Abstract
Pancreatic cancer is one of the most lethal malignancies. Significant progresses have been made in understanding of pancreatic cancer pathogenesis, including appreciation of precursor lesions or premalignant pancreatic intraepithelial neoplasia (PanINs), description of sequential transformation from normal pancreatic tissue to invasive pancreatic cancer and identification of major genetic and epigenetic events and the biological impact of those events on malignant behavior. However, the currently used therapeutic strategies targeting tumor epithelial cells, which are potent in cell culture and animal models, have not been successful in the clinic. Presumably, therapeutic resistance of pancreatic cancer is at least in part due to its drastic desmoplasis, which is a defining hallmark for and circumstantially contributes to pancreatic cancer development and progression. Improved understanding of the dynamic interaction between cancer cells and the stroma is important to better understanding pancreatic cancer biology and to designing effective intervention strategies. This review focuses on the origination, evolution and disruption of stromal molecular and cellular components in pancreatic cancer, and their biological effects on pancreatic cancer pathogenesis.
Keywords: Cytokines, Growth factors, Immunology, Stroma, Therapy, Transcriptional factors
Introduction
Pancreatic cancer is one of the most lethal malignancies with a 5-year survival rate below 5%.1, 2 Although surgery remains the best choice for pancreatic cancer treatment, most cases are diagnosed at an advanced stage, making patients poor candidates for surgical treatment.3, 4, 5, 6 Major reasons for the dismal prognosis for pancreatic cancer include lack of early appreciable symptoms, tendency of rapid local or distant metastasis, and intrinsic resistance to conventional chemotherapeutics.4, 5, 6, 7 Because effective systemic therapy capable of controlling the aggressive pancreatic cancer biology is currently lacking, the need for a better understanding of detailed mechanisms underlying pancreatic cancer development and progression is urgent.3, 8, 9, 10
Recent studies on pancreatic cancer genetics and epigenetics have led to the identification of notable genetic alterations, such as K-ras, p53, Smad4, and p16.11, 12, 13, 14, 15, 16 These signature genetic events, combined with accompanying histopathological alterations, suggest a sequential transformation roadmap of pancreatic cancer from normal pancreatic epithelium to increasing grades of pancreatic intraepithelial neoplasia to, ultimately, invasive pancreatic adenocarcinoma.17 However, targeting these signature genetic and epigenetic alterations has not resulted in useful preventive and/or therapeutic modalities in clinic, while gemcitabine remains the first-line chemotherapeutic agent for pancreatic cancer.3, 18
Recently, paradigm of pancreatic cancer research has shifted from parenchyma to stroma.19, 20, 21 Evidently, tumors with identical germline mutations exhibit diverse formations of stroma and the degrees of stromal reaction predict aggressive phenotype.11, 12, 13, 14 In fact, histological hallmark of pancreatic cancer is its pronounced desmoplastic reactions (Fig. 1). In general, pancreatic cancer stroma could account for more than 90% of the total tumor volume. Many signaling pathways have been proposed to mediate interactions between cancer cells and stroma.21, 22 Identification of the pivotal role that the stroma plays in pancreatic cancer development and progression has led to the development of potential targeted therapies for pancreatic cancer, some of which appear to be promising and exhibit synergistic efficacy in combination with gemcitabine.18 This review focuses on the origination, evolution and disruption of stromal molecular and cellular components in pancreatic cancer, and their biological effects on pancreatic cancer pathogenesis.
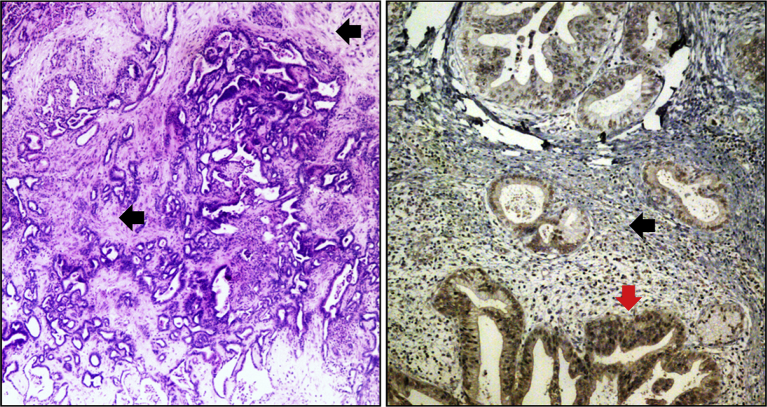
Figure 1.
Pancreatic cancer stroma. Shown are tissue sections of both mouse (left panel) and human (right panel) pancreatic cancer. The mouse pancreatic cancer is from a L-KrasG12D/+; L-p53R172H; pdx1-Cre+ (KPC) mouse (stroma is indicated by black arrows). In human pancreatic cancer, FOXM1 is highly expressed in the invasive lesion (indicated by a red arrow, see Ref. 10 for more information).
Pathogenetic basis of pancreatic cancer
Histopathological studies on pancreatic neoplasms have identified three major precursor lesions, which have the potential to evolve into highly malignant and invasive pancreatic cancer (PDAC): pancreatic intraepithelial neoplasia (PanIN), mucinous cystic neoplasms (MCN), and intraductal papillary mucinous neoplasms (IPMN).23, 24 PanIN is the most common precursor pancreatic lesion.25 It is believed that the precursor lesions evolve step-wisely into invasive pancreatic cancer.17 This PanIN-to-PDAC progression model has been supported by thorough genetic analyses and molecular profiling studies.26, 27
Mutational activation of K-ras is the most notable oncogene identified in pancreatic cancer cells. Although occasionally occurring in normal pancreatic tissue and only about 30% of pancreatic cancer lesions at the earliest stage,28 the frequency of K-ras activation increases as the disease progresses and is found in nearly all pancreatic cancer cases.29 Other major genetic alterations include inactivation of tumor-suppressive genes, e.g., p16/CDKN2A, TP53, and SMAD4. Most recently, a landmark study of sequencing of 23,219 transcripts representing 20,661 protein-coding genes in 24 pancreatic cancer cases has detailed a large number of genetic alterations (an average of 63) and a core set of 12 signaling pathways and processes that have an altered gene expression in 67%–100% of pancreatic cancer cases.30
However, identification and experimental validation of a tremendous number of molecular events and aberrant activated signal transduction pathways have failed to be translated into the clinic as either reliable early detection markers or effective therapies for pancreatic cancer.31, 32, 33, 34, 35, 36, 37, 38, 39 Arguably, both malignant cells and stroma are responsible for the extreme lethality and general therapy resistance of pancreatic cancer, and their highly complex interplays are as important.20, 21, 40 Knowledge of the cancer cell parenchyma and surrounding stroma as well as the molecules and signaling pathways that mediate their interactions will improve our reevaluation and optimization of current therapeutic strategies for pancreatic cancer. Those interactions are further subject to temporal and spatial changes during pancreatic cancer progression. Therefore, it is very difficult to simplify what the functions of any particular molecules and/or cells are in the tumor stroma (Fig. 2).
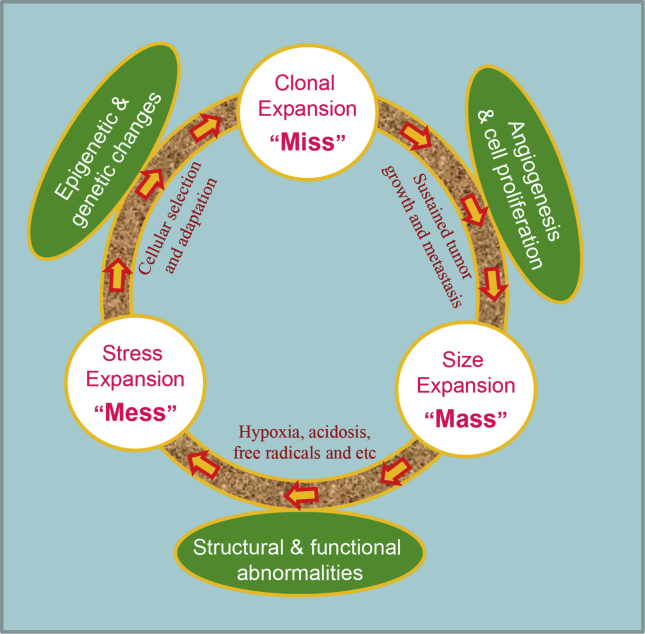
Figure 2.
Pancreatic cancer progression. Pancreatic cancer with distinct desmoplasia differs from normal tissues structurally and functionally, leading to imbalanced oxygen perfusion, radical generation and growth factor production. This extremely chaotic tumor environment (“Mess”) causes the majority of tumor cells to die (“Miss” in action) and only few to survive cellular selection and adaptation through genetic and epigenetic changes. The surviving tumor cells with new genetic and epigenetic makeup proliferate and induce vascular formation, leading to an increased tumor size (“Mass”). The cycle repeats and tumor cells become more malignant, invasive and metastatic. The temporal and spatial changes of tumor cells and their surrounding stroma impose tremendous problems for designing effective therapeutic strategies.
Biology of pancreatic cancer stroma
It is understandable that initiation and progression of malignant cells are not fully determined by the molecular genetic determinants of cancer cells.40 Histologically, a tumor consists of far more than a collection of homogenous cancer cells; it also includes the stroma, the extracellular and cellular tissue framework that surrounds and interacts with cancer cells.40 Our failure to eliminate tumors therapeutically by targeting those events in the clinic is at least in part due to our lack of detailed appreciation of the role of tumor cell microenvironment in tumor pathogenesis and therapy resistance. There are many lines of evidence to support the pivotal role of stroma in pancreatic cancer development and progression.40
Normal pancreatic tissue suppresses pancreatic tumor formation. Physiologically, pancreatic stroma is essential to maintain pancreatic tissue homeostasis, as reflected by reciprocity among the cells in the pancreas and the surrounding microenvironment via communication with each other and the extracellular matrix (ECM) via junctions, receptors, hormones, and other soluble factors.40 Also, normal stroma may protect non-malignant pancreatic cells from developing into malignant cells. For example, pancreatic ductal hyperplasia, commonly considered a precancerous condition or carcinoma in situ, precedes pancreatic carcinoma.41 In fact, autopsy studies have shown that around 30% of cases harbor ductal hyperplasia in pancreatic tissue in those presumably having no malignant pancreatic diseases.41, 42 Although pancreatic ductal hyperplasia possesses genetic alterations, some of which are even more chaotic than those in pancreatic cancer cells, it does not transform into malignancy in most cases. Given the vital impact of the stroma on pancreatic cancer development as well as the similarities between organ development and carcinogenesis, it is reasonable to state that alterations of the stroma are actively involved in pancreatic cancer development and progression.
Several studies have supported the suppressive impact of normal stroma on pancreatic cancer. In a three-dimensional tissue culture system model, co-culture of pancreatic cancer cells with “normal” stromal cells reduced the total number of tumor cells, indicating the protective effects of normal stroma against pancreatic cancer development.43, 44 Similarly, the normal adipose-derived stromal cells inhibited pancreatic cancer cell viability and proliferation in vitro and pancreatic tumor growth in an animal model.45 Therefore, normal stromal cells could be potentially used as cytotoxic agents targeting malignant ductal cells for pancreatic cancer treatment.
Pancreatic inflammation regulates pancreatic carcinogenesis. Chronic pancreatitis is a well-defined disease induced by repetitive acute injury or a self-perpetuating inflammatory process.46, 47, 48, 49 Constant tissue damage in cases of this disease leads to excessive stromal formation and, ultimately, exocrine insufficiency.50 Chronic pancreatitis and pancreatic cancer have the similar property in that they bear large portions of the stroma. Epidemiological studies have provided strong evidence that chronic pancreatitis is a major risk factor for pancreatic cancer.51 In one prospective study, pancreatic cancer incidence was strikingly 27-fold higher in patients with chronic pancreatitis than in disease-free individuals in a common population.52 Patients with topical pancreatitis have a 100-fold increase in risk of pancreatic cancer, and onset of malignant transformation in such patients is approximately 14 years earlier than in patients with sporadic pancreatitis.51, 53 A recent study has further confirmed the link between pancreatic inflammation and pancreatic cancer.54
The pancreatic stroma is relevant in hereditary pancreatic cancer. More than 10% of pancreatic cancer cases are hereditary,11 and most of those cases result from progression from hereditary pancreatitis to chronic pancreatitis to, finally, pancreatic cancer. Previous studies demonstrated that an Arg-His substitution at residue 117 of the cationic trypsinogen gene (PRSS1) was associated with the hereditary pancreatic cancer phenotype. However, despite the presence of mutations of PRSS1 in all 10 trillion human cells of a human body, they only cause hereditary cancer specifically in the pancreas.55 Given the fact that tumors caused by such mutations not only are tissue- and individual-specific but also are formed from just one or a few cells in pancreatic tissue, it is logical to believe that aberrant stroma has a deciding impact on pancreatic carcinogenesis.
Tumor-associated stromal cells promote pancreatic cancer progression. Epidemiological and histological analyses described above strongly support the potential for the pancreatic stroma to promote pancreatic cancer development and progression, and prompt biologists to seek direct evidence of it. Hwang et al first identified and isolated immortalized primary human pancreatic stellate cells (hPSCs) from fresh pancreatic adenocarcinoma samples.56 In vivo studies showed that hPSCs in conditioned medium increased pancreatic tumor cell proliferation, migration, invasion, and colony formation. Furthermore, treatment with hPSCs in conditioned medium rendered pancreatic cancer cells more resistant to gemcitabine and radiation therapy. Co-injection of pancreatic tumor cells and hPSCs in an orthotopic model of pancreatic cancer resulted in increased primary tumor incidence, size, and metastasis, which corresponded with the proportion of hPSCs in the injections.56 Other group confirmed this finding.57 These data indicate that stellate cells play an important role in supporting and promoting multiple aspects of pancreatic cancer (e.g., proliferation, migration, invasion, colony formation, and angiogenesis). However, there are many other studies, which have shown that stromal cells, such as myofibroblasts and immune cells may be more an inhibitory than promoting factor to tumor development and progression.58, 59 Understandably, the impacts of stroma on pancreatic cancer are highly circumstantial, depending on the temporal and spatial existence and functional statuses of those stromal components and the malignant cells themselves.
Origination of pancreatic cancer stroma
The presence of a large amount of stroma in tumor samples is the most prominent histological feature of pancreatic cancer, much more so than in other tumor types. Among the complexity and heterogeneity of pancreatic tumor stromal cells are: mesenchymal, endothelial, and inflammatory/immune cells.40 Presumably, different stromal components restrain or support the growth and metastasis of pancreatic cancer cells, while the origins of those phenotypically diverse stromal cells remain a subject of heated debate.40 Clearly defining the sources of pancreatic stromal cells may help enhance our understanding of stroma functions and improve our current therapeutic strategies for pancreatic cancer.60, 61, 62, 63 There are four major sources of stromal cells: 1) recruitment of pre-existing stromal cells, 2) transdifferentiation from quiescent precursors, 3) generation via epithelial-to-mesenchymal transition, and 4) derivation from cancer stem cells.
Generation of stromal cells via recruitment of pre-existing stromal cells. Morphological similarities between myofibroblasts and pre-existing tissue fibroblasts suggest that myofibroblasts are derived from those fibroblasts. Under culture conditions with specific cytokines and growth factors, such as transforming growth factor (TGF)-β, fibroblasts can be induced to express myofibroblast markers and obtain morphological properties of myofibroblasts.64, 65 In addition to local activation of quiescent stromal cells in cancerous regions, tumor cells are also able to recruit stromal cells to adjacent regions and organize them into tumor vessels.66 Therefore, activating pre-existing stromal cells may be the initial and most efficient method for tumor cells to form an extensive stroma.
Generation of stromal cells via transdifferentiation from mesenchymal stem cells (MSCs). MSCs represent another potential source of pancreatic cancer stromal cells.60, 67 MSCs are heterogeneous connective tissue progenitors found in various locations, such as bone marrow, dermis, and adipose tissue.68 Upon secretion of chemotactic factors by pancreatic cancer cells, MSCs may exhibit innate tropism for those locations, migrate to the cancer stroma, and exert their multipotent capacity to transdifferentiate into osteocytes, adipocytes, chondrocytes, or myocytes.40 Lineage-tracing studies confirmed the function of MSCs as potential sources of cancer stroma.60, 61 Studeny et al first tested the use of MSCs to induce overexpression of cytotoxic agents in certain tumors, and found that after intravenous administration, MSCs were able to integrate and persist in the tumor stroma of pre-established lung cancers and suppress the growth of the tumor cells.61 Similar concepts are confirmed in other tumor models.60, 69, 70 Also, Karnoub et al reported that tumor cells recruit MSCs to tumor xenografts54 and are addicted to the chemokine CCL5 secreted from MSCs for their metastatic spread.71 The ability of MSCs to travel to solid tumors after intravenous administration further supports that activated hPSCs are derived from MSCs.
Generation of stromal cells via epithelial-to-mesenchymal transition. Myofibroblasts are the most abundant stromal cells and are actively involved in the development of pancreatic cancer stroma.64, 72, 73, 74, 75, 76 However, recent discoveries that myofibroblasts can be derived from epithelial cells have provided a new impetus for investigating the processes involved in myofibroblast formation in the fibrotic and malignant contexts.76, 77, 78, 79, 80, 81, 82, 83, 84 Several lines of evidence support that epithelial cells are important sources of myofibroblasts in pancreatic fibrosis and cancer.83 First, epithelium-to-myofibroblast transition can be induced in cultured epithelial cells in a number of organ systems.80, 82 Second, histopathological analysis revealed that stromal cells from different tissues shared many characteristics with epithelial cells derived tissues.81 Third, genetic tests of malignant tissues have shown that isolated mesenchymal cells with myofibroblast characteristics were derived from tumor epithelial cells.76, 85 Finally, in a genetic mouse model of TGF-β-induced pulmonary fibrosis, increases in the number of myofibroblasts largely resulted from transdifferentiation from epithelial cells.82
Generation of stromal cells via transdifferentiation from cancer stem cells. Blood vessels play vital roles in growth and metastasis of cancer cells.86, 87 It is generally accepted that tumor angiogenesis is the formation of new blood vessels from existing ones and new circulating endothelial progenitor cells from bone marrow.88 However, recent evidences suggest that a proportion of the endothelial cells of blood vessels formed in certain tumors are derived from the tumors themselves, having differentiated from stem-like tumor cells.89, 90 These findings raise further questions. Can this concept be generalized to other cancer types, such as pancreatic cancer? Also, what factors are engaged in tumor stem cell transdifferentiation into endothelial cells? Answering these questions could help improve our designing of new therapies for pancreatic cancer.40
Regulation of pancreatic stroma formation
Although the biological impact of pancreatic cancer stroma on tumor cells has been investigated for some time, it is still too early to develop stroma-eliminating agents that indirectly target pancreatic cancer before the molecular mechanisms underlying stromal formation are well understood. Indeed, various autocrine and paracrine loops are engaged in the process of stromal formation.40 Here we describe our current knowledge on and the supportive evidence of the contribution of three signaling pathways—TGF-β, platelet-derived growth factor (PDGF), and Hedgehog (Hh)—to the initiation of pancreatic cancer desmoplastic reactions.
The TGF-β signaling pathway is commonly deregulated in pancreatic cancer cells. Alteration of this pathway has an important function in cancer stroma formation. For example, ligands secreted by tumor cells can activate the TGF-β pathway in the stromal cells in a paracrine manner, leading to downregulation of known antitumor factors and upregulation of protumor factors and resulting in increased ECM deposition.91 Pancreatic cancer cells overexpressing TGF-β1 may also promote fibroblast proliferation.92 Moreover, TGF-β can influence angiogenesis directly or indirectly by stimulating the vascular endothelial growth factor (VEGF) pathway. SMAD4 mutation is the most common genetic event in the TGF-β pathway.30 SMAD4 deficiency combined with activated K-ras mutation accelerated PSCs activation and ECM production,93 whereas restoration of SMAD4 expression suppressed PDAC xenograft tumor growth in part by modulation of ECM turnover.94, 95 Both IL-1 and IL-6 activate PSC in part via modulation of TGF-β1 production,96 and anti-TGF-β1 neutralizing antibody-attenuated α-smooth muscle actin expression induced by IL-1 and IL-6.96 Recent study has indicated that TGF-β regulates desmoplastic responses by carcinoma cells.96, 97 This supports that TGF-β inhibitors have potential as adjuncts to treatment of pancreatic cancer with gemcitabine in that they eliminate stroma-associated chemoresistance. TGF-β-based therapeutic strategies for pancreatic cancer are promising and currently in development, including those with inhibitors of TGFBR1 and TGFBR2.98, 99 LY2157299, a potent TGF-β type I receptor kinase inhibitor that can reverse TGF-β-mediated biological activity, is currently under phase 1–2, study in pancreatic cancer. Researchers are also examining an antisense oligonucleotide agent specific to TGF-β2, named AP 12009, in a phase 1–2, study.100
The PDGF family members are the most extensively investigated regulators of mesenchymal cell proliferation and migration during development.101 They are also highly expressed in tumors and are among the strongest mediators of desmoplastic reactions.102 Upon stimulation, pancreatic cancer cells can secrete PDGF into the surrounding microenvironment and recruit stromal fibroblasts to facilitate tumor cell growth and migration.103 Furthermore, PDGF is a required element in division of fibroblasts and confers a more efficient cell-cycle transition from G1 to S phase in those cells.104 Soluble PDGF receptor (PDGFR)-IgG significantly reduced pancreatic tumor growth by disrupting the paracrine PDGFR signaling among tumor cells and stromal fibroblasts.102 Therefore, PDGF plays an important role in the activation of PSCs and the initiation of pancreatic desmoplastic reactions.102
The role of Hh signaling pathway in pancreatic cancer development remains controversial. The Hh signaling pathway is one of the most fundamental factors in embryonic development and takes part in patterning of numerous tissue structures105 and aberrant Hh signaling is involved in numerous types of cancer,106 including pancreatic cancer.107 Differential expressions of the core components of the pathway are found in normal pancreatic tissues and pancreatic cancers.108 For example, expression of sonic Hh (SHH), a secreted Hh ligand, becomes dysregulated as early as in PanIN lesions, whereas expression of this ligand is completely absent from normal human pancreatic cells.107 However, elucidation of the roles of Hh signaling in pancreatic cancer development is far from complete.107, 108, 109 For instance, a paracrine is required for the Hh pathway in human pancreatic cancer xenograft models and autochthonous murine pancreatic tumor models, in which the Hh ligand is produced by tumor cells and the pathway is activated in the tumor stroma.110, 111 Consistently, Bailey et al reported that expression of SHH increased tumor growth by contributing to the formation of desmoplasia in pancreatic tumors; and mechanistically, SHH increased the differentiation and motility of hPSCs and fibroblasts.112 Conversely, cyclopamine inhibits Hh signaling pathway by binding to and suppressing SMO, resulting in the depletion of tumor-associated stroma and increase of the intratumoral concentration of gemcitabine.113 These data indicate that Hh signaling pathway plays an important role in pancreatic cancer desmoplasia and inhibition of this pathway may constitute a novel therapeutic strategy. Indeed, IPI-926, a semisynthetic derivative of cyclopamine, dramatically depleted stromal components in the pancreas and increased intratumoral vascular density. Also, co-administration of gemcitabine and IPI-926 significantly enhanced the intratumoral concentration of a gemcitabine metabolite, produced transient disease stabilization, and prolonged survival in mice with pancreatic cancer.113 Another study has shown that combination treatment with cyclopamine and an EGFR inhibitor had a better antitumor activity than either drug alone.114 Hh inhibitors are now being tested in treatment of pancreatic cancer in a phase 2 clinical trial.
Clinical significance of stromal markers of pancreatic cancer
Although various treatment modalities have been developed and tested for pancreatic cancer, surgical resection remains the most effective treatment. To that end, great efforts have been made to identify efficient markers for detecting pancreatic cancer before it becomes unresectable. However, early-stage pancreatic cancer could remain a “silent” disease in the clinic and the disease only becomes apparent after the tumor invades surrounding tissues or metastases to distant organs.115 In a retrospective review of patients with pancreatic cancer diagnosed by chance, tumors at the onset of certain subtle symptoms are still resectable.116 However, the symptoms appeared to be too nonspecific and vague and opportunities for early detection are easily missed. Development of efficient detection methods for pancreatic cancer is an urgent need.
Search for curable pancreatic precursor lesions. Pancreatic cancer stromal markers are potentially very significant biomarker for early pancreatic cancer detection and diagnosis. Normal tissue stroma strictly protects cells bearing genetic or epigenetic mutations (initiators) from malignant transformation, while aberrant tissue stroma may act as a tumor promoter indispensable for the carcinogenic process. Because surgical resection generates the best survival benefits in pancreatic cancer patients, current screening efforts are mainly directed at individuals with an inherited predisposition for curable early-stage disease.40 Indeed, screening has identified silent pancreatic neoplasia in many individuals with strong family histories of pancreatic cancer.117, 118 However, such screening based solely on identification of genetic or epigenetic mutations (initiators) will definitely bring with it risk of overtreatment. Defects in current screening modalities for pancreatic cancer highlight the importance of screening for tumor-promoting factors, such as differentially expressed molecules in the pancreatic tumor stromal environment.
Stromal preponderance in sampled pancreatic tissue is used for cancer diagnosis. Because of its ability to detect small preinvasive lesions (∼1 cm in diameter), endoscopic ultrasound is used widely as a screening test for pancreatic cancer.40 Clinical trials demonstrated that endoscopic ultrasound detected more pancreatic cystic lesions (93%) than did magnetic resonance imaging (81%) or computed tomography (27%).119 Focal preinvasive lesions evident on endoscopic sonograms, such as IPMN, are probably most readily sampled using fine-needle aspiration. However, because pancreatic cancer is characterized by pronounced desmoplasia, many pancreatic tumor specimens obtained using fine-needle aspiration come from the stromal compartment, making accurate diagnosis difficult.40 Identification and validation of stroma-related markers of pancreatic cancer will definitely aid enhancing current parenchyma-based diagnostic modalities.
Distinctions between normal stroma in the pancreas and tumor stroma. Arguably, pancreatic stromal samples can have ample traits sufficient to distinguish malignant and normal tissue. Although clinical trials are lacking in this regard of using stromal markers to diagnose pancreatic cancer, oncologists have done much work in searching for candidate stromal markers of pancreatic cancer.40 Based on the finding that interactions among cancer and stromal cells play critical roles in tumor invasion, metastasis, and chemoresistance, it is reasonable to believe that the gene expression profiles of the stromal components in pancreatic cancer differ from those in chronic pancreatitis and reflect the interaction of stroma with tumor cells. In a study of gene expression profiles of pancreatic stromal tissue samples obtained from patients with PDAC and chronic pancreatitis, and pancreatic cell lines of stromal origin, 255 genes were expressed at higher levels and 61 genes were expressed at lower levels in the PDAC samples than in the chronic pancreatitis samples.120 Binkley et al reported similar results.121 Distinct gene expression patterns in tumor and normal stromal samples are reported for other cancer types, such as breast cancer.122 These studies demonstrate the potential application of stromal markers for pancreatic cancer detection.
Development of stroma-targeting therapy
Only about 10% of patients with newly diagnosed pancreatic cancer are candidates for surgical resection, whereas the remaining 90% undergo combination treatment including gemcitabine-based chemotherapy.123, 124, 125 However, large percentage of pancreatic cancers is resistant to gemcitabine, while the underlying mechanisms of resistance are unclear. Evidently, the pancreatic cancer stroma plays critical roles in gemcitabine resistance of pancreatic cancer. In a xenograft pancreatic cancer model with little stromal formation, the intracellular metabolite of gemcitabine is readily detected at a relatively high concentration and it exerts optimal tumor-suppressive effects.113 In contrast, in pancreatic tumors in KPC mice, which are characterized by pronounced desmoplastic reactions, the metabolite is almost undetectable and has little effect on tumors.113 Multiple components in the pancreatic tumor stroma, such as abnormal vasculature and myofibroblasts, are believed to contribute to chemoresistance. The functional redundancy exists among various signaling molecules and pathways that regulate tumor stromal formation and maintenance. Therefore, targeting single molecules would unlikely be effective in control pancreatic cancer (Fig. 3).
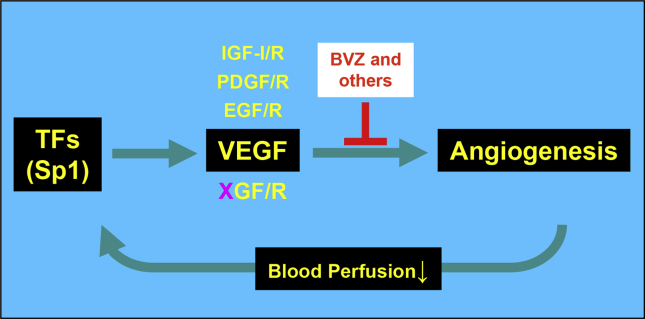
Figure 3.
VEGF inhibitors resistance model. The use of a VEGF neutralizing antibody (“BVZ” or others) decreases tumor angiogenesis of and reduces blood perfusion to tumor tissues. Increased hypoxia and other changes aggravate the stressful microenvironment in the tumor. The tumor cells counteract by inducing the expression of critical transcription factors (“TFs”) like Sp1, which upregulates VEGF expression and renders BVZ resistance. Permanent resistance to BVZ occurs when other functionally redundant factors, e.g., IFG-I/R, PDGF/R, EGF/R and other unknown factors (“XGF/R”), are also unregulated by the increased expression of Sp1, which is initiated by the treatment of the VEGF neutralizing antibody.
Growth factor receptor pathways. Overexpression of epidermal growth factor receptor (EGFR) and its ligands is frequently observed in pancreatic cancer and correlates with poor prognosis and disease progression.126 Erlotinib is an orally active small molecule that binds to the ATP-binding site of EGFR. A phase 3 trial of erlotinib in combination with gemcitabine produced a small but significant increase in survival durations in patients with advanced pancreatic cancer.127 However, the precise mechanisms by which EGFR inhibitors exert their clinical activity remain undefined. That EGFR activation produces both chemoattraction and stimulation of proliferation of hPSCs suggests a potential involvement of stromal regulation in the tumor-suppressive efficacy of EGFR inhibitors.102 However, a recent phase 3 trial of EGFR inhibitors have shown to be ineffective, including the use of the monoclonal antibody cetuximab in patients with late-stage pancreatic cancer. Several other clinical trials examining EGFR tyrosine kinase inhibitors are under way.40 Therefore, the results of clinical trials examining EGFR inhibitors seem to be promising, while the clinical relevance and cost-effectiveness remains debatable.
On the other hand, hepatocyte growth factor (HGF) is also overexpressed in 78% of pancreatic cancer cells.128 Mesenchymal cells normally constitute the major source of HGF, whereas under hypoxic conditions, activated myofibroblasts overproduce HGF and subsequently enhance the malignant phenotypes of pancreatic cancer cells and render them resistant to conventional therapy. Preclinical evaluations suggested that targeting the HGF pathway is of potential value in pancreatic cancer treatment.40 In addition, ARQ 197 is a MET receptor tyrosine kinase inhibitor that is currently being tested in treatment of pancreatic cancer in a phase 2 trial.40
Angiogenesis and extracellular remodeling. Angiogenesis is indispensable to tumor development and progression and is mainly mediated by the VEGF family of proteins and receptors. VEGF is overexpressed in more than 90% of pancreatic cancers, making it an appealing target for therapy.40 Treatment of other tumor types showed that VEGF-targeted therapy has optimal antitumor efficacy.129 However, a phase 3 trial in patients with advanced pancreatic cancer failed to show a survival benefit for bevacizumab, a humanized monoclonal antibody.130 The AVITA (BO17706) phase 3 study testing the antitumor efficacy of the addition of bevacizumab to gemcitabine and erlotinib in patients with metastatic pancreatic cancer had similar results.131 Although there are still clinical trials examining the potential benefits of VEGF inhibitors in combination with other agents, the data currently available do not seem to justify their use for pancreatic cancer.
The large gap between experimental data and clinical reality prompts biologists to explore the underlying mechanisms. Olive et al showed that extensive desmoplastic reactions in pancreatic tumors render blood vessels sparse and functionally abnormal,113 thus imposing a strong barrier to drug delivery. Therefore, an excessive destruction of the vasculature would severely compromise the delivery of oxygen and therapeutics to solid tumors, producing hypoxia that would decrease the effectiveness of many chemotherapeutics. Based on this rationale, a delicate balance between vascular normalization and excessive vascular regression is needed, which may confer substantial benefits to pancreatic cancer patients.8, 40
On the other hand, experimental results indicate that overexpression of matrix metalloproteinases (MMPs) in pancreatic cancer cells plays an important role in tumor cell migration and invasion,132 making MMPs ideal candidate therapeutic targets for preventing the promotion of pancreatic cancer progression. However, clinical trials questioned their effectiveness as potential targets in pancreatic cancer chemotherapy. Marimastat is a broad-spectrum synthetic MMP inhibitor (MMPI) that was first tested in a large randomized phase 3 trial in patients with advanced pancreatic cancer.40 Inconsistent with preclinical studies, marimastat, neither alone nor combined with gemcitabine, improved overall survival durations over that produced by treatment with gemcitabine alone.133, 134 In another phase 3 trial, investigators studied BAY-12-9566, a specific inhibitor of MMP-2, MMP-3, MMP-9, and MMP-13, in patients with locally advanced or metastatic pancreatic cancer. Disappointingly, interim analysis showed that treatment with BAY-12-9566 was not superior to that with gemcitabine but rather undermined its survival benefits.135 Promising preclinical results but contradictory clinical findings have indicated that the roles of MMPs in cancer biology are very complex and far from being elucidated fully. Besides protumorigenic functions, various studies showed that MMPs could act as tumor suppressors in certain contexts.40 The circumstantial functions of MMPs as well as the disappointing clinical trial results cast doubts for future applications of their inhibitors in pancreatic cancer therapy.
Other stromal components. The abundance of pancreatic tumor stroma and its major implications for cancer promotion inspired us to identify stromal markers to help enrich cytotoxic agents in certain tumor compartments. Secreted protein acidic and rich in cysteine (SPARC) is an important component of the pancreatic tumor stroma and has a distinct overexpression pattern.136 Previous studies found that albumin holds some affinity for SPARC and that this property may facilitate intratumoral accumulation of albumin-bound drugs.137 For example, nab-paclitaxel is a 130-nm albumin-bound formulation of paclitaxel particles. In vivo experiments showed that the pancreatic cancer stroma enriched the pattern of nab-paclitaxel distribution, significantly increasing the intratumoral concentration of gemcitabine over that with administration of gemcitabine alone, and partly reversing the innate gemcitabine resistance of pancreatic cancer cells.138 Nab-paclitaxel further stabilized intratumoral gemcitabine levels by promoting oxidative degradation of cytidine deaminase, which is the primary enzyme responsible for gemcitabine metabolism.139, 140, 141, 142 Combination therapy for pancreatic cancer with nab-paclitaxel and gemcitabine is currently under investigation in a late-stage phase 3 clinical trial.
Conclusions and future directions
Massive stromal formation is a histological hallmark of pancreatic cancer, making the pancreatic cancer an outstanding model for exploring the temporal and spatial interplays among cancer cells and the stroma. Increasing experimental and clinical evidence indicate that the pancreatic tumor stroma actively regulates tumor development and progression. Clearly, the stroma consists of highly heterogeneous components, e.g., various cell types, diverse matrix compositions, and complex signaling networks, mediating the initiation, evolution and perpetuation of the desmoplastic reactions. Evidently, it would be naïve to judge the stroma as either a promoter or an inhibitor of carcinogenesis, thus being premature to target the stroma indiscriminationally as therapeutic strategies. However, the presence of a unique gene expression profiles within the stroma suggests that differentially expressed molecules may be used as detecting markers to aid currently inefficient parenchyma-based methods of detection, diagnosis and prognosis. Furthermore, detailed evaluation and validation of a large number of those stroma-related markers would help identify potential therapeutic targets. Systematical studies of the cross-talk between pancreatic cancer cells and the stroma will further our understanding of pancreatic cancer pathogenesis and categorizing molecular pathways for designing more effective preventive and therapeutic modalities.
Conflict of interest
All authors declare no conflict of interest.
Acknowledgements
We thank Dr. Xiangyu Kong for his thoughtful suggestions; and Don Norwood for editorial comments. The work was supported in part by grants R01-CA129956, R01-CA148954, R01CA152309 and R01CA172233 (to K. Xie) from the National Cancer Institute, National Institutes of Health. This research is supported in part by the National Institutes of Health through MD Anderson's Cancer Center Support Grant CA016672.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Lin C.C., Mariotto A.B. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 4.Bednar F., Simeone D.M. Recent advances in pancreatic surgery. Curr Opin Gastroenterol. 2014;30:518–523. doi: 10.1097/MOG.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 5.Karachristos A., Esnaola N.F. Surgical management of pancreatic neoplasms: what's new? Curr Gastroenterol Rep. 2014;16:397. doi: 10.1007/s11894-014-0397-x. [DOI] [PubMed] [Google Scholar]
- 6.Bliss L.A., Witkowski E.R., Yang C.J., Tseng J.F. Outcomes in operative management of pancreatic cancer. J Surg Oncol. 2014;110:592–598. doi: 10.1002/jso.23744. [DOI] [PubMed] [Google Scholar]
- 7.McCarroll J.A., Naim S., Sharbeen G. Role of pancreatic stellate cells in chemoresistance in pancreatic cancer. Front Physiol. 2014;5:141. doi: 10.3389/fphys.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xie K., Wei D., Huang S. Transcriptional anti-angiogenesis therapy of human pancreatic cancer. Cytokine Growth Factor Rev. 2006;17:147–156. doi: 10.1016/j.cytogfr.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Huang C., Xie K. Crosstalk of Sp1 and Stat3 signaling in pancreatic cancer pathogenesis. Cytokine Growth Factor Rev. 2012;23:25–35. doi: 10.1016/j.cytogfr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quan M., Wang P., Cui J., Gao Y., Xie K. The roles of FOXM1 in pancreatic stem cells and carcinogenesis. Mol Cancer. 2013;12:159. doi: 10.1186/1476-4598-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad A., Kowdley G.C., Pawlik T.M., Cunningham S.C. Hereditary pancreatic and hepatobiliary cancers. Int J Surg Oncol. 2011;2011:154673. doi: 10.1155/2011/154673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brentnall T.A. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol. 2005;6:437–445. doi: 10.1007/s11864-005-0046-6. [DOI] [PubMed] [Google Scholar]
- 13.Kimura W., Kuroda A., Makuuchi M. Problems in the diagnosis and treatment of a so-called mucin-producing tumor of the pancreas. Pancreas. 1998;16:363–369. doi: 10.1097/00006676-199804000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Neesse A., Michl P., Frese K.K. Stromal biology and therapy in pancreatic cancer. Gut. 2011;60:861–868. doi: 10.1136/gut.2010.226092. [DOI] [PubMed] [Google Scholar]
- 15.Cowan R.W., Maitra A. Genetic progression of pancreatic cancer. Cancer J. 2014;20:80–84. doi: 10.1097/PPO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 16.Rustgi A.K. Familial pancreatic cancer: genetic advances. Genes Dev. 2014;28:1–7. doi: 10.1101/gad.228452.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood L.D., Hruban R.H. Pathology and molecular genetics of pancreatic neoplasms. Cancer J. 2012;18:492–501. doi: 10.1097/PPO.0b013e31827459b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kindler H.L., Ioka T., Richel D.J. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 19.Stromnes I.M., DelGiorno K.E., Greenberg P.D., Hingorani S.R. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014;35:1451–1460. doi: 10.1093/carcin/bgu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neesse A., Krug S., Gress T.M., Tuveson D.A., Michl P. Emerging concepts in pancreatic cancer medicine: targeting the tumor stroma. Onco Targets Ther. 2013;7:33–43. doi: 10.2147/OTT.S38111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu G.C., Kimmelman A.C., Hezel A.F., DePinho R.A. Stromal biology of pancreatic cancer. J Cell Biochem. 2007;101:887–907. doi: 10.1002/jcb.21209. [DOI] [PubMed] [Google Scholar]
- 22.McCleary-Wheeler A.L., McWilliams R., Fernandez-Zapico M.E. Aberrant signaling pathways in pancreatic cancer: a two compartment view. Mol Carcinog. 2012;51:25–39. doi: 10.1002/mc.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brugge W.R., Lauwers G.Y., Sahani D., Fernandez-del Castillo C., Warshaw A.L. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–1226. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 24.Maitra A., Fukushima N., Takaori K., Hruban R.H. Precursors to invasive pancreatic cancer. Adv Anat Pathol. 2005;12:81–91. doi: 10.1097/01.pap.0000155055.14238.25. [DOI] [PubMed] [Google Scholar]
- 25.Bardeesy N., DePinho R.A. Pancreatic cancer biology and genetics. Nat Rev Cancer. 2002;2:897–909. doi: 10.1038/nrc949. [DOI] [PubMed] [Google Scholar]
- 26.Heinmoller E., Dietmaier W., Zirngibl H. Molecular analysis of microdissected tumors and preneoplastic intraductal lesions in pancreatic carcinoma. Am J Pathol. 2000;157:83–92. doi: 10.1016/S0002-9440(10)64520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamano M., Fujii H., Takagaki T., Kadowaki N., Watanabe H., Shirai T. Genetic progression and divergence in pancreatic carcinoma. Am J Pathol. 2000;156:2123–2133. doi: 10.1016/S0002-9440(10)65083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klimstra D.S., Longnecker D.S. K-ras mutations in pancreatic ductal proliferative lesions. Am J Pathol. 1994;145:1547–1550. [PMC free article] [PubMed] [Google Scholar]
- 29.Rozenblum E., Schutte M., Goggins M. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 30.Jones S., Zhang X., Parsons D.W. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim Y., Han D., Min H., Jin J., Yi E.C., Kim Y. Comparative proteomic profiling of pancreatic ductal adenocarcinoma cell lines. Mol Cells. 2014;37:888–898. doi: 10.14348/molcells.2014.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilarsky C., Grutzmann R. Genomics of pancreatic ductal adenocarcinoma. Hepatobiliary Pancreat Dis Int. 2014;13:381–385. doi: 10.1016/s1499-3872(14)60281-2. [DOI] [PubMed] [Google Scholar]
- 33.Caba O., Prados J., Ortiz R. Transcriptional profiling of peripheral blood in pancreatic adenocarcinoma patients identifies diagnostic biomarkers. Dig Dis Sci. 2014;59:2714–2720. doi: 10.1007/s10620-014-3291-3. [DOI] [PubMed] [Google Scholar]
- 34.Lili L.N., Matyunina L.V., Walker L.D., Daneker G.W., McDonald J.F. Evidence for the importance of personalized molecular profiling in pancreatic cancer. Pancreas. 2014;43:198–211. doi: 10.1097/MPA.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shain A.H., Salari K., Giacomini C.P., Pollack J.R. Integrative genomic and functional profiling of the pancreatic cancer genome. BMC Genomics. 2013;14:624. doi: 10.1186/1471-2164-14-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang G., He P., Tan H. Integration of metabolomics and transcriptomics revealed a fatty acid network exerting growth inhibitory effects in human pancreatic cancer. Clin Cancer Res. 2013;19:4983–4993. doi: 10.1158/1078-0432.CCR-13-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J., Wu W., Chen L. Profiling the potential tumor markers of pancreatic ductal adenocarcinoma using 2D-DIGE and MALDI-TOF-MS: up-regulation of complement C3 and alpha-2-HS-glycoprotein. Pancreatology. 2013;13:290–297. doi: 10.1016/j.pan.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 38.Bond T. Identification of chemosensitive pancreatic cancer with gene profiling. Biomark Med. 2013;7:200. [PubMed] [Google Scholar]
- 39.Brandi J., Dando I., Palmieri M., Donadelli M., Cecconi D. Comparative proteomic and phosphoproteomic profiling of pancreatic adenocarcinoma cells treated with CB1 or CB2 agonists. Electrophoresis. 2013;34:1359–1368. doi: 10.1002/elps.201200402. [DOI] [PubMed] [Google Scholar]
- 40.Kong X., Li L., Li Z., Xie K. Targeted destruction of the orchestration of the pancreatic stroma and tumor cells in pancreatic cancer cases: molecular basis for therapeutic implications. Cytokine Growth Factor Rev. 2012;23:343–356. doi: 10.1016/j.cytogfr.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birnstingl M. A study of pancreatography. Br J Surg. 1959;47:128–139. doi: 10.1002/bjs.18004720203. [DOI] [PubMed] [Google Scholar]
- 42.Kozuka S., Sassa R., Taki T. Relation of pancreatic duct hyperplasia to carcinoma. Cancer. 1979;43:1418–1428. doi: 10.1002/1097-0142(197904)43:4<1418::aid-cncr2820430431>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 43.Petersen O.W., Ronnov-Jessen L., Howlett A.R., Bissell M.J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci USA. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Froeling F.E., Mirza T.A., Feakins R.M. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, beta-catenin, and Ezrin expression in tumor cells. Am J Pathol. 2009;175:636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cousin B., Ravet E., Poglio S. Adult stromal cells derived from human adipose tissue provoke pancreatic cancer cell death both in vitro and in vivo. PLoS One. 2009;4:e6278. doi: 10.1371/journal.pone.0006278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupte A.R., Forsmark C.E. Chronic pancreatitis. Curr Opin Gastroenterol. 2014;30:500–505. doi: 10.1097/MOG.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 47.Hyun J.J., Lee H.S. Experimental models of pancreatitis. Clin Endosc. 2014;47:212–216. doi: 10.5946/ce.2014.47.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Issa Y., Bruno M.J., Bakker O.J. Treatment options for chronic pancreatitis. Nat Rev Gastroenterol Hepatol. 2014;11:556–564. doi: 10.1038/nrgastro.2014.74. [DOI] [PubMed] [Google Scholar]
- 49.Pinho A.V., Chantrill L., Rooman I. Chronic pancreatitis: a path to pancreatic cancer. Cancer Lett. 2014;345:203–209. doi: 10.1016/j.canlet.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Kloppel G. Chronic pancreatitis, pseudotumors and other tumor-like lesions. Mod Pathol. 2007;20:113–131. doi: 10.1038/modpathol.3800690. [DOI] [PubMed] [Google Scholar]
- 51.Whitcomb D.C. Inflammation and Cancer V. Chronic pancreatitis and pancreatic cancer. Am J Physiol Gastrointest Liver Physiol. 2004;287:G315–G319. doi: 10.1152/ajpgi.00115.2004. [DOI] [PubMed] [Google Scholar]
- 52.Malka D., Hammel P., Maire F. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Whitcomb D.C., Pogue-Geile K. Pancreatitis as a risk for pancreatic cancer. Gastroenterol Clin North Am. 2002;31:663–678. doi: 10.1016/s0889-8553(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 54.Rhim A.D., Mirek E.T., Aiello N.M. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Whitcomb D.C., Gorry M.C., Preston R.A. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 56.Hwang R.F., Moore T., Arumugam T. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68:918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu Z., Vonlaufen A., Phillips P.A. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am J Pathol. 2010;177:2585–2596. doi: 10.2353/ajpath.2010.090899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Z., Jia Z., Gao Y. Activation of Vitamin D receptor signaling downregulates the expression of nuclear FOXM1 protein and suppresses pancreatic cancer cell stemesss. Clin Cancer Res. 2015;21(4):844–853. doi: 10.1158/1078-0432.CCR-14-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Özdemir B.C., Pentcheva-Hoang T., Carstens J.L. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Studeny M., Marini F.C., Dembinski J.L. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst. 2004;96:1593–1603. doi: 10.1093/jnci/djh299. [DOI] [PubMed] [Google Scholar]
- 61.Studeny M., Marini F.C., Champlin R.E., Zompetta C., Fidler I.J., Andreeff M. Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res. 2002;62:3603–3608. [PubMed] [Google Scholar]
- 62.Kallifatidis G., Beckermann B.M., Groth A. Improved lentiviral transduction of human mesenchymal stem cells for therapeutic intervention in pancreatic cancer. Cancer Gene Ther. 2008;15:231–240. doi: 10.1038/sj.cgt.7701097. [DOI] [PubMed] [Google Scholar]
- 63.Ghaedi M., Soleimani M., Taghvaie N.M. Mesenchymal stem cells as vehicles for targeted delivery of anti-angiogenic protein to solid tumors. J Gene Med. 2011;13:171–180. doi: 10.1002/jgm.1552. [DOI] [PubMed] [Google Scholar]
- 64.Ronnov-Jessen L., Petersen O.W., Koteliansky V.E., Bissell M.J. The origin of the myofibroblasts in breast cancer. Recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J Clin Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tomasek J.J., Gabbiani G., Hinz B., Chaponnier C., Brown R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 66.Udagawa T., Puder M., Wood M., Schaefer B.C., D'Amato R.J. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20:95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- 67.Ogawa M., LaRue A.C., Drake C.J. Hematopoietic origin of fibroblasts/myofibroblasts: Its pathophysiologic implications. Blood. 2006;108:2893–2896. doi: 10.1182/blood-2006-04-016600. [DOI] [PubMed] [Google Scholar]
- 68.Spaeth E.L., Dembinski J.L., Sasser A.K. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. doi: 10.1371/journal.pone.0004992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakamizo A., Marini F., Amano T. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005;65:3307–3318. doi: 10.1158/0008-5472.CAN-04-1874. [DOI] [PubMed] [Google Scholar]
- 70.Hung S.C., Deng W.P., Yang W.K. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11:7749–7756. doi: 10.1158/1078-0432.CCR-05-0876. [DOI] [PubMed] [Google Scholar]
- 71.Karnoub A.E., Dash A.B., Vo A.P. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 72.Faouzi S., Le Bail B., Neaud V. Myofibroblasts are responsible for collagen synthesis in the stroma of human hepatocellular carcinoma: an in vivo and in vitro study. J Hepatol. 1999;30:275–284. doi: 10.1016/s0168-8278(99)80074-9. [DOI] [PubMed] [Google Scholar]
- 73.Powell D.W., Mifflin R.C., Valentich J.D., Crowe S.E., Saada J.I., West A.B. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277:C1–C9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- 74.Phan S.H. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 75.Desmouliere A., Darby I.A., Gabbiani G. Normal and pathologic soft tissue remodeling: role of the myofibroblast, with special emphasis on liver and kidney fibrosis. Lab Invest. 2003;83:1689–1707. doi: 10.1097/01.lab.0000101911.53973.90. [DOI] [PubMed] [Google Scholar]
- 76.Petersen O.W., Nielsen H.L., Gudjonsson T. Epithelial to mesenchymal transition in human breast cancer can provide a nonmalignant stroma. Am J Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee E.H., Joo C.K. Role of transforming growth factor-beta in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci. 1999;40:2025–2032. [PubMed] [Google Scholar]
- 78.Oldfield M.D., Bach L.A., Forbes J.M. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li J.H., Wang W., Huang X.R. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nightingale J., Patel S., Suzuki N. Oncostatin M, a cytokine released by activated mononuclear cells, induces epithelial cell-myofibroblast transdifferentiation via Jak/Stat pathway activation. J Am Soc Nephrol. 2004;15:21–32. doi: 10.1097/01.asn.0000102479.92582.43. [DOI] [PubMed] [Google Scholar]
- 81.Willis B.C., Liebler J.M., Luby-Phelps K. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol. 2005;166:1321–1332. doi: 10.1016/s0002-9440(10)62351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim K.K., Kugler M.C., Wolters P.J. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci USA. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Selman M., Pardo A. Role of epithelial cells in idiopathic pulmonary fibrosis: from innocent targets to serial killers. Proc Am Thorac Soc. 2006;3:364–372. doi: 10.1513/pats.200601-003TK. [DOI] [PubMed] [Google Scholar]
- 84.Gudjonsson T., Adriance M.C., Sternlicht M.D., Petersen O.W., Bissell M.J. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–272. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moinfar F., Man Y.G., Arnould L., Bratthauer G.L., Ratschek M., Tavassoli F.A. Concurrent and independent genetic alterations in the stromal and epithelial cells of mammary carcinoma: implications for tumorigenesis. Cancer Res. 2000;60:2562–2566. [PubMed] [Google Scholar]
- 86.Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–18. doi: 10.1053/sonc.2002.37263. [DOI] [PubMed] [Google Scholar]
- 87.Folkman J. Fundamental concepts of the angiogenic process. Curr Mol Med. 2003;3:643–651. doi: 10.2174/1566524033479465. [DOI] [PubMed] [Google Scholar]
- 88.Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- 89.Wang R., Chadalavada K., Wilshire J. Glioblastoma stem-like cells give rise to tumour endothelium. Nature. 2010;468:829–833. doi: 10.1038/nature09624. [DOI] [PubMed] [Google Scholar]
- 90.Ricci-Vitiani L., Pallini R., Biffoni M. Tumour vascularization via endothelial differentiation of glioblastoma stem-like cells. Nature. 2010;468:824–828. doi: 10.1038/nature09557. [DOI] [PubMed] [Google Scholar]
- 91.Vogelmann R., Ruf D., Wagner M., Adler G., Menke A. Effects of fibrogenic mediators on the development of pancreatic fibrosis in a TGF-beta1 transgenic mouse model. Am J Physiol Gastrointest Liver Physiol. 2001;280:G164–G172. doi: 10.1152/ajpgi.2001.280.1.G164. [DOI] [PubMed] [Google Scholar]
- 92.Lohr M., Schmidt C., Ringel J. Transforming growth factor-beta1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 2001;61:550–555. [PubMed] [Google Scholar]
- 93.Bardeesy N., Cheng K.H., Berger J.H. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duda D.G., Sunamura M., Lefter L.P. Restoration of SMAD4 by gene therapy reverses the invasive phenotype in pancreatic adenocarcinoma cells. Oncogene. 2003;22:6857–6864. doi: 10.1038/sj.onc.1206751. [DOI] [PubMed] [Google Scholar]
- 95.Schwarte-Waldhoff I., Volpert O.V., Bouck N.P. Smad4/DPC4-mediated tumor suppression through suppression of angiogenesis. Proc Natl Acad Sci USA. 2000;97:9624–9629. doi: 10.1073/pnas.97.17.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aoki H., Ohnishi H., Hama K. Cyclooxygenase-2 is required for activated pancreatic stellate cells to respond to proinflammatory cytokines. Am J Physiol Cell Physiol. 2007;292:C259–C268. doi: 10.1152/ajpcell.00030.2006. [DOI] [PubMed] [Google Scholar]
- 97.Levy L., Hill C.S. Smad4 dependency defines two classes of transforming growth factor β (TGF-β) target genes and distinguishes TGF-β-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Melisi D., Ishiyama S., Sclabas G.M. LY2109761, a novel transforming growth factor beta receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther. 2008;7:829–840. doi: 10.1158/1535-7163.MCT-07-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Medicherla S., Li L., Ma J.Y. Antitumor activity of TGF-beta inhibitor is dependent on the microenvironment. Anticancer Res. 2007;27:4149–4157. [PubMed] [Google Scholar]
- 100.Schlingensiepen K.H., Schlingensiepen R., Steinbrecher A. Targeted tumor therapy with the TGF-beta 2 antisense compound AP 12009. Cytokine Growth Factor Rev. 2006;17:129–139. doi: 10.1016/j.cytogfr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 101.Hoch R.V., Soriano P. Roles of PDGF in animal development. Development. 2003;130:4769–4784. doi: 10.1242/dev.00721. [DOI] [PubMed] [Google Scholar]
- 102.Blaine S.A., Ray K.C., Branch K.M., Robinson P.S., Whitehead R.H., Means A.L. Epidermal growth factor receptor regulates pancreatic fibrosis. Am J Physiol Gastrointest Liver Physiol. 2009;297:G434–G441. doi: 10.1152/ajpgi.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong J., Grunstein J., Tejada M. VEGF-null cells require PDGFR alpha signaling-mediated stromal fibroblast recruitment for tumorigenesis. EMBO J. 2004;23:2800–2810. doi: 10.1038/sj.emboj.7600289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yu J., Liu X.W., Kim H.R. Platelet-derived growth factor (PDGF) receptor-alpha-activated c-Jun NH2-terminal kinase-1 is critical for PDGF-induced p21WAF1/CIP1 promoter activity independent of p53. J Biol Chem. 2003;278:49582–49588. doi: 10.1074/jbc.M309986200. [DOI] [PubMed] [Google Scholar]
- 105.Xie K., Abbruzzese J.L. Developmental biology informs cancer: the emerging role of the hedgehog signaling pathway in upper gastrointestinal cancers. Cancer Cell. 2003;4:245–247. doi: 10.1016/s1535-6108(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 106.Teglund S., Toftgard R. Hedgehog beyond medulloblastoma and basal cell carcinoma. Biochim Biophys Acta. 2010;1805:181–208. doi: 10.1016/j.bbcan.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 107.Thayer S.P., di Magliano M.P., Heiser P.W. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berman D.M., Karhadkar S.S., Maitra A. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 109.Feldmann G., Dhara S., Fendrich V. Blockade of hedgehog signaling inhibits pancreatic cancer invasion and metastases: a new paradigm for combination therapy in solid cancers. Cancer Res. 2007;67:2187–2196. doi: 10.1158/0008-5472.CAN-06-3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yauch R.L., Gould S.E., Scales S.J. A paracrine requirement for hedgehog signalling in cancer. Nature. 2008;455:406–410. doi: 10.1038/nature07275. [DOI] [PubMed] [Google Scholar]
- 111.Tian H., Callahan C.A., DuPree K.J. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bailey J.M., Swanson B.J., Hamada T. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995–6004. doi: 10.1158/1078-0432.CCR-08-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olive K.P., Jacobetz M.A., Davidson C.J. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu W.G., Liu T., Xiong J.X., Wang C.Y. Blockade of sonic hedgehog signal pathway enhances antiproliferative effect of EGFR inhibitor in pancreatic cancer cells. Acta Pharmacol Sin. 2007;28:1224–1230. doi: 10.1111/j.1745-7254.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 115.Kelsen D.P., Portenoy R., Thaler H., Tao Y., Brennan M. Pain as a predictor of outcome in patients with operable pancreatic carcinoma. Surgery. 1997;122:53–59. doi: 10.1016/s0039-6060(97)90264-6. [DOI] [PubMed] [Google Scholar]
- 116.Pelaez-Luna M., Takahashi N., Fletcher J.G., Chari S.T. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 117.Szafranska A.E., Doleshal M., Edmunds H.S. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–1724. doi: 10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li A., Omura N., Hong S.M. Pancreatic cancers epigenetically silence SIP1 and hypomethylate and overexpress miR-200a/200b in association with elevated circulating miR-200a and miR-200b levels. Cancer Res. 2010;70:5226–5237. doi: 10.1158/0008-5472.CAN-09-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vincent A., Herman J., Schulick R., Hruban R.H., Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pilarsky C., Ammerpohl O., Sipos B. Activation of Wnt signalling in stroma from pancreatic cancer identified by gene expression profiling. J Cell Mol Med. 2008;12:2823–2835. doi: 10.1111/j.1582-4934.2008.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Binkley C.E., Zhang L., Greenson J.K. The molecular basis of pancreatic fibrosis: common stromal gene expression in chronic pancreatitis and pancreatic adenocarcinoma. Pancreas. 2004;29:254–263. doi: 10.1097/00006676-200411000-00003. [DOI] [PubMed] [Google Scholar]
- 122.Allinen M., Beroukhim R., Cai L. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 123.Sultana A., Tudur Smith C., Cunningham D. Systematic review, including meta-analyses, on the management of locally advanced pancreatic cancer using radiation/combined modality therapy. Br J Cancer. 2007;96:1183–1190. doi: 10.1038/sj.bjc.6603719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huguet F., Andre T., Hammel P. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–331. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 125.Burris H.A., 3rd, Moore M.J., Andersen J. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 126.Ueda S., Ogata S., Tsuda H. The correlation between cytoplasmic overexpression of epidermal growth factor receptor and tumor aggressiveness: poor prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2004;29:e1–e8. doi: 10.1097/00006676-200407000-00061. [DOI] [PubMed] [Google Scholar]
- 127.Moore M.J., Goldstein D., Hamm J. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 128.Furukawa T., Duguid W.P., Kobari M., Matsuno S., Tsao M.S. Hepatocyte growth factor and Met receptor expression in human pancreatic carcinogenesis. Am J Pathol. 1995;147:889–895. [PMC free article] [PubMed] [Google Scholar]
- 129.Hurwitz H., Fehrenbacher L., Novotny W. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 130.Kindler H.L., Niedzwiecki D., Hollis D. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303) J Clin Oncol. 2010;28:3617–3622. doi: 10.1200/JCO.2010.28.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Van Cutsem E., Vervenne W.L., Bennouna J. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 132.Bramhall S.R., Neoptolemos J.P., Stamp G.W., Lemoine N.R. Imbalance of expression of matrix metalloproteinases (MMPs) and tissue inhibitors of the matrix metalloproteinases (TIMPs) in human pancreatic carcinoma. J Pathol. 1997;182:347–355. doi: 10.1002/(SICI)1096-9896(199707)182:3<347::AID-PATH848>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 133.Bramhall S.R., Rosemurgy A., Brown P.D., Bowry C., Buckels J.A. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447–3455. doi: 10.1200/JCO.2001.19.15.3447. [DOI] [PubMed] [Google Scholar]
- 134.Bramhall S.R., Schulz J., Nemunaitis J., Brown P.D., Baillet M., Buckels J.A. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161–167. doi: 10.1038/sj.bjc.6600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Moore M.J., Hamm J., Dancey J. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296–3302. doi: 10.1200/JCO.2003.02.098. [DOI] [PubMed] [Google Scholar]
- 136.Infante J.R., Matsubayashi H., Sato N. Peritumoral fibroblast SPARC expression and patient outcome with resectable pancreatic adenocarcinoma. J Clin Oncol. 2007;25:319–325. doi: 10.1200/JCO.2006.07.8824. [DOI] [PubMed] [Google Scholar]
- 137.Nyman D.W., Campbell K.J., Hersh E. Phase I and pharmacokinetics trial of ABI-007, a novel nanoparticle formulation of paclitaxel in patients with advanced nonhematologic malignancies. J Clin Oncol. 2005;23:7785–7793. doi: 10.1200/JCO.2004.00.6148. [DOI] [PubMed] [Google Scholar]
- 138.Von Hoff D.D., Ramanathan R.K., Borad M.J. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J Clin Oncol. 2011;29:4548–4554. doi: 10.1200/JCO.2011.36.5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borazanci E., Von Hoff D.D. Nab-paclitaxel and gemcitabine for the treatment of patients with metastatic pancreatic cancer. Expert Rev Gastroenterol Hepatol. 2014;8:739–747. doi: 10.1586/17474124.2014.925799. [DOI] [PubMed] [Google Scholar]
- 140.Porter C.E., Waddell J.A., Solimando D.A., Jr. Nab-paclitaxel plus gemcitabine regimen for pancreatic Cancer. Hosp Pharm. 2014;49:18–22. doi: 10.1310/hpj4901-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Von Hoff D.D., Ervin T., Arena F.P. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Frese K.K., Neesse A., Cook N. nab-Paclitaxel potentiates gemcitabine activity by reducing cytidine deaminase levels in a mouse model of pancreatic cancer. Cancer Discov. 2012;2:260–269. doi: 10.1158/2159-8290.CD-11-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]