Abstract
In humans, anabolic androgenic steroid (AAS) use has been associated with hyperactivity and disruption of circadian rhythmicity. We used an animal model to determine the impact of AAS on the development and expression of circadian function. Beginning on day 68 gonadally intact male rats received testosterone, nandrolone, or stanozolol via constant release pellets for 60 days; gonadally intact controls received vehicle pellets. Wheel running was recorded in a 12:12 LD cycle and constant dim red light (RR) before and after AAS implants. Post-AAS implant, circadian activity phase, period and mean level of wheel running wheel activity were compared to baseline measures. Post-AAS phase response to a light pulse at circadian time 15h was also tested. To determine if AAS differentially affects steroid receptor coactivator (SRC) expression we measured SRC-1 and SRC-2 protein in brain. Running wheel activity was significantly elevated by testosterone, significantly depressed by nandrolone, and unaffected by stanozolol. None of the AAS altered measures of circadian rhythmicity or phase response. While SRC-1 was unaffected by AAS exposure, SRC-2 was decreased by testosterone in the hypothalamus. Activity levels, phase of peak activity and circadian period all changed over the course of development from puberty to adulthood. Development of activity was clearly modified by AAS exposure as testosterone significantly elevated activity levels and nandrolone significantly suppressed activity relative to controls. Thus, AAS exposure differentially affects both the magnitude and direction of developmental changes in activity levels depending in part on the chemical composition of the AAS.
Keywords: anabolic androgenic steroids, circadian rhythms, rats, running wheel activity, SRC-2, body weight
INTRODUCTION
Abuse of anabolic androgenic steroids (AAS) has increased dramatically in recent years [1,2,3,4,5]. AAS abuse has become a public health issue, and evidence of numerous undesirable behavioral changes have been reported. For example, AAS use reportedly induces hyperactivity and sleep disturbances [2,5,6,7,8]. Since these disorders are associated with changes in circadian rhythmicity [9,10,11,12] as well as with human AAS abuse, it is quite plausible that these effects are mediated by AAS-induced changes in biological clock function. Because the human data are largely subjective or based on single case reports and uncontrolled conditions, it is essential to establish animal models to confirm the validity of the findings for humans. Human alterations in circadian rhythms associated with psychiatric disorders are quantitative in nature, typically represented by relatively small but significant differences in phase or amplitude of various daily rhythms [9,10,11,12]. Thus, even relatively minor changes in circadian rhythm expression can serve as markers of certain psychopathological states [10].
Studies using various mammalian species have demonstrated effects of replacement doses of testosterone on circadian rhythmicity in castrated males [13,14,15,16]. For example, testosterone increased activity levels and elevated nocturnal amplitude of activity in castrated male voles [16]. In hamsters, castration increased the variability and duration of running wheel activity [15]. The diurnal rodent, Octodon degus exhibited significantly reduced wheel-running activity and increased phase angle of entrainment after castration [14]. Castrated male mice displayed a slightly shorter free-running period and increased activity following treatment with exogenous testosterone [13]. These data, along with the human studies discussed above, suggest that chronic exposure to very high doses of androgens such as those employed by human AAS users, may indeed affect circadian rhythms and mean levels of wheel-running activity. To our knowledge, the current study is the first to determine the impact of AAS exposure on the chronobiological regulation of activity.
In order to provide a comprehensive assessment of the effects of AAS on the chronobiological regulation of activity, there were three hypotheses that guided this study. The first hypothesis was that exposure to AAS would influence wheel running activity and circadian rhythms. The rational for this hypothesis is predicated on the hyperactivity and altered sleep patterns reported for humans following AAS use [2,5,6,7,8], and data showing that endogenous levels of testosterone may influence circadian rhythms in other rodent species [13,14,15,16,17]. The second hypothesis was that AAS with dissimilar chemical characteristics would differentially affect both the level of wheel running activity and circadian parameters. This is based on behavioral research indicating that three of the most commonly abused AAS, testosterone, nandrolone and stanozolol [8,18], all have markedly different effects on a variety of behavior patterns. For example testosterone potentiates aggressiveness, nandrolone has little effect on aggression and stanozolol actually inhibits aggression [19]. The third hypothesis was that chronic AAS exposure would have an impact on the ontogenetic development of wheel running activity and circadian rhythms. This was predicted on the basis of work in Octodon degus showing that the development of adult circadian rhythm patterns are age and hormone dependent [17]. Because our baseline measures of wheel-running activity began prior to puberty and continued through adolescence and into adulthood we were able to examine the impact of these three distinct AAS on running wheel activity and circadian rhythms over the course of development.
Several measures were obtained in order to examine how AAS influence circadian rhythms and the expression of wheel running activity. Specifically, we assessed the effects of chronic AAS exposure on running wheel activity in 12:12 LD and constant dim red light (RR), peak time of activity in LD, circadian period in RR and phase-response to a light pulse at circadian time 15 hours. These parameters represent central circadian clock properties that play a critical role in the normal expression of circadian rhythms [20]. To better understand the biological consequences of AAS exposure on activity levels and circadian rhythms, we measured body and tissue weights, serum testosterone levels, and two nuclear receptor coactivators, SRC-1 and SRC-2 which have been shown to dramatically enhance the transcriptional activity of nuclear receptors, including androgen receptors (AR) [21,22,23].
MATERIALS AND METHODS
Subjects and Treatment Groups
Forty-Eight male Long-Evans rats, approximately 25 days old, were purchased from Charles River Labs (North Wilmington, MA, 01887, USA). Each rat was randomly assigned to one of four treatment groups: testosterone propionate (T), nandrolone (N), stanozolol (S) and control (C). Body weights were recorded twice: once when hormone pellets were implanted and again at the time of sacrifice. All procedures were conducted in accordance with the guidelines established for the care and use of laboratory animals by the National Institute of Health.
Housing
The rats were placed directly into transparent plastic cages, 47 x 27 x 20 cm (length x width x height) with ad lib food and water, and a 34 cm diameter running wheel on the day of their arrival in the lab in a 12:12 LD cycle with lights on at 0900 hours. Cages were arranged on open racks, alternating by drug treatment in a random block design. Trays suspended below wire mesh cage floors contained crushed corncob bedding. Bedding was changed, and water and food replenished approximately once per week. During 12:12 LD entrainment, light was provided by overhead fluorescent ceiling fixtures. The light intensity at the bottom center of each cage averaged 59 +/− 7 lux, with no significant difference among the four treatment groups. During free-running circadian period assays and phase-response assays in constant dim red light (RR) the red light was provided by three safelights, each with a number one Kodak red monochromatic filter and a 15 watt bulb in a light-tight room measuring 16 ft. long by 8 ft. wide and 8 ft. High. Red light intensity was less than one lux in all cages.
AAS Treatment
The three AAS, testosterone (T), nandrolone (N) and stanozolol (S), were chosen for several reasons: 1) they are structurally distinct in their chemical composition, 2) they have differing affinities for the androgen receptor [24], 3) they are markedly different in their behavioral consequences (for review, see [19]), and 4) they are highly abused by humans. Previous studies [25,26,27,28,29,30] have employed daily subcutaneous injections to provide chronic exposure to high doses of AAS. In order to eliminate the effects of repeated handling and injections of the animals while in the running wheels, we used timed-release pellets (Innovative Research of America, Sarasota, FL, USA) designed to administer 200 mg of hormone at a constant rate for 60 days. This dosage reportedly provides approximately 3.3 mg AAS/rat/day (Innovative Research of America, Sarasota, FL, USA). Interscapular subcutaneous implants were inserted under a ketamine (100 mg/ml) and xylazine (18 mg/ml) anesthesia mix injected ip at 0.75 ml per kg body weight with wound clip sutures.
Schedule of Wheel-Running Assays and AAS Implantation
The rats were allowed to acclimate to the running wheels for six days from age 25 days to age 31 days. All rats were assayed for baseline wheel-running activity in the 12:12 LD cycle for 14 days from age 32 days to age 46 days, and in RR for 12 days from age 47 days to age 59 days. Rats were removed from running-wheel cages from age 60 days to age 83 days and housed individually in plastic cages with ad lib food and water in 12:12 LD with lights on at 0600h. During this time, while the rats re-entrained to the 12:12 LD cycle, they were implanted with timed-release hormone pellets at age 68 days, and the running wheel cages were cleaned.
The rats were assayed for post-treatment wheel-running activity in 12:12 LD for 12 days from age 84 days to age 96 days, and in RR from age 97 to 115 days. A 30 minute white light pulse was presented to all rats simultaneously on day 97 at approximately circadian time fifteen (ct15) (27 hours after lights-off on the last day of LD entrainment). The pulse source was a 15-watt fluorescent lamp centered and aligned front to back approximately six inches over the top of each cage. Light intensity at the center of each cage during light-pulses averaged 502 +/− 28 lux, and was not significantly different among treatment groups. The 12:12 LD photoperiod was restored at age 116 days, and all the rats were sacrificed at age 118 days.
Wheel Running Assays
Wheel revolutions were recorded with magnetic switches, using VitalView software (cages, wheels, computer interface and software were purchased from Minimitter Co., Inc., Bend, OR, 97701, USA), as total revolutions per successive ten-minute interval. Mean number of wheel-revolutions per ten-minute interval, for the duration of LD and RR testing, was used to determine mean activity levels in each condition. Actiview software (Minimitter) was used to construct computer-generated actograms for each subject.
Circadian phase was determined using the cosinor function in Actiview and best eye-fit regression lines through activity onsets to estimate baseline and post-AAS peak activity time in the 12:12 LD photoperiod. The periodogram function in Actiview was used to estimate baseline and post AAS treatment circadian periods for each rat in LD and RR by finding the best-fit period between 22 and 26 hours sampling successive one-minute intervals. Best-fit regression lines drawn on actograms through successive activity onsets were also used to estimate baseline and post-treatment circadian periods in LD and RR. The post-AAS circadian period in RR was estimated from the last ten days of the 18 days in RR following the ct15 light pulse.
Phase of activity onset following the light pulse was estimated by projecting onsets from days 10–19 in RR back to last day of prior entrainment, and using the clock time of the projected phase on the last day of entrainment as the measure of post-light pulse phase position. This post light-pulse phase value was compared to a control phase measurement (same procedure but no light pulse) for each rat from the pre-AAS assay of RR activity projected back to the last day of baseline LD activity to estimate the phase shift induced by the light pulse.
Blood and Tissue Collection
At the conclusion of testing, the animals were weighed and sacrificed by decapitation. Trunk blood was collected and serum was removed and frozen at −20 degrees. Serum levels of testosterone were determined using an I125 kit from ICN Biomedicals, Inc. (Costa Mesa, CA). Testes and seminal vesicles were removed and weighed.
Western Blot Analysis of SRC-1 and SRC-2
Immediately after decapitation, the entire hypothalamus and amygdala brain tissues were excised, placed in chilled microfuge tubes, snap frozen on dry ice and stored at −80 C. Tissue was homogenized in TEDG (consisting of 10 mM Tris-base, 1 mM EDTA, 1 mM Dithiothreitol (DTT), 10% glycerol 400 mM NaCl, pH = 7.4) and protease inhibitors (P2714, 1:10 dilution, Sigma, Saint Louis, MO) using a Teflon homogenizer. After tissue homogenization, samples were centrifuged at 12,000 g for 30 min at 4 C to sediment cellular debris and nuclei. The supernatant fraction was collected, and the protein concentration was determined by Bradford assay. Eighty ug of total protein from each tissue sample was gel electrophoresed on 7.5% polyacrylamide gels containing 1% SDS and transferred to a polyvinylidene difluoride membrane (Millipore, Bedford, MA). Samples were analyzed by Western blot for detection of SRC-1 as described previously [31]. Briefly, SRC-1 from brain was probed by using a mouse monoclonal antibody generated against amino acids 477–947 of human SRC-1 (1135-H4, 0.5 ug/ml. Membranes were incubated in a horseradish peroxidase-linked sheep-anti-mouse secondary antibody (1:6000, Amersham, Piscataway, NJ) for one hour. Immunoreactive bands were detected with an enhanced chemiluminescence kit (ECL; New England Biolabs, Ipswich, MA) and membranes exposed to a Storm 860 PhosphorImager (Molecular Dynamics, Piscataway, NJ) for analysis and then exposed to film (Blue Sensitive X-ray film, Laboratory Products Sales, Rochester, NY) for image collection. Membranes were stripped with stripping buffer (62.5 mM Tris, pH=6.7, containing 100 mM 2-mercaptoethanol and 2% SDS) and then re-probed for actin to normalize for differences in total protein loaded. Membranes were incubated overnight in a mouse monoclonal antibody generated against chicken actin (1:75,000, MAB1501, Chemicon, Temecula, CA) and then membranes were incubated in a horseradish peroxidase linked sheep-anti-mouse secondary antibody (1:10,000, Amersham) for one hour. For analysis of SRC-2, samples were gel electrophoresed as described above, and membranes were incubated in a mouse monoclonal antibody generated against amino acids 959–1067 of human SRC-2 (TIF2, clone 29, 1.0 ug/ml, BD Transduction Lab, San Jose, CA) overnight and then incubated in horseradish peroxidase linked sheep-anti-mouse secondary antibody (1:6000, Amersham) for one hour. Immunoreactive bands were detected and blots were stripped and probed for actin as described above. The total volume of the immunoreactive bands (total area of immunoreactive pixels X average optical density) detected on the PhosphorImager were analyzed using Image Quant software (v. 5.2, Molecular Dynamics).
Statistical Analysis
Main effects of AAS treatment and post-hoc comparisons among specific AAS groups were derived from analysis of variance using the GLM procedure in SAS (SAS Institute, Carey, NC, USA). Values are reported as mean +/− SEM. Mean differences from baseline within groups were tested against the null hypothesis of no change with the paired t-test. Body and tissue weights, serum testosterone levels and nuclear receptor coactivator expression levels were analyzed using ANOVA followed by Fisher's PLSD or Bonferroni’s test for post hoc comparisons.
RESULTS
Wheel running activity in LD and RR
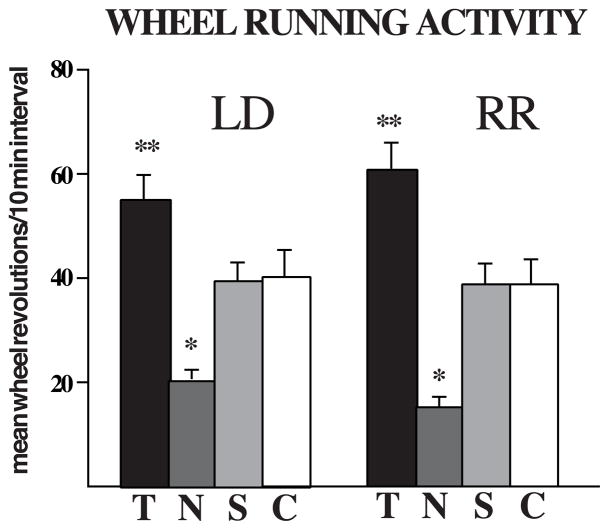
AAS treatment significantly altered wheel running activity in LD and RR photoperiods (Figure 1). Baseline (pre-AAS) measures of activity in both conditions were not significantly different among AAS treatment groups for both LD and RR conditions (p>0.76). However, after AAS treatment there was a significant overall effect of AAS exposure on running wheel activity for LD and RR (p<0.0001). Post-hoc analysis revealed that T significantly (p<0.05) increased mean activity levels in both LD and RR conditions relative to controls. In contrast, N treatment resulted in a significant (p<0.05) decrease in wheel running activity in both LD and RR conditions. Mean activity levels of S-treated males were similar to controls.
Figure 1.
Mean (±SEM) running wheel activity in LD and RR showing post-AAS values. Chronic exposure to testosterone significantly increased (** p< 0.05) running wheel activity compared to gonadally intact controls. This occurred in both the LD and RR conditions. In contrast, chronic exposure to nandrolone significantly decreased (* p< 0.05) running wheel activity relative to gonadally intact control levels. N = 12 rats/group
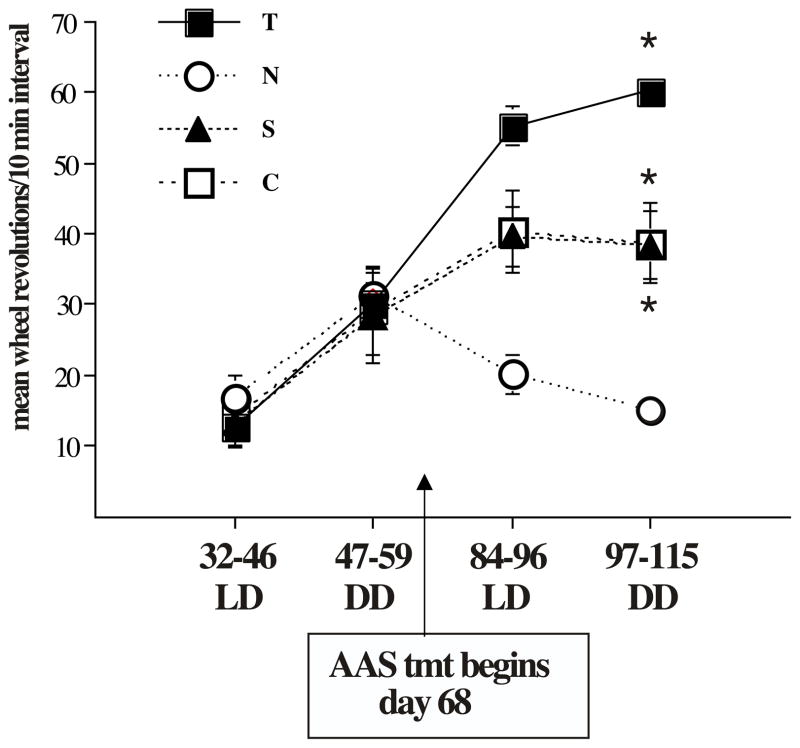
There was also a highly significant (p< .001) effect of mean running wheel activity over time (Figure 2). Control rats displayed a three-fold increase in wheel running activity over the course of development (p< 0.01: days 84–96 vs days 32–46 (LD) and days 97–115 vs days 32–46 (RR)). Stanozolol treated males exhibited essentially the same pattern with a significant increase in activity over the course of development (p<0.001: days 84–96 vs days 32–46 (LD) and days 97–115 vs days 32–46 (RR)). Testosterone-treated males showed the greatest increase in wheel running activity, but the same pattern of increase over time was observed (p<0.001: days 84–96 vs days 32–46 (LD) and days 97–115 vs days 32–46 (RR)). Notably, nandrolone-treated males were similar to all other groups prior to AAS exposure, but after AAS exposure these animals exhibited no significant developmental increase in wheel running activity.
Figure 2.
Mean (±SEM) activity levels over time. Compared to baseline (day 32–46) there was a highly significant increase in activity levels in testosterone-treated males (p< 0.001). Controls and stanozolol-treated males were similar and also displayed a significant increase in activity compared to baseline (p< 0.001). In contrast, nandrolone-treated males did not show an increase in activity over the course of development.
Circadian Period
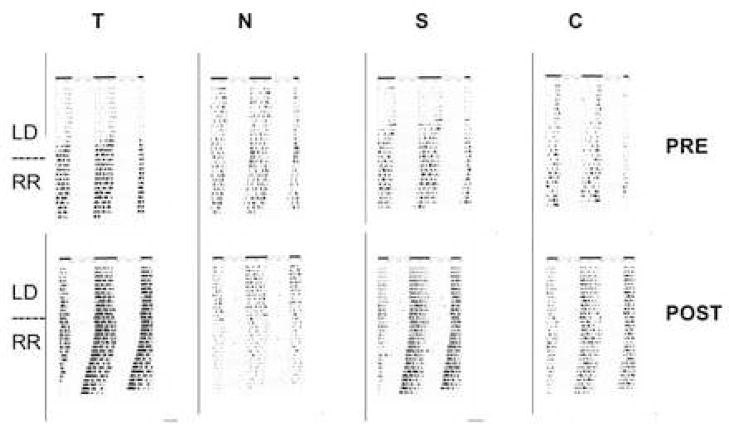
Sample actograms are shown in Figure 3 for baseline and post-treatment in LD and RR for each treatment group. There were no statistically significant effects of AAS treatment on circadian period. The mean baseline period from periodogram analysis in RR was 23.89 +/− 0.04 h RR (p>0.87), and 23.75 +/− 0.04 h post treatment (p>0.43). Comparable values from best-fit regression line estimates were 23.86 +/− 0.02 h for baseline period in RR (p>0.71), and 23.80 +/− 0.03 h post-treatment in RR (p>0.41).
Figure 3.
Sample actograms for each treatment group showing pre-AAS and post-AAS activity patterns. Pre-AAS treatment activity is shown in the top figure for each group, and post-AAS activity is shown below for the same subject. The dotted line separates the 12:12 LD activity from constant dim red light (RR).
There was a significant decrease in circadian period over the course of development in all groups. The mean changes in circadian period from baseline to RR was −0.15 +/− 0.04 h from periodogram estimates (p<0.001) and −0.07 +/− 0.03 h for best fit regression line estimates (p<0.05). There was no significant effect of AAS exposure on the shortening of tauRR over time.
Phase of Peak and Phase of Activity Onset
The phase of peak is shown in Table 1. There were no significant differences among the AAS groups for either baseline or post-treatment means for phase. There was, however, an overall significant (p< 0.001) advance in the mean time of peak of nearly three hours over the course of development (age 32–46 days to age 84–96). The change from pre-AAS to post-AAS values was significant for controls (p< 0.01) as well as for all AAS treatment groups (p< 0.001). The advance in time of peak was not affected by AAS treatment. There was no significant difference between groups for phase of activity onset over development (Table 1).
Table 1.
Mean baseline and post-AAS peak phase of activity and phase of activity onset over development. Values are given in hours relative to time of lights off (8 pm). N= 12 animals/group.
| Group | days 32–46 (preAAS) | days 84–96 (post-AAS) |
|---|---|---|
| PEAK PHASE OF ACTIVITY | ||
| Testosterone (T) | −6.7 ± .68 | −4.7 ±.18 ** |
| Nandrolone (N) | −7.7 ± .40 | −5.0 ± .31 ** |
| Stanozolol (S) | −7.9 ± .57 | −5.3 ± .28 ** |
| Gonadally intact (C) | −7.6 ± .53 | −4.7 ± .12 * |
| PHASE OF ACTIVITY ONSET | ||
| Testosterone (T) | 21.07 ± .03 | 21.00 ±.00 |
| Nandrolone (N) | 21.07 ± .03 | 20.00 ± .01 |
| Stanozolol (S) | 21.07 ± .03 | 21.00 ± .00 |
| Gonadally intact (C) | 21.15 ± .10 | 21.01 ± .01 |
p < 0.01 compared to days 32–46
p < 0.001 compared to days 32–46
Ct15 Phase Response
The overall mean phase response to the ct15 light pulse was a phase delay of 1.82 +/− 0.23h (p<0.001). There were no significant differences in magnitude of the response among any of the AAS treatment groups.
Body and Tissue Weights
Body weights are shown in Table 2. Body weights did not differ between groups at the start of AAS treatment. However, by the end of the experiment, the body weights of the T group were significantly lower than gonadally intact controls (p<.05). There were no significant differences in body weights of either N or S groups compared to controls. Testes weights (Table 3) were significantly lower than gonadally intact controls in all three AAS groups. Seminal vesicle weights in testosterone-exposed males were significantly elevated compared to gonadally intact controls (Table 3).
Table 2.
Mean baseline and post-AAS body weights over development. Values are given in grams. N= 12 animals/group.
| Group | days 32–46 (pre-AAS) | days 97–115 (post-AAS) |
|---|---|---|
| Testosterone (T) | 366 ± 11 | 411 ±.14 * |
| Nandrolone (N) | 342 ± 9 | 482 ± 15 |
| Stanozolol (S) | 358 ± 8 | 478 ± 13 |
| Gonadally intact (C) | 362 ± 9 | 479 ± 13 |
There were no significant differences between groups on days 32–46.
Weights of testosterone-treated males were significantly lighter (* p < 0.01) when compared to controls on days 97–115
Table 3.
Mean (±sem) values for for testes weights, seminal vesicles weights and serum testosterone levels for males receiving testosterone (T), nandrolone (N), or stanozolol (S) and gonadally intact control animals (C). N= 12 for all groups.
| Tissue | TP | ND | ST | C |
|---|---|---|---|---|
| Testes wt (g) | 3.31±.13** | 3.23±.09*** | 3.51±.12* | 3.85±.08 |
| Seminal vesicle wt (g) | 2.18±.17** | 1.80±.12 | 1.15±.08 | 1.41±.14 |
| Serum testosterone (ng/ml) | 6.37±13** | 0.18±.07** | 0.78±.27 | 3.25±.69 |
p< .05 compared to gonadally intact controls
p< .01 compared to gonadally intact controls
p< .001 compared to gonadally intact controls
Serum Testosterone Levels
Serum testosterone levels (Table 3) in males exposed to testosterone were significantly higher than controls, reflecting the level of exogenous AAS treatment. Nandrolone exposure resulted in serum testosterone levels that were significantly below gonadally intact levels. Serum levels of testosterone in stanozolol-treated males were not statistically different from controls. Control levels (gonadally intact) of serum testosterone were within the normal range [32].
Western Blot Analysis of SRC-1 and SRC-2
Testosterone exposure significantly decreased expression of SRC-2 in hypothalamus in comparison to controls (Figure 4). There was no effect of AAS treatment on SRC-2 expression in the amygdala (data not shown). In contrast to SRC-2, no effects of AAS exposure on SRC-1 expression were detected in the hypothalamus or amygdala.
Figure 4.
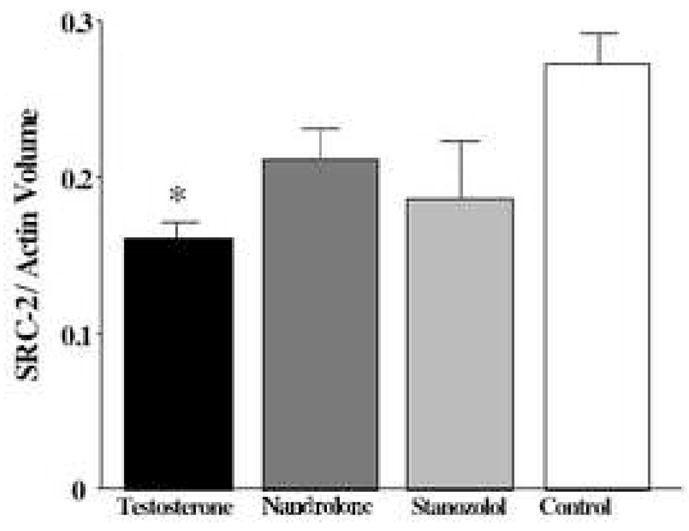
Western blot analysis of SRC-2 expression in the hypothalamus. Testosterone treatment decreased mean SRC-2 protein levels in the hypothalamus compared to control animals (mean + SEM, * p < 0.03). Densitometric analysis of SRC-2 immunoreactive bands and Actin immunoreactive bands expressed as a ratio. N = 6 animals/group.
DISCUSSION
A major goal of this study was to determine the impact of three different AAS on both running wheel activity levels and circadian rhythms in gonadally intact male rats. An important feature of the experimental paradigm was that we were also able to assess ontogenetic changes in circadian function from adolescence to adulthood.
Effects of AAS on circadian rhythms
Pubertal exposure to chronic high levels of testosterone to mimic AAS exposure, significantly increased running wheel activity. Previous studies have shown that testosterone plays a role in maintaining running wheel activity levels in rodents, as castration reduces running wheel activity [13,14,16]. In the current study, the running wheel activity of males chronically exposed to high levels of testosterone was significantly increased in comparison to intact control males. It is important to note that this increase is in comparison to gonadally intact males- not castrates. This suggests that very high testosterone levels can influence running wheel activity and may be responsible for some of the side effects of AAS reported in humans such as hyperactivity and disturbances in sleep patterns [2,5,6,7,8]. The increased running wheel activity is consistent with earlier studies showing that exposure to chronic high levels of testosterone increases other androgen-dependent behaviors such as aggression, sexual behaviors and scent marking [25,26,27,28,29,30]. However, locomotor (open field) activity is not increased by exposure to testosterone [33,34,35,36,37]. Thus, AAS effects on running wheel activity appear to be specific to androgenic influences, rather than a generalized, nonspecific increase in arousal.
Consistent with our previously published studies, differential effects of testosterone, nandrolone and stanozolol were quite evident. However, the effects did not parallel those of AAS effects on sociosexual and aggressive behaviors. For example, with the exception of a suppression of ultrasonic vocalizations, nandrolone has typically had either no effect or a stimulatory effect on behavior [25,26,27,28,29,30,38]. On the other hand, stanozolol, which has been found to inhibit most androgen-dependent behaviors, such as sexual, aggressive, and sociosexual behaviors [25,26,27,28,29,30], had no effect on wheel running activity. Our current data clearly show that running wheel activity is influenced by AAS. The differential effects of individual AAS may explain the common practice in AAS abusers of using several AAS simultaneously (“stacking”: [2,5]) as some AAS may enhance anabolic responses while simultaneously attenuating or even blocking potentially pernicious effects of others. This view is supported in animal models in which the stacking of stanozolol with testosterone has been found to prevent the inhibitory effects of stanozolol on several androgen-dependent behaviors [30].
In contrast to the effect of AAS on wheel running activity, there were no effects of AAS on circadian period, phase or phase response. Although Daan et al. [13] reported that castrated male house mice treated with replacement levels of exogenous testosterone displayed a slightly shorter circadian period relative to controls, studies in other rodent species have reported that testosterone does not alter circadian period [14,15,16]. The effects of castration on circadian period have not been studied in rats, but it is possible that androgens do not play a major role in circadian clock function in this rodent species. The large (n=12) numbers of animals per group, the lengthy AAS exposure (7 weeks), and the small standard errors all suggest that subtle changes in rhythmicity would be detected if they were present. One important factor, however, is the dose of AAS. Because we did not wish to disturb the rats in the running wheels, we used AAS pellets rather than injections and the dosing was somewhat lower as a result. Human AAS users often report using extremely high doses of multiple AAS, and it may be that even higher AAS doses than those employed in this study would modify circadian clock function. The dose administered in the present study still exceeded control males serum T levels, and was sufficient to alter running wheel activity. If it were possible to administer a higher dose in the running wheels, perhaps AAS would have modified the central clock.
Previous studies in adult male rats have reported that AAS have no effect on body weight [25,39]. In the current study we found that males receiving chronic exposure to high doses of testosterone had significantly lower body weights than all other groups. Our data suggest that, in adult rats, the testosterone-induced increase in running wheel activity may be responsible for the suppression of body weight.
One notable difference between the present study and previous studies in AAS animals is the use of pellets rather than injections to maintain chronic high levels of AAS. In the current study it was necessary to deliver the AAS on a relatively constant basis without disturbing the rats in the running wheels. In order to assess the effectiveness of the AAS pellets, we measured testes and seminal vesicle weights and body weights. Chronic exposure to high doses of testosterone, nandrolone and stanozolol have all been previously shown to decrease testes weights [30,40] and testosterone has been shown to increase seminal vesicle weights [30]. Our endocrine data show that the pellets were effective in suppressing testes weights in all AAS groups compared to gonadally intact males. Moreover, the seminal vesical weights of testosterone-treated males were significantly increased. Although the AAS pellets yielded lower serum testosterone levels than AAS injections [30], these levels were clearly sufficient to reduce body weight and to have effects on endocrine measures similar to injections. Most importantly, the doses used in this study were sufficient to induce clear behavioral changes in the expression of wheel running activity directly attributable to specific the AAS administered.
Androgen receptor (AR) activation in brain is believed to be involved in mediating male reproductive and aggressive behaviors [41]. However, in animals exposed to AAS, AR) binding and affinity may not be directly correlated with changes in behavior [24,30]. This suggests that other aspects of AR action may be affected by chronic exposure to very high levels of androgens. For example, it has recently been shown that nuclear receptor coactivators may contribute to nuclear receptor transcription through processes such as acetylation, methylation, phosphorylation and chromatin remodeling [23]. Thus, a possible explanation for the differences in AAS effects is via alterations in AR cofactors [42]. To address this question, we measured levels of steroid receptor coactivator-1 (SRC-1, also known as NcoA-1) [43] and SRC-2 (also known as NCoA-2, TIF-2, GRIP-1) [44,45]. SRC-1 and SRC-2 have both been shown to play a profound role in steroid hormone action in brain [31,46,47,48,49]. Both SRC-1 and SRC-2 are expressed at high levels in the hypothalamus of rodents [46,47,48,50,51,52]. However, to date, only SRC-1 has been examined in amygdala. Both the hypothalamus and amygdala contain high levels of androgen receptors [53]. SRC-1 expression in brain appears to be regulated by a variety of factors, including hormones [54,55] and daylength [56]. However, SRC-1 was not altered by exposure to AAS in the present study. On the other hand, SRC-2 was significantly increased in males chronically exposed to high levels of testosterone. This effect was brain-region specific as the increase was found only in the hypothalamus, and not in the amygdala. Thus, the behavioral effects of specific AAS differ with respect to their interaction with steroid receptor cofactors and with respect to brain region. SRC-2 is expressed in androgen-responsive motoneurons [42] and as been shown to be critical for AR action in testes [57]. While, to the best of our knowledge, the role of SRC-2 on wheel running activity has not been investigated, one study in SRC-1 knock-out mice reveals that this coactivator is involved in motor function [58]. These findings suggest that chronic exposure to AAS may influence androgen-dependent behaviors, such as wheel running activity, by modulating expression of SRC-2 in brain. For example, down-regulation of SRC-2 in the hypothalamus may be a potential compensatory mechanism to decrease steroid receptor activity in the presence of elevated T levels. The present study of nuclear receptor coactivator expression analyzed the entire hypothalamus by Western Blot. In future studies, it will be important to investigate coactivator expression with techniques that provide cellular resolution (eg. immunohistochemistry), thus allowing a more detailed neuroanatomical analysis.
Developmental changes in circadian function
One of the most striking and consistent findings from our study was a change in circadian phase and period over the course of development. From day 32 (prior to puberty) to day 115, there was a significant increase in running wheel activity, a significant phase advance in peak levels and a significant shortening of the circadian period. Recent work in both humans [59] and the diurnal rodent, Octodon degus [17] suggests that the maturation of adult patterns of circadian function develop during puberty. Octodon showed a phase delay and lengthened circadian period following puberty. A similar phase delay has also been reported in humans [59]. These changes mirror the phase advance and shortened circadian period observed in the present study. Although humans and other species such as Octodon and rats show changes in circadian phase across puberty, the differences in direction may reflect species-specific mechanisms. The lack of effect on phase of activity onset in our study suggests that the time of activity onset does not shift, but the distribution of activity does.
In our gonadally intact control males, running wheel activity increased during puberty, (from days 32–59) and then following a further elevation during young adulthood (days 84–96), remained stable thereafter (days 97–115). These changes in wheel running activity parallel rising serum T levels. For example, it has been found that there is a marked increase in serum T between days 21 and 43 [60] and between days 48 and 56 [61] which is consistent our wheel running activity changes. In addition, the serum T levels of our control males taken at sacrifice are within the range (2–5 ng/ml) previously reported for adult males [25,29,62]. Taken together these data suggest that the developmental changes in wheel running activity may be a reflection of changes in serum testosterone levels.
The AAS treatments began on day 68 and it is during this period of AAS exposure that the expression the wheel running activity clearly began to diverge between groups. Specifically, testosterone-treated males showed a significant increase in wheel running activity, while the nandrolone-treated rats displayed a significant decrease in running wheel activity. Stanozolol-treated males did not differ from controls. This supports our hypothesis that the impact of the AAS on wheel running activity is influenced by the chemical composition of the AAS. This hypothesis is further substantiated by the differences in serum T levels obtained for each of our treatment groups. Serum T levels were significantly elevated in testosterone-treated males relative to controls. Nandrolone-treated males, which showed a marked decrease in wheel running activity also had significantly lower serum T levels than controls. Stanozolol-treated males did not differ significantly from control males with regard to either their running wheel activity or serum T levels. The alterations in wheel running activity after exposure to AAS in late puberty underscores the point that AAS exposure can have a profound impact on the development of adult activity levels.
Conclusions
This study constitutes a comprehensive developmental analysis of circadian rhythmicity and wheel running activity in gonadally intact male rats exposed to three different anabolic androgenic steroids (AAS). Three major findings resulted from this study. First, chronic exposure to AAS affects wheel running activity without altering circadian clock regulation of activity. Second, the expression of running wheel activity in male rats is explicitly modified by the specific chemical nature of the AAS. Third, adult patterns of activity and circadian rhythms change over the course of adolescent development.
Acknowledgments
We thank Mr. Albert Davis, Ms. Mbaira Maorongarti, Ms. Cheryl Jenks for technical assistance. The mouse monoclonal antibody of human SRC-1 was kindly provided by Dean Edwards, Bert O’Malley, Ming Tsai and Sergio Onate, Baylor College of Medicine. This research was supported by NIH grants DA10886 (MYM), DK61935 (MJT), Skidmore College Summer Student-Faculty Collaborative Research Award funded in part by the W. M. Keck Foundation, and Skidmore College Faculty Research Grants to BP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DuRant RH, Escobedo LG, Heath GW. Anabolic steroid use, strength training, and multiple drug use among adolescents in the United States. Pediatrics. 1995;96:23–28. [PubMed] [Google Scholar]
- 2.Haupt H, Rovere G. Anabolic steroids: a review of the literature. Am J Sports Med. 1984;12:469–489. doi: 10.1177/036354658401200613. [DOI] [PubMed] [Google Scholar]
- 3.Lovstakken K, Peterson L, Homer AL. Risk factors for anabolic steroid use in college students and the role of expectancy. Addict Behav. 1999;24:425–430. doi: 10.1016/s0306-4603(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 4.Stilger VG, Yesalis CE. Anabolic–androgenic steroid use among high school football players. J Community Health. 1999;24:131–145. doi: 10.1023/a:1018706424556. [DOI] [PubMed] [Google Scholar]
- 5.Uzych L. Anabolic-androgenic steroids and psychiatric-related effects: a review. Can J Psychiatry. 1992:37. doi: 10.1177/070674379203700106. [DOI] [PubMed] [Google Scholar]
- 6.Pope HGJ, Katz DL. Body builder's psychosis. Lancet. 1987;1:863. doi: 10.1016/s0140-6736(87)91642-4. [DOI] [PubMed] [Google Scholar]
- 7.Pope HGJ, Katz DL. Affective and psychotic symptoms associated with anabolic steroid use. Am J Psychiat. 1988;145:487–490. doi: 10.1176/ajp.145.4.487. [DOI] [PubMed] [Google Scholar]
- 8.Pope HGJ, Katz DL. Psychiatric and medical effects of anabolic-androgenic steroid use. A controlled study of 160 athletes. Arch Gen Psychiat. 1994;51:375–382. doi: 10.1001/archpsyc.1994.03950050035004. [DOI] [PubMed] [Google Scholar]
- 9.Anderson JL, Wirz-Justice A. Biological rhythms in the pathophysiology and treatment of affective disorders. In: Horton R, Katona C, editors. Biological Aspects of Affective Disorders. New York: Academic Press; 1991. pp. 223–269. [Google Scholar]
- 10.Rossenwasser A, Wirz-Justice A. Circadian rhythms and depression: clinical and experimental models. In: Redfern P, Lemmer B, editors. Handbook of Experimental Pharmacology. Berlin: Springer-Verlag; 1997. pp. 125pp. 457–486. [Google Scholar]
- 11.Teicher M, Lawrence J, Barber N, Finkelstein S, Liebermen H, Baldessarini R. Increased activity and phase delay in circadian motility rhythms in geriatric depression. Arch Gen Psychiat. 1988;45:913–917. doi: 10.1001/archpsyc.1988.01800340039005. [DOI] [PubMed] [Google Scholar]
- 12.Tsujimoto T, Yamada N, Shimoda K, Hanada K, Takahashi S. Circadian rhythms in depression, Part I: circadian rhythms in inpatients with various mental disorders. J Affective Disorders. 1990;18:199–210. doi: 10.1016/0165-0327(90)90037-9. [DOI] [PubMed] [Google Scholar]
- 13.Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on as circadian pacemaker in mice (Mus musculus) Proc Nat Acad Sci. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jechura T, Walsh J, Lee T. Testicular hormones modulate circadian rhythms of the diurnal rodent, Ocotodon degus. Horm Behav. 2000;38:243–249. doi: 10.1006/hbeh.2000.1624. [DOI] [PubMed] [Google Scholar]
- 15.Morin L, Cummings L. Effect of surgical or photoperiodic castration, testosterone replacement or pinealectomy on male hamster running rhythmicity. Physiol Behav. 1981;6:825–838. doi: 10.1016/0031-9384(81)90106-2. [DOI] [PubMed] [Google Scholar]
- 16.Rowsemitt C. Activity of castrated male voles: rhythms of responses to testosterone replacement. Physiol Behav. 1989;45:7–13. doi: 10.1016/0031-9384(89)90159-5. [DOI] [PubMed] [Google Scholar]
- 17.Lee T, Hummer D, Jechura T, Mahoney M. Pubertal development of sex differences in circadian function. Ann NY Acad Sci. 2004;1021:262–275. doi: 10.1196/annals.1308.031. [DOI] [PubMed] [Google Scholar]
- 18.Mottram DR, George AJ. Anabolic steroids. Bailliere's Clin Endocrinol Metab. 2000;14:55–69. doi: 10.1053/beem.2000.0053. [DOI] [PubMed] [Google Scholar]
- 19.McGinnis MY. Anabolic androgenic steroids and aggression: studies using animal models. NY Acad Sci. 2004;1036:399–415. doi: 10.1196/annals.1330.024. [DOI] [PubMed] [Google Scholar]
- 20.Pittendrigh C. Circadian systems: General Perspective. In: Aschoff J, editor. Handbook of Behavioral Neurobiology. Vol. 4. New York: Plenum Press; 1981. pp. 57–77. [Google Scholar]
- 21.Edwards D. The role of coactivators and corepressors in the biology and mechanism of action of steroid hormone receptors. J Mammary Gland Biol Neoplasia. 2000;5:307–324. doi: 10.1023/a:1009503029176. [DOI] [PubMed] [Google Scholar]
- 22.Glass C, Rosenfeld M. The coregulator exchange in transcriptional function of nuclear receptors. Genes Dev. 2000;14:121–141. [PubMed] [Google Scholar]
- 23.Lonard D, O'Malley B. The expanding cosmos of nuclear receptor coactivators. Cell. 2006;125:411–414. doi: 10.1016/j.cell.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Roselli CE. The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area. Brain Research. 1998;792:271–276. doi: 10.1016/s0006-8993(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 25.Breuer ME, McGinnis MY, Lumia AR, Possidente BP. Aggression in male rats receiving anabolic androgenic steroids: effects of social and environmental provocation. Horm Behav. 2001;40:409–418. doi: 10.1006/hbeh.2001.1706. [DOI] [PubMed] [Google Scholar]
- 26.Farrell SF, McGinnis MY. Effects of pubertal anabolic-androgenic steroid (AAS) administration on reproductive and aggressive behaviors in male rats. Behav Neurosci. 2003;117:904–911. doi: 10.1037/0735-7044.117.5.904. [DOI] [PubMed] [Google Scholar]
- 27.Farrell SF, McGinnis MY. Long-term effects of pubertal anabolic-androgenic steroid exposure on reproductive and aggressive behaviors in male rats. Horm Behav. 2004;46:193–203. doi: 10.1016/j.yhbeh.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 28.McGinnis MY, Lumia AR, Breuer ME, Possidente BP. Physical provocation potentiates aggression in male rats receiving anabolic androgenic steroids. Horm Behav. 2002;41:101–110. doi: 10.1006/hbeh.2001.1742. [DOI] [PubMed] [Google Scholar]
- 29.McGinnis MY, Lumia AR, Possidente BP. Effects of withdrawal from anabolic androgenic steroids on aggression in adult male rats. Physiol Behav. 2002;75:541–549. doi: 10.1016/s0031-9384(02)00657-1. [DOI] [PubMed] [Google Scholar]
- 30.Wesson DW, McGinnis MY. Stacking anabolic androgenic steroids (AAS) during puberty in rats: A neuroendocrine and behavioral assessment. Pharmacol Biochem Behav. 2006;83:410–419. doi: 10.1016/j.pbb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Molenda-Figueira H, Williams D, Griffin A, Rutledge E, Blaustein J, Tetel M. Nuclear receptor coactivators function in estrogen receptor- and progestin receptor-dependent aspects of sexual behavior in female rats. Horm Behav. 2006;50:383–392. doi: 10.1016/j.yhbeh.2006.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krey LC, McGinnis MY. Time courses of the appearance/disappearance of nuclear androgen+receptor complexes in the brain and adenohypophysis following testosterone administration/withdrawal to castrated male rats: relationships with gonadotropin secretion. J Steroid Biochem. 1990;35:403–408. doi: 10.1016/0022-4731(90)90247-p. [DOI] [PubMed] [Google Scholar]
- 33.Clark AS, Barber DM. Anabolic-androgenic steroids and aggression in castrated male rats. Physiol Behav. 1994;56:1107–1113. doi: 10.1016/0031-9384(94)90351-4. [DOI] [PubMed] [Google Scholar]
- 34.Keleta Y, Lumia A, Anderson G, McGinnis M. Behavioral effects of pubertal anabolic androgenic steroid in male rats with low serotonin. Brain Res. 2007;1132:129–138. doi: 10.1016/j.brainres.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 35.Lindqvist AS, Johansson-Steensland P, Nyberg F, Fahlke C. Anabolic androgenic steroid affects competitive behaviour, behavioural response to ethanol and brain serotonin levels. Behav Brain Res. 2002;133:21–29. doi: 10.1016/s0166-4328(01)00408-9. [DOI] [PubMed] [Google Scholar]
- 36.Martinez Sanchis S, Argon CM, Salvador A. Cocaine-induced locomotor activity is enhanced by exogenous testosterone. Physiol Behav. 2002;76:605–609. doi: 10.1016/s0031-9384(02)00764-3. [DOI] [PubMed] [Google Scholar]
- 37.Salvador A, Moya–Albiol L, Martinez-Sanchis S, Simon VM. Lack of effects of anabolic androgenic steroids on locomotor activity in intact male mice. Percep Motor Skills. 1999;88:319–328. doi: 10.2466/pms.1999.88.1.319. [DOI] [PubMed] [Google Scholar]
- 38.Long SF, Wilson MC, Sufka KJ, Davis WM. The effects of cocaine and nandrolone co administration on aggression in male rats. Prog Neuro-Psychopharmacol Biol Psychiat. 1996;20:839–856. doi: 10.1016/0278-5846(96)00063-2. [DOI] [PubMed] [Google Scholar]
- 39.Lumia AR, Thorner KM, McGinnis MY. Effects of chronically high doses of the anabolic androgenic steroid, testosterone, on intermale aggression and sexual behavior in male rats. Physiol Behav. 1994;55:331–335. doi: 10.1016/0031-9384(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 40.Feinberg MJ, Lumia AR, McGinnis MY. The effects of anabolic-androgenic steroids on sexual behavior and reproductive tissues in male rats. Physiol Behav. 1997;62:23–30. doi: 10.1016/s0031-9384(97)00105-4. [DOI] [PubMed] [Google Scholar]
- 41.McGinnis MY, Marcelli M, Lamb DJ. Consequences of mutations in androgen receptor genes: molecular biology and behavior. In: Pfaff DW, editor. Hormones, Brain and Behavior. Vol. 5. New York: Academic Press; 2002. pp. 347–380. [Google Scholar]
- 42.O'Bryant E, Jordan C. Expression of nuclear receptor coactivators in androgen-responsive and -unresponsive motoneurons. Horm Behav. 2005;47:29–38. doi: 10.1016/j.yhbeh.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 43.Onate S, Tsai S, Tsai M, O'Malley B. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 44.Hong H, Kohli K, Garabedian M, Stallcup M. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receprors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voegel J, Heine M, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 46.Apostolakis E, Ramamurphy M, Zhou D, Onate S, O'Malley B. Acute disruption of select steroid receptor coactivators prevents reproductive behavior in rats and unmasks genetic adaptation in knockout mice. Mol Endo. 2002;16:1511–1523. doi: 10.1210/mend.16.7.0877. [DOI] [PubMed] [Google Scholar]
- 47.Auger A, Tetel M, McCarthy M. Steroid receptor co-activator-1 mediates the development of sex specific brain morphology and behavior. Proc Nat Acad Sci. 2000;97:7551–7555. doi: 10.1073/pnas.97.13.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Molenda H, Griffin A, Auger A, McCarthy M, Tetel M. Nuclear receptor coactivators modulate hormone-dependent gene expression in brain and female reproductive behavior in rats. Endocrinol. 2002;143:436–444. doi: 10.1210/endo.143.2.8659. [DOI] [PubMed] [Google Scholar]
- 49.Tetel M, Yore M, Webb L, Chadwick J, Molenda H. Steroid receptor coactivator-2 is expressed in female rat brain and physically interacts with estrogen receptor (ER) alpha, but not ER-beta, in a ligand-dependent manner. Society for Neuroscience (abst) 2006;258:9. [Google Scholar]
- 50.Martinez de Arrieta C, Koibuchi N, Chin W. Coactivator and corepressor gene expression in rat cerebellum during postnatal development and the effect of altered thyroid status. Endocrinol. 2000;141:1693–1698. doi: 10.1210/endo.141.5.7467. [DOI] [PubMed] [Google Scholar]
- 51.Meijer O, Steenbergen P, de Kloet E. Differential expression and regional distribution of steroid receptor coactivators SRC-1 and SRC-2 in brain and pituitary. Endocrinol. 2000;141:2192–2199. doi: 10.1210/endo.141.6.7489. [DOI] [PubMed] [Google Scholar]
- 52.Misiti S, Schomburg L, Yen P, Chin W. Expression and hormonal regulation of coactivator and corepressor genes. Endocrinol. 1998;139:2493–2500. doi: 10.1210/endo.139.5.5971. [DOI] [PubMed] [Google Scholar]
- 53.McGinnis MY, Dreifuss RM. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinol. 1989;124:618–626. doi: 10.1210/endo-124-2-618. [DOI] [PubMed] [Google Scholar]
- 54.Camacho-Arroyo I, Neri-Gomez T, Gonzalez-Arenas A, Guerra-Araiza C. Changes in the content of steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid hormone receptors in the rat brain during the estrous cycle. J Steroid Biochem Mol Biol. 2005;94:267–272. doi: 10.1016/j.jsbmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 55.Iannacone E, Yan A, Gauger K, Dowling A, Zoeller R. Thyroid hormone exerts site-specific effects on SRC-1 and NCoR expression selectively in the neonatal rat brain. Mol Cell Endocrinol. 2002;186:49–59. doi: 10.1016/s0303-7207(01)00672-4. [DOI] [PubMed] [Google Scholar]
- 56.Tetel M, Ungar T, Hassan Z, Bittman E. Photoperiodic regulation of androgen receptor and steroid receptor coactivator-1 in Siberian hamster brain. Mol Brain Res. 2004;131:79–87. doi: 10.1016/j.molbrainres.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ye X, Han S, Tsai S, Demayo F, Xu J, Tsai M, O'Malley B. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc Natl Scad Sci USA. 2005;102:9487–9492. doi: 10.1073/pnas.0503577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nishihara E, Yoshida-Kimoya H, Chan C, Liao L, Davis RL, O'Malley BW, Xu J. SRC-1 null mice exhibit moderate motor dysfunction and delayed development of cerebellar Purkinje cells. J Neurosci. 2003;23:213–222. doi: 10.1523/JNEUROSCI.23-01-00213.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carskadon M, Acebo C. Regulation of sleepiness in adolescents: Updates, insights, and speculation. Sleep. 2002;25:606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 60.Ohno S, Nakajima Y, Inoue K, Nakazaya H, Nakajin S. Genistein administration decreases serum corticosterone and testosterone levels in rats. Life Sci. 2003;74:733–742. doi: 10.1016/j.lfs.2003.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Huhtaniemi IT, Nevo N, Amsterdam A, Naor Z. Effect of postnatal treatment with a gonadotropin-releasing hormone antagonist on sexual maturation of male rats. Biol Reprod. 1986;35:501–507. doi: 10.1095/biolreprod35.3.501. [DOI] [PubMed] [Google Scholar]
- 62.Murono E, Derk R, Akgul Y. In vivo exposure of young adult male rats to methoxychlor reduces serum testosterone levels and ex vivo Leydig cell testosterone formation and cholesterol side-chain cleavage activity. Reproductive Toxicol. 2006;21:148–153. doi: 10.1016/j.reprotox.2005.08.005. [DOI] [PubMed] [Google Scholar]