scholar]
- Mehta KM, Yaffe K, Langa KM, et al. Additive effects of cognitive function and depressive symptoms on mortality in elderly community-living adults. J Gerontol A Biol Sci Med Sci. 2003;58:M461–467. doi: 10.1093/gerona/58.5.m461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Richards L, Lynch CF, et al. The effect of gender and age at onset of depression on mortality. J Clin Psychiatry. 1997;58:355–360. doi: 10.4088/jcp.v58n0805. [DOI] [PubMed] [Google Scholar]
- Radloff LS, Teri L. Use of the Center for Epidemiological Studies Depression Scale with older adults. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. Haworth Press; New York: 1986. pp. 119–135. [Google Scholar]
- Royall DR, Chiodo LK, Mouton C, et al. Cognitive predictors of mortality in elderly retirees: Results from the Freedom House Study. Am J Geriatr Psychiatry. 2007;15:243–251. doi: 10.1097/01.JGP.0000240824.84867.02. [DOI] [PubMed] [Google Scholar]
- Royall DR, Chiodo LK, Polk MJ. Correlates of disability among elderly retirees with “sub-clinical” cognitive Impairment. J Gerontol A Biol Sci Med Sci. 2000;55:M541–546. doi: 10.1093/gerona/55.9.m541. [DOI] [PubMed] [Google Scholar]
- Royall DR, Cordes J, Polk MJ. CLOX: An executive clock-drawing task. J Neurol Neurosurg Psychiatry. 1998;64:588–594. doi: 10.1136/jnnp.64.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall DR, Espino DV. Not all clock-drawing tasks are the same. J Am Geriatr Soc. 2002;50:1166–1167. doi: 10.1046/j.1532-5415.2002.50282.x. [DOI] [PubMed] [Google Scholar]
- Royall DR, Espino DV, Polk M, et al. Validation of a Spanish Translation of the CLOX for use in Hispanic Samples: The Hispanic EPESE Study. Int J Geriatr Psychiatry. 2003;18:135–141. doi: 10.1002/gps.804. [DOI] [PubMed] [Google Scholar]
- Royall DR, Gao J-H, Kellogg DL., Jr. Insular Alzheimer's disease pathology as a cause of “age-related” autonomic dysfunction and mortality in the non-demented elderly. Med Hypotheses. 2006;67:747–758. doi: 10.1016/j.mehy.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Royall DR, Mulroy A, Chiodo LK, et al. Clock drawing is sensitive to executive control: A comparison of six methods. J Gerontol B Psychol Sci Soc Sci. 1999;54:P328–333. doi: 10.1093/geronb/54b.5.p328. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, et al. Executive control mediates memory’s association with change in functional status: The Freedom House Study. J Am Geriatr Soc. 2005;53:11–17. doi: 10.1111/j.1532-5415.2005.53004.x. [DOI] [PubMed] [Google Scholar]
- Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDSs): Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. The Haworth Press; New York: 1986. pp. 165–173. [Google Scholar]
- Takeshita J, Masaki K, Ahmed I, et al. Are depressive symptoms a risk factor for mortality in elderly Japanese-American Men?: The Honolulu-Asia Aging Study. Am J Psychiatry. 2002;159:1127–1132. doi: 10.1176/appi.ajp.159.7.1127. [DOI] [PubMed] [Google Scholar]
- Ventura R, Maas R, Ruppel R, Stuhr U, et al. Psychiatric conditions in patients with recurrent unexplained syncope. Europace. 2001;3:311–316. doi: 10.1053/eupc.2001.0182. [DOI] [PubMed] [Google Scholar]
- Whang W, Albert CM, Sears SF, et al. Depression as a predictor for appropriate shocks among patients with implantable cardioverter-defibrillators: Results from the triggers of ventricular arrhythmias (TOVA) study. J Am Coll Cardiol. 2005;45:1090–1095. doi: 10.1016/j.jacc.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Whooley MA, Browner WS. Association between depressive symptoms and mortality in older women. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1998;158:2129–2135. doi: 10.1001/archinte.158.19.2129. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Evans JC, Vasan RS, et al. Depressive symptoms, coronary heart disease, and overall mortality in the Framingham Heart Study. Psychosom Med. 2005;67:697–702. doi: 10.1097/01.psy.0000181274.56785.28. [DOI] [PubMed] [Google Scholar]