Abstract
Cardiac tissue engineering aims to create functional tissue constructs that can reestablish the structure and function of injured myocardium. Although bioreactors have facilitated the engineering of cardiac patches of clinically relevant size in vitro, a major drawback remains the transportation of the engineered tissues from a production facility to a medical operation facility while maintaining tissue viability and preventing contamination. Furthermore, after implantation, most of the cells are endangered by hypoxic conditions that exist before vascular flow is established. We developed a portable device that provides the perfusion and electrical stimulation necessary to engineer cardiac tissue in vitro, and to transport it to the site where it will be implantated. The micropump-powered perfusion apparatus may additionally function as an extracorporeal active pumping system providing nutrients and oxygen supply to the graft post-implantation. Such a system, through perfusion of oxygenated media and bioactive molecules (e.g. growth factors), could transiently support the tissue construct until it connects to the host vasculature and heart muscle, after which it could be taken away or let biodegrade.
I. INTRODUCTION
A biomimetic approach to cardiac tissue engineering aims to recapitulate relevant aspects of the in vivo environment, including 3-dimensional (3D) structure and/or biochemical cues, and biophysical cues (electrical and/or mechanical) [1]. Our laboratory’s overall approach includes: i. physiologic density of cell subpopulations in a 3D setting to enable cell communication and coupling, ii. convective-diffusive oxygen supply by medium perfusion to mimic the role of capillary network, iii. induction of macroscopic synchronous contractions of cultured constructs by electrical signals designed to mimic those in native heart [2–3]. These approaches, after only 8 days in vitro, result in constructs of clinically-relevant size (1 mm thick), increased amplitude of synchronous contractions and in a remarkable level of ultrastructural organization. Indeed, there also appears to be a synergistic effect with the application of two such conditioning stimuli (perfusion and electrical stimulation), leading to enhanced organization and functionality of engineered cardiac tissue [4].
Even as bioreactors have improved for the engineering of tissues in vitro for potential transplantation, a major drawback remains the long-distance transportation of viable, sterile engineered three-dimensional tissues from the production facility to either medical operation chambers for implantation, or collaborating laboratories (e.g. for advanced imaging, physiological analysis etc) [5].
Furthermore, although the implanted engineered cardiac tissue may improve the systolic and diastolic left ventricular function [6], neoangiogenesis of vessels growing into the implanted tissue has been shown to be too slow to prevent cell death due to host hypoxic conditions after myocardial infarction [7–8]. Going forward, therefore, any scenario for implantation of thicker (> 100 μm) engineered tissue graft needs to provide a means for effective and timely connection with the host blood supply.
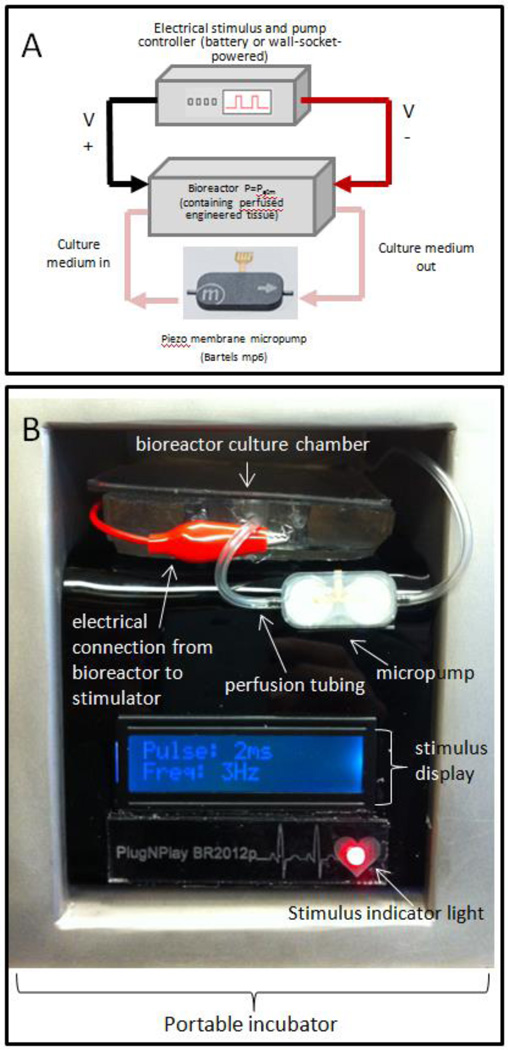
We present here a low-power consumption portable bioreactor system delivering electrical stimulation and perfusion of oxygenated media through biodegradable tubes for the growth and maintenance of 3D engineered cardiac tissue (see Figure 1). This system may be transported to an operating theater whilst continuing to apply the electrical stimulation and perfusion that are critical for the maintenance of the engineered cardiac tissue without the exposure of the sterile tissue and cell culture media to the external environment during transportation. Furthermore, we propose that the pumping system, if connected to the engineered tissue post-implantation, may also potentially serve as an extracorporeal active system pumping oxygen and active molecules through the tissue. In this way, it may also serve to transiently support the engineered tissue as it connects with host vasculature and heart tissue, after which point it could be cut off, and all remaining tubing would subsequently biodegrade.
Figure 1.
(A) Experimental setup incorporating micropump, electrical stimulation, control circuitry, bioreactor housing electrodes and microdialysis tubing for cultivation of engineered tissues composed of encapsulated cardiac cells, (B) closeup of bioreactor, and stimulation/perfusion controller inside portable incubator
II. METHODS
A. Cardiac Patch Preparation
Cardiac constructs were prepared as previously described [2–3] by seeding collagen sponges (8 mm × 10 mm × 1.5 mm, Figure 2A) with cell populations isolated from 2-day old Sprague Dawley rats (108 cells/cm3, 5 μl Matrigel/106 cells). The collagen sheet was folded around an autoclaved bundle of 14 hollow fibers (Spectrum Lab), manufactured from regenerated cellulose (see Figure 2B), and secured using a single stitch suture (see Figure 2C). Cells were cultured in high-glucose DMEM + 10% FBS, 1% HEPES, and 1% penicillin (10,000 U/ml)/streptomycin (10,000 μg/ml) at all times (Invitrogen).
Figure 2.
(A) drawing of collagen scaffold dimensions (B) drawing of scaffold folded around an autoclaved bundle of 14 hollow fibers and fastened with single suture stitch (C) closeup of cardiac construct composed of collagen scaffold seeded with cardiac cells, and fastened by single suture stitch (scale bar = 4 mm) (D) closeup of bioreactor outfitted with electrodes and perfusion tubing, with scaffold tubings fastened into place for perfusion and maintenance of scaffold between carbon rod electrodes
B. Electrical Stimulation and Perfusion of Cardiac Patches
Cardiac constructs in electrically stimulated groups were exposed to pulsatile electric field stimuli (monophasic pulses of 2 ms duration, 3 Hz, 3 V/cm) for 5 days using square waves after 3 days of preculture without electrical stimulation, as previously described [9].
The cellulose tubing running through the cardiac tissues was connected to a micropump (Bartels mp6 controlled via OEM controller chip) 3 days after seeding, in a closed loop configuration containing 25 mL of cell culture media, at a perfusion rate of 0.5 mL/min (see Figure 1), in a custom-built bioreactor (see Figure 2D, Figure 3A-B). The tubing and pump were sterilized prior to exposure to cells via perfusion of ethanol.
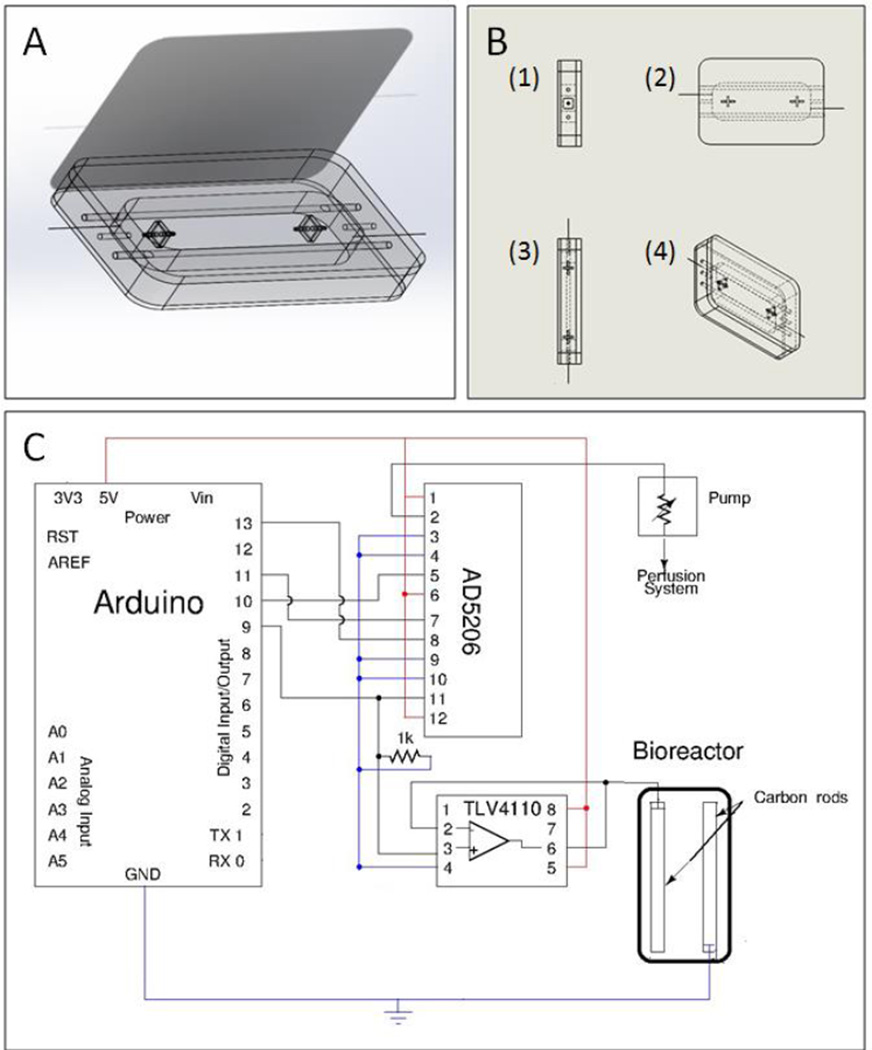
Figure 3.
(A) third angle projected view of final assembly of the bioreactor with connectors to connect to perfusion tubing and hold perfused construct in place and carbon rod electrodes connected to platinum wires to connect electrodes to a cardiac stimulator. (B) orthographic (1) side, (2) front, (3) top edge, and (4) third angle projection views of assembled bioreactor (C) Circuit diagram for the implementation of electrical stimulation and media perfusion in the bioreactor. The Arduino microcontroller outputs a pulse wave signal which is passed through a high-output current amplifier (TLV4110) in unity gain mode to preserve the signal strength across the carbon rods of the bioreactor. The pump is controlled by a digital potentiometer IC (AD5206) which is set by the microcontroller
C. Modeling of oxygen distribution
Finite element modeling was used to solve for oxygen distribution using a commercially available software package (COMSOL, Burlington MA). Studies involving two- (2D) and three-dimensional (3D) mathematical modeling of a representative construct were performed. Constructs were approximated as radially symmetric with evenly distributed, radially symmetric channels. To obtain concentration profiles within the constructs, the Navier-Stokes equations for incompressible fluids, mass balance equations, and fluid velocity profiles for a convective-diffusive regime were numerically solved. A defined concentration was used as initial condition within the construct and as boundary condition at the inlets and external surfaces in direct contact with the medium bath (C0=1.85e-1 mol/m3 [10] ; convective flux at the outlet; a specific flux within the construct (cells uptake) and insulation/symmetry elsewhere.
The 3D domains of the constructs were geometrically modeled and non-structured meshes were generated with triangular elements. No-slip boundary conditions were used for the perfusion tubing walls, a fixed velocity for the inlet channel, and zero pressure for the outlet. Values were taken from literature for the fluid properties viscosity and density [11]. The oxygen diffusion coefficient used was DO2=2.7 10−9 m2/s [10], the cells’ oxygen uptake rate was Rup=6.33 10−17 mol/(s*cell) [10]; cell density was ρc=11011 cell/m2 (calculated); resulting in an approximated 2D cells oxygen consumption rate of Rc=Rup* ρc/2=3.165 10−6 mol/(m2*s). Similarly, the 3D cell consumption rate was Rc=6.3310−3 mol/(m3*s). The inflow linear velocity was Uin=3.8 10−3 m/s.
D. Cardiac Patch Preparation
Engineered tissues were first fixed in a 4% paraformaldehyde solution for 24 h before being dehydrated, embedded in paraffin, and sectioned into 5 μm thickness sheets perpendicularly to the perfusion tubings. The sections were then stained with haematoxylin and eosin (H&E) for general evaluation, as well as for distributions of cell nuclei (DAPI), as previously described [2].
E Quantification of cell number and distribution
For quantification of cell distribution, perpendicular sections of each scaffold were divided into 3 equal concentric regions. After enhancing the appearance of cell nuclei, the total number of cells in each region was counted using a custom-developed script running image analysis commands (MATLAB). The number of cells in each region was then divided by the total number of cells in the cross section, to obtain the percentage of cells as a function of depth in the tissue.
III RESULTS/DISCUSSION
A. Bioreactor Design
The main cell culture housing of the bioreactor is made of custom-casted polydimethylsiloxane (PDMS), which is a biocompatible, autoclavable silicone that is permeable to diffusion but maintains sterility via sealed lid [12]. Sealed holes on both sides allow tubings and electrical wires to connect to the stimulator and perfusion controllers (see Figure 3 A-B) using standard connectors, for easy fabrication and assembly. Inside the culture chamber, hollow microdialysis cellulose tubing mimics capillaries with interstitial medium flow that protect cells from shear stress: they bathe the interior of the cardiac construct with cell culture media with nutrients picked up via diffusion in the other parts of the closed loop. The hollow, hydrophilic, fibers have outside diameter of 280 μm, inside diameter of: 200 μm, and molecular weight cut off (MWCO) of 18 kDa. They provide a clear, flexible, autoclavable network of permeable channels throughout the scaffold. Advantages of this system are its horizontal configuration, which facilitates transport, as compared to vertical perfusion loops requiring stands [3], and the stability provided by the tubings for maintaining the tissue in the center of the electric field stimulus provided by the carbon rod electrodes. The system also fits neatly into portable incubator systems such as the Kivex Biotec G95 shown in Figure 1B, and the scaffold is easy to remove from the bioreactor.
B. Electrical Stimulator/Perfusion Controller Design
Voltage-controlled electrical stimulation is performed via a custom-built stimulator consisting of a microprocessor (Arduino UNO®) running custom software, digital potentiometer chips (AD5206) for amplitude control, and an operational amplifier (TLV 4110) in unity gain mode for preservation of signal strength. In comparison to commercially available electrical stimulation systems, the custom microcontroller-controlled system presented here offers more control (e.g. ability to program low duty cycles, and custom waveforms) at a favorable price. Furthermore, in comparison to our previously- published custom computer-controlled electrical stimulation systems, the microcontrollers allow for portability at further reduced price [2].
The pump employed is a piezo-actuated micropump (Bartels mp6 [13]) which is composed of injection molded parts for housing and pump chamber, piezo actuators and passive valves. All plastic parts are made from Poly Phenyl Sulphone, a highly resistant polymer used in medical applications [11], which is the only material which is in contact with the pumped cell culture medium. Perfusion speed is set by the microcontroller via control of the digital potentiometer and an OEM pump controller chip. In this configuration, a 22.5 KHz pump frequency was utilized, which, along with potentiometer, allows pump flow rates of 250 μL/min to 1 mL/min, at low energy consumption (< 200 mW), which facilitates batter operation (see Table I for a more complete list of parameter specifications).
TABLE I.
Bioreactor specificatoins (Nina please complete)
| Parameter | Min. Value |
|---|---|
| Power consumption | 200–800 mW |
| Electrical stimulation amplitude range | 0 – 5V |
| Pump flow rate | 0.25 – 1.0 mL/min |
| Battery Life (with two 9V alkaline batteries) | 23 – 90 h |
In addition to the cost, size, low power consumption and flexibility afforded by this system, another advantage is that it utilizes many open source hardware elements, facilitating the modular addition of additional elements (e.g. mechanical stimulation via linear actuators etc) or customization (e.g. of user interface, stimulation regimes etc).
C. Modeling of oxygen distribution
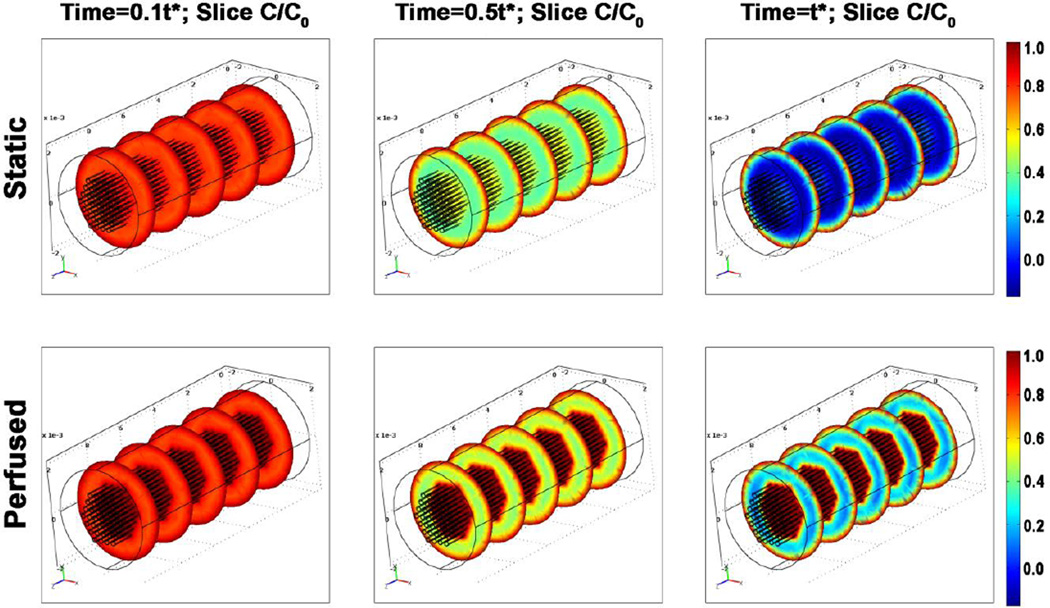
In past studies with early bioreactors providing static culture conditions, the thickness of viable tissues has been limited by the penetration depth of oxygen diffusion, corresponding to a ~100 μm thick surface layer of compact tissue on statically grown constructs and a relatively a-cellular interior [14]. In this study, a set of simulations was run based on the assumption that perfusion ensured maintenance of a constant oxygen concentration within the dialysis tubings. The relative oxygen concentration heat maps at relevant time points during culture (Figure 4) show that although oxygen concentration along the outermost surface is comparable for all constructs, in the tissue space closest to the perfusion tubings oxygen concentration is lower in the control group as compared to the perfused group. Furthermore, within the intermediate spacing between perfusion tubings and the outer surface of the construct, the perfused constructs show higher oxygen concentration as compared to controls, demonstrating the benefits of perfusion for the maturation of thicker constructs. However, in the tissue space, the oxygen concentration of ~30% of saturation also indicates that perfusion in this configuration may not be sufficient to completely avoid oxygen transport limitations. In fact, when the venous blood oxygen saturation is less than 30%, tissue oxygen balance has been shown to be compromised, and anaerobic metabolism ensues [10]. Optimization of channel configuration therefore remains an important area of future work.
Figure 4.
Model solutions for the distribution of normalized oxygen concentration of sequential cross-sections of engineered cardiac tissue for constructs cultured under static (top) and perfused (bottom) conditions at (L-R) t = 10%, 50%, and 100% of t* (the time the system takes to reach steady state), respectively.
D. System Validation: electrical stimulation, perfusion
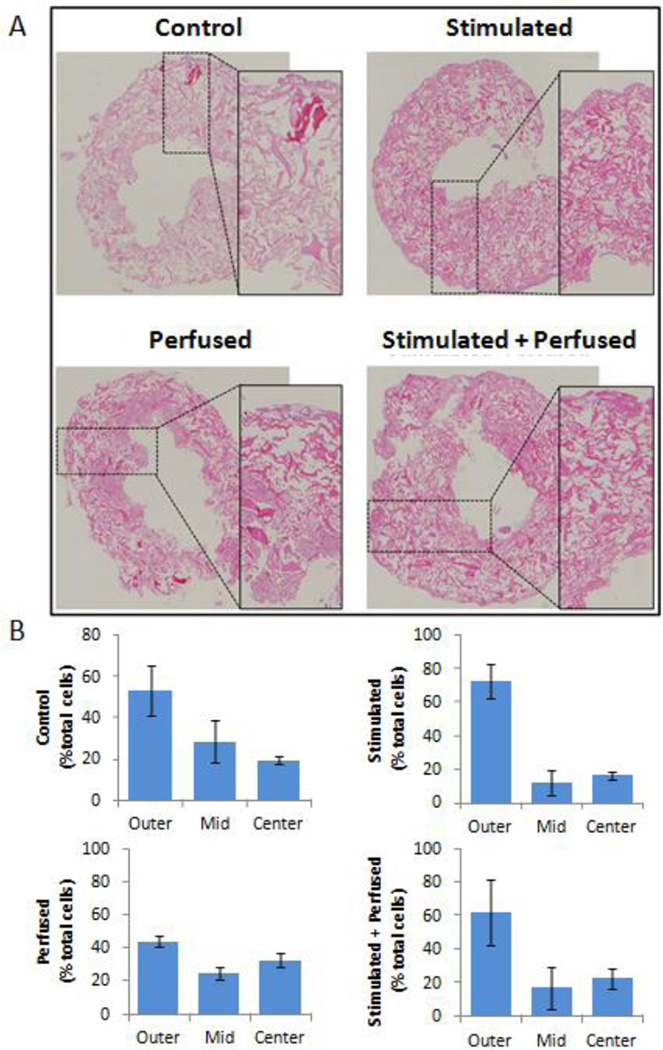
A challenge in cardiac tissue engineering has been the growth and maintenance of high, physiological cell densities (~108 cells/cm3): even though cells may be seeded at a high density, cells may nonetheless be lost during culture, likely because unattached cells cannot survive and are washed away. Construct cultivation with perfusion, which enhances transport of vital nutrients and oxygen to cultured tissues, has been found to yield higher cell densities and more compact tissues [3–4, 14]. In this study, as expected, application of perfusion maintained more uniform, compact tissue, as compared to other groups (Figure 5A). In non-perfused cultures, a higher percentage of the cells clustered near the outer surface of the construct than along the inner surface in contact with the perfusion tubings (Figure 5B). Also interesting to note is that electrically stimulated groups maintained more cells in the most superficial portion of the scaffold, suggesting there may be a synergistic effect of the application of electrical stimulation and cell distribution. Electrical stimulation has also been shown to affect cardiac cell morphology in tissue engineered systems [15], but further studies would be needed to validate whether electrical stimulation affected cell attachment.
Figure 5.
Cell distribution changes associated with perfusion and stimulation. (A) bright-field images of Hematoxylyn/Eosin staining for unstimulated (control) or exposed to a electric field stimuli (stimulated), perfusion, or both, respectively for a period of 5 days, magnification 40x. Images in inserts are zoomed visions of representative scaffolds’ sections. (B) Histograms show the percentage of cells in each third of the scaffold depth (outer, mid, and inner, respectively) after 8 days of culture (5 days with electrical stimulation and/or perfusion). N=2 from two separate experiments.
An important advantage of this cell culture configuration, as compared to published systems with perfusion of cardiac constructs, is that it overcomes limitations associated with channel blockages when seeding channeled scaffolds with hydrogels at high cell density. Some of these problems may be mitigated by presoaking of scaffolds with laminin and by seeding scaffolds via perfusion [16], but these systems require two sets of bioreactors (one for construct seeding, another for construct cultivation) and still produce only limited cardiomyocyte elongation and formation of intracellular connections, even with the simultaneous application of electrical stimulation [4]. This system, by providing a substrate for attachment and trapping secreted cell products, facilitates the formation of mature cardiac tissue in a short period of time via the use of hydrogel, but without the problem of blocked small-diameter channels.
E. Future Work
The system presented here provides improved support for the transport of engineered cardiac tissues for later implantation or analysis. Future work includes tissue culture optimization via programming of custom electrical stimulation waveforms, developing suture-free methods of tissue connection to perfusion tubings, and optimization of cellulose tubing configuration and MWCO for oxygen and nutrient delivery to engineered tissues. Further studies would be needed to validate whether electrical stimulation affected cell attachment. Another area of interesting future work will be the subcutaneous implantation studies on animal models to determine whether the perfusion component of the system may also function as a transient support device after implantation. For these studies, the controlled and real time release of oxygen and nutrients, angiogenic factors, and local drug delivery will be critical.
ACKNOWLEDGMENT
The authors would like to thank Chuck Murry, Elio De Berardinis, Anastasia Felker, Keith Yeager, and Adrienne Drenckhahn for their help and advice. This work was funded by NIH (grant EB015888).
Research supported by NIH Grant 5 R21 RR026244-02R1 5 R01 HL076485-05)
REFERENCES
- 1.Vunjak-Novakovic G, et al. Bioengineering heart muscle: a paradigm for regenerative medicine. Annu Rev Biomed Eng. 2011 Aug 15;13:245–267. doi: 10.1146/annurev-bioeng-071910-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tandon N, et al. Electrical stimulation systems for cardiac tissue engineering. Nat Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Radisic M, et al. Cardiac tissue engineering using perfusion bioreactor systems. Nat Protoc. 2008;3:719–738. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maidhof R, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. J Tissue Eng Regen Med. 2011 Dec 13; doi: 10.1002/term.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tandon N, et al. Feasibility of Long-Distance Transfer for High Resolution Optical Mapping of Cardiac Tissue Constructs. Biophysical journal. 2012;102:676a. [Google Scholar]
- 6.Zimmermann WH, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006 Apr;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu T, et al. Polysurgery of cell sheet grafts overcomes diffusion limits to produce thick, vascularized myocardial tissues. FASEB J. 2006 Apr;20:708–710. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- 8.Zimmermann WH, et al. Cardiac grafting of engineered heart tissue in syngenic rats. Circulation. 2002 Sep 24;106:I151–I157. [PubMed] [Google Scholar]
- 9.Tandon N, et al. Optimization of electrical stimulation parameters for cardiac tissue engineering. J Tissue Eng Regen Med. 2011 Jun;5:e115–e125. doi: 10.1002/term.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown DA, et al. Analysis of Oxygen Transport in a Diffusion-Limited Model of Engineered Heart Tissue. Biotechnology and Bioengineering. 2007;97:962–975. doi: 10.1002/bit.21295. [DOI] [PubMed] [Google Scholar]
- 11.Cimetta E, et al. Microfluidic device generating stable concentration gradients for long term cell culture: application to Wnt3a regulation of b-catenin signaling. Lab on a Chip. 2010;10:3277–3283. doi: 10.1039/c0lc00033g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coles A, Curtis J. Silicone Biomaterials: History and Chemistry. In: Ratner B, editor. Biomaterials Science: An Introduction to Materials in Medicine. 2nd Ed. San Diego, CA: Elsevier Academic Press; 2004. pp. 80–86. [Google Scholar]
- 13.Bartels-Mikrotechnik. 10/1/2012) Technical Data Micropump mp6. Available: http://www.micro-components.com/index.php/micropump-information/technical-data-mp6.
- 14.Radisic M, et al. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am J Physiol Heart Circ Physiol. 2004 Feb;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 15.Radisic M, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings of the National Academy of Sciences. 2004 Dec 28;101:18129–18134. doi: 10.1073/pnas.0407817101. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maidhof R, et al. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010 Mar-Apr;26:565–572. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]