Abstract
This study evaluates the migratory potential of monocytes isolated from two groups of human subjects: naïve and non-naïve to Cannabis. Phytocannabinoids (pCB), the bioactive agents produced by the plant Cannabis, regulate the phenotype and function of immune cells by interacting with CB1 and CB2 receptors. It has been shown that agents influencing the phenotype of circulating monocytes influence the phenotype of macrophages and the outcome of immune responses. To date, nothing is known about the acute and long-term effects of pCB on human circulating monocytes. Healthy subjects were recruited for a single blood draw. Monocytes were isolated, fluorescently labeled and their migration quantified using a validated assay that employs near infrared fluorescence and modified Boyden chambers. CB1 and CB2 receptor mRNA expression was quantified by qPCR. Monocytes from all subjects (n = 10) responded to chemokine (c–c motif) ligand 2 (CCL2) and human serum stimuli. Acute application of pCB significantly inhibited both the basal and CCL2-stimulated migration of monocytes, but only in subjects non-naïve to Cannabis. qPCR analysis indicates that monocytes from subjects non-naïve to Cannabis express significantly more CB1 mRNA. The phenotype of monocytes isolated from subjects non-naïve to Cannabis is significantly different from monocytes isolated from subjects naïve to Cannabis. Only monocytes from subjects non-naïve to Cannabis respond to acute exposure to pCB by reducing their overall migratory capacity. Our study suggests that chronic exposure to Cannabis affects the phenotype of circulating monocytes and accordingly could influence outcome of inflammatory responses occurring in injured tissues.
Keywords: Monocyte, Cannabis, Cannabinoid, CCL2, Transmigration, Neuroinflammation
Introduction
Circulating monocytes migrate into damaged tissues where they differentiate into macrophages of two major phenotypes, M1 and M2 (Garden and Moller 2006; Colton 2009; van de Veerdonk and Netea 2010). M1 and M2 macrophages release distinct cytokines, chemokines and other mediators, and thus play a critical role in the outcome of immune responses. Cannabinoids regulate many aspects of monocyte biology, including their differentiation into M1 and M2 phenotypes, as well as their ability to produce immune modulators, indicating that these compounds represent promising therapeutics for regulating immune responses (Montecucco et al. 2008; Roth et al. 2002; Klein and Cabral 2006; Raborn and Cabral 2010; Patinkin et al. 2008; Raborn et al. 2008; Miller and Stella 2008; Walter et al. 2003). Accordingly, regimented cannabinoid treatments alleviate the pathogenesis and symptoms measured in animal models of various human immune diseases, both in the periphery and CNS, with a portion of these therapeutic effects due to cannabinoids regulating monocyte biology. Based on this evidence, significant effort was invested in better understanding how cannabinoids regulate monocyte biology with the aim of developing novel therapeutic approaches designed to treat immune diseases (Baker et al. 2000; Maresz et al. 2007; Ni et al. 2004; Zhang et al. 2009; Castillo et al. 2010; Landucci et al. 2011). Despite this effort, little is known about how cannabinoids regulate monocyte migration, an early and fundamental step of immune response implementation.
Endogenous cannabinoids (endocannabinoids, eCB) are lipids that activate cannabinoid receptors expressed by many cell types (Mach and Steffens 2008). The two primary eCBs, arachidonoylethanolamine (AEA or anandamide) and 2-arachidonoyl glycerol (2-AG), are produced by many cell types, including endothelial cells and T cells (Rossi et al. 2010; Zhang et al. 2011; Huang et al. 2010). pCB encompass ~70 terpenophenolic compounds isolated from the Cannabis plant and include Δ9-tetrahydrocannabinol (THC), cannabinol (CBN) and cannabidiol (CBD). Both eCBs and some pCBs activate CB1 and CB2 receptors (both are G protein-coupled receptors, GPCRs) (Pertwee et al. 2010). THC and CBD are the two most abundant cannabinoids produced by many Cannabis varieties, while CBN is a degradation product of THC (Izzo et al. 2009).
Several laboratories reported that activation of CB1 and CB2 receptors regulates both the basal and chemokine-stimulated migration of cells belonging to the monocytic lineage. However, it is difficult to draw a conclusion on whether cannabinoids truly regulate human monocyte migration because there are striking discrepancies in the results reported dependent upon the cell lines (Kishimoto et al. 2003; Franklin and Stella 2003; Walter et al. 2003; Patinkin et al. 2008; Montecucco et al. 2008; Sacerdote et al. 2000). Here, we sought to revisit this question using freshly isolated human monocytes since they have a more direct relevance to human health. In the first set of experiments, we validated both the use of freshly isolated CD14+ monocytes and the quantification of their migratory response using an unbiased fluorescent technique (Miller and Stella 2009). Using this approach, we then asked whether monocytes isolated from Cannabis naïve and non-naïve subjects exhibit differential sensitivity to various modulators of cell migration, including cannabinoids.
Materials and methods
Patients and study design
Subjects were recruited for this pilot study according to the rules prescribed by the Human Subjects Committee at the University of Washington. Blood was obtained by vein-puncture from the ante-cubital vein of healthy human subjects under a protocol approved by the Human Subjects Committee at the University of Washington. Donors provided prior written informed consent to the procedure and use of the sample. (Table 1) No Cannabis was administered to subjects in this study; these data are based on the report of the subjects in an intake questionnaire.
Table 1.
Human subjects data
| Gender | Female | Male |
|---|---|---|
| Number of subjects | 11 | 14 |
| Average age (±SD) | 36 (±12.2) | 31.3 (± 4.4) |
Materials
Gibco® RPMI1640 (Invitrogen, Carlsbad, CA). DRAQ-5™ (Axxora, San Diego, CA). CP55940, JWH-015, SR144528 (SR2), THC, CBD and CBN (NIDA). AEA and 2-AG (Cayman Chemical, Ann Arbor, MI, USA). CCL2 and CCL2 neutralizing antibody (Calbiochem®/EMD Chemicals, Gibbstown, NJ, USA). NucleoSpin® RNA XS kit (Macherey–Nagel, Düren, Germany/Chlontech, Mountainview, CA, USA); Human spleen and human brain total RNA (Zyagen, San Diego, CA, USA). Sprint RT Complete-Double PrePri-med (Clontech, Mountain View, CA, USA). LNA-based FAM-labeled Universal Probe Library Human set (Roche Applied Science (Indianapolis, IN, USA). Miltenyi LD Midi Macs® separation unit and monocyte isolation kit II (Miltenyi Biotech Inc, Auburn, CA, USA).
Monocyte isolation
The buffy coat was collected after centrifugation of whole blood using BD Vacutainer® CPT Cell Preparation Tubes with sodium citrate (VWR Scientific, San Francisco, CA). The buffy coat was rinsed with PBS (30 ml, centrifuged at 3,000 rpm × 15 min). The resulting pellet was resuspended in buffer and monocytes isolated using an ex vivo deletion strategy developed by Miltenyi Biotech (Midi Macs™ cocktail kit). This strategy uses negative selection (indirect cell labeling) according to the manufacturer’s instructions. The cells retained on a magnetized column are positive for CD3, CD7, CD16, CD19, CD56, CD123 and CD235a, while the unlabeled cells which pass through the magnet are purportedly CD14+ and negative for the other markers.
Migration quantification
To quantify cell migration, we used a method that we recently developed and is based on the modified Boyden chamber (Miller and Stella 2009). Briefly, filters (pore diameter = 5 μm) were coated with human fibronectin (10 μg/ml in PBS for 30 min). Isolated CD14 + monocytes were fluorescently labeled with DRAQ-5 (700 nM, 10 min at 37 °C in RPMI 1640 supplemented with 0.1 % BSA). Cells were then rinsed in RPMI (0.1 % BSA) and re-suspended for a final density of 104 cells per 390 μl per upper well. Lower wells were loaded with media (82 μl) containing vehicle (0.1 % DMSO for basal migration) or the chemo-attractant tested. When cannabinoids were tested for their effect on a chemo-attractant, these compounds were added to both the upper and lower wells. Fluorescence emitted by the cells that had migrated toward the bottom filter area was detected with an Odyssey® Imaging system (Li-COR Biosciences, Lincoln, NE) (Miller and Stella 2009). To validate this method, we performed manual counting by imaging the bottom of the filter with a Zeiss Axiovert microscope (Carl Zeiss, Thornwood, NY) in three high-powered fields (320×). Here, cells that were counted were defined as identifiable by DRAQ-5-stained nuclei and phalloydin-stained actin filaments. Cells were counted in three fields (area 0.1 mm2) from each condition, these numbers were averaged and multiplied by 320 (area of one entire well was 32 mm2).
Cannabinoid mixture
Phytocannabinoids were incubated both individually and in a mixture (Mix) with human monocytes. The ratio for this Mix was calculated as THC + CBN/CBD = 5.2. Final concentrations were: THC = 1 μM, CBN = 300 nM and CBD = 250 nM, which is based on a phenotype system in which any plant analysis sample with a value >1 is considered a ‘drug type’ plant as opposed to a ‘fiber type’ plant (Doorenbos 1971; Fetterman et al. 1971).
qPCR
Total RNA was isolated and purified with the NucleoSpin® RNA XS kit. The RNA quality and concentration was assessed spectrophotometrically (A260/A280 >1.9) by a NanoDrop instrument (Thermo Scientific, Rockland, DE, USA). Total RNA from human spleen and brain were used as reference (Zyagen, San Diego, CA, USA). Specifically, 250 ng of total RNA were reverse-transcribed into cDNA with Sprint RT Complete-Double Pre-Primed (according to the manufacturer’s instructions). Probe based qPCR was performed in triplicate using the LNA-based FAM-labeled Universal Probe Library Human set combined with target-specific primers suggested by the freeware tool Universal Probe Library Design Center and FastStart Universal Probe Master (ROX) Mix (Roche) using a StepOnePlus Real-Time PCR System (ABI, Foster City, CA, USA). The best primers/probe set tested for each target was selected by comparing amplification consistency in human brain and spleen samples. Primers and probes for CNR1 and CNR2 are in Online Resource 1A. To compare samples from different origins (i.e., monocytes, brain and spleen), we quantified several housekeeping genes and assessed the consistency of their relative Ct values for normalization. We determined the Ct values of the following mRNAs: 1-hypoxanthine phosphoribosyl-transferase (HPRT), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YW-HAZ), beta-actin (ACTB), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and TATA box binding protein (TBP) (Online Resource 1B). From this panel we selected TBP because its amplification levels in the samples that we were comparing were within the same range (Online Resource 2). Thus, the target/TBP ratio was used to compare the relative target expression in different samples using the modified ΔΔCT method (Pfaffl 2001). In all samples, CNR1 expression was compared to its level in brain and CNR2 compared to its level in spleen. Data are displayed as direct fold change (RQ) and represent the mean of triplicate qPCR per target.
Data analysis
Data were analyzed with Prism 4.0 (GraphPad, San Diego, CA) and results are expressed as mean ± SEM. For migration, the effect of each compound on basal migration was calculated (after systematically subtracting the background fluorescent signal) as: Migration = (migration with conditioned media/basal migration) × 100. In the case of CCL2 stimulation: migration = (migration with CCL2 + CB/migration stimulated by CCL2) × 100.
Results
Validation: CCL2 increases the migration of freshly isolated human monocytes
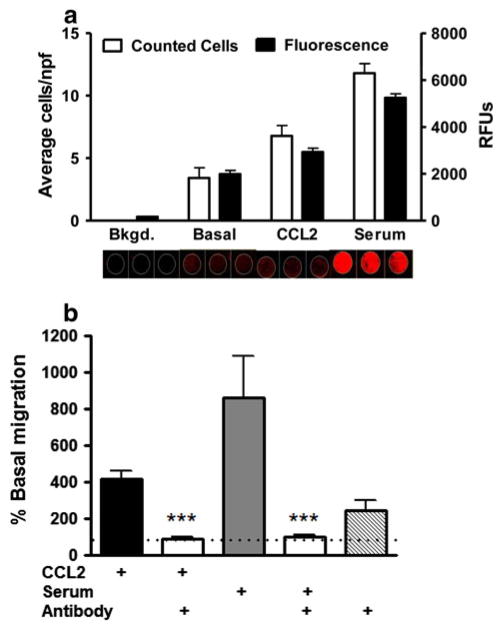
To validate the method used in this study, we used freshly isolated monocytes from a healthy subject and confirmed a correlation between the fluorescence signal (near-infrared) emitted by cells that had migrated through the filter and actual number of cells by counting them (Fig. 1a). This result extends our previous study in which we also found a correlation between the fluorescent signal and actual cell number in mouse microglial cells in culture (Miller and Stella 2009). To stimulate the migration of human monocytes, we tested both CCL2 (monocyte chemoattractant protein 1) and serum freshly isolated from this subject (Table 2), and found that both stimuli enhanced cell migration (419 ± 38 % and 861 ± 253 %, respectively; n = 17). To determine whether the stimulatory response induced by freshly isolated serum is due to CCL2, we incubated serum with a CCL2 neutralizing antibody (10 μg/ml) and found that indeed this antibody blocked the stimulatory response induced by serum (Fig. 1b). This result indicates that the majority of the migratory stimulatory response induced by freshly isolated serum on monocyte migration requires CCL2, confirming and extending previous reports (Weiss et al. 1998; Conductier et al. 2010; Prinz and Priller 2010; Mahad and Ransohoff 2003). Together these results validate our approach to study ex vivo migratory response of human monocytes.
Fig. 1.
CCL2 increases the migration of freshly isolated human monocytes. a Correlation between cell quantity and fluorescence units: freshly isolated monocytes were labelled with DRAQ5 and phalloydin. After incubation and migration, non-migrated cells were wiped from the top side of the filter, rinsed with PBS and fluorescence values for migrated cells (bottom of the filter) were quantified on the Odyssey® imaging system. After fluorescence scanning, membranes were manually counted on a Zeiss Axiovert microscope. Average number of cells in three representative fields per condition correlated well with fluorescence values. b CCL2 is necessary for serum-induced migration. Using the potent chemo-attractant, CCL2, and human serum, we established positive controls with CCL2 and serum. Applying a CCL2 neutralizing antibody to the conditioned media in the lower chambers inhibited this migratory induction to basal levels. As a control, neutralizing antibody on its own showed no inhibitory activity on migration
Table 2.
Human subjects data 2
| Gender | Female | Male |
|---|---|---|
| Number of subjects | 5 | 5 |
| Average age (±SD) | 28.6 (±3.5) | 32.8 (±3.4) |
| Naïve to Cannabis | 3 | 2 |
| Non-naïve to Cannabis | 2 | 3 |
Differential migratory responses of monocytes: Cannabis naïve and non-naïve subjects
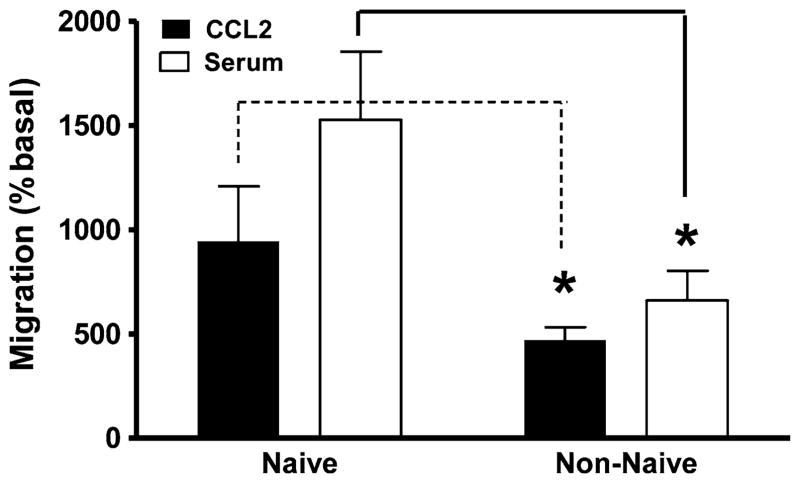
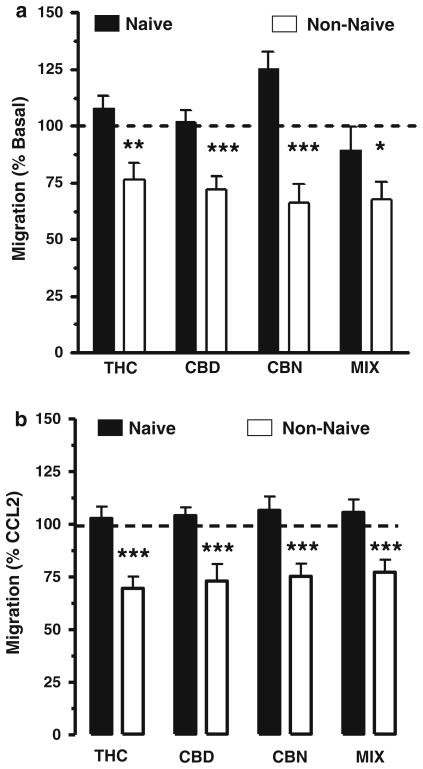
We isolated monocytes from ten healthy human subjects: five subjects were naïve to Cannabis (i.e., either never used this plant or had been naïve for more than 3 years), and five subjects were non-naïve (current use of Cannabis >twice a week) (Table 2). We then tested how CCL2, serum and pCB regulate their cell migration. We found that monocytes isolated from Cannabis non-naïve subjects exhibit a reduced response to both CCL2 and serum compared to monocytes isolated from naïve subjects (Fig. 2). We also found that both basal and stimulated (CCL2 and serum) migration of monocytes isolated from Cannabis non-naïve subjects were inhibited by pCB, whereas these responses measured in monocytes isolated from Cannabis naïve subjects were not affected (Fig. 3a, b). Remarkably, synthetic compounds and eCBs (other than PEA) did not affect the migration of monocytes isolated from both Cannabis naïve and non-naïve subjects (Online Resource 3a–d). Together these results suggest that monocytes isolated from Cannabis naïve and non-naïve subjects exhibit different migratory responses and that only monocytes isolated from Cannabis non-naïve subjects are sensitive to pCB. This selective sensitivity to pCB is specific, because, both synthetic cannabinoids and eCB do not affect the migratory response of monocytes isolated from both groups of subjects.
Fig. 2.
Differential migratory responses of monocytes isolated from Cannabis naïve and non-naïve subjects. Monocytes isolated from five subjects (n = 3 for each condition), who were naïve to Cannabis use, have a significantly increased response to CCL2 and serum compared to monocytes isolated from subjects who were non-naïve to Cannabis use. Significance p < 0.05 (students’ t test,)
Fig. 3.
Differential sensitivity to pCB between Cannabis naïve and non-naïve subjects. (a) Basal migration of freshly isolated human monocytes was significantly reduced by addition of phytocannabinoids (pCB) to the lower wells of the migration chamber: THC, cannabidiol (CBD), cannabinol (CBN) and a set ratio of these compounds (MIX). Basal migration was significantly inhibited in the non-naïve cohort. (b) Additionally, the migratory response toward CCL2 was also reduced by ~25 % when the pCBs were added to CCL2 in the lower chambers. In control experiments (not shown) pCBs alone in the upper chamber with cell loading did not affect basal migration. Statistical significance ***p < 0.001; **p < 0.001; *p < 0.05 (unpaired, two-tailed t test calculated by GraphPad)
Differential cannabinoid receptor expression
Is this differential sensitivity of monocytes to pCB due to changes in CB receptor expression? Because of the small number of monocytes isolated from each subject, as well as the absence of antibodies that specifically label cannabinoid receptors (Atwood and Mackie 2010), we measured changes in CB receptor mRNA expression by qPCR using TBP mRNA as the house-keeping gene (Online Resource 1). We found that monocytes isolated from Cannabis non-naïve subjects express fourfold higher amounts of CB1 receptor mRNA as compared to cells isolated from Cannabis naïve subjects, whereas CB2 receptor mRNA remained unchanged between the two cohorts (Fig. 4a, b). These results show that the sensitivity of monocytes isolated from non-naïve subjects to pCB correlates with enhanced expression of CB1 receptors.
Fig. 4.
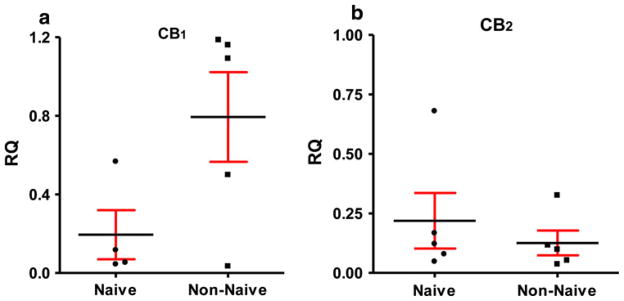
Cannabinoid receptor message in human monocytes: a subjects non-naïve to Cannabis have fourfold increase in CB1 receptor mRNA compared to naïve subjects. Total RNA was amplified using qPCR and ΔΔCT calculations using TBS as a control housekeeping gene was used for quantification. Human brain RNA was used as a positive control and calibration sample. The difference did not reach statistical significance. b No difference was found in mRNA for CB2 receptor between the cohorts. Human spleen RNA was used as a positive control (RQ relative quantification)
Discussion
Circulating monocytes play a fundamental role in the initiation of inflammatory and immune responses (Geissmann et al. 2003). The molecular components controlling the ability of circulating monocytes to infiltrate diseased tissue, including crossing the blood brain barrier, represent promising targets for drugs designed to treat diseases involving chronic inflammatory and immune responses. Here we report that monocytes isolated from human subjects naïve and non-naïve to Cannabis respond differentially to specific modulators of cell migration.
CCL2, a potent chemo-attractant, is produced by various cell types (including endothelial cells, fibroblasts, astrocytes and microglia) in response to growth factors, cytokines and oxidative stress (Deshmane et al. 2009). Thus, CCL-2 gradients participate in directing circulating monocytes toward injured tissue, such as the CNS in the case of multiple sclerosis (MS), cerebral ischemia and brain tumors (Conductier et al. 2010; Gonzalez-Navarro et al. 2008; Kulkarni and Anders 2008; Fujita et al. 2011). Indeed, CCL2 production increases in diseased tissue and many immune cells express CCL2 receptors (Dawson et al. 2003). We found that the CCL2-stimulated migration of monocytes is attenuated in non-naïve subjects. While we still do not understand the molecular details of this reduced response (e.g., whether it is due to decreases in CCL2 receptor expression and/or coupling), this result suggests that chronic use of Cannabis affects the ability of circulating monocytes to respond to a potent chemo-attractant known to recruit monocytes toward damaged and inflamed tissues. This result is remarkable when considering that numerous patients regularly use Cannabis as medicine (an estimated 20 % of patients with MS) (Chong et al. 2006).
Most subtypes of immune cells express cannabinoid receptors (Miller and Stella 2008). We found that monocytes isolated from subjects non-naïve to Cannabis express more CB1 receptors than monocytes isolated from subjects naïve to Cannabis, a result that corroborated a study reporting a similar response in PBMCs (Nong et al. 2002). To our knowledge, only one study tested whether cannabinoids affect the migration of freshly isolated human monocytes (Montecucco et al. 2008) (Online Resource 3). Note that the cannabinoid compound tested was JWH-015 at 20 μM, a concentration that leads to both CB1, CB2 and GPR55 cross-activation (Lauckner et al. 2008) and to receptor-independent effects (Stella 2010). Here we tested JWH-015 at 3 μM, a concentration that still cross-activates CB1, CB2 and GPR55 receptors, while minimizing receptor-independent effects, and found that this aminoalkylindole compound does not regulate the migration of freshly isolated monocytes. In fact, none of the synthetic cannabinoids that we tested affected monocyte migration. Considering this lack of response to synthetic cannabinoids, the inhibitory response induced by phyto-CB that we measured only in monocytes isolated from non-naïve subjects suggests a mechanism involving the selective sensitization to this subfamily of compounds acting on CB receptors. To our knowledge, this is the first example of Cannabis use leading to this selective sensitizing of immune cells to pCBs.
In conclusion, our study suggests that chronic Cannabis use affects key aspects of monocyte migration; both their basal migration and response to potent chemo-attractant stimulants. Accordingly, the ability of circulating monocytes to invade tissues under healthy and diseased conditions, and how therapeutic compounds control this step, may differ when considering subjects naïve or not to Cannabis, as in the case in patients with MS.
Supplementary Material
Online Resource 3: Endo-cannabinoids and synthetic cannabinoid ligands have no effect on basal or serum-induced migration of human monocytes.
Acknowledgments
Eric Horne and Jonathan Coy for assistance with microscopy. Funding to NS (DA014486) and to MS (F32AT005046, NCCAM).
Abbreviations
- CB
Cannabinoid
- eCB
Endocannabinoid
- pCB
Phytocannabinoid
- AEA
Arachidonoylethanolamine
- PEA
Palmioylethanolamine
- THC
Tetrahydrocannabinol
- CBN
Cannabinol
- CBD
Cannabidiol
- PBMC
Peripheral blood mononuclear cell
- CCL2
C–C motif ligand 2 or monocyte chemo-attractant protein 1
Footnotes
Conflict of interest The authors declare that they have no competing interests.
Electronic supplementary material The online version of this article (doi:10.1007/s10787-012-0133-9) contains supplementary material, which is available to authorized users.
Contributor Information
Michelle Sexton, Email: msexton@bastyr.edu, Department of Pharmacology, University of Washington, 1959 NE Pacific St., BB-1538, HSC, Box 357280, Seattle, WA 98195, USA. Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA.
Aurelio Silvestroni, Department of Neurology, University of Washington, Seattle, WA, USA.
Thomas Möller, Department of Neurology, University of Washington, Seattle, WA, USA.
Nephi Stella, Department of Pharmacology, University of Washington, 1959 NE Pacific St., BB-1538, HSC, Box 357280, Seattle, WA 98195, USA. Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, WA, USA.
References
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- Castillo A, Tolon MR, Fernandez-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- Chong MS, Wolff K, Wise K, Tanton C, Winstock A, Silber E. Cannabis use in patients with multiple sclerosis. Mult Scler. 2006;12:646–651. doi: 10.1177/1352458506070947. [DOI] [PubMed] [Google Scholar]
- Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. doi: 10.1007/s11481-009-9164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Dawson J, Miltz W, Mir AK, Wiessner C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin Ther Targets. 2003;7:35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorenbos NJ. Cultivation, extraction and analysis of Cannabis sativa L. Ann NY Acad Sci. 1971;191:3–14. [Google Scholar]
- Fetterman PS, Keith ES, Waller CW, Guerrero O, Doorenbos NJ, Quimby MW. Mississippi-grown Cannabis sativa L: preliminary observation on chemical definition of phenotype and variations in tetrahydrocannabinol content versus age, sex, and plant part. J Pharm Sci. 1971;60:1246–1249. doi: 10.1002/jps.2600600832. [DOI] [PubMed] [Google Scholar]
- Franklin A, Stella N. Arachidonylcyclopropylamide increases microglial cell migration through cannabinoid CB2 and abnormal-cannabidiol-sensitive receptors. Eur J Pharmacol. 2003;474:195–198. doi: 10.1016/s0014-2999(03)02074-0. [DOI] [PubMed] [Google Scholar]
- Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Navarro H, Vinue A, Vila-Caballer M, Fortuno A, Beloqui O, Zalba G, Burks D, Diez J, Andres V. Molecular mechanisms of atherosclerosis in metabolic syndrome: role of reduced IRS2-dependent signaling. Arterioscler Thromb Vasc Biol. 2008;28:2187–2194. doi: 10.1161/ATVBAHA.108.175299. [DOI] [PubMed] [Google Scholar]
- Huang NL, Juang JM, Wang YH, Hsueh CH, Liang YJ, Lin JL, Tsai CT, Lai LP. Rimonabant inhibits TNF-alpha-induced endothelial IL-6 secretion via CB1 receptor and cAMP-dependent protein kinase pathway. Acta Pharmacol Sin. 2010;31:1447–1453. doi: 10.1038/aps.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo AA, Borrelli F, Capasso R, Dimarzo V, Mechoulam R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol Sci. 2009;30:515–527. doi: 10.1016/j.tips.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Kishimoto S, Gokoh M, Oka S, Muramatsu M, Kajiwara T, Waku K, Sugiura T. 2-Arachidonoylglycerol induces the migration of HL-60 cells differentiated into macrophage-like cells and human peripheral blood monocytes through the cannabinoid CB2 receptor-dependent mechanism. J Biol Chem. 2003;278:24469–24475. doi: 10.1074/jbc.M301359200. [DOI] [PubMed] [Google Scholar]
- Klein TW, Cabral GA. Cannabinoid-induced immune suppression and modulation of antigen-presenting cells. J Neuroimmune Pharmacol. 2006;1:50–64. doi: 10.1007/s11481-005-9007-x. [DOI] [PubMed] [Google Scholar]
- Kulkarni O, Anders HJ. CCL2/MCP1: a novel target in systemic lupus erythematosus and lupus nephritis. Z Rheumatol. 2008;67:220–224. doi: 10.1007/s00393-008-0283-8. [DOI] [PubMed] [Google Scholar]
- Landucci E, Scartabelli T, Gerace E, Moroni F, Pellegrini-Giampietro DE. CB1 receptors and post-ischemic brain damage: studies on the toxic and neuroprotective effects of cannabinoids in rat organotypic hippocampal slices. Neuropharmacology. 2011;60:674–682. doi: 10.1016/j.neuropharm.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. 2008;105:2699–2704. doi: 10.1073/pnas.0711278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mach F, Steffens S. The role of the endocannabinoid system in atherosclerosis. J Neuroendocrinol. 2008;20(Suppl 1):53–57. doi: 10.1111/j.1365-2826.2008.01685.x. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, Shriver LP, Ledent C, Cheng X, Carrier EJ, Mann MK, Giovannoni G, Pertwee RG, Yamamura T, Buckley NE, Hillard CJ, Lutz B, Baker D, Dittel BN. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–497. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Miller AM, Stella N. CB2 receptor-mediated migration of immune cells: it can go either way. Br J Pharmacol. 2008;153:299–308. doi: 10.1038/sj.bjp.0707523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AM, Stella N. Microglial cell migration stimulated by ATP and C5a involve distinct molecular mechanisms: quantification of migration by a novel near-infrared method. Glia. 2009;57:875–883. doi: 10.1002/glia.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecucco F, Burger F, Mach F, Steffens S. CB2 cannabinoid receptor agonist JWH-015 modulates human monocyte migration through defined intracellular signaling pathways. Am J Physiol Heart Circ Physiol. 2008;294:H1145–H1155. doi: 10.1152/ajpheart.01328.2007. [DOI] [PubMed] [Google Scholar]
- Ni X, Geller EB, Eppihimer MJ, Eisenstein TK, Adler MW, Tuma RF. Win 55212–2, a cannabinoid receptor agonist, attenuates leukocyte/endothelial interactions in an experimental autoimmune encephalomyelitis model. Mult Scler. 2004;10:158–164. doi: 10.1191/1352458504ms1009oa. [DOI] [PubMed] [Google Scholar]
- Nong L, Newton C, Cheng Q, Friedman H, Roth MD, Klein TW. Altered cannabinoid receptor mRNA expression in peripheral blood mononuclear cells from marijuana smokers. J Neuroimmunol. 2002;127:169–176. doi: 10.1016/s0165-5728(02)00113-3. [DOI] [PubMed] [Google Scholar]
- Patinkin D, Milman G, Breuer A, Fride E, Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur J Pharmacol. 2008;595:1–6. doi: 10.1016/j.ejphar.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Dimarzo V, Elphick MR, Greasley PJ, Hansen HS, Kunos G, Mackie K, Mechoulam R, Ross RA. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Tickets to the brain: role of CCR2 and CX3CR1 in myeloid cell entry in the CNS. J Neuroimmunol. 2010;224:80–84. doi: 10.1016/j.jneuroim.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Raborn ES, Cabral GA. Cannabinoid inhibition of macrophage migration to the trans-activating (Tat) protein of HIV-1 is linked to the CB(2) cannabinoid receptor. J Pharmacol Exp Ther. 2010;333:319–327. doi: 10.1124/jpet.109.163055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raborn ES, Marciano-Cabral F, Buckley NE, Martin BR, Cabral GA. The cannabinoid delta-9-tetrahydrocannabinol mediates inhibition of macrophage chemotaxis to RANTES/CCL5: linkage to the CB2 receptor. J Neuroimmune Pharmacol. 2008;3:117–129. doi: 10.1007/s11481-007-9077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Bernardi G, Centonze D. The endocannabinoid system in the inflammatory and neurodegenerative processes of multiple sclerosis and of amyotrophic lateral sclerosis. Exp Neurol. 2010;224:92–102. doi: 10.1016/j.expneurol.2010.03.030. [DOI] [PubMed] [Google Scholar]
- Roth MD, Baldwin GC, Tashkin DP. Effects of delta-9-tetrahydrocannabinol on human immune function and host defense. Chem Phys Lipids. 2002;121:229–239. doi: 10.1016/s0009-3084(02)00159-7. [DOI] [PubMed] [Google Scholar]
- Sacerdote P, Massi P, Panerai AE, Parolaro D. In vivo and in vitro treatment with the synthetic cannabinoid CP55, 940 decreases the in vitro migration of macrophages in the rat: involvement of both CB1 and CB2 receptors. J Neuroimmunol. 2000;109:155–163. doi: 10.1016/s0165-5728(00)00307-6. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. 2010;58:1017–1030. doi: 10.1002/glia.20983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Veerdonk FL, Netea MG. Diversity: a hallmark of monocyte society. Immunity. 2010;33:289–291. doi: 10.1016/j.immuni.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Walter L, Franklin A, Witting A, Wade C, Xie Y, Kunos G, Mackie K, Stella N. Nonpsychotropic cannabinoid receptors regulate microglial cell migration. J Neurosci. 2003;23:1398–1405. doi: 10.1523/JNEUROSCI.23-04-01398.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JM, Downie SA, Lyman WD, Berman JW. Astrocyte-derived monocyte-chemoattractant protein-1 directs the transmigration of leukocytes across a model of the human blood-brain barrier. J Immunol. 1998;161:6896–6903. [PubMed] [Google Scholar]
- Zhang M, Martin BR, Adler MW, Razdan RJ, Kong W, Ganea D, Tuma RF. Modulation of cannabinoid receptor activation as a neuroprotective strategy for EAE and stroke. J Neuroimmune Pharmacol. 2009;4:249–259. doi: 10.1007/s11481-009-9148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Hilton DA, Hanemann CO, Zajicek J. Cannabinoid receptor and N-acyl phosphatidylethanolamine phospholipase D-evidence for altered expression in multiple sclerosis. Brain Pathol. 2011;21(5):544–557. doi: 10.1111/j.1750-3639.2011.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 3: Endo-cannabinoids and synthetic cannabinoid ligands have no effect on basal or serum-induced migration of human monocytes.