Abstract
The influx of cytosolic Ca2+ into mitochondria is mediated primarily by the mitochondrial calcium uniporter (MCU)1, a small-conductance, Ca2+-selective channel2-6. MCU modulates intracellular Ca2+ transients and regulates ATP production and cell death1. Recently, Joiner et al. reported that MCU is regulated by mitochondrial CaMKII, and this regulation determines stress response in heart7. They reported a very large current putatively mediated by MCU that was about two orders of magnitude greater than the MCU current (IMCU) that we previously measured in heart mitochondria3. Also, the current traces presented by Joiner et al. showed unusually high fluctuations incompatible with the low single-channel conductance of MCU. Here we performed patch-clamp recordings from mouse heart mitochondria under the exact conditions used by Joiner et al. We confirmed that IMCU in cardiomyocytes is very small and showed that it is not directly regulated by CaMKII. Thus the currents presented by Joiner et al. do not correspond to MCU, and there is no direct electrophysiological evidence that CaMKII regulates MCU.
The main differences in the experimental conditions used by Joiner et al7 and in our previous study3 were: the use of hypotonic shock to prepare mitoplasts (vs. French Press in our study), the presence of high Na+ concentration in recording solutions (vs. Na+-free solutions), and the age of the mice (2–3 months vs. 3–4 weeks).
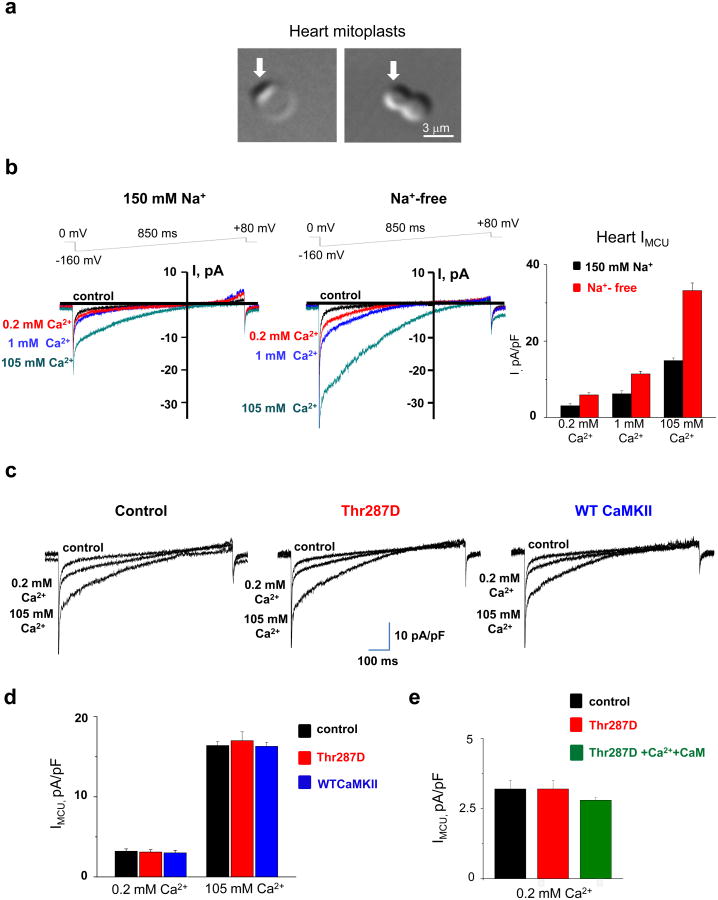
Fig. 1a shows mouse heart mitoplasts obtained by exposure of mitochondria to hypotonic shock. The measured average membrane capacitance (Cm) was 0.65±0.03 pF (±SEM, n=65), which correlates well with Cm measurements reported for heart mitoplasts obtained with French press3, as well as with measurements of the inner mitochondrial membrane surface area using EM8,9 and with estimated measurements of idealized cardiac mitochondria10. Therefore, the values reported by Joiner et al. are abnormally high (5–9 pF), indicating inaccuracy in monitoring Cm leading to faulty values of IMCU densities throughout the paper.
Fig. 1. Heart MCU current and CaMKII.
(a) Transmitted image of heart mitoplasts obtained by exposure of mitochondria to 5-minute hypotonic shock. Both round (left panel) and figure 8-shaped (right panel) mitoplasts were present in this preparation and used for electrophysiological experiments. Arrows indicate remnants of the outer mitochondrial membrane. Note that the average diameter of heart mitoplasts in this preparation is ∼4.5 μm (n=65), which corresponds well with the average membrane capacitance (Cm) measurements of 0.67 pF that we previously reported. (b) Representative heart whole-mitoplast MCU currents (IMCU) recorded in the presence (left panel) or absence (middle panel) of 150 mM NaGluconate in both the pipette and bath solutions. IMCU was recorded with different bath Ca2+ concentrations: 0.2 mM (red), 1 mM (blue), and 105 mM (green). IMCU was blocked by 50 nM RuR added to the 0.2 mM Ca2+ bath solution (control, black). Currents in left and middle panels are not normalized and were recorded from two different mitoplasts with comparable membrane capacitance (Cm= 0.80 pF and 0.84 pF, respectively). The voltage ramp protocol used to elicit IMCU is indicated at the top. Note that with Na+ in the recording solutions we also observed a small outward current at high positive voltages. This current was absent in Na+-free conditions (middle panel and Fieni et al3). Pipette solution, in mM: 150 NaGluconate, 40 HEPES, 2 NaCl, 1.5 EGTA, tonicity 450 mmol per kg with sucrose, pH 7.2 with NaOH. Bath Ca2+ solutions with 0.2 and 1 mM Ca2+ were prepared by addition of 1 M stock solution of CaCl2 into the bath solution containing, in mM: 150 NaGluconate, 40 HEPES, tonicity 300 mmol per kg, pH 7.4 with NaOH. The bath solution with 105 mM Ca2+ contained 105 mM CaCl2 and 10 mM HEPES, pH 7.2 with Tris base. Right panel, histogram representing average MCU current densities (IMCU normalized to the Cm) obtained in the presence (black) or absence (red) of 150 mM NaGluconate in recording solutions with different bath Ca2+ concentrations (0.2, 1, and 105 mM). Current amplitudes were measured at 5 ms after stepping from 0 to −160 mV. IMCU densities were as follows: at bath 0.2 mM Ca2+, 3.3±0.4 pA/pF (n = 8) with 150 NaGluconate in recording solutions and 6±0.7 pA/pF without NaGluconate in recording solutions; at bath 1 mM Ca2+, 6.2±0.7 pA/pF (n = 9) with NaGluconate and 11.4±0.7 pA/pF (n=6) without NaGluc; at bath 105 mM Ca2+, 14.2±0.7 pA/pF (n = 12) with NaGluconate and 33.2±2 pA/pF (n=7) without NaGluconate in the pipette solution. Statistical data are presented as mean ± SEM. (c) Representative IMCU in control (left panel), in the presence of a constitutively active monomeric CaMKII (Thr287D mutant) in the patch pipette (middle panel), and in the presence of wild-type monomeric CaMKII previously activated (autophosphorylated) with Ca2+/calmodulin (CaM) and Mg2+/ATP (γ-thiol-ATP) (right panel) in the patch pipette. IMCU was elicited by a voltage ramp protocol (see panel b) in the presence of 0.2 and 105 mM Ca2+. IMCU amplitude was monitored for up to 35 min after formation of the whole-mitoplast configuration as in Joiner et al. (However, the calculated diffusion time15 for the 35-kDa monomer of CaMKII from the pipette into the mitoplast is only ∼25 seconds.) Pipette solution contained, in mM: 150 NaGluconate, 40 HEPES, 2 NaCl, 1.5 EGTA, tonicity 450 mmol per kg with sucrose, pH 7.2 with NaOH. The recombinant Thr287D and wild-type CaMKII were added to the control solution at 0.5 or 1 μM, in the presence of 2 mM Na2ATP and 3 mM MgCl2. (Addition of ATP and Mg2+ alone did not affect IMCU.) (d) Histogram showing average IMCU current densities obtained in the absence (black, control) or presence of Thr287D (red) or wild-type monomeric CaMKII pre-autophosphorylated with thiol-ATP (blue) in the pipette. Currents were measured in 0.2 and 105 mM Ca2+ as described in (c), and amplitudes were determined at 5 ms after stepping from 0 to −160 mV. IMCU densities were as follows: at bath 0.2 mM Ca2+, 3.2±0.3 pA/pF (n=17) in control, 3.2±0.3 pA/pF (n=14) for Thr287D, and 3.0±0.3 pA/pF (n=8) for autophosphorylated wild-type CaMKII; at bath 105 mM Ca2+, 16.4±0.5 pA/pF (n=16) in control, 17.9±1.1 pA/pF (n=11) for Thr287D, and 16.2±0.5 pA/pF (n=5) for autophosphorylated wild-type CaMKII. Statistical data are presented as mean ± SEM. (e) Histogram showing average IMCU current densities in control (black) and in the presence of a constitutively active monomeric CaMKII (Thr287D mutant) in the patch pipette either alone (red) or with 1 μM CaM and 5–10 μM free Ca2+ (green). IMCU densities were as follows: at bath 0.2 mM Ca2+, 3.2±0.3 pA/pF (n=17) in control, 3.2±0.3 pA/pF (n=14) for Thr287D, and 2.8±0.1 pA/pF (n=5) for Thr287D in the presence of 1 μM CaM and 5–10 μM free Ca2+. Current amplitudes were measured at 5 ms after stepping from 0 to −160 mV. Statistical data are presented as mean ± SEM.
We recorded IMCU from heart mitoplasts isolated by hypotonic shock with 150 mM NaGluconate in the pipette and bath solutions (as in Joiner et al., Fig. 1b, left panel) and without Na+ (conditions previously used by us3, Fig. 1b, middle panel). Interestingly, IMCU recorded in the presence of NaGluconate was significantly smaller than in its absence (Fig. 1b). Our data support the observation that elevated Na+ may regulate heart mitochondrial [Ca2+]11,12. Importantly, the whole-mitoplast IMCU was about two orders of magnitude lower than the current reported by Joiner et al. (∼2 pA at -160 mV in 0.2 mM Ca2+ vs. ∼180 pA) and did not exhibit high fluctuations as expected for a small-conductance channel. Also, the current reported by Joiner et al. was not inhibited by Ru360 in the same fashion as the IMCU2. In 10 nM Ru360, IMCU shows no immediate inhibition upon stepping from 0 mV to -120 mV2, and the inhibition develops slowly over time2, whereas the current of Joiner et al. was inhibited immediately upon stepping from 0 to -160 mV. All these observations indicate that Joiner et al. did not record IMCU. We suggest that either they did not record from inner mitochondrial membrane or the integrity of their mitoplasts was compromised.
Next, we tested whether IMCU is directly regulated by CaMKII as claimed by Joiner et al., who reported that addition of a constitutively active monomeric form of CaMKII (T287D mutant) to the patch pipette potentiated their currents. When we applied T287D, we failed to observe any functional change in IMCU, either without (Fig 1c middle panel, and d) or with Ca2+ plus calmodulin (Fig. 1e). We further verified these results using wild-type monomeric CaMKII pre-autophosphorylated with thiol-ATP to prevent de-autophosphorylation and again observed no change in IMCU (Fig. 1c right panel and d).
In conclusion, the noisy currents presented by Joiner et al. are not carried by MCU, and their extremely high amplitude misrepresents the actual MCU activity in heart. Heart, with abundant mitochondria and frequently elevated cytosolic Ca2+, has very low MCU current3, which is likely critical for avoiding disruption of cytosolic Ca2+ signaling and preventing mitochondrial Ca2+ overload and cell death. Finally, our electrophysiological experiments with MCU currents did not indicate that MCU is regulated by CaMKII.
Methods
Electrophysiological experiments were performed as in Fieni et al3. Recombinant δ–human monomeric CaMKII (1-137) was purified from baculovirus using an N-terminal 6X-HN tag and Ni chromatography followed by gel filtration. Activity of recombinant CaMKII was measured in NaGluconate pipette solution using the peptide substrate AC-213. Constitutive activity (no Ca2+ /calmodulin) was undetectable for wild-type CaMKII and 4.6 μmol/min/mg for T287D. The Ca2+/calmodulin stimulated activity of T287D CaMKII was 9.7 μmol/min/mg. Wild-type CaMKII was autophosphorylated in γ-thiol-ATP to promote Thr287 autophosphorylation, which allows CaMKII to be active without Ca2+/calmodulin (i.e., autonomous activity)14. The autonomous activity of wild-type CaMKII was 19.4 μmol/min/mg (∼91% of the Ca2+/calmodulin stimulated activity).
References
- 1.Rizzuto R, Bernardi P, Pozzan T. Mitochondria as all-round players of the calcium game. J Physiol. 2000;529 Pt 1:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. doi:PHY_1167 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 3.Fieni F, Bae Lee S, Jan YN, Kirichok Y. Activity of the mitochondrial calcium uniporter varies greatly between tissues. Nat Commun. 2012;3:1317. doi: 10.1038/ncomms2325. doi:10.1038/ncomms2325 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaudhuri D, Sancak Y, Mootha VK, Clapham DE. MCU encodes the pore conducting mitochondrial calcium currents. eLife. 2013;2:e00704. doi: 10.7554/eLife.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. doi:10.1038/nature10230 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baughman JM, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. doi:10.1038/nature10234 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joiner ML, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. doi:10.1038/nature11444 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. The American Journal of Physiology. 1978;235:C147–158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- 9.Smith HE, Page E. Morphometry of rat heart mitochondrial subcompartments and membranes: application to myocardial cell atrophy after hypophysectomy. Journal of Ultrastructure Research. 1976;55:31–41. doi: 10.1016/s0022-5320(76)80079-2. [DOI] [PubMed] [Google Scholar]
- 10.Williams GS, Boyman L, Chikando AC, Khairallah RJ, Lederer WJ. Mitochondrial calcium uptake. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10479–10486. doi: 10.1073/pnas.1300410110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Rourke B, Maack C. The role of Na dysregulation in cardiac disease and how it impacts electrophysiology. Drug Discovery Today Disease Models. 2007;4:207–217. doi: 10.1016/j.ddmod.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maack C, et al. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashpole NM, Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Molecular and cellular neurosciences. 2011;46:720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Rokita AG, Anderson ME. New therapeutic targets in cardiology: arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Archiv : European Journal of Physiology. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]