To the Editor:
We report a series of patients with surfactant protein C (SFTPC) dysfunction mutations who presented with severe and persistent respiratory failure in early infancy, resulting in discussions of lung transplantation and withdrawal of support. All three patients were successfully chronically ventilated and weaned off mechanical ventilation (MV). Institutional review board consent was obtained at the University of Colorado.
SFTPC mutations were first recognized in 2001, when a 6-week-old child presented with respiratory distress and a family history of lung disease, and genetic studies revealed the SFTPC mutation (1). Symptom onset in SFTPC dysfunction mutations varies from during the neonatal period to in senior citizens (2–4). In addition, outcomes vary tremendously, from asymptomatic family members to respiratory failure leading to transplant or death (5–9). Hydroxychloroquine has been associated with weaning patients off oxygen, significant weight gain, and improvements in chest radiography (10). However, once a child requires persistent MV, typically discussions arise around withdrawal of support versus transplantation. A case report was recently published of an infant who required persistent MV and was able to wean completely off all support (11).
Patient 1
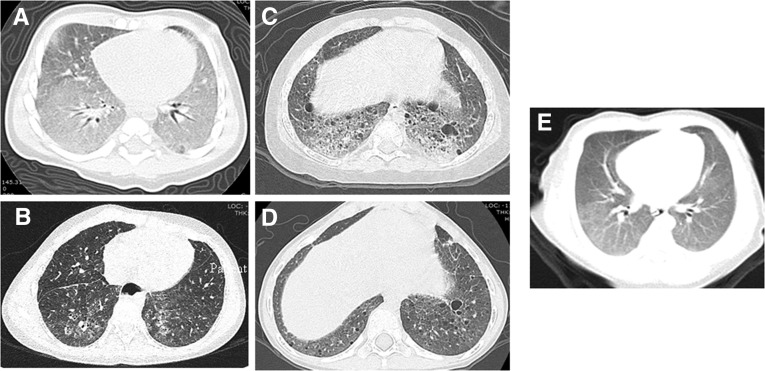
Patient 1 was born full-term, with no perinatal complications. She was noted to be cyanotic at birth but was stable for discharge at 3 days of life. She had multiple admissions for respiratory distress, and at 2.5 months of age, a chest computed tomography (CT) scan (Figure 1) showed diffuse ground-glass opacities and septal thickening; a biopsy was consistent with a mutation in SFTPC: diffuse alveolar proteinosis with type II cell hyperplasia, lipid and iron-laden macrophages, septal widening and fibrosis, and scattered necrotic debris. With this diagnosis, she was started on medical therapies (Table 1). The patient was unable to be weaned from MV. After discussions about tracheostomy with chronic MV versus withdrawal of support versus transplantation, the family elected to proceed with chronic MV at 3.5 months of age. She was ultimately discharged on a home ventilator at 19 months of age and was decannulated by age 6 years (Table 1). She has had subsequent illness but never required reintubation. She has autism and has been unable to cooperate with pulmonary function tests. Her most recent CT scan showed nonspecific interstitial pneumonia findings, diffuse ground-glass opacities, and bronchiectasis (Figure 1). The diagnosis of a surfactant protein C mutation (c.545 G>C, .pGly182Arg) was confirmed by genetic testing once available. Patient 1 had a family history of a maternal uncle dying of pneumonia at 6 months of age and a maternal grandfather dying of lung disease at 49 years of age, but neither one had genetic studies performed.
Figure 1.
Initial and most recent computed tomography (CT) scan for each patient. (A) Patient 1 initial CT scan with diffuse ground-glass opacities and septal thickening. (B) Patient 1 recent CT scan with nonspecific interstitial pneumonia findings, diffuse ground-glass opacities, and bronchiectasis. (C) Patient 2 initial CT scan with marked interstitial prominence, cystic change, and interstitial/alveolar infiltrate. (D) Patient 2 recent CT scan with interstitial prominence and increased number of parenchymal and subpleural cysts. (E) Patient 3 only CT scan with diffuse ground-glass opacities.
Table 1.
Summary of Patients’ Clinical Courses
| Patient | Age at Presentation | SFTPC Mutation | Family History | Age at MV Initiation | Maximum Respiratory Support | Age at Tracheostomy (mo) | LOS (mo) | Age at Decannulation (yr) | Respiratory Treatments | Support at Last Contact (Age) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 d | c.545 G>C, .pGly182Arg | Positive | 2 mo | CMV | 3.5 | 19 | 6 | Clearance, steroids, hydroxychloroquine | Room air (10 yr) |
| 2 | 4 h | c.563 T>A, p. Leu188Gln | Negative | 1 d | HFOV | 3 | 10 | 5 | Clearance, steroids, azathioprine | 0.25 L NC (5 yr) |
| 3 | 10 min | c.567 C>G, p.Cys189Trp | Negative | 4 mo | HFOV | 4.5 | 8 | 2 | Steroids, azithromycin, hydroxychloroquine | Room air (3 yr) |
Definition of abbreviations: CMV = conventional mechanical ventilation; HFOV = high-frequency oscillatory ventilation; LOS = hospital length of stay; MV = mechanical ventilation; NC = nasal cannula; SFTPC = surfactant protein C.
Patient 2
Patient 2 was born at 36 weeks and 6 days with an antenatal course complicated by preterm labor at 27 weeks and preeclampsia. At 4 hours of life, she developed respiratory failure necessitating intubation and high-frequency oscillation for 3 months. After discussions about tracheostomy with ventilation versus withdrawal of support versus transplantation, the family elected to proceed with chronic ventilation. Her initial hospitalization (at an outside facility) lasted 10 months, and she was ultimately discharged on a home ventilator. Because her lung disease remained undiagnosed, she had a repeat CT scan at 22 months (Figure 1), which showed marked interstitial prominence with alveolar disease and a subsequent lung biopsy. The biopsy showed focal mild fibrosis in areas of endogenous lipoid pneumonia, consistent with a mutation in SFTPC. She was started on therapies and successfully decannulated by 5 years (Table 1). She was subsequently lost to follow up. She initially had delayed milestones, but she was catching up rapidly. Genetic testing, once available, confirmed the SFTPC mutation (c.563 T>A, p. Leu188Gln). No family history of lung disease was reported.
Patient 3
Patient 3 was born at 39 weeks and 3 days with a perinatal course complicated by chorioamnionitis. He was noted to be tachypneic at 10 minutes of life and required noninvasive ventilation. A CT scan (Figure 1) revealed ground-glass opacities, and subsequent genetic testing revealed a SFTPC mutation (c.567 C>G, p.Cys189Trp). He was discharged home on medical therapies with nasal cannula oxygen (Table 1). In the subsequent months, he developed respiratory failure requiring high-frequency oscillation. After discussions about tracheostomy with MV versus withdrawal of support versus transplantation, the family elected to proceed with chronic MV. He was discharged home at 8 months on a home ventilator with medical therapies and was decannulated at age 3 years (Table 1). Since decannulation, he has not had any pulmonary-related hospitalizations, he takes only azithromycin, and he does not require any oxygen therapy. His development is unremarkable. There is no family history of lung disease.
Discussion
We present three patients with SFTPC mutations and respiratory failure. Withdrawal of support and lung transplantation were offered to each family, and each family elected to proceed with chronic MV. Different medication regimens were used in these patients, although systemic steroids in some form, dose, and duration were universal to all. Expert opinion and case reports (10, 11) have governed disease treatment, with attention to antiinflammatory agents (steroids, azathioprine, and hydroxychloroquine). The natural history of patients with SFTPC mutations is poorly understood and varies considerably, even within families (2–4). Early respiratory failure appears to resolve over time, and it is unknown whether this is secondary to medical therapies or just the natural history of the disease.
Lung transplantation has been a solution for infants with SFTPC mutations and persistent respiratory failure. Because lung transplantation has a high risk for complication and mortality, with a median survival of 4.9 years for pediatric lung transplants (12), we propose that chronic MV may be a better option for infants and young children with SFTPC mutations because of this potential for recovery over time. With chronic MV, infants with respiratory failure can grow, develop, and ultimately be at home weaning off of ventilatory support without the chronic medications and potential for fatal outcomes with lung transplantation.
Acknowledgments
Acknowledgment
The authors acknowledge Dr. Lawrence Nogee from Johns Hopkins School of Medicine for his assistance with interpreting genetic testing.
Footnotes
Supported by the Academic Training Program in Pediatric Pulmonary Disease 5T32HL007670 (D.R.L.).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 2.Wert SE, Whitsett JA, Nogee LM. Genetic disorders of surfactant dysfunction. Pediatr Dev Pathol. 2009;12:253–274. doi: 10.2350/09-01-0586.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas AQ, Lane K, Phillips J, III, Prince M, Markin C, Speer M, Schwartz DA, Gaddipati R, Marney A, Johnson J, et al. Heterozygosity for a surfactant protein C gene mutation associated with usual interstitial pneumonitis and cellular nonspecific interstitial pneumonitis in one kindred. Am J Respir Crit Care Med. 2002;165:1322–1328. doi: 10.1164/rccm.200112-123OC. [DOI] [PubMed] [Google Scholar]
- 4.Abou Taam R, Jaubert F, Emond S, Le Bourgeois M, Epaud R, Karila C, Feldmann D, Scheinmann P, de Blic J. Familial interstitial disease with I73T mutation: A mid- and long-term study. Pediatr Pulmonol. 2009;44:167–175. doi: 10.1002/ppul.20970. [DOI] [PubMed] [Google Scholar]
- 5.Percopo S, Cameron HS, Nogee LM, Pettinato G, Montella S, Santamaria F. Variable phenotype associated with SP-C gene mutations: fatal case with the I73T mutation. Eur Respir J. 2004;24:1072–1073. doi: 10.1183/09031936.04.00092304. [DOI] [PubMed] [Google Scholar]
- 6.Poterjoy BS, Vibert Y, Sola-Visner M, McGowan J, Visner G, Nogee LM. Neonatal respiratory failure due to a novel mutation in the surfactant protein C gene. J Perinatol. 2010;30:151–153. doi: 10.1038/jp.2009.97. [DOI] [PubMed] [Google Scholar]
- 7.Gower WA, Nogee LM. Surfactant dysfunction. Paediatr Respir Rev. 2011;12:223–229. doi: 10.1016/j.prrv.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avital A, Hevroni A, Godfrey S, Cohen S, Maayan C, Nusair S, Nogee LM, Springer C. Natural history of five children with surfactant protein C mutations and interstitial lung disease. Pediatr Pulmonol. 2014;49:1097–1105. doi: 10.1002/ppul.22971. [DOI] [PubMed] [Google Scholar]
- 9.Hamvas A. Inherited surfactant protein-B deficiency and surfactant protein-C associated disease: clinical features and evaluation. Semin Perinatol. 2006;30:316–326. doi: 10.1053/j.semperi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Rosen DM, Waltz DA. Hydroxychloroquine and surfactant protein C deficiency. N Engl J Med. 2005;352:207–208. doi: 10.1056/NEJM200501133520223. [DOI] [PubMed] [Google Scholar]
- 11.van Hoorn J, Brouwers A, Griese M, Kramer B. Successful weaning from mechanical ventilation in a patient with surfactant protein C deficiency presenting with severe neonatal respiratory distress. BMJ Case Rep. 2014;2014:bcr2013203053. doi: 10.1136/bcr-2013-203053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benden C, Edwards LB, Kucheryavaya AY, Christie JD, Dipchand AI, Dobbels F, Kirk R, Lund LH, Rahmel AO, Yusen RD, et al. International Society for Heart and Lung Transplantation. The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Lung and Heart-Lung Transplantation Report—2013; focus theme: age. J Heart Lung Transplant. 2013;32:989–997. doi: 10.1016/j.healun.2013.08.008. [DOI] [PubMed] [Google Scholar]