Abstract
Rationale: Structural risk factors for obstructive sleep apnea syndrome (OSAS) in adolescents have not been well characterized. Because many adolescents with OSAS are obese, we hypothesized that the anatomic OSAS risk factors would be more similar to those in adults than those in children.
Objectives: To investigate the anatomic risk factors in adolescents with OSAS compared with obese and lean control subjects using magnetic resonance imaging (MRI).
Methods: Three groups of adolescents (age range: 12–16 yr) underwent MRI: obese individuals with OSAS (n = 49), obese control subjects (n = 38), and lean control subjects (n = 50).
Measurements and Main Results: We studied 137 subjects and found that (1) obese adolescents with OSAS had increased adenotonsillar tissue compared with obese and lean control subjects; (2) obese OSAS adolescents had a smaller nasopharyngeal airway than control subjects; (3) the size of other upper airway soft tissue structures (volume of the tongue, parapharyngeal fat pads, lateral walls, and soft palate) was similar between subjects with OSAS and obese control subjects; (4) although there were no major craniofacial abnormalities in most of the adolescents with OSAS, the ratio of soft tissue to craniofacial space surrounding the airway was increased; and (5) there were sex differences in the pattern of lymphoid proliferation.
Conclusions: Increased size of the pharyngeal lymphoid tissue, rather than enlargement of the upper airway soft tissue structures, is the primary anatomic risk factor for OSAS in obese adolescents. These results are important for clinical decision making and suggest that adenotonsillectomy should be considered as the initial treatment for OSAS in obese adolescents, a group that has poor continuous positive airway pressure adherence and difficulty in achieving weight loss.
Keywords: adenoid, adolescents, MRI, obstructive sleep apnea syndrome, tonsils
At a Glance Commentary
Scientific Knowledge on the Subject
Although several studies have evaluated the structural basis for obstructive sleep apnea in children and in adults, very few studies have specifically addressed the important transitional stage of adolescence. This study provides comprehensive, magnetic resonance imaging–based soft tissue and craniofacial measurements of the upper airway in obese adolescents with obstructive sleep apnea syndrome and compares them with those in both obese and lean age-matched control subjects.
What This Study Adds to the Field
The data we report indicate that adolescents with obstructive sleep apnea have an anatomic risk profile similar to that of children, not that of adults, and that lymphoid tissue, rather than other soft tissue components, is the primary structural abnormality. This finding is important for clinical management and suggests that, in adolescents who are obese, adenotonsillectomy should be considered as a primary treatment for obstructive sleep apnea, especially because continuous positive airway pressure adherence tends to be poor in this age group.
The obstructive sleep apnea syndrome (OSAS) is common in children and adults, but it has not been well studied in adolescents. In one study, researchers reported the prevalence of OSAS in adolescents to be 2% (1), but the prevalence is probably higher in the United States because of the adolescent obesity epidemic (2). OSAS in children is thought to be secondary to a combination of enlargement of the lymphoid tissue (tonsils and adenoid) (3) and, sometimes, obesity, as well as to reductions in neuromuscular tone (4). In adults, there are known anatomic risk factors for OSAS, including enlargement of the tongue, soft palate, parapharyngeal fat pads, and lateral pharyngeal walls (5) in conjunction with craniofacial restriction (retrognathia) (6). In addition to anatomic factors, physiologic mechanisms increase OSAS risk in both children and adults (7–12). Although these risk factors for OSAS have been well described in children and adults, few studies have addressed the important transitional developmental phase of adolescence.
Given the obesity epidemic in adults and adolescents (2) and the decline in lymphoid tissue growth with age (13), we suspected that the anatomic risk factors for OSAS in adolescents would be more similar to those of adults than to those of children. Accordingly, obesity-related anatomic risk factors for OSAS, including enlargement of the parapharyngeal fat pads, tongue (including tongue fat), lateral pharyngeal walls, and total upper airway soft tissues could play an important role in the pathogenesis of OSAS in adolescents. Moreover, the anatomic risk factors for OSAS in adults are thought to be different in men versus women (14). It is not known whether anatomic risk factors for OSAS in adolescents, including the size of lymphoid tissue, are different between boys and girls.
To determine the anatomic risk factors for OSAS in adolescents, we studied participants by using magnetic resonance imaging (MRI). MRI allowed us to determine upper airway sizes, surrounding soft tissue volumes, adenotonsillar tissue volumes, and craniofacial structures. Although enlargement of upper airway soft tissue structures and reduction in the craniofacial skeleton increase the risk of developing OSAS (5, 6), it is likely that a combination of these structures confers additional increased risk (15). Specifically, a smaller craniofacial area combined with larger upper airway soft tissue volume should increase the severity of OSAS. Therefore, in addition to the specific upper airway soft tissue volumes and craniofacial structures that we examined in the investigation, we also studied a combined measure: the ratio of the total upper airway soft tissue volume to the combined nasopharyngeal and oropharyngeal craniofacial space (ratio of soft tissue to craniofacial space [STCF]). The STCF ratio measures how much soft tissue occupies the space within the limits of the craniofacial structure. Such a measure has never been studied before, and it may provide important insight into the pathogenesis of sleep apnea in adolescents.
The objectives of this study were to examine anatomic risk factors for OSAS in three groups (obese patients with OSAS, obese control subjects, and lean control subjects) of adolescents (age range: 12–16 yr) by MRI. We hypothesized that in the adolescents with OSAS compared with the obese and lean control subjects: (1) upper airway caliber would be smaller, (2) upper airway soft tissues structures would be larger, (3) adenotonsillar tissue would be larger, (4) the STCF ratio would be greater, and (5) the craniofacial structures would be smaller. We also hypothesized that, in all participant groups, the boys would have larger upper airway soft tissue and craniofacial structures than the girls.
Portions of this investigation have been presented previously in abstract form (16–18).
Methods
The Institutional Review Board at the Children’s Hospital of Philadelphia (CHOP) approved the study. Informed consent was obtained from the parents and/or guardians of the participants, and assent was obtained from the participants. Additional information on the methods used in this study is presented in the online supplement.
Participants
Adolescents aged 12–16 years were recruited as part of a larger study evaluating the pathophysiology of OSAS (19–22). Adolescents with OSAS were recruited from the Sleep Center at CHOP. Control subjects (obese and lean) were recruited from the CHOP Healthy Weight Program and the general population via advertisements. All control subjects were nonsnorers without symptoms of OSAS. Participants were defined as obese if their body mass index (BMI) was above the 95th percentile and lean if their BMI was below the 85th percentile (23). Participants with OSAS were eligible if they were obese and had an apnea–hypopnea index (AHI) of more than five events per hour (i.e., mild to moderate pediatric OSAS), and control participants were eligible if they had an AHI of less than 1.5 events/h (24–27). Adenotonsillectomy was an exclusion criterion. Lean adolescents with OSAS were not included, as OSAS is very uncommon in lean individuals in this age group (28).
Sleep Study
Baseline in-laboratory polysomnography was performed using standard pediatric recording and scoring techniques (29).
MRI
Upper airway MRI was performed using a 3T scanner (MAGNETOM Sonata; Siemens Healthcare, Malvern, PA) equipped with a prototype enhanced gradient system.
Upper Airway Analysis
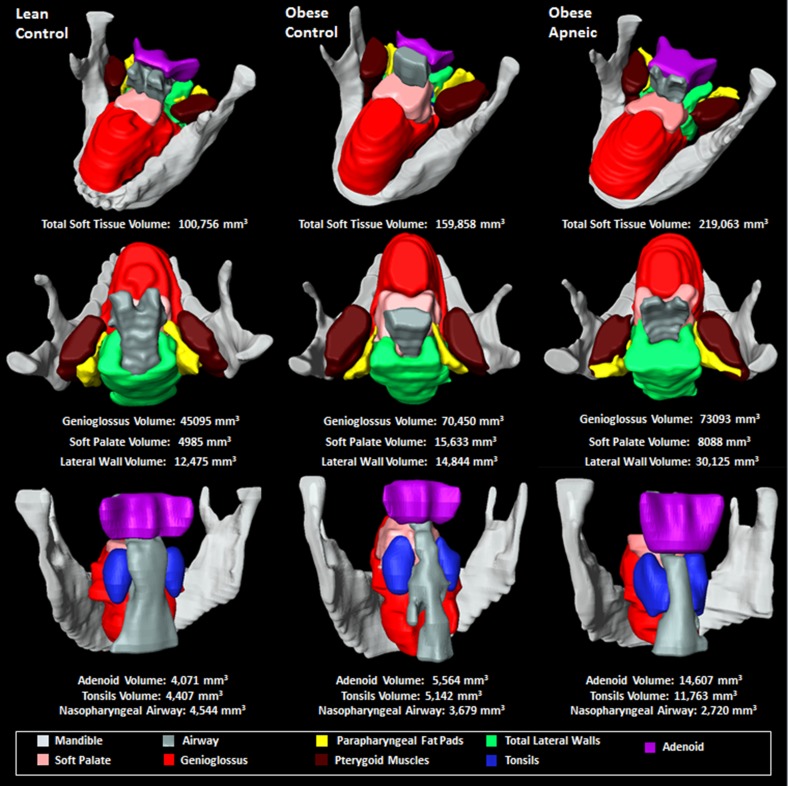
Using the Amira 4.1.2 image analysis software program (Visage Imaging, San Diego, CA), we manually segmented and analyzed each slice of the axial upper airway MRI scan. There were four general analysis domains: airway, soft tissue, adenotonsillar tissue, and craniofacial structures. We measured airway volume, cross-sectional area, and minimum airway area in the retropalatal (RP), retroglossal (RG), and nasopharyngeal (NP) regions, as well as minimum anteroposterior airway width and minimum lateral airway width in the RP and RG regions (Figure 1). Airway length was measured as the distance between the palatal plane and a parallel plane through the base of the epiglottis (see Figure E2B) (14, 30–32). Airway length was adjusted for height (14, 30–32). Volumetric analysis of the upper airway soft tissue structures was performed on the axial T1-weighted MRI scans and included the soft palate, tongue genioglossus muscle, other tongue (geniohyoid, hyoglossus, myohyoid, digastric, and mylohyoid) muscles, parapharyngeal fat pads, lateral pharyngeal walls (which included the tonsils), pterygoid muscle, epiglottis, and total sum of soft tissue volumes (Figure 2). Axial T2-weighted MRI scans were used for measurements of the tonsils (right and left combined) and adenoid as they provided better resolution of lymphoid tissue than T1-weighted images did (Figure 3).
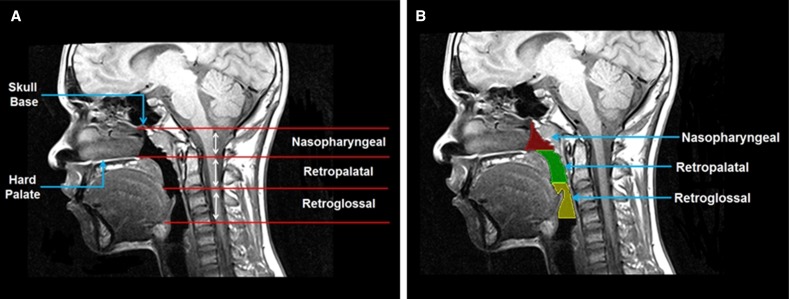
Figure 1.
(A) Anatomic definitions of the upper airway regions on a midsagittal magnetic resonance (MR) image are demonstrated: nasopharyngeal (from level of skull base to level of hard palate), retropalatal (from level of hard palate to caudal margin of soft palate), and retroglossal (from caudal margin of soft palate to base of tongue). (B) Upper airway segmented into three regions on a midsagittal MR slice. Red = nasopharyngeal airway; green = retropalatal airway; yellow = retroglossal airway. The participant was a lean control with an apnea–hypopnea index of 0.12/h and a body mass index z-score of −0.86.
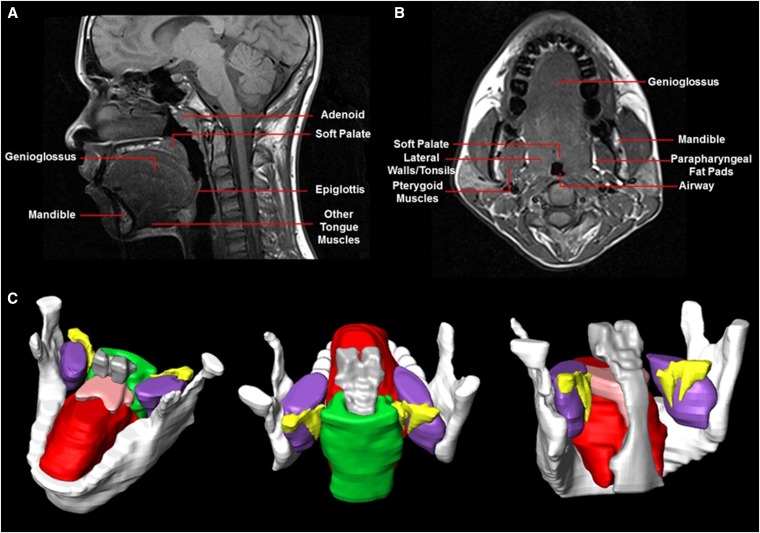
Figure 2.
Magnetic resonance imaging analysis of upper airway soft tissue structures. (A) Upper airway anatomy on a sagittal magnetic resonance (MR) image. (B) Upper airway anatomy on an axial MR image. (C) Three-dimensional reconstruction of upper airway soft tissue structures using Amira software. White = mandible; pink = soft palate; yellow = parapharyngeal fat pads; green = lateral pharyngeal walls; red = genioglossus; purple = pterygoid muscles; gray = airway. Images show the same participant as in Figure 1.
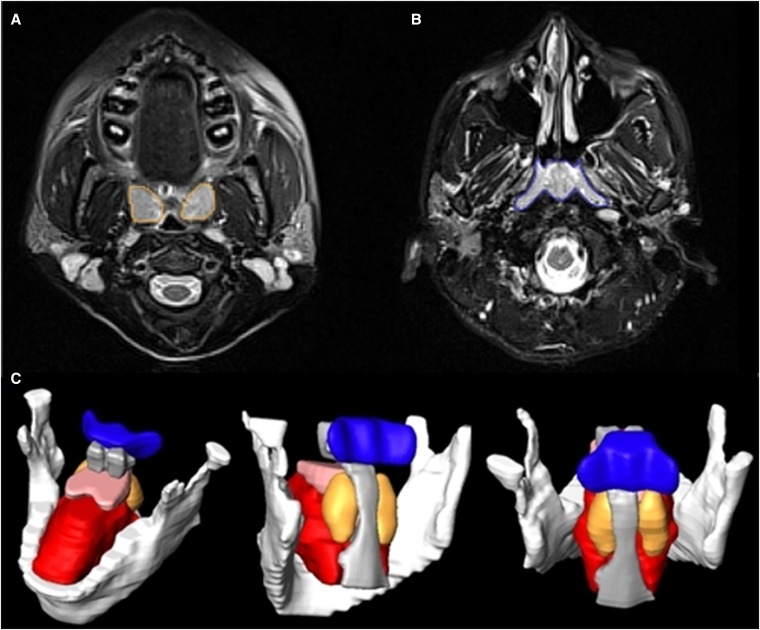
Figure 3.
Magnetic resonance imaging analysis highlighting the upper airway lymphoid tissue. (A) Axial T2-weighted magnetic resonance (MR) image with palatine tonsil tissue segmentation (orange). (B) Axial T2-weighted MR image with adenoid tissue segmentation (blue). (C) Three-dimensional reconstruction of adenoid (blue) and tonsils (orange/rust). White = mandible; pink = soft palate; red = genioglossus; gray = airway. Images show the same participant as in Figure 1.
Primary cephalometric analysis of craniofacial structures was analyzed based on five subdomains (as in our previous studies [6]): (1) mandibular width measured in two dimensions and mandibular length and depth measured in three dimensions (Figure E1), (2) hyoid measurements of the distance from hyoid to nasion, sella, and supramentale (Figure E2A), (3) craniofacial angles, (4) craniofacial heights and areas, and (5) maxillary measurements (Figure E2B).
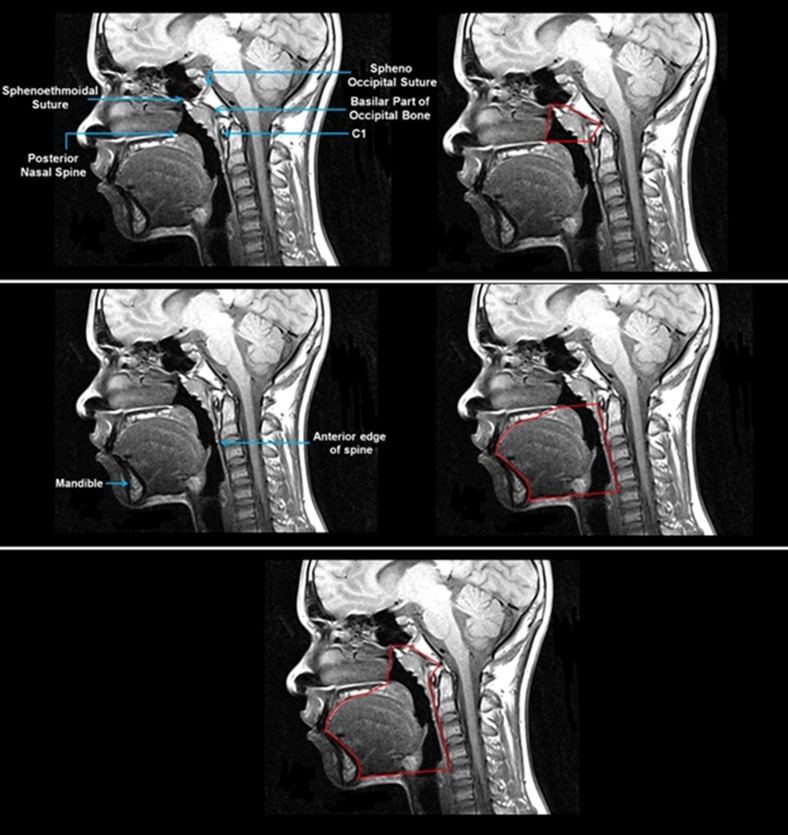
In addition to the above-described measures, we examined a combined measure: the STCF ratio. Total soft tissue volume included the volumes of the genioglossus, other tongue muscles, soft palate, retropalatal and retroglossal lateral pharyngeal walls, parapharyngeal fat pads, epiglottis, pterygoid muscle, palatine tonsils, and adenoid. The oropharyngeal portion of the craniofacial structure was delineated by the mandible and maxilla and bound posteriorly by the spine (Figure 4). The nasopharyngeal portion of this craniofacial structure was delineated by four boundaries (Figure 4): (1) the anterior boundary, extending from the posterior nasal spine to the sphenoethmoidal suture; (2) the posterior border, forming a plane following the basilar part of the occipital bone; (3) the superior boundary, constituting a straight line from the sphenoethmoidal suture to the sphenooccipital suture; and (4) the inferior border, which was a straight line from the posterior nasal spine to the C1 vertebra.
Figure 4.
Midsagittal magnetic resonance image showing anatomic landmarks and outline of the combined nasopharyngeal and oropharyngeal craniofacial structure. Top panel depicts anatomic landmarks and segmentation of the nasopharyngeal portion of the craniofacial structure. Middle panel shows anatomic landmarks and segmentation of the oropharyngeal portion of the craniofacial structure. Bottom panel depicts the combined craniofacial structure outlined in red. Images show the same participant as in Figure 1.
The technician performing the MRI analyses was blinded to the polysomnography results.
Statistical Analysis
See the online supplement for information about the statistical analysis. In brief, an adjusted analysis of covariance was used to compare the three groups with a subdomain-specific, Bonferroni-corrected level of significance.
Results
Participant Characteristics
Demographic comparisons of the groups are presented in Table 1. There were no significant differences in most demographic variables, but, by study design, there were significant differences in AHI and weight-related variables between the three participant groups. Of note, there were no significant differences in BMI z-scores between obese participants with OSAS and obese control subjects, and there was no significant difference in AHI between the obese and lean control groups.
Table 1.
Demographic Characteristics of the Study Groups
| Characteristic* | Mean ± SD or N (%) |
P Value† | Pairwise Comparisons |
||||
|---|---|---|---|---|---|---|---|
| OSAS (n = 49) | Obese Controls (n = 38) | Lean Controls (n = 50) | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | ||
| Age, yr | 14.6 ± 1.4 | 14.2 ± 1.5 | 14.7 ± 1.5 | 0.1631 | — | — | — |
| Males | 35 (71.4%) | 24 (63.2%) | 35 (70.0%) | 0.6872 | — | — | — |
| Height, cm | 165.4 ± 9.4 | 165.3 ± 11.1 | 164.2 ± 10.8 | 0.8268 | — | — | — |
| Weight, kg | 101.6 ± 36.3 | 94.5 ± 27.0 | 55.1 ± 11.9 | <0.0001 | 0.1431 | <0.0001 | <0.0001 |
| BMI, z-score | 2.39 ± 0.39 | 2.24 ± 0.34 | 0.16 ± 0.98 | <0.0001 | 0.3008 | <0.0001 | <0.0001 |
| AHI, events/h | 20.1 ± 25.9 | 0.52 ± 0.39 | 0.37 ± 0.43 | <0.0001 | <0.0001 | <0.0001 | 0.9655 |
| SpO2 nadir, % | 83.1 ± 9.3 | 93.2 ± 2.4 | 93.0 ± 4.7 | <0.0001 | <0.0001 | <0.0001 | 0.9051 |
| % total sleep time with ET CO2 > 50 mm Hg | 17.6 ± 27.4 | 8.2 ± 16.4 | 5.9 ± 18.8 | 0.0219 | 0.0472 | 0.0083 | 0.6229 |
| Race | |||||||
| African American | 37 (75.5%) | 33 (86.8%) | 34 (68.0%) | 0.1255 | — | — | — |
| White/other | 12 (24.5%) | 5 (3.7%) | 16 (32.0%) | ||||
| Tanner stage‡ | |||||||
| Early, ≤3 | 12 (28.6%) | 10 (32.3%) | 15 (30.0%) | 0.9439 | — | — | — |
| Late, ≥4 | 30 (71.4%) | 21 (67.7%) | 35 (70.0%) | ||||
Definition of abbreviations: AHI = apnea–hypopnea index; BMI = body mass index; ET = end-tidal; OSAS = obstructive sleep apnea syndrome; SpO2 = oxygen saturation as measured by pulse oximetry.
Demographics are presented as mean ± SD or frequency (percentage).
P value derived from analysis of variance or χ2 test comparing values across the three subject groups.
n = 14 (7 with OSAS, 7 obese control subjects) missing information on Tanner stage.
We compared boys and girls within each of the participant groups (see Table E1). Boys were taller than girls in each group (P < 0.025 for each comparison). Boys in the obese control group were slightly younger (P = 0.044) than girls and had a lower percentage of late Tanner stage (P = 0.013). Boys in the lean control group also had a slightly higher mean AHI than girls in the lean control group (P = 0.017), but this value was still within the normal range. There were no other differences in demographic characteristics between boys and girls within each participant group.
The results presented below are adjusted for relevant covariates, including age, race, and Tanner stage (see online supplement).
Differences between Participant Groups
Upper airway caliber
Figure 5 shows a reduction in the size of the nasopharyngeal airway for representative OSAS, obese control, and lean control participants. Similar findings were noted when all participants were examined. Comparisons of upper airway caliber between participant groups are presented in Table 2 and Figure 6. The OSAS group had a smaller nasopharyngeal volume, nasopharyngeal mean cross-sectional area, and nasopharyngeal minimum airway area, and a larger retroglossal anteroposterior dimension at the minimum airway area, than both obese and lean control subjects. Participants with OSAS and obese control subjects had significantly smaller retropalatal lateral dimensions than lean control subjects, but there was no difference between participants with OSAS and obese control subjects. Airway volume and mean cross-sectional area in the retropalatal and retroglossal regions were not significantly different across participant groups. There was a trend for airway length adjusted for height to be longer (P = 0.053) in the OSAS group than in obese control subjects, and this length was significantly longer (P = 0.003) in participants with OSAS than in lean control subjects, but there was no difference in adjusted or unadjusted airway length between obese and lean control subjects.
Figure 5.
Upper airway anatomy shown in three participants, all girls. (Left) Lean control participant with an apnea–hypopnea index (AHI) of 0.0/h. (Middle) Obese control participant with AHI of 0.3/h. (Right) Obese participant with obstructive sleep apnea syndrome (OSAS) with an AHI of 9.0/h. The adolescent with OSAS had larger adenotonsillar tissue, total soft tissue, and lateral wall volumes, as well as a smaller nasopharyngeal airway, than the obese and lean control subjects.
Table 2.
Comparison of Upper Airway Caliber between Individuals with OSAS, Obese Control Subjects, and Lean Control Subjects in All Participants
| Domain | Variable | Adjusted Mean ± SE* |
P Value† | Pairwise Comparisons‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OSAS | Obese Controls | Lean Controls | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | |||
| Nasopharyngeal§ | Volume, mm3 | 2,352 ± 248 | 3,557 ± 275 | 4,190 ± 233 | 2.0 × 10−6 | 0.0014 | 4.4 × 10−7 | 0.0897 |
| Cross-sectional area, mm2 | 472.5 ± 47.3 | 761.6 ± 52.4 | 750.7 ± 44.4 | 1.8 × 10−5 | 7.0 × 10−5 | 4.2 × 10−5 | 0.8773 | |
| Minimum area, mm2 | 79.2 ± 8.4 | 109.8 ± 9.3 | 104.4 ± 7.9 | 0.0282 | 0.0156 | 0.0327 | 0.6626 | |
| Retropalatal║ | Volume, mm3 | 2,879 ± 212 | 2,535 ± 238 | 2,957 ± 202 | 0.3897 | — | — | — |
| Cross-sectional area, mm2 | 370.5 ± 30.1 | 358.7 ± 33.7 | 420.4 ± 28.6 | 0.3290 | — | — | — | |
| Minimum area, mm2 | 62.7 ± 8.1 | 60.5 ± 9.1 | 86.6 ± 7.7 | 0.0508 | — | — | — | |
| Minimum AP dimension, mm | 8.07 ± 0.50 | 7.46 ± 0.55 | 7.38 ± 0.47 | 0.5597 | — | — | — | |
| Minimum lateral dimension, mm | 10.31 ± 0.68 | 10.34 ± 0.75 | 14.50 ± 0.64 | 1.2 × 10−5 | 0.9730 | 2.0 × 10−5 | 7.2 × 10−5 | |
| Retroglossal¶ | Volume, mm3 | 5,584 ± 344 | 4,776 ± 386 | 4,807 ± 327 | 0.1778 | — | — | — |
| Cross-sectional area, mm2 | 646.2 ± 43.6 | 570.6 ± 48.9 | 578.3 ± 41.5 | 0.4120 | — | — | — | |
| Minimum area, mm2 | 119.4 ± 9.1 | 100.9 ± 10.3 | 110.4 ± 8.7 | 0.3986 | — | — | — | |
| Minimum AP dimension, mm | 13.30 ± 0.68 | 10.50 ± 0.75 | 9.55 ± 0.64 | 0.0004 | 0.0063 | 0.0001 | 0.3503 | |
| Minimum lateral dimension, mm | 14.08 ± 0.95 | 13.03 ± 1.05 | 16.08 ± 0.90 | 0.0887 | — | — | — | |
| Airway length** | Airway length, mm | 59.5 ± 1.1 | 57.5 ± 1.2 | 55.5 ± 1.0 | 0.0289 | 0.2120 | 0.0079 | 0.2059 |
| Airway length/height, mm/cm | 0.361 ± 0.005 | 0.346 ± 0.006 | 0.339 ± 0.005 | 0.0106 | 0.0532 | 0.0032 | 0.3893 | |
Definition of abbreviations: AP = anteroposterior; OSAS = obstructive sleep apnea syndrome.
Least squares mean and SE estimates from regression model adjusted for age at consent, race, and Tanner stage.
Analysis of variance (PANOVA).
P values for pairwise comparisons (P < 0.0167 statistically significant after Bonferroni correction), applicable when PANOVA suggests significant differences between groups.
Bonferroni-corrected significance level of PANOVA < 0.0167 (equals 0.05/3).
Bonferroni-corrected significance level of PANOVA < 0.01 (equals 0.05/5).
Bonferroni-corrected significance level of PANOVA < 0.01 (equals 0.05/5).
Bonferroni-corrected significance level PANOVA < 0.025 (equals 0.05/2).
Figure 6.
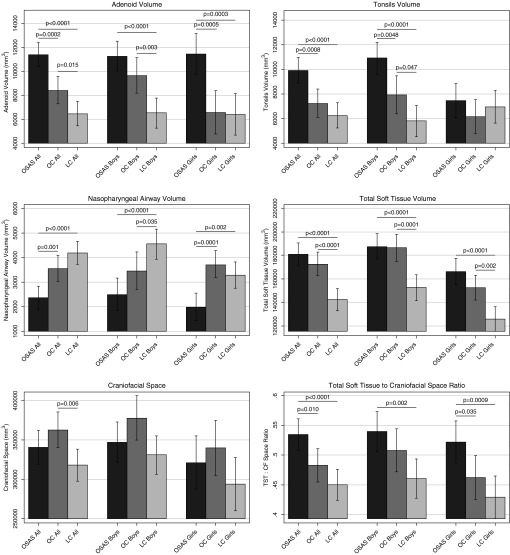
Bar graphs showing differences in adenoid volume, tonsil volume, nasopharyngeal airway volume, total soft tissue volume, craniofacial space, and ratio of total soft tissue to craniofacial space (TST:CF) in the three participant groups and separated into boys and girls. LC = lean control subjects; OC = obese control subjects; OSAS = obstructive sleep apnea syndrome.
Lymphoid tissue volume
Figure 5 shows larger adenoid and tonsils in a representative participant with OSAS than in an obese control and a lean control. Similar findings were noted when all participants were examined (Table 3 and Figure 6). The OSAS group had larger volumes of the adenoid and tonsils than both obese and lean control subjects. Obese control subjects also had a larger volume of the adenoids, but not of the tonsils, than lean control subjects.
Table 3.
Comparisons of Lymphoid Tissue Volumes between Participants with OSAS, Obese Control Subjects, and Lean Control Subjects
| Population | Variable (mm3) | Adjusted Mean ± SE* |
P Value† | Pairwise Comparisons‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OSAS | Obese Controls | Lean Controls | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | |||
| All participants§ | Adenoid | 11,398 ± 514 | 8,429 ± 583 | 6,464 ± 518 | 3.1 × 10−9 | 0.0002 | 6.1 × 10−10 | 0.0153 |
| Tonsils | 9,915 ± 528 | 7,205 ± 591 | 6,233 ± 521 | 6.4 × 10−6 | 0.0008 | 2.4 × 10−6 | 0.2325 | |
| Boys§ | Adenoid | 11,257 ± 635 | 9,643 ± 756 | 6,503 ± 637 | 5.2 × 10−6 | 0.1094 | 1.1 × 10−6 | 0.0025 |
| Tonsils | 10,887 ± 661 | 7,899 ± 782 | 5,783 ± 651 | 2.5 × 10−6 | 0.0048 | 4.6 × 10−7 | 0.0465 | |
| Girls§ | Adenoid | 11,440 ± 878 | 6,563 ± 916 | 6,402 ± 875 | 0.0002 | 0.0005 | 0.0003 | 0.9040 |
| Tonsils | 7,453 ± 704 | 6,120 ± 711 | 6,926 ± 678 | 0.4359 | — | — | — | |
Definition of abbreviation: OSAS = obstructive sleep apnea syndrome.
Least squares mean and SE estimates from regression model adjusted for age at consent, race, Tanner stage, and sex (in all participants only).
Analysis of variance (PANOVA).
P values for pairwise comparisons (P < 0.0167, indicating statistically significant after Bonferroni correction), applicable when PANOVA suggests significant differences between groups.
Bonferroni-corrected significance level of PANOVA < 0.025 (equals 0.05/2).
Upper airway soft tissue structures
Figure 5 shows that the volume of the lateral walls (which are composed of pharyngeal muscle and palatine tonsils) is larger in a representative participant with OSAS than in an obese control and a lean control. Similar findings were noted when all participants were examined (Table 4). The volume of the total lateral walls and, specifically, the volume of the retropalatal lateral walls were larger in the OSAS group than in the obese control subjects and lean control subjects. However, this was not true for the volume of the lateral walls minus the tonsillar volume, which indicates that the changes in the lateral walls between participants with OSAS and obese control subjects were related to the palatine tonsils. Most of the upper airway soft tissue volumes (excluding soft palate, epiglottis, and tongue fat volumes) were larger in the OSAS group than in the lean control subjects, and these volumes were larger in the obese control subjects than in the lean control subjects (except for retropalatal lateral wall volume).
Table 4.
Comparison of the Volumes of the Upper Airway Soft Tissue Structures between Individuals with OSAS, Obese Control Subjects, and Lean Control Subjects in All Participants
| Domain | Variable (mm3) | Adjusted Mean ± SE* |
P Value† | Pairwise Comparisons‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OSAS | Obese Controls | Lean Controls | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | |||
| Total volumes§ | Total tongue volume | 105,922 ± 3,185 | 106,887 ± 3,427 | 88,044 ± 3,167 | 8.0 × 10−5 | 0.8353 | 0.0001 | 0.0001 |
| Soft palate volume | 8,916 ± 410 | 9,271 ± 455 | 8,281 ± 392 | 0.2571 | — | — | — | |
| Total lateral wall volume | 27,627 ± 997 | 23,486 ± 1,091 | 19,506 ± 972 | 4.4 × 10−7 | 0.0056 | 6.7 × 10−8 | 0.0090 | |
| Fat pad volume | 6,096 ± 351 | 5,421 ± 384 | 3,649 ± 335 | 7.6 × 10−6 | 0.1932 | 2.1 × 10−6 | 0.0010 | |
| Pterygoid volume | 20,170 ± 800 | 18,497 ± 885 | 15,954 ± 774 | 0.0013 | 0.1595 | 0.0003 | 0.0370 | |
| Epiglottis volume | 1,312 ± 82 | 1,319 ± 90 | 1,210 ± 79 | 0.5933 | — | — | — | |
| Partial volumes║ | Genioglossus volume | 77,064 ± 2,643 | 80,855 ± 2,888 | 66,667 ± 2,546 | 0.0012 | 0.3302 | 0.0060 | 0.0005 |
| Tongue fat volume (Dixon) | 21,100 ± 1,505 | 25,061 ± 1,496 | 18,525 ± 1,327 | 0.0069 | 0.0671 | 0.1966 | 0.0017 | |
| Other tongue volume | 28,783 ± 1,125 | 26,133 ± 1,210 | 22,231 ± 1,118 | 0.0004 | 0.1082 | 8.6 × 10−5 | 0.0225 | |
| RP lateral wall volume | 16,778 ± 690 | 13,538 ± 754 | 11,860 ± 666 | 6.2 × 10−6 | 0.0018 | 1.6 × 10−6 | 0.1063 | |
| RG lateral wall volume | 10,854 ± 594 | 9,962 ± 650 | 7,618 ± 579 | 0.0007 | 0.3093 | 0.0002 | 0.0010 | |
| Lateral wall volume: tonsils | 17,853 ± 737 | 16,368 ± 778 | 13,180 ± 694 | 4.9 × 10−5 | 0.1657 | 1.2 × 10−5 | 0.0036 | |
Definition of abbreviations: OSAS = obstructive sleep apnea syndrome; RP = retropalatal; RG = retroglossal.
Least squares mean and SE estimates from regression model adjusted for age at consent, race, Tanner stage, and sex.
Analysis of variance (PANOVA).
P values for pairwise comparisons (P < 0.0167, indicating statistically significant after Bonferroni correction), applicable when PANOVA suggests significant differences between groups.
Bonferroni-corrected significance level of PANOVA < 0.0083 (equals 0.05/6).
Bonferroni-corrected significance level of PANOVA < 0.0083 (equals 0.05/6).
Craniofacial structures
Comparisons of the craniofacial structures between participant groups are presented in Table 5. We examined several craniofacial domains (see the Methods section and the online supplement). The only measure that was nominally smaller (P = 0.038) in the participants with OSAS than in obese control subjects was total mandibular length. There were no other differences between the participants with OSAS and obese control subjects. However, there were many statistically or nominally significant differences between patients with OSAS and lean control subjects (Table 5). Compared with lean control subjects, participants with OSAS had a larger saddle angle, nasion-sella to horizontal plane, palatal plane to nasion-sella, lower facial height, anterior facial height, posterior nasal spine to anterior arch atlas, oropharyngeal area, distance from hyoid to third cervical vertebra, hyoid to retropogonion and retropogonion to C3, mandibular depth, mandibular corpus length, mandibular width (at the first and premolars and at the gonion), and maxillary depth. The upper to anterior facial height ratio, mandibular ramus length, and maxillary divergence were smaller in participants with OSAS than in lean control subjects. Similarly, many, but not all, the findings for these measures were the same when we compared obese control subjects with lean control subjects.
Table 5.
Comparison of Craniofacial Structures between OSAS, Obese Control Subjects, and Lean Control Subjects in All Participants
| Domain | Variable | Adjusted Mean ± SE* |
P Value† | Pairwise Comparisons‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OSAS | Obese Controls | Lean Controls | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | |||
| Craniofacial angles§ | SNA angle, ° | 87.34 ± 0.62 | 88.72 ± 0.71 | 88.48 ± 0.62 | 0.2710 | — | — | — |
| SNB angle, ° | 83.13 ± 0.59 | 83.77 ± 0.67 | 83.20 ± 0.59 | 0.7462 | — | — | — | |
| ANB angle, ° | 4.19 ± 0.38 | 4.95 ± 0.43 | 5.28 ± 0.38 | 0.1176 | — | — | — | |
| Saddle angle, ° | 130.38 ± 0.84 | 129.48 ± 0.97 | 126.28 ± 0.84 | 0.0025 | 0.4841 | 0.0008 | 0.0162 | |
| ANS-PNS to Na-sella, ° | 9.07 ± 0.87 | 6.51 ± 1.00 | 8.17 ± 0.87 | 0.1565 | — | — | — | |
| Craniofacial heights and areas║ | Lower facial height (LFH), mm | 7.08 ± 0.08 | 7.12 ± 0.09 | 6.74 ± 0.08 | 0.0033 | 0.7560 | 0.0037 | 0.0031 |
| Upper facial height (UFH), mm | 4.62 ± 0.06 | 4.57 ± 0.07 | 4.62 ± 0.06 | 0.7675 | — | — | — | |
| Anterior facial height (AFH), mm | 11.69 ± 0.11 | 11.70 ± 0.12 | 11.33 ± 0.11 | 0.0374 | 0.9615 | 0.0230 | 0.0326 | |
| UFH/AFH | 0.39 ± 0.00 | 0.39 ± 0.00 | 0.40 ± 0.00 | 0.0167 | 0.3699 | 0.0403 | 0.0062 | |
| PNS to anterior arch atlas, mm | 3.93 ± 0.07 | 3.82 ± 0.08 | 3.63 ± 0.07 | 0.0092 | 0.2729 | 0.0023 | 0.0817 | |
| Nasooropharyngeal area, mm2 | 23.99 ± 0.48 | 24.6 ± 0.56 | 22.79 ± 0.48 | 0.0492 | 0.4078 | 0.0833 | 0.0183 | |
| Oropharyngeal area, mm2 | 16.71 ± 0.35 | 17.47 ± 0.41 | 15.71 ± 0.36 | 0.0071 | 0.1565 | 0.0494 | 0.0019 | |
| Nasopharyngeal area, mm2 | 7.22 ± 0.18 | 7.21 ± 0.21 | 7.05 ± 0.18 | 0.7771 | — | — | — | |
| Hyoid distances¶ | Hyoid to sella distance, mm | 10.21 ± 0.14 | 10.18 ± 0.16 | 9.82 ± 0.14 | 0.0912 | — | — | — |
| Hyoid to third cervical vertebra, mm | 3.59 ± 0.07 | 3.62 ± 0.08 | 3.25 ± 0.07 | 0.0003 | 0.8212 | 0.0004 | 0.0006 | |
| Hyoid to retropogonion, mm | 4.44 ± 0.10 | 4.17 ± 0.11 | 3.70 ± 0.10 | 1.3 × 10−6 | 0.0643 | 2.3 × 10−7 | 0.0019 | |
| Retropogonion to C3, mm | 7.60 ± 0.11 | 7.60 ± 0.13 | 6.86 ± 0.12 | 1.1 × 10−5 | 0.9898 | 1.4 × 10−5 | 7.3 × 10−5 | |
| Mandibular measures** | Mandibular divergence, mm | 60.47 ± 0.71 | 60.16 ± 0.81 | 61.06 ± 0.71 | 0.7022 | — | — | — |
| Mandibular depth, mm | 6.53 ± 0.09 | 6.74 ± 0.10 | 6.16 ± 0.09 | 0.0002 | 0.1285 | 0.0043 | 5.9 × 10−5 | |
| Mandibular length corpus, mm | 8.70 ± 0.12 | 8.89 ± 0.14 | 7.96 ± 0.12 | 1.8 × 10−6 | 0.2875 | 3.4 × 10−5 | 2.0 × 10−6 | |
| Mandibular length ramus, mm | 5.05 ± 0.09 | 5.22 ± 0.10 | 5.45 ± 0.09 | 0.0010 | 0.2069 | 0.0024 | 0.1123 | |
| Total mandibular length, mm | 13.73 ± 0.13 | 14.13 ± 0.14 | 13.38 ± 0.12 | 0.0009 | 0.0382 | 0.0510 | 0.0002 | |
| Mandibular width first molar, mm | 4.53 ± 0.05 | 4.51 ± 0.06 | 4.31 ± 0.05 | 0.0032 | 0.8437 | 0.0017 | 0.0075 | |
| Mandibular width second premolar, mm | 3.86 ± 0.04 | 3.88 ± 0.05 | 3.72 ± 0.04 | 0.0233 | 0.7370 | 0.0202 | 0.0155 | |
| Mandibular width condyle, mm | 9.57 ± 0.08 | 9.82 ± 0.10 | 9.55 ± 0.08 | 0.0833 | — | — | — | |
| Mandibular width gonion, mm | 8.25 ± 0.10 | 8.33 ± 0.11 | 7.90 ± 0.10 | 0.0086 | 0.5820 | 0.0116 | 0.0051 | |
| Maxillary measures†† | Maxillary divergence, mm | 57.6 ± 0.84 | 58.98 ± 0.97 | 61.26 ± 0.84 | 0.0108 | 0.2801 | 0.0028 | 0.0861 |
| Maxillary unit depth, mm | 4.87 ± 0.06 | 4.75 ± 0.07 | 4.54 ± 0.06 | 0.0012 | 0.1886 | 0.0003 | 0.0344 | |
| Maxillary width first molar, mm | 4.65 ± 0.05 | 4.74 ± 0.06 | 4.55 ± 0.05 | 0.0576 | — | — | — | |
| Maxillary width second premolar, mm | 4.18 ± 0.05 | 4.22 ± 0.06 | 4.06 ± 0.05 | 0.1020 | — | — | — | |
Definition of abbreviations: ANB = difference between SNA and SNB; ANS = anterior nasal spine; Na = nasion; OSAS = obstructive sleep apnea syndrome; PNS = posterior nasal spine; SNA = sella (S)–nasion (N)–subspinale (A); SNB = sella (S)–nasion (N)–supramentale (B).
Least squares mean and SE estimates from regression model adjusted for age at consent, race, Tanner stage, and sex.
Analysis of variance (PANOVA).
P values for pairwise comparisons (P < 0.0167, indicating statistically significant after Bonferroni correction), applicable when P ANOVA suggests significant differences between groups.
Bonferroni-corrected significance level of PANOVA < 0.001 (equals 0.05/5).
Bonferroni-corrected significance level of PANOVA < 0.00625 (equals 0.05/8).
Bonferroni-corrected significance level of PANOVA < 0.0125 (equals 0.05/4).
Bonferroni-corrected significance level of PANOVA < 0.0056 (equals 0.05/9).
Bonferroni-corrected significance level of PANOVA < 0.0125 (equals 0.05/4).
Comparisons of global measures of soft tissue volumes and craniofacial space
Comparisons of total soft tissue volume, craniofacial space, and the STCF ratio among the three participant groups are presented in Table 6 and Figure 6. Total soft tissue was significantly larger in the participants with OSAS and obese control subjects than in the lean control subjects, but there were no significant differences between the OSAS and obese control groups. Obese control subjects had a larger craniofacial space than lean control subjects, but there were no significant differences between participants with OSAS and obese or lean control subjects. However, participants with OSAS had a significantly larger STCF ratio than both obese and lean control subjects.
Table 6.
Comparisons of Global Measures of Soft Tissue Volume and Ratio of Craniofacial Volume to Space between Participants with OSAS, Obese Control Subjects, and Lean Control Subjects
| Population | Variable | Adjusted Mean ± SE* |
P Value† | Pairwise Comparisons‡ |
||||
|---|---|---|---|---|---|---|---|---|
| OSAS | Obese Controls | Lean Controls | OSAS vs. Obese Controls | OSAS vs. Lean Controls | Obese vs. Lean Controls | |||
| All participants | Total soft tissue (TST), mm3 | 180,962 ± 4,964 | 172,351 ± 5,243 | 142,223 ± 4,905 | 7.5 × 10−7 | 0.2303 | 2.9 × 10−7 | 8.0 × 10−5 |
| CF space, mm3 | 340,372 ± 10,780 | 362,261 ± 11,519 | 317,673 ± 10,391 | 0.0236 | 0.1669 | 0.1361 | 0.0064 | |
| TST:CF space ratio | 0.534 ± 0.013 | 0.483 ± 0.014 | 0.449 ± 0.013 | 0.0001 | 0.0099 | 2.6 × 10−5 | 0.1019 | |
| Boys | Total soft tissue (TST), mm3 | 187,552 ± 5,593 | 186,469 ± 6,000 | 152,428 ± 5,515 | 3.2 × 10−5 | 0.8965 | 4.4 × 10−5 | 0.0001 |
| CF space, mm3 | 346,919 ± 13,178 | 377,695 ± 14,363 | 330,857 ± 12,220 | 0.0603 | — | — | — | |
| TST:CF space ratio | 0.540 ± 0.017 | 0.508 ± 0.018 | 0.460 ± 0.017 | 0.0073 | 0.2165 | 0.0019 | 0.0680 | |
| Girls | Total soft tissue (TST), mm3 | 166,282 ± 5,521 | 152,459 ± 5,525 | 125,625 ± 5,267 | 2.9 × 10−5 | 0.0881 | 6.9 × 10−6 | 0.0019 |
| CF space, mm3 | 321,176 ± 17,399 | 339,667 ± 17,918 | 293,499 ± 17,231 | 0.2196 | — | — | — | |
| TST:CF space ratio | 0.521 ± 0.018 | 0.462 ± 0.019 | 0.429 ± 0.018 | 0.0032 | 0.0347 | 0.0009 | 0.2453 | |
Definition of abbreviations: CF = craniofacial; OSAS = obstructive sleep apnea syndrome; TST = total soft tissue volume.
Least squares mean and SE estimates from regression model adjusted for age at consent, race, Tanner stage, and sex (in all patients only).
Analysis of variance (PANOVA).
P values for pairwise comparisons (P < 0.0167, indicating statistically significant after Bonferroni correction), applicable when PANOVA suggests significant differences between groups.
Associations between continuous AHI and the MRI variables that were significantly different between participants with OSAS and obese control subjects
We performed a correlational analysis between continuous AHI and the MRI measurements that were significantly different between participants with OSAS and obese control subjects to examine the relationship with OSAS severity. We restricted this analysis to only the OSAS group. Overall, we observed significant or suggestive (P < 0.05) correlations between AHI severity and the following MRI measures (see Table E2): adenoid volume, tonsillar volume, nasopharyngeal cross-sectional area, and both total and retropalatal lateral wall volumes.
Sex differences in lymphoid tissue between and within participant groups
For detailed results, see Tables E1 and E3–E13, Table 3, and Figure 6. In brief, among boys, those with OSAS had significantly larger tonsillar but not adenoid volumes than obese controls, whereas among the girls, the OSAS group had significantly larger adenoid but not tonsillar volumes than the obese and lean control subjects. Among subjects with OSAS, boys had nominally larger tonsil volumes than girls (P = 0.032), but there were no differences in adenoid volumes. Among obese control subjects, boys had nominally larger tonsils (P = 0.011) and significantly larger adenoids (P = 0.003) than girls did. There were no significant differences between boys and girls in lymphoid tissue volumes among lean control subjects. Consequently, in between-group comparisons of boys (Table E3), there was no difference in nasopharyngeal airway size between boys with OSAS and obese control subjects, whereas in between-group comparisons in girls (Table E4), nasopharyngeal airway measures were significantly smaller in those with OSAS than in obese and lean control subjects. In all subject groups, most soft tissue volumes were larger in boys than in girls. Among subjects with OSAS, there was no difference in the STCF ratio between boys and girls.
Discussion
This large study of adolescents with OSAS compared with obese and lean control subjects is one of the few to specifically target the pathophysiology of OSAS in adolescents. The primary findings are as follows: (1) Obese adolescents with OSAS had increased adenotonsillar tissue compared with obese and lean control subjects without OSAS; (2) obese adolescents with OSAS had a smaller nasopharyngeal airway than the control groups; (3) the sizes of other upper airway soft tissue structures (tongue, parapharyngeal fat pads, lateral walls, and soft palate) were similar between participants with OSAS and obese control subjects; (4) although there were no major craniofacial abnormalities in most adolescent participants with OSAS, the STCF ratio was increased; and (5) there were sex differences in the pattern of lymphoid proliferation between participants with OSAS and obese control subjects, with boys with OSAS having larger tonsils and girls with OSAS having larger adenoids.
In recent years, it has become recognized that OSAS is a common cause of morbidity in both children and adults. However, adolescence—the transition from childhood to adulthood and a period of development known to be associated with major sleep issues (33)—remains virtually unstudied in relation to OSAS. Adolescence is a critical time of transition characterized not only by changes in sexual development but also by changes in somatic growth and cortical processing (34). It is characterized by an increasingly more collapsible upper airway, with attenuation of upper airway reflexes (35) as well as overall ventilatory control (36). The upper airway changes markedly during adolescence, especially in boys who develop a laryngeal prominence (the “Adam’s apple”) and a change in voice. Despite the importance of this developmental stage, the structural changes associated with OSAS have not been specifically studied in this age group.
Obesity is thought to be an etiologic factor for OSAS that is more common in adolescents than in younger children, although few studies have been performed to support this assertion. However, many adolescents do not have resolution of OSAS despite substantial weight loss (37), suggesting that obesity alone may not be the primary factor in the development of OSAS. Thus, a detailed evaluation of the upper airway in obese adolescents with OSAS is warranted. To determine the anatomic risk factors for OSAS in adolescents, and to distinguish the effects of obesity from those of OSAS, we studied obese adolescents with OSAS, obese control subjects, and lean control subjects.
The prevalence of adolescent obesity in the United States is currently 20.5% (2). Obesity is associated with an increased risk of OSAS throughout the age spectrum, from infancy through adulthood (38–42). It is unclear how obesity interacts with adenotonsillar hypertrophy, as well as with ventilatory drive and pulmonary mechanics, as a risk factor for OSAS. Although not studied well in the literature, the prevailing belief is that adolescents with OSAS are usually obese, and thus the pathophysiology and management of OSAS are more similar to those noted in adults than in younger children. Thus, these patients are often treated with continuous positive airway pressure (CPAP) rather than with adenotonsillectomy. However, the present study shows that adolescents with OSAS still had large tonsils and adenoids, resulting in a narrower upper airway. Thus, these obese adolescents had airway structural factors similar to those of younger children rather than of adults.
Little is known about the growth of upper airway structures during adolescence. Arens and coworkers showed that adenotonsillar tissue continues to increase, along with upper airway size, in healthy children until 11 years of age; older adolescents were not studied (43). In contrast, Papaioannou and colleagues found that adenotonsillar tissue began to regress after 8 years of age in children and adolescents without snoring, but it continued to increase in size in those with snoring (44). This suggests that adolescents who develop OSAS may deviate from the normal tissue growth pattern. The present study supports the concept that adolescents with OSAS have abnormal upper airway lymphoid proliferation.
Nonlymphoid soft tissue was increased in both the obese OSAS and obese control groups compared with lean control subjects, but it did not differ between the two obese groups (obese OSAS and obese control subjects). Thus, deposition of fat within the tongue or parapharyngeal soft tissues does not appear to be a major risk factor for OSAS in adolescents. This is different from OSAS in adults, where participants with OSAS have larger lateral pharyngeal walls and more tongue fat volume than control subjects (5, 45). One untested possibility is that adipose cells are deposited within lymphoid tissue in obese adolescents with OSAS. Alternatively, it is possible that the obese control subjects did not develop OSAS despite enlargement of upper airway soft tissues, owing to the presence of compensatory upper airway neuromotor reflexes during sleep. It has been shown that healthy children have increased upper airway reflexes to stimuli such as subatmospheric pressure and carbon dioxide during sleep (9) and that these reflexes decline during adolescence (35). However, the rate of decline during adolescence is variable, and we have shown that obese adolescents without OSAS have increased upper airway reflexes during sleep compared with BMI-matched adolescents with OSAS (19).
Although enlargement of upper airway soft tissue structures and reduction in the craniofacial skeletal size increases the risk of developing OSAS, it is likely that a combination of these structures confers additional increased risk. A smaller craniofacial area and larger upper airway soft tissue volume should increase the severity of OSAS. Therefore, we examined the STCF ratio. This was found to be increased in the adolescents with OSAS, suggesting that increasing tissue within the craniofacial space increases the risk for OSA. In fact, the nasopharyngeal airway was smaller in the OSAS group than in the control group secondary to increased lymphoid tissue. Airway length has also been shown to be increased in adults and children with OSAS compared with normal control subjects (14, 32). Moreover, studies have shown that healthy men and boys have a longer airway than women and girls do (14, 30–32). Our data indicate that, in adolescents, airway length adjusted for height was borderline longer in the OSAS group than in obese control subjects, but unadjusted airway length was not different between these groups. We did not find sex-related differences in airway length in the OSAS group, but airway length in the obese and lean control subjects was significantly larger in the boys than in the girls.
Our study has shown important anatomic differences between adolescents with OSAS and control subjects. In addition, we observed “dose–response” relationships between AHI severity and adenoid volume, tonsillar volume, nasopharyngeal cross-sectional area, total lateral wall volume, and retropalatal lateral wall volume. Previous studies done with this cohort have also demonstrated that obese adolescents with OSAS have decreased upper airway reflexes in response to subatmospheric pressure loads (19), as well as a decreased ventilatory response to hypercapnia during sleep (20), compared with either lean or obese age-matched control subjects. Thus, the pathophysiology of OSAS in adolescents is complex and involves both anatomic and neuromotor abnormalities. Further research is needed to determine the relative contributions of anatomic and neuromotor dysfunction to the pathophysiology of OSAS in this age group.
This study shows interesting differences between boys and girls with OSAS. Both had increased lymphoid tissue, but the boys had predominantly larger tonsils, whereas the girls had predominantly larger adenoids. The reason for these sex differences is unknown, but it may be related to differences in estrogen receptors in lymphoid tissue (46, 47); further study is needed in this area. Clinically, this difference may not be important, as usual surgical treatment includes both adenoidectomy and tonsillectomy.
There are few studies reported in the literature in which researchers have examined upper airway structure specifically in adolescents. Arens and colleagues (3) evaluated a cohort of both children and adolescents that was younger (age range: 8–17 yr; mean age: 12 yr) than the sample in the present study. In their study, participants with OSAS were found to have larger tonsils and adenoids than control subjects did, similar to other studies in younger children. In addition, they noted increased size of the parapharyngeal fat pads in the OSAS group; a nonobese group was not available for comparison. In the present study, we did not find a difference in fat pads between participants with OSAS and obese control subjects, although both obese groups had significantly larger fat pads than the lean control subjects did.
A limitation of this study, as with most other published MRI-based studies, is that anatomic measurements were made during wakefulness. It is possible that upper airway hypotonia during sleep would affect upper airway muscle bulk and volumes, resulting in some differences from the present study.
In summary, the present study shows that lymphoid tissue, rather than other soft tissue components, is the primary structural abnormality in obese adolescents with OSAS. This finding is important for clinical management and suggests that, even in obese adolescents, adenotonsillectomy should be considered as an initial treatment for OSAS. This is particularly important when one considers that in this age group CPAP adherence tends to be poor (48, 49) and achieving weight loss is very difficult. However, further clinical studies, including pre- and postoperative polysomnography, are needed to confirm the results of this study in the adolescent population.
Acknowledgments
Acknowledgment
The authors thank all of the Children’s Hospital of Philadelphia sleep laboratory and radiology technologists who helped conduct this study. The authors are grateful to the children and their families for their enthusiastic participation in this study. The authors also thank Dana Zakrzewski for submitting the manuscript.
Footnotes
Supported by grants from the National Institutes of Health (R01 HL058585, R01 HL089447, and P01 HL094307).
Author Contributions: Conception and design: R.J.S., R.M.B., S.H., and C.L.M.; data acquisition: R.M.B., S.H., I.E.T., and C.L.M.; analysis and interpretation: R.J.S., C.K., S.B., B.T.K., F.-L.C., S.W., J.T., D.A.T., and C.L.M.; drafting of the manuscript: R.J.S., B.T.K., and C.L.M.; critical revision: R.J.S., B.T.K., D.A.T., F.-L.C., and C.L.M.; and final approval of the version to be published: R.J.S., B.T.K., and C.L.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201501-0169OC on April 2, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Sánchez-Armengol A, Fuentes-Pradera MA, Capote-Gil F, García-Díaz E, Cano-Gómez S, Carmona-Bernal C, Castillo-Gómez J. Sleep-related breathing disorders in adolescents aged 12 to 16 years: clinical and polygraphic findings. Chest. 2001;119:1393–1400. doi: 10.1378/chest.119.5.1393. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arens R, McDonough JM, Costarino AT, Mahboubi S, Tayag-Kier CE, Maislin G, Schwab RJ, Pack AI. Magnetic resonance imaging of the upper airway structure of children with obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2001;164:698–703. doi: 10.1164/ajrccm.164.4.2101127. [DOI] [PubMed] [Google Scholar]
- 4.Marcus CL, Katz ES, Lutz J, Black CA, Galster P, Carson KA. Upper airway dynamic responses in children with the obstructive sleep apnea syndrome. Pediatr Res. 2005;57:99–107. doi: 10.1203/01.PDR.0000147565.74947.14. [DOI] [PubMed] [Google Scholar]
- 5.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 6.Chi L, Comyn FL, Mitra N, Reilly MP, Wan F, Maislin G, Chmiewski L, Thorne-FitzGerald MD, Victor UN, Pack AI, et al. Identification of craniofacial risk factors for obstructive sleep apnoea using three-dimensional MRI. Eur Respir J. 2011;38:348–358. doi: 10.1183/09031936.00119210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marcus CL, McColley SA, Carroll JL, Loughlin GM, Smith PL, Schwartz AR. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol (1985) 1994;77:918–924. doi: 10.1152/jappl.1994.77.2.918. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Colrain IM, Melendres MC, Karamessinis LR, Pepe ME, Samuel JM, Abi-Raad RF, Trescher WH, Marcus CL. Cortical processing of respiratory afferent stimuli during sleep in children with the obstructive sleep apnea syndrome. Sleep. 2008;31:403–410. doi: 10.1093/sleep/31.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marcus CL, Lutz J, Hamer A, Smith PL, Schwartz A. Developmental changes in response to subatmospheric pressure loading of the upper airway. J Appl Physiol (1985) 1999;87:626–633. doi: 10.1152/jappl.1999.87.2.626. [DOI] [PubMed] [Google Scholar]
- 10.Marcus CL, Lutz J, Carroll JL, Bamford O. Arousal and ventilatory responses during sleep in children with obstructive sleep apnea. J Appl Physiol (1985) 1998;84:1926–1936. doi: 10.1152/jappl.1998.84.6.1926. [DOI] [PubMed] [Google Scholar]
- 11.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sands SA, Eckert DJ, Jordan AS, Edwards BA, Owens RL, Butler JP, Schwab RJ, Loring SH, Malhotra A, White DP, et al. Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014;190:930–937. doi: 10.1164/rccm.201404-0783OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughn VC.Growth and development Behrman RE, Vaughn VC.editors. Nelson textbook of pediatrics. 12th ed.Philadelphia: W.B. Saunders; 198310–38. [Google Scholar]
- 14.Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP. The male predisposition to pharyngeal collapse: importance of airway length. Am J Respir Crit Care Med. 2002;166:1388–1395. doi: 10.1164/rccm.2112072. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165:260–265. doi: 10.1164/ajrccm.165.2.2009032. [DOI] [PubMed] [Google Scholar]
- 16.Kim C, Marcus CL, Bradford R, Gallagher PR, Torigian D, Victor U, Udupa JK, Schwab RJ. Upper airway soft tissue difference in apneic adolescent children compared to BMI matched controls [abstract] Am J Respir Crit Care Med. 2010;181:A2196. [Google Scholar]
- 17.Kim C, Huang J, Gallagher PR, Schwab RJ, Bradford R, Pepe M, Ward M, Lee NY, Marcus CL. Relationship between upper airway collapsibility and the size of upper airway soft tissues in obese adolescents with OSAS and obese controls [abstract] Am J Respir Crit Care Med. 2011;183:A5331. [Google Scholar]
- 18.Kim C, Marcus CL, Bradford R, Schwab RJ. Relationship between upper airway lymphoid tissues (adenoid, palatine and lingual tonsils) in adolescents [abstract] Am J Respir Crit Care Med. 2012;185:A6661. [Google Scholar]
- 19.Huang J, Pinto SJ, Yuan H, Katz ES, Karamessinis LR, Bradford RM, Gallagher PR, Hannigan JT, Nixon T, Ward MB, et al. Upper airway collapsibility and genioglossus activity in adolescents during sleep. Sleep. 2012;35:1345–1352. doi: 10.5665/sleep.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan H, Pinto SJ, Huang J, McDonough JM, Ward MB, Lee YN, Bradford RM, Gallagher PR, Shults J, Konstantinopoulou S, et al. Ventilatory responses to hypercapnia during wakefulness and sleep in obese adolescents with and without obstructive sleep apnea syndrome. Sleep. 2012;35:1257–1267. doi: 10.5665/sleep.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan H, Schwab RJ, Kim C, He J, Shults J, Bradford R, Huang J, Marcus CL. Relationship between body fat distribution and upper airway dynamic function during sleep in adolescents. Sleep. 2013;36:1199–1207. doi: 10.5665/sleep.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koren D, Marcus CL, Kim C, Gallagher PR, Schwab R, Bradford RM, Zemel BS. Anthropometric predictors of visceral adiposity in normal-weight and obese adolescents. Pediatr Diabetes. 2013;14:575–584. doi: 10.1111/pedi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Himes JH, Dietz WH Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. Guidelines for overweight in adolescent preventive services: recommendations from an expert committee. Am J Clin Nutr. 1994;59:307–316. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- 24.Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–1239. doi: 10.1164/ajrccm/146.5_Pt_1.1235. [DOI] [PubMed] [Google Scholar]
- 25.Witmans MB, Keens TG, Davidson Ward SL, Marcus CL. Obstructive hypopneas in children and adolescents: normal values. Am J Respir Crit Care Med. 2003;168:1540. doi: 10.1164/ajrccm.168.12.954. [DOI] [PubMed] [Google Scholar]
- 26.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2–9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40:22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 27.Uliel S, Tauman R, Greenfeld M, Sivan Y. Normal polysomnographic respiratory values in children and adolescents. Chest. 2004;125:872–878. doi: 10.1378/chest.125.3.872. [DOI] [PubMed] [Google Scholar]
- 28.Redline S, Storfer-Isser A, Rosen CL, Johnson NL, Kirchner HL, Emancipator J, Kibler AM. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–408. doi: 10.1164/rccm.200703-375OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iber C, Ancoli-Israel S, Chesson AL, Jr, Quan SFAmerican Academy of Sleep MedicineThe AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications.Westchester, IL: American Academy of Sleep Medicine; 2007 [Google Scholar]
- 30.Segal Y, Malhotra A, Pillar G. Upper airway length may be associated with the severity of obstructive sleep apnea syndrome. Sleep Breath. 2008;12:311–316. doi: 10.1007/s11325-008-0191-9. [DOI] [PubMed] [Google Scholar]
- 31.Genta PR, Schorr F, Eckert DJ, Gebrim E, Kayamori F, Moriya HT, Malhotra A, Lorenzi-Filho G. Upper airway collapsibility is associated with obesity and hyoid position. Sleep. 2014;37:1673–1678. doi: 10.5665/sleep.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronen O, Malhotra A, Pillar G. Influence of gender and age on upper-airway length during development. Pediatrics. 2007;120:e1028–e1034. doi: 10.1542/peds.2006-3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens JA, Belon K, Moss P. Impact of delaying school start time on adolescent sleep, mood, and behavior. Arch Pediatr Adolesc Med. 2010;164:608–614. doi: 10.1001/archpediatrics.2010.96. [DOI] [PubMed] [Google Scholar]
- 34.Chugani HT. Biological basis of emotions: brain systems and brain development. Pediatrics. 1998;102(5 Suppl E):1225–1229. [PubMed] [Google Scholar]
- 35.Bandla P, Huang J, Karamessinis L, Kelly A, Pepe M, Samuel J, Brooks L, Mason TA, II, Gallagher PR, Marcus CL. Puberty and upper airway dynamics during sleep. Sleep. 2008;31:534–541. doi: 10.1093/sleep/31.4.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marcus CL, Glomb WB, Basinski DJ, Davidson SL, Keens TG. Developmental pattern of hypercapnic and hypoxic ventilatory responses from childhood to adulthood. J Appl Physiol (1985) 1994;76:314–320. doi: 10.1152/jappl.1994.76.1.314. [DOI] [PubMed] [Google Scholar]
- 37.Verhulst SL, Franckx H, Van Gaal L, De Backer W, Desager K. The effect of weight loss on sleep-disordered breathing in obese teenagers. Obesity (Silver Spring) 2009;17:1178–1183. doi: 10.1038/oby.2008.673. [DOI] [PubMed] [Google Scholar]
- 38.Kahn A, Mozin MJ, Rebuffat E, Sottiaux M, Burniat W, Shepherd S, Muller MF. Sleep pattern alterations and brief airway obstructions in overweight infants. Sleep. 1989;12:430–438. doi: 10.1093/sleep/12.5.430. [DOI] [PubMed] [Google Scholar]
- 39.Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol. 1996;21:176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 40.Redline S, Tishler PV, Schluchter M, Aylor J, Clark K, Graham G. Risk factors for sleep-disordered breathing in children: associations with obesity, race, and respiratory problems. Am J Respir Crit Care Med. 1999;159:1527–1532. doi: 10.1164/ajrccm.159.5.9809079. [DOI] [PubMed] [Google Scholar]
- 41.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 42.Chay OM, Goh A, Abisheganaden J, Tang J, Lim WH, Chan YH, Wee MK, Johan A, John AB, Cheng HK, et al. Obstructive sleep apnea syndrome in obese Singapore children. Pediatr Pulmonol. 2000;29:284–290. doi: 10.1002/(sici)1099-0496(200004)29:4<284::aid-ppul8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 43.Arens R, McDonough JM, Corbin AM, Hernandez ME, Maislin G, Schwab RJ, Pack AI. Linear dimensions of the upper airway structure during development: assessment by magnetic resonance imaging. Am J Respir Crit Care Med. 2002;165:117–122. doi: 10.1164/ajrccm.165.1.2107140. [DOI] [PubMed] [Google Scholar]
- 44.Papaioannou G, Kambas I, Tsaoussoglou M, Panaghiotopoulou-Gartagani P, Chrousos G, Kaditis AG. Age-dependent changes in the size of adenotonsillar tissue in childhood: implications for sleep-disordered breathing. J Pediatr. 2013;162:269–274.e4. doi: 10.1016/j.jpeds.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 45.Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37:1639–1648. doi: 10.5665/sleep.4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shim GJ, Gherman D, Kim HJ, Omoto Y, Iwase H, Bouton D, Kis LL, Andersson CT, Warner M, Gustafsson JA. Differential expression of oestrogen receptors in human secondary lymphoid tissues. J Pathol. 2006;208:408–414. doi: 10.1002/path.1883. [DOI] [PubMed] [Google Scholar]
- 47.Evagelatou M, Farrant J. Effect of oestradiol-17β on the expression of oestrogen receptor mRNA in human tonsillar cells. J Mol Endocrinol. 1995;14:13–19. doi: 10.1677/jme.0.0140013. [DOI] [PubMed] [Google Scholar]
- 48.DiFeo N, Meltzer LJ, Beck SE, Karamessinis LR, Cornaglia MA, Traylor J, Samuel J, Gallagher PR, Radcliffe J, Beris H, et al. Predictors of positive airway pressure therapy adherence in children: a prospective study. J Clin Sleep Med. 2012;8:279–286. doi: 10.5664/jcsm.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prashad PS, Marcus CL, Maggs J, Stettler N, Cornaglia MA, Costa P, Puzino K, Xanthopoulos M, Bradford R, Barg FK. Investigating reasons for CPAP adherence in adolescents: a qualitative approach. J Clin Sleep Med. 2013;9:1303–1313. doi: 10.5664/jcsm.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]