Abstract
During critical periods of development, the brain easily changes in response to environmental stimuli, but this neural plasticity declines by adulthood. By acutely disrupting paired immunoglobulin-like receptor B(PirB) function at specific ages, we show that PirB actively represses neural plasticity throughout life. We disrupted PirB function either by genetically introducing a conditional PirB allele into mice or by minipump infusion of a soluble PirB ectodomain (sPirB) into mouse visual cortex. We found that neural plasticity, as measured by depriving mice of vision in one eye and testing ocular dominance, was enhanced by this treatment both during the critical period and when PirB function was disrupted in adulthood. Acute blockade of PirB triggered the formation of new functional synapses, as indicated by increases in miniature excitatory postsynaptic current (mEPSC) frequency and spine density on dendrites of layer 5 pyramidal neurons. In addition, recovery from amblyopia— the decline in visual acuity and spine density resulting from long-term monocular deprivation— was possible after a 1-week infusion of sPirB after the deprivation period. Thus, neural plasticity in adult visual cortex is actively repressed and can be enhanced by blocking PirB function.
INTRODUCTION
During postnatal development, the capacity of the brain to undergo experience-dependent changes in synaptic strength and circuit connectivity is dynamically regulated, with plasticity peaking during developmental critical periods and then decreasing with maturation (1–3). Critical periods are key times when sensory experience is necessary for normal circuit development and when abnormal experience can generate enduring anomalies in brain structure and function (2,4). Ocular dominance (OD) plasticity is a graphic example of experience-driven synaptic and circuit plasticity. Children born with congenital cataract in one eye will suffer amblyopia—a loss of visual acuity—if not corrected early in life (5, 6). Monocular visual deprivation (MD) has been used in animal models of amblyopia to understand underlying mechanisms. After a brief period of MD or enucleation (ME) during juvenile life, visually driven responses of neurons in the binocular zone of mammalian primary visual cortex (V1) shift toward the open eye, and cortical territory containing neurons responding to open-eye stimulation expands, where as closed-eye responses weaken and territory shrinks (3,7,8). These effects are maximal around postnatal day 28 (P28) in mice and decrease thereafter; by adulthood, little OD plasticity resulting from eye closure can be detected, particularly with shorter periods of deprivation (7–11). Furthermore, a long-term period of MD (LTMD) spanning the entire critical period (for example, P19 to P47) generates an enduring loss of acuity and cortical function in the deprived eye—even if binocular vision is restored in adulthood (4, 12, 13). This normal decrease in plasticity by adulthood, although important for stabilizing neural circuits, acts as a barrier to recovery after injury because it limits cortical reorganization, can lock in effects of dysfunctional development, and even opposes acquisition of new learning. If adult neural circuits could be returned to an immature state, critical periods might be effectively reopened, facilitating recovery after nervous system damage, leading to new treatments for neurodegenerative or developmental disorders, or even enhancing learning in healthy individuals.
A limited number of candidate molecules that appear to act as endogenous negative regulators of cortical plasticity have been identified (14–18). One such molecule, paired immunoglobulin-like receptor B(PirB), is expressed in cortical and hippocampal neurons as well as in some immune cells (14). In the nervous system, PirB binds several ligands, including major histocompatibility complex (MHC) class I proteins, NogoA, and myelin components (19,20). Both in immune cells and neurons, ligand binding recruits SHP-1 and SHP-2 phosphatases (14, 21). SHP recruitment requires PirB phosphorylation on its ITIM (immunoreceptor tyrosine-based inhibitory motif) domains (21, 22). In neurons, cofilin is also recruited to PirB, leading to changes in the actin cytoskeleton (20).
Germline PirB−/− mice have enhanced OD plasticity not only during the critical period but also beyond, and they recover more rapidly in a stroke model (14, 23, 24). PirB and its human ortholog, leukocyte immunoglobulin-like receptor, subfamily B, member 2 (LilrB2), bind soluble b amyloid oligomers, and germline PirB deletion rescues OD plasticity and hippocampal deficits in a mouse model of Alzheimer’s disease (AD) (20). However, it remains unknown whether the enhanced OD plasticity and stroke recovery in germline PirB−/− mice are due to early developmental changes, or whether PirB acts at all ages to limit plasticity, which would make it an attractive therapeutic target for drug development. Because PirB is a receptor, signaling can be modulated by conditional genetic knockout or by interfering with ligand binding (19). If PirB functions throughout life, disrupting PirB should enhance plasticity or facilitate recovery at any age.
RESULTS
Genetic deletion of PirB enhances OD plasticity
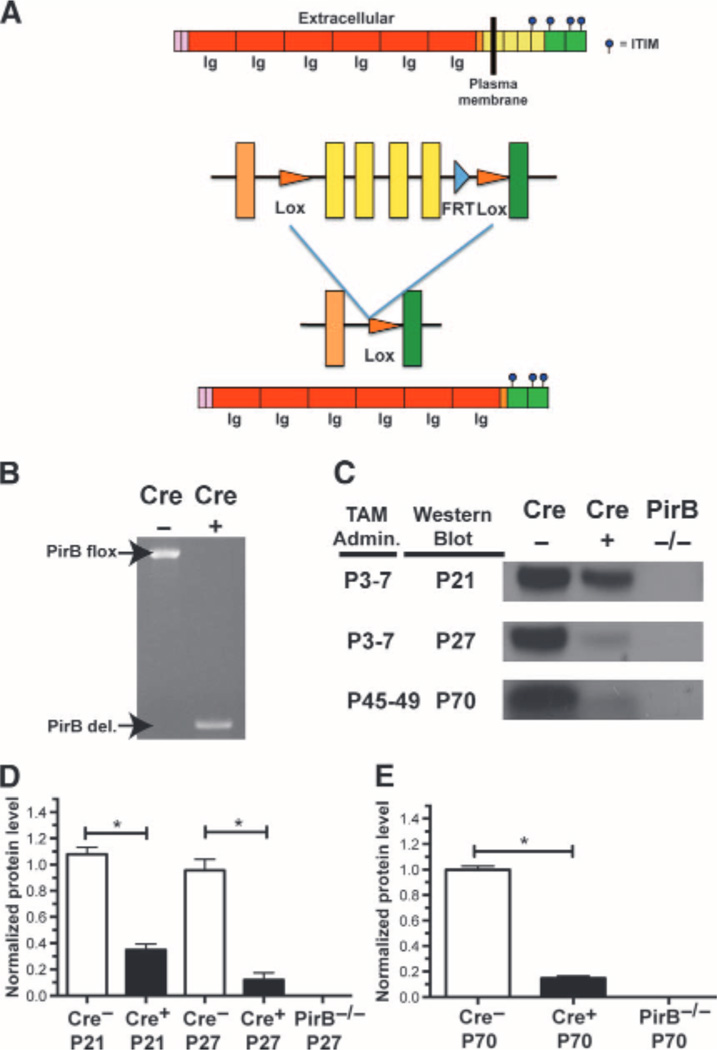
To disrupt PirB function with temporal control, a conditional allele of PirB was generated by inserting loxP sites surrounding exons 10 to 13, which contain the transmembrane domain and first ITIM domain of PirB (14) (Fig. 1A). To obtain robust widespread deletion, this PirB flox mouse line was crossed with a transgenic mouse line expressing tamoxifen-inducible CreERT2 on a ubiquitin C promoter (25). The resulting Ubc-CreERT2; PirB flox/flox mice were bred with PirB flox/flox mice, producing experimental Ubc-CreERT2; PirB flox/flox animals (henceforth called Cre+) as well as PirB flox/flox (Cre−) littermate controls. Tamoxifen injections given either neonatally or after critical period closure induced robust deletion of the floxed allele from genomic DNA within 1 week (Fig. 1B). PirB protein loss was more gradual; for example, daily tamoxifen treatment from P3 to P7 diminished PirB protein in the forebrain by ~90% by P27 (Fig. 1, C and D). A similar gradual loss of protein was seen at P70 after tamoxifen treatment from P45 to P49 (Fig. 1, C and E). Thus, tamoxifen administration substantially reduced PirB protein levels by the peak of the OD critical period at P28 (7), as well as in adulthood by P70.
Fig. 1.
A Tamoxifen-inducible Cre-dependent strategy for deletion of PirB with temporal control. (A) Schematic of PirB protein structure (top) and floxed PirB allele (bottom) before and after Cre-mediated excision. (B) Daily tamoxifen given via injection of nursing mother (P3 to P7) induces deletion of the floxed region at P21 as detected by polymerase chain reaction (PCR). (C) Western blots for PirB protein in forebrain at ages (left) of tamoxifen (TAM) administration and Western blotting. (D) Quantification of PirB protein in forebrain after tamoxifen administration (P3 to P7), normalized to average Cre− levels across all ages assayed: Cre− P21: n = 4 mice versus Cre+ P21: n = 5, P = 0.02, U test. Cre− P27: n = 5 versus Cre+ P27: n = 4, P = 0.02, U test. (E) Quantification of PirB protein in forebrain at P70 (adult) after tamoxifen injection from P45 to P49. Cre− P70: n = 4 versus Cre+ P70: n = 4, P = 0.03, U test. *P < 0.05.
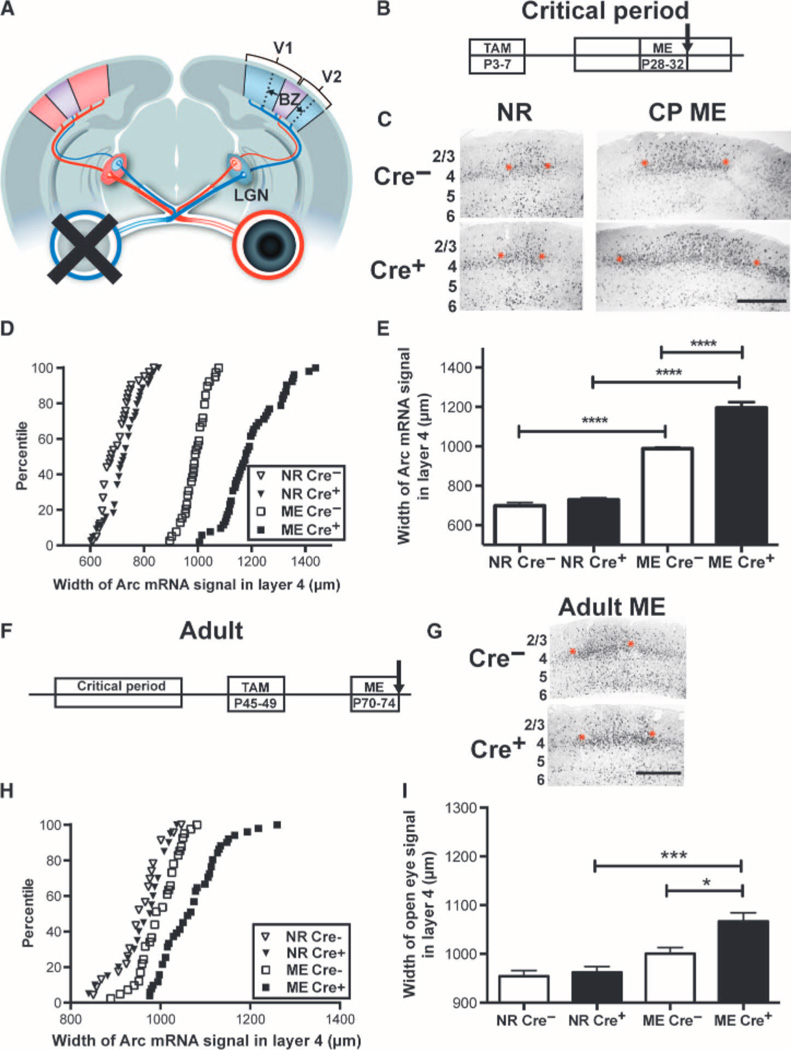
To assess whether OD plasticity during the critical period is increased by acute postnatal removal of PirB, mice received ME from P28 to P32. At P32, we used the method of Arc mRNA induction to asses show much the functional representation of the spared eye had expanded within visual cortex (8). Arcis an immediate early gene induced within minutes of visual stimulation, and the up-regulated mRNA can be detected in cortical neurons functionally driven by the stimulated eye (26). We measured the horizontal extent of the Arc mRNA in situ hybridization signal along L4 of visual cortex ipsilateral to the spared, stimulated eye (Fig.2, A to C, and fig. S1). This expansion in width of Arc mRNA signal is a reliable measure of open-eye strengthening after visual deprivation and correlates well with other methods used to assess OD plasticity including single unit electrophysiology (7, 8, 27, 28), visual evoked potentials (VEPs) (8, 11, 29), or intrinsic signal imaging (8, 24, 30–33). The width of Arc mRNA induction does not expand in transgenic mice known to lack OD plasticity as measured by other methods (20, 28, 33), whereas there is an increase in width of Arc mRNA signal in mice known to have increased OD plasticity (14, 15, 24).
Fig. 2.
Timed genetic deletion of PirB enhances OD plasticity. (A) Schematic of mouse visual system. Each retina (right: red, left: blue) projects primarily contralaterally to the lateral geniculate nucleus (LGN), which projects to visual cortex (V1). A small binocular zone (BZ, purple) in V1 receives input from both eyes; in response to deprivation of one eye (for example, left), the representation of the open (right; ipsilateral) eye expands (arrows). (B) Timeline of inducible knockout of PirB and assessment of OD plasticity via Arc mRNA induction. (C) Example micrographs of in situ hybridizations for Arc mRNA induced in BZ of visual cortex after open-eye stimulation. Each black dot is a cell. ME from P28 to P32 results in expansion of the ipsilateral (open) eye representation (between red asterisks), as compared with normal rearing (NR). Cre− = PirB flox/flox. Cre+ = UbC-CreERT2; PirB flox/flox. Width of Arc signal in L4 was measured (see fig. S1). Cortical layers indicated at left; scale bar, 500 mm. CP, critical period. (D) Cumulative histograms of width of Arc mRNA signal by individual section. NR Cre−: n = 41 sections; NRCre+: n = 44; MECre−: n = 39; MECre+: n = 52. (E) Graph of data in (D), with mean and SEM by animal: deletion of PirB during the critical period enhances OD plasticity. NRCre−: n = 7 mice versus NRCre+: n = 7, P = 0.65; MECre−: n = 7 versus MECre+: n = 7, **** indicates P < 0.0001, two-way ANOVA with Tukey post hoc test. (F) Timeline of inducible deletion of PirB and assessment of OD plasticity in adults. (G) Example Arc mRNA in situ hybridization micrographs at P74, as in (C). (H) Cumulative histograms of width of Arc mRNA induction from individual sections. NRCre−: n = 23 sections; NRCre+: n = 20; MECre−: n = 41; MECre+: n = 51. (I) Graph of data shown in (G) (mean and SEM by animal): deletion of PirB in adulthood enhances OD plasticity. NRCre−: n = 5 mice versus NRCre+: n = 5, P = 0.99; MECre−: n = 7 versus MECre+: n = 8, P = 0.013; MECre− versus NRCre−: P = 0.18; MECre+ versus NRCre+: P = 0.0004, by two-way ANOVA with Tukey post test. *P < 0.05, ***P < 0.001.
As expected during the critical period, 4 days of ME in either Cre+ or Cre− mice caused substantial expansion in width of Arc mRNA signal as compared to normally reared controls (Fig. 2, B to E). However, in mice lacking PirB (Cre+), the spared-eye representation expanded 21% more than control (Cre−) littermates, where as in normally reared mice, there was no difference between genotypes in the width of Arc mRNA signal induced by stimulation of the ipsilateral eye (Fig. 2E). A two-way analysis of variance (ANOVA) confirmed a significant interaction effect between visual manipulation and genotype (P < 0.0001). To further facilitate comparisons between genotypes, a plasticity index was calculated by normalizing the width of Arc mRNA induction after ME to the normally reared value for each genotype. The plasticity index in Cre+ mice is 23% higher than that in Cre− mice (fig. S2A), consistent with the absence of PirB. These observations imply that PirB does not act only early in fetal life, but rather actively represses OD plasticity during the critical period.
In developing wild-type animals, OD plasticity decreases between P35 and P40 (the end of the critical period), and short periods of MD or ME (3 to 4 days) thereafter have little effect on OD (7–11). To determine whether decreasing PirB function might enhance plasticity in adulthood, tamoxifen was administered from P45 to P49 (Fig. 2F), which resulted in an almost complete loss of PirB protein by P70 (Fig. 1, C and E). Then, these adult mice received ME from P70 to P74, weeks after the critical period has normally closed. As with juvenile mice, at P74, there was no significant difference in baseline width of Arc mRNA signal between genotypes in normally reared controls (Fig. 2, G to I). There was also no significant difference in width of Arc mRNA signal after ME from P70 to P74 in Cre− controls, as expected, given the brief 4-day period of deprivation at this older age. However, we observed a significant expansion of open-eye representation (P = 0.0004) in Cre+ mice (which lacked PirB) with 4 days of ME (Fig. 2, H and I, and fig. S2B). In adults, as in juveniles, a two-way ANOVA confirmed that there was a significant interaction between visual manipulation and genotype (P = 0.04). Although the magnitude of adult OD plasticity was reduced in all genotypes as compared to that observed during the critical period, deletion of PirB in adults was sufficient to enhance OD plasticity and to cause a significant expansion in open-eye representation not observed in adult wild-type littermate controls with normal PirB levels.
Postnatal deletion of PirB from excitatory neurons enhances adult OD plasticity
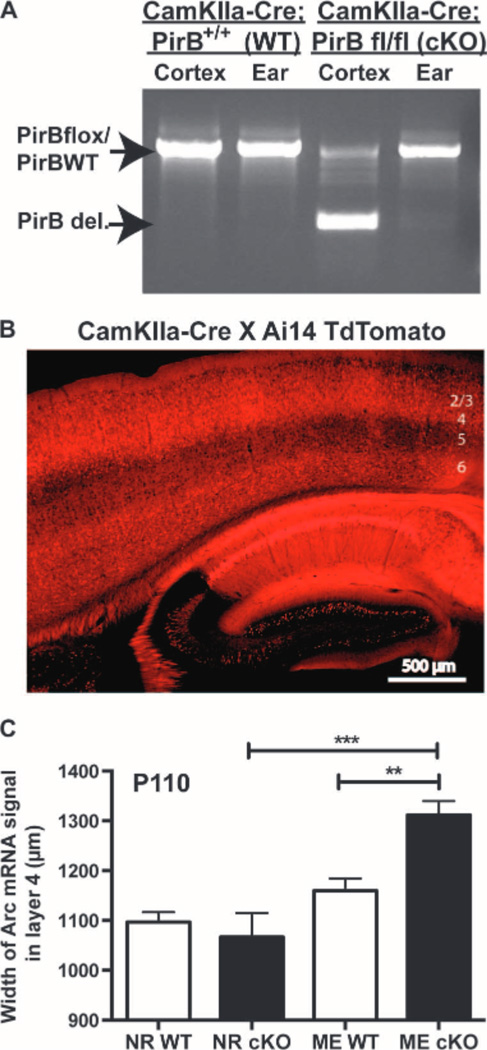
In the experiments above, a ubiquitously expressed Cre recombinase was used to achieve robust deletion of PirB in all cells. Next, we investigated whether loss of PirB specifically in forebrain excitatory neurons was sufficient to enhance OD plasticity. PirB flox/flox mice were crossed with a CamKIIa-Cre line, which expresses Cre exclusively in forebrain excitatory neurons (34, 35), generating CamKIIa-Cre; PirB flox/flox conditional knockouts, and CamKIIa-Cre; PirB+/+ littermate controls. PCR genotyping of brain and ear confirms brain-specific deletion of the floxed region of PirB (Fig. 3A). To confirm the spatial pattern of Cre deletion, CamKIIa-Cre mice were also crossed to the Ai14 TdTomato reporter line (36). Results show faithful Cre activity at P30 in pyramidal neurons of hippocampus and cortex (Fig. 3B). Previous studies have shown that excision of floxed regions of DNA in this Cre line is gradual, with complete deletion occurring around 3months of age (35), permitting us to examine effects of PirB deletion in adulthood.
Fig. 3.
Cre-mediated deletion of PirB from forebrain excitatory neurons enhances adult OD plasticity. (A) Genotyping of samples from ear and cerebral cortex from P100 CamKIIa-Cre; PirB flox/flox (cKO) or CamKIIa-Cre; PirB WT (wild type), showing deletion of floxed PirB in cortex but not ear. (B) CamKIIa-Cre; PirB flox/+ breeders were crossed with the Ai14 TdTomato reporter line, generating red fluorescence in the presence of Cre. Sagittal section through visual cortex (layers indicated at right) and hippocampus of a P30 mouse shows Cre present in pyramidal neurons. (C) Graphs of width of L4 region activated by stimulation of ipsilateral (open) eye in visual cortex, assessed using Arc mRNA induction. Deletion of PirB from forebrain excitatory neurons increases open-eye expansion in adult mice after ME from P100 to P110. NRWT: n = 5 mice versus NRcKO: n = 4, P = 0.91. MEWT: n = 8 mice versus MEcKO: n=5, P = 0.006. NR versus MEWT: P = 0.39, NR versus MEcKO: P = 0.0002,by two-way ANOVA with Tukey post hoc test.**P < 0.01,***P < 0.001.
CamKIIa-Cre; PirB flox/flox conditional knockouts, and CamKIIa-Cre; PirB+/+ littermate controls received 10 days of ME from P100 to P110, followed by assessment of OD plasticity. Open-eye expansion in visual cortex in CamKIIa conditional knockout mice was ~13% greater than in littermate controls (Fig. 3C and fig. S2C), suggesting that postnatal deletion of PirB from excitatory neurons is sufficient to increase OD plasticity by P110. A two-way ANOVA confirmed significant interaction between visual manipulation and genotype (P = 0.007). Furthermore, this increase is similar in magnitude to previous observations of enhanced OD plasticity in adult mice with PirB germline deletion (14), implying that loss of PirB function in excitatory neurons may be largely responsible for the observed PirB−/− phenotype.
Blockade of PirB ligand binding rapidly enhances OD plasticity
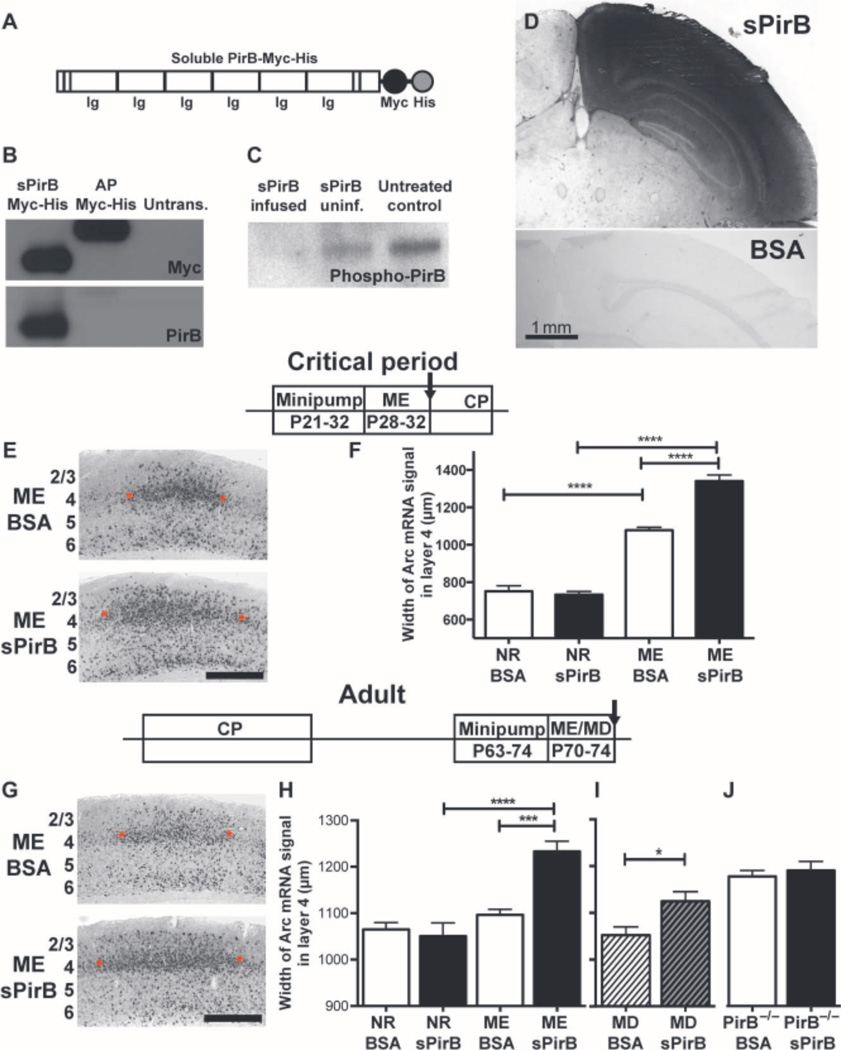
The genetic approaches used above excise PirB from the genome postnatally, but the ensuing loss of PirB protein is gradual and widespread. These experiments establish that PirB actively functions throughout the animals’ lives. Next, a molecular-pharmacological approach was used as a proof-of-concept for a therapeutic reagent that blocks PirB function and to achieve rapid and local disruption of PirB function within visual cortex. We generated a soluble PirB ectodomain (sPirB) protein, containing the first six immunoglobulin G (Ig)–like domains and His and Myc tags for purification and detection (Fig. 4A). Soluble ectodomains of receptors act as “decoys” to sequester endogenous ligands, thus reducing ligand binding and subsequent receptor signaling. They have been used frequently in experimental contexts, and several are currently on the market as therapeutics (37–39). Previous studies have demonstrated that soluble versions of the full PirB ectodomain with similar sequence to the one used here can bind known PirB ligands including MHC class I (14, 21, 40); myelin components NogoA, OMgp, and MAG (19); and b amyloid oligomers (20). However, the PirB ectodomain has never been used therapeutically. sPirB would be expected to block PirB binding to endogenous ligands and therefore reduce downstream signaling.
Fig. 4.
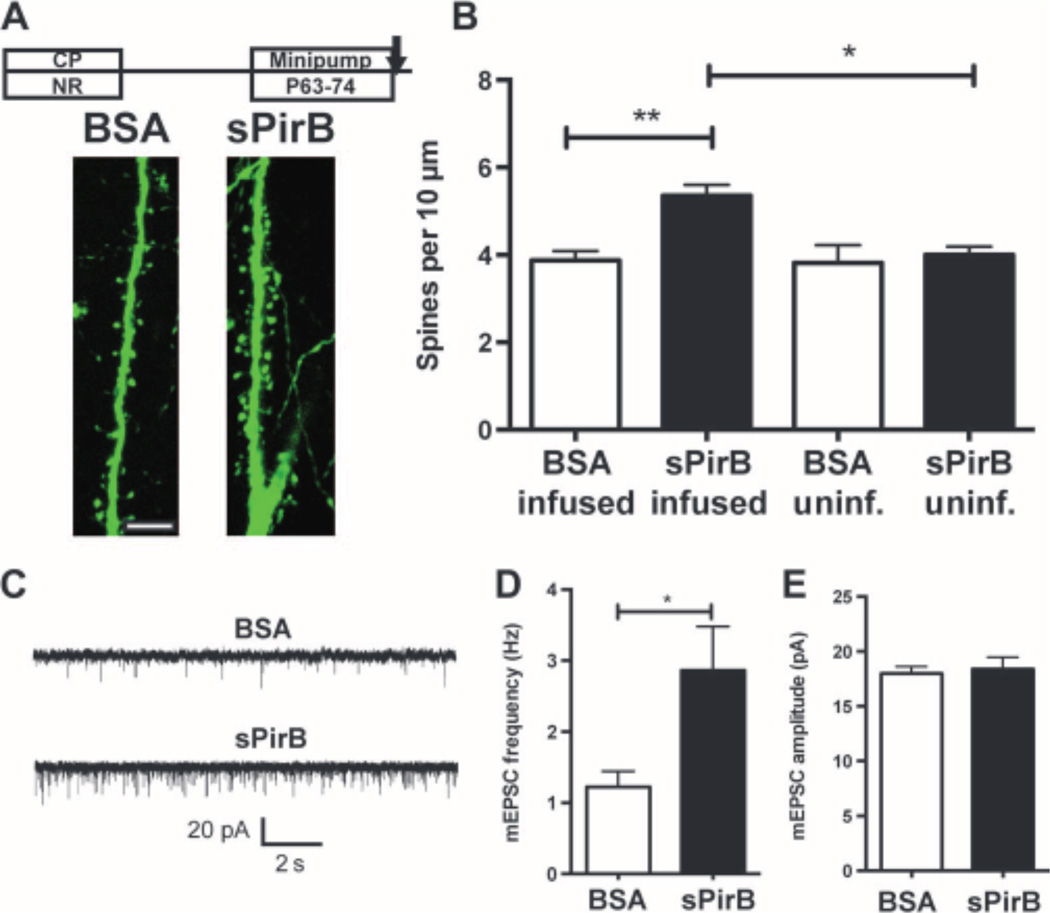
Blockade of PirB binding enhances OD plasticity in WT visual cortex. (A) Schematic of soluble PirB-Myc-His (sPirB) fusion protein, indicating extracellular Ig-like domains plus Myc and His tags. (B) Western blot of culture supernatant from sPirB-transfected HEK293 cells, detecting Myc tag and PirB ectodomain; Myc-His–tagged alkaline phosphatase (AP-Myc-His) is a positive control. (C) PirB phosphorylation is decreased after 7 days (P21 to P28) of sPirB infusion into WT mouse cortex, as shown by phospho-tyrosine IP and PirB Western blot of cortical lysates from infused (sPirB infused), uninfused (sPirB uninf.) hemispheres, or untreated littermate controls. (D) Section of visual cortex immunostained with anti-Myc antibody after 11 days (P21toP32)of sPirB or BSA infusion (1 mg/ml). Scale bar, 1 mm. (E and F) Minipump infusions of sPirB during critical period (CP). Timeline as shown. (E) Example Arc mRNA in situ hybridization micrographs of visual cortex after BSA (top)or sPirB (bottom) treatment. Scale bar, 500 mm. Red asterisks indicate borders of Arc mRNA signal induced by stimulating the ipsilateral (open) eye in layer 4. (F) Graphs comparing width of Arc mRNA signal in L4 after open-eye stimulation. Width of territory activated by open-eye stimulation after ME is greater after sPirB infusion than with BSA. NRBSA: n = 4 mice, NR sPirB: n = 4, MEBSA: n = 5 versus ME sPirB: n = 6, P < 0.0001, by two-way ANOVA and Tukey post hoc test for all comparisons indicated. (Gto I) sPirB infusions into adult WT visual cortex; timeline as shown. (G) Example of Arc mRNA situ hybridization micrographs at P74. Scale bar, 500 mm. (H) Graphs comparing width of Arc mRNA signal in L4 after stimulation of the ipsilateral (open) eye. sPirB infusion from P63 to P74 enhances open-eye expansion after ME. NRBSA: n = 4 mice versus NR sPirB: n = 4, P = 0.99. MEBSA: n = 4 versus ME sPirB: n = 5, P = 0.0004. NR versus MEBSA: P = 0.88, NR versus ME sPirB: P < 0.0001. (I) sPirB infusion coupled with 3 days of MD also enhances OD plasticity. (J) sPirB infusion has no effect on OD plasticity when infused into visual cortex of PirB−/− mice. ME PirB−/− BSA: n = 5 mice versus ME PirB−/− sPirB: n = 5, P = 0.95, ME PirB−/− BSA versus MEWTBSA: P = 0.034. MD BSA versus MD sPirB: n = 4 mice per group, P = 0.036. *P < 0.05, ***P < 0.001, ****P < 0.0001, by two-way ANOVA and Tukey post hoc test.
We transfected a plasmid coding for sPirB into human embryonic kidney (HEK) 293 cells, and the recombinant protein was then secreted into culture supernatant. We detected sPirB by Western blotting against both the Myc tag and the PirB ectodomain (Fig. 4B). sPirB was then produced at large scale and purified via Ni-His column. Next, either sPirB or bovine serum albumin (BSA) was infused into visual cortex (V1) of wild-type mice during the critical period via osmotic minipumps. To assess efficacy of sPirB infusions, we implanted minipumps at P21. At P28, cortical tissue was harvested posterior to the implantation site in the infused and contralateral hemispheres, as well as in uninfused littermates. PirB phosphorylation (14) was decreased in visual cortex posterior to the infusion site compared to both the contralateral hemisphere and untreated littermate controls (Fig. 4C). Eleven days after implantation, extensive diffusion of sPirB can be detected by anti-Myc immunostaining of sections (Fig. 4D) across visual cortex as far as 2 mm posterior to the infusion site (fig. S3B); no anti-Myc staining was detected in BSA-infused control brains (Fig. 4D and fig. S3C).
Minipump infusion of sPirB into visual cortex of wild-type mice for 11 days during the critical period (P21 to P32), combined with ME from P28 to P32, resulted in a pronounced expansion in width of visual cortex containing neurons functionally driven by the open (ipsilateral) eye, as assessed with Arc mRNA induction (Fig. 4E). By this measure, OD plasticity was 24% greater with sPirB infusion than in controls infused with an equivalent concentration of BSA (Fig. 4F and fig. S2D); a two-way ANOVA confirmed significant interaction between visual manipulation and treatment (P < 0.0001). Because the infusion was local and limited to an 11-day period beginning at the onset of the critical period (7, 8), the results of this experiment help to narrow considerably the spatiotemporal window in which PirB acts to suppress plasticity. Together with the tamoxifen-inducible PirB knockout results presented above, our data suggest that PirB actively suppresses plasticity locally in visual cortex during the critical period for OD plasticity.
Substantial OD plasticity could be restored to the visual cortex of adult wild-type mice after minipump infusions of sPirB from P63 to P74. ME from P70 to P74 resulted in significant expansion of the functional representation of the open eye in the presence of sPirB, but not BSA (Fig. 4, G and H, and fig. S2E). This effect of sPirB was twice as large as that of tamoxifen-driven PirB excision in adult mice (compare Fig. 2I). In contrast, there was no significant effect of 11-day minipump infusions of either BSA or sPirB on the width of Arc mRNA induction after stimulation of the ipsilateral eye in mice reared with normal vision (Fig. 4H, NR sPirB). Consistent with the idea that sPirB affects the expansion rather than the baseline width of Arc induction, a two-way ANOVA detects a significant interaction effect between visual deprivation and treatment (P = 0.001).
We further assessed the effect of sPirB on adult OD plasticity using MD (as opposed to ME). In adult mice, a brief period of MD such as 3 days does not usually produce a shift in OD favoring the open eye (confer Fig. 4,H and I) (7, 10, 11, 41). Therefore, we examined whether MD from P70 to P73, coupled with sPirB treatment, would result in open-eye expansion. Similar to results with ME, sPirB-treated adult mice receiving MD had a significant increase in open-eye expansion, with no change in BSA-treated controls (Fig. 4I and fig. S2F). Therefore, in adult visual cortex, sPirB treatment during brief periods of either MD or ME generates detectable OD plasticity.
PirB binds multiple ligands (19, 40), which themselves can bind to other receptors (19, 40), possibly accounting for the observation that sPirB has a larger effect on OD plasticity than genetically induced deletion of PirB. To test this idea, we infused sPirB or BSA by minipumps into adult germline PirB−/− mice, which also received 4-day ME (P70 to P74). Germline PirB−/− mice implanted with BSA minipumps demonstrated the expected increase in OD plasticity (14, 24) when compared to wild-type mice receiving BSA (Fig. 4, H and J). However, there was no additional effect of sPirB infusion compared to BSA infusion in PirB−/− mice (Fig. 4J). This lack of effect of sPirB infusion in adult PirB−/− visual cortex suggests that endogenous PirB is required to enhance OD plasticity and that the pharmacological blockade is PirB-specific. Together with results from the tamoxifen-inducible PirB mice, these findings indicate that acute, specific manipulations that delete or block PirB function are sufficient to enhance OD plasticity even when administered well beyond the end of the critical period.
sPirB increases spine density and functional synapses on L5 pyramidal neurons
There is a significant increase in spine density on the apical tufts of L5 pyramidal neurons in the visual cortex of germline PirB knockout mice reared with normal vision. It has been proposed that these extra spines represent a preexisting structural substrate that is then recruited for the more rapid and robust OD plasticity observed in these mice (24). Indeed, previous sensory experience in visual or auditory systems increases plasticity, and is accompanied by increased structural connectivity (41–44). We tested whether PirB might contribute to these structural changes.
sPirB infusion might trigger an increase in spine density even without visual deprivation. To test this hypothesis, visual cortex of normally reared wild-type Thy-1 YFP-H transgenic mice (45), in which cortical L5 pyramidal neurons are yellow fluorescent protein (YFP)–labeled, received minipump infusions of either sPirB or BSA from P63 to P74 (Fig. 5, A and B). Spines on apical dendrites of L5 pyramidal neurons were examined in the binocular zone at a distance posterior to the infusion site comparable to that studied above for assessment of OD plasticity. In this region after sPirB infusion, pyramidal neuron somata, dendrites, and spines appeared intact and healthy, without fragmentation or blebbing (Fig. 5A). Spine density on L5 apical dendritic tufts of animals reared with normal vision was 38% greater in the presence of sPirB than of BSA (Fig. 5B). Spine density on L5 neurons in the un-infused hemisphere was not altered, and a two-way ANOVA confirms a significant interaction effect between hemisphere and treatment (P = 0.03). The observed density increase could arise if sPirB acts on a subclass of dendritic spines. However, after an 11-day infusion of either sPirB or BSA, there was no significant difference in the proportion of spines classified as mushroom, thin, or stubby (46) (fig. S4B). Together, results show that in adult visual cortex, it is possible to generate a local increase in spine density on L5 neurons by infusing sPirB, even in the absence of a visual manipulation or deprivation.
Fig. 5.
sPirB increases spine density and functional synapses on L5 pyramidal neurons of normally reared mice. (A) Timeline of minipump infusions [BSA (1 mg/ml) or sPirB from P63 to P74] and example dendrites of YFP-labeled L5 pyramidal neurons in binocular zone of visual cortex in WT Thy-1 YFP-H animals reared with normal visual experience. Scale bar, 10 mm. (B) Histograms of spine density on apical tufts of L5 neurons in sPirB infused versus in the uninfused (unif.) contralateral hemisphere, or in BSA controls: BSA infused: n = 5mice versus sPirB infused: n = 5, P = 0.01, one to two cells per animal. BSA uninf.: n = 5 versus sPirB uninf.: n = 5, P = 0.96, BSA inf. versus uninf.: P = 0.99, sPirB inf. versus uninf.: P = 0.016, by two-way ANOVA and Tukey post hoc test. (C) Example traces of mEPSC responses recorded from visual cortical slices (P70 to P77) from L5 pyramidal neurons after BSA or sPirB infusion, as in (A).(D) Increased mEPSC frequency with sPirB infusion: BSA: n = 12 neurons versus sPirB n = 13, P = 0.046, by Mann-Whitney U test. (E) No change in mEPSC amplitude: BSA: n = 12 neurons versus sPirB n = 13, P = 0.70, by Mann-Whitney U test.
To examine whether the increase in spine density represents new functional synapses, miniature excitatory postsynaptic currents (mEPSCs) were recorded from L5 pyramidal neurons in slices of visual cortex (P70 to P77), after 7 to 11 days of sPirB minipump infusion in vivo, in mice reared with normal binocular vision (Fig. 5C). mEPSC frequency was significantly greater after sPirB treatment than in BSA littermates (Fig. 5D),with no change in mEPSC amplitude (Fig. 5E). This finding is consistent with the idea that sPirB infusion causes an increase in synaptic connectivity, suggesting that newly formed spines represent sites of functional synapses.
sPirB treatment after LTMD enables recovery of spine density
LTMD is a well-studied animal model of amblyopia because it involves an experience-dependent developmental loss of function in the deprived eye (47, 48). In rodents, LTMD profoundly decreases visual acuity, as well as the number of cortical neurons visually driven by the deprived eye. There is little, if any, recovery, even after restoration of binocular vision (4, 12, 13, 17, 49). It has been proposed that a decrease in dendritic spine density underlies these functional deficits (4, 49). For example, LTMD generates a significant decline in spine density on basolateral dendrites of L5 pyramidal neurons contralateral to the deprived eye (49).
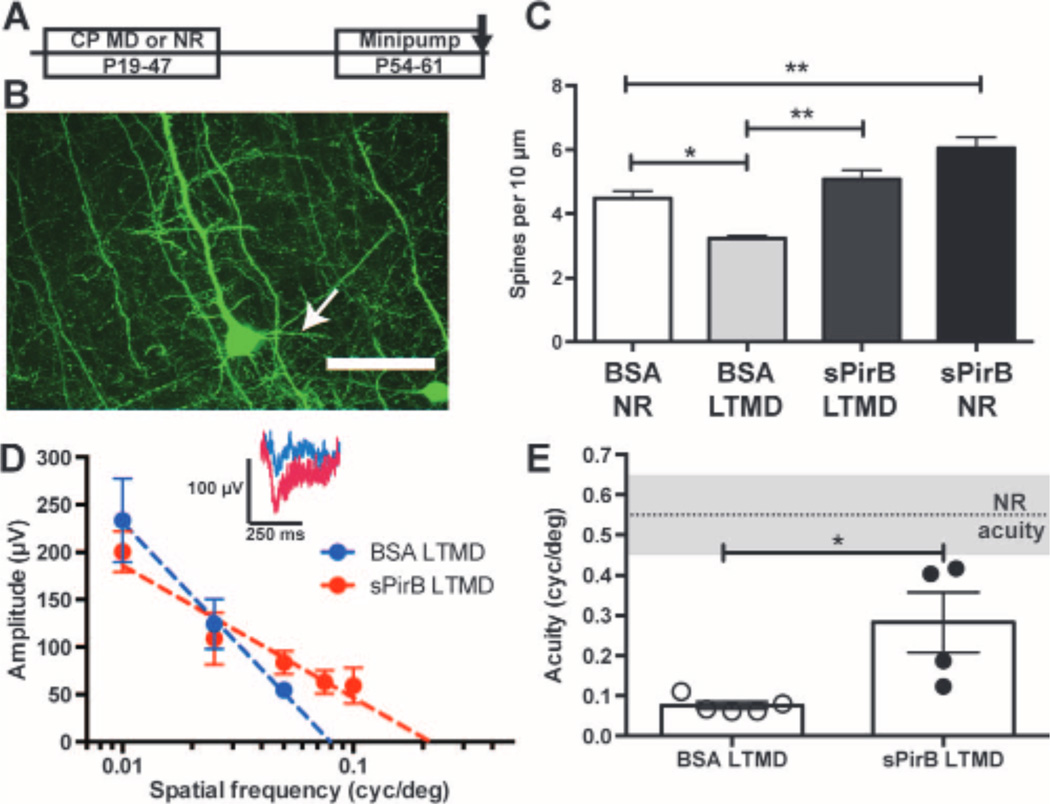
Given the rapid and generative effect of sPirB on spine density and mEPSC frequency described above in normal visual cortex, we wondered whether sPirB treatment might generate a spine density increase that could facilitate recovery from LTMD. Thy-1 YFP wild-type mice were either normally reared or received LTMD spanning the entire critical period for OD plasticity (P19 to P47). At P47, the deprived eye was reopened to restore binocular vision for 1 week. Then at P54, minipumps containing either sPirB or BSA were implanted in the visual cortex contralateral to the deprived eye until P61 (Fig. 6A), at which time, spine density on L5 basolateral dendrites was measured.
Fig. 6.
sPirB allows structural and functional recovery from amblyopia after LTMD. (A) Experimental timeline: LTMD from P19 to P47, eye reopening at P47, and minipump infusion from P54 to P61. (B) Representative YFP-labeled L5 cell soma and basolateral (arrow) dendrites in visual cortex of WT Thy-1 YFP-H mice. Scale bar, 50 mm. (C) Bar graphs showing changes in basolateral dendritic spine density: LTMD causes a significant decline in spine density (BSA LTMD) that can be fully reversed with sPirB infusion (sPirB LTMD) (BSA NR: n = 5 mice versus BSA LTMD: n = 4, P = 0.02. sPirB LTMD: n = 5, sPirB versus BSA LTMD, P = 0.001, sPirB NR: n = 5 animals, one to two cells per animal, sPirB versus BSA NR: P = 0.003). *P < 0.05,**P < 0.01,by two-way ANOVA and Tukey post hoc test. (D) Averaged cortical VEP response amplitudes (microvolts) to stimuli at a range of spatial frequencies (cycles per degree) after LTMD in mice receiving minipump infusion of either sPirB or BSA. Dotted line: Semilogarithmic regression of visual responses. Inset: Population average traces at 0.05 cycle per degree. (E) Bar graphs showing spatial acuity after LTMD plus infusion of either BSA or sPirB. Gray shaded region indicates mean acuity ± SEM of normally reared (NR) controls. Measurements from individual mice are plotted (circles). Loss of acuity with LTMD is reversed after just 1weekof sPirB infusion (NR, n = 6 mice versus BSA LTMD, n = 5 mice, P = 0.004. sPirB LTMD, n = 4 mice versus BSA LTMD, P = 0.016). *P < 0.05, **P < 0.01, by U test.
LTMD caused a 28% decrease in spine density along L5 basolateral dendrites in BSA-treated controls despite 2weeks of subsequent binocular vision (Fig. 6, B and C), as expected from previous studies (4, 49). In contrast, the spine loss accompanying LTMD was almost entirely reversed by minipump infusion of sPirB. There was a 57% greater basolateral dendritic spine density in sPirB-treated animals than in LTMD BSA-treated controls, essentially restoring spine density to levels of BSA-treated controls reared with normal vision (Fig. 6C). There was no detectable change in overall distribution of basolateral spine types (fig. S4D), similar to observations above for L5 apical dendrites. In addition, in normally reared littermates infused with sPirB, spine density increased on basolateral dendrites compared to normally reared BSA-infused controls, demonstrating that sPirB infusion can increase spine density not only on apical tufts of L5 pyramidal neurons (compare Fig. 5B) but also on their basolateral dendrites (Fig. 6C). Together, these results demonstrate that sPirB treatment just for 1 week can reverse spine loss resulting from LTMD even when infused after a delay following eye reopening.
sPirB induces recovery of visual acuity after LTMD
To assess whether the recovery in spine density could mediate recovery of visual function, VEP recordings were made from cortical layer 4/5 to measure spatial frequency acuity. This method is well established, is highly sensitive, and tightly correlates with behavioral and single unit measurements of visual recovery after LTMD (4, 12, 17, 49, 50). Moreover, because the effects of sPirB minipump infusions are restricted to a local region of visual cortex (Fig.5B and fig. S3B), VEPs are well suited to assess recovery of deprived eye function specifically within the infused region. Visual acuity is measured by varying the spatial frequency of visual stimuli and monitoring the amplitude of VEP responses (Fig. 6D) (4, 12).
In normally reared Thy-1 YFP wild-type mice, visual acuity measurements were similar to previously reported values, about 0.55 ± 0.1 cycles per degree (Fig. 6E, shaded region) (17, 29, 50). After 4weeks of LTMD from P19 to P47, BSA-treated control animals experienced a marked loss of visual acuity, with responses above noise level only to a maximum spatial frequency of 0.05 cycle per degree (Fig. 6, D and E). This severe (85 to 90%) reduction in acuity (Fig. 6E) persisted for 2 weeks even after restoration of binocular vision, including 1 week of BSA minipump treatment. The lack of recovery is consistent with previous results, suggesting that binocular vision alone is not sufficient for recovery of visual function after the critical period (4, 12, 13, 17, 49).
In contrast, there was significant improvement in visual acuity in mice receiving a 1-week minipump infusion of sPirB (Fig. 6, D and E). Although the degree of recovery was variable among animals, 50% of the sPirB-treated mice recovered visual acuity to 0.4 cycle per degree or higher, nearly the normal range for unmanipulated mice (Fig. 6E). Even the remaining sPirB-treated mice regained acuity higher than that measured in the highest LTMDBSA-treated controls. These results suggest that after just 1week of sPirB treatment, there was significant functional recovery of visual acuity in the deprived eye and that, in some cases, visual function rapidly recovered to nearly normal levels.
DISCUSSION
There are several major findings from this study. First, by acutely disrupting the function of the endogenous receptor PirB, we find that OD plasticity in visual cortex of adult mice can be enhanced long after critical period closure. Two independent but complementary methods were used: genetic deletion of PirB with temporal control and blockade of ligand binding with a sPirB decoy protein. Both methods generated enhanced OD plasticity, not only during the critical period but also in adulthood, phenocopying germline PirB knockouts (14, 24). These observations suggest that endogenous PirB actively acts to repress cortical plasticity throughout life, validating PirB as a target for therapeutic interventions that could improve recovery from injury, correct dysfunctional developmental plasticity, or perhaps even temporarily enhance learning in normal individuals.
Second, we report that in a model of amblyopia, it is possible to reverse the loss of both the spines and the visual acuity in cortex after LTMD by restoring binocular vision coupled with an infusion of sPirB. Last, sPirB infusion into the visual cortex of normally reared mice produced rapid increases in spine density and functional synapses on L5 pyramidal neurons. Although motor learning (51, 52), sensory enrichment (52), or brief MD(44) all have been shown to produce rapid increases in spine density, sPirB treatment produces a larger magnitude change and does so in the absence of novel stimuli or training. Together, our observations imply that targeting and disrupting PirB function increase synaptic connectivity and plasticity, even after the critical period. Because PirB is expressed by pyramidal neurons throughout the neocortex (14), our results may also apply to cortical areas other than the visual system.
sPirB as an acute regulator of spine and synapse density
Infusion of sPirB decreases PirB downstream signaling (Fig. 4C), consistent with its previously demonstrated sequestration of endogenous PirB ligands (14, 19, 20, 23). In adulthood, acute blockade with sPirB results in enhanced OD plasticity and produces a rapid increase in spine density and mEPSC frequency, even in the absence of altered vision. Many interventions that affect synaptic connectivity and spine dynamics also enhance OD plasticity, including transplantation of inhibitory neuron progenitors (53) or disruption ofNgR1-NogoAfunction (16, 54, 55). Spine density increases also correlate with enhanced subsequent plasticity (41, 44): new spines generated during an initial MD can be co-opted for more robust OD plasticity during a subsequent MD (24, 41, 44). Furthermore, in germline PirB−/− mice, enhanced OD plasticity is associated with a large increase in spine density on L5 neurons and an increase in the magnitude of L4 to L2/3 long-term potentiation (LTP) in visual cortex (24). Collectively, these experiments connect an increase in spine density and functional connectivity to enhanced synaptic plasticity. Thus, sPirB may generate greater OD plasticity by creating a more highly interconnected structural substrate that can be accessed for more rapid and robust synaptic change.
sPirB as a potential therapy for recovery from amblyopia
LTMD throughout the critical period, used here as an animal model of amblyopia, leads to a profound loss of visual acuity, as well as to loss of visual responsiveness of cortical neurons to stimulation of the deprived eye; both are highly resistant to recovery even when binocular vision is subsequently restored (4, 12, 13, 17, 49). Decreases in spine density on both L2/3 pyramidal cells (4) and pyramidal neurons throughout cortex have also been reported after LTMD or chronic MD (49). Although reversal of this spine loss on cortical pyramidal neurons has been seen, reversal required that the formerly open eye be sutured closed in combination with fairly disruptive treatments such as chondroitinase ABC to digest extracellular matrix (4), or 10 days of dark exposure followed by visual stimulation (49, 56). Recovery of spines in both of these cases was accompanied by robust recovery of VEP acuity. In our study, we found that sPirB infusion, combined with binocular vision, was sufficient by itself to bring spine density values and VEP acuity estimates close to normal. Visual acuity as measured by VEPs predicts physiologically relevant recovery of visual function in the deprived eye, indicating that vision through the deprived eye in sPirB-treated mice has greatly improved (4, 43, 44). Together with the data on spine recovery, these results suggest that sPirB can enable significant structural and functional recovery from amblyopia after LTMD within just 7 days of treatment.
These observations imply that sPirB—a soluble receptor ectodomain— is a potential therapeutic agent, and they provide proof-of-concept for generating other PirB blocking reagents. The standard treatment for human amblyopic patients mandates early intervention during a developmental critical period and involves alternating patching between the two eyes to strengthen the amblyopic eye, but this treatment interferes with development of binocular depth perception (6). There are several PirB homologs in humans (LilrBs), and LilrB2 protein is expressed in the human brain (25). Targeting LilrB2 or other members of the LilrB receptor family might permit recovery from amblyopia without requiring eye patching, as implied by the results of the LTMD experiments in mice.
There are a number of limitations and issues to consider in translating our findings. First, it would be important to determine whether the increase in spine density and functional recovery from amblyopia persists stably beyond the period of sPirB infusion. Second, it is possible that a longer infusion or higher concentration of sPirB would produce a more robust recovery for all animals. In addition, rather than minipump infusions, it would be preferable to develop a small-molecule drug that can cross the blood-brain barrier. Finally, as mentioned above, LilrB2 is present in human brain, but because there are other family members, it will be important to characterize their expression and function in human central nervous system.
PirB: An endogenous target for manipulations of synapse and systems-level plasticity
Our observations add to a growing body of research that has unmasked active roles for molecules in the brain acting as negative regulators of functional and structural plasticity both in development and in adulthood (17, 50, 54). In the case of PirB, this negative regulation may also be hijacked, as in AD, where b amyloid oligomers bind to PirB/LilrB2 with nanomolar affinity, resulting in loss of OD plasticity and deficits in cortical and hippocampal synaptic plasticity (20). Thus, sPirB and human soluble receptor homologs might even be viable therapeutics for AD. By generating a recombinant sPirB protein, we have demonstrated a use for selectively blocking PirB receptor interaction with endogenous ligands. These results further support the value of creating PirB/LilrB antagonists that cross the blood-brain barrier, enhancing plasticity and increasing synapse and spine density in cases of disease, dysfunction, injury, or even for cognitive enhancement in normal individuals.
MATERIALS AND METHODS
Study design
The objective of this study was to devise methods to delete PirB function acutely, then monitor the effects on measures of synaptic and OD plasticity, and recovery from LTMD. Two methods were used: tamoxifen-induced PirB deletion via a PirB conditional allele, or sPirB minipump infusion. Because OD plasticity is induced by changes in visual experience, experiments were designed to capture an interaction effect between genotype/treatment and visual manipulation; four groups and a two-way ANOVA design were used to test for interactions. All experiments were performed blind to genotype and/or treatment. Littermates were used to control for genetic variation, and mice were randomly assigned to different visual manipulations and treatments within a litter. To detect genotype effects similar or greater than those previously reported, sample sizes were chosen on the basis of a statistical power of 80% with an a value of 0.05 (14, 24). The number of replicate measurements and animals is given in each figure legend.
Mouse strains
PirB−/− and PirB flox/flox mice were generated as described (14). A PirB WT line was maintained on the same background and used for all minipump infusion experiments performed during the critical period (P21 to P32). For adult minipump experiments (P63 to P74), PirB WT and PirB−/− mice were crossed with the Thy-1 YFP-H transgenic line (JAX#003782),which expresses YFP in a subset of L5 pyramidal neurons (45). For inducible knockout experiments, UbC-CreERT2 mice (JAX #007001) (25) were bred with PirB flox mice to generate UbC-CreERT2; PirB flox/flox mice and PirB flox/flox littermates. For conditional knockout experiments, CamKIIa-Cre; PirB flox/+ mice (57) were bred with PirB flox/+ mice to generate CamKIIa-Cre; PirB flox/flox mice and CamKIIa-Cre; PirB+/+ littermates. CamKIIa-Cre mice were also bred with Ai14 TdTomato reporter mice (36). All experiments were performed in accordance with protocols approved by Stanford University Animal Care and Use Committee in keeping with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
sPirB protein product ion
To create a sPirB mimic, the PirB ectodomain was cloned into a plasmid containing a His tag for purification and a Myc tag for antibody detection with a sequence identical to previous publications (14, 19, 20, 40). For minipump infusions, Invitrogen Custom Services produced sPirB in larger quantities in FreeStyle HEK293 cells and purified it on a nickel column (Ni-His, Invitrogen).
Osmotic minipump implantations and sPirB infusion
Craniotomies were performed, and minipumps (ALZET model 1002; 0.25 ml/hour, 100-ml capacity) containing either sPirB (1 mg/ml) or BSA (1 mg/ml) (VWR EM-2930) in 0.1 M phosphate-buffered saline were implanted subcutaneously, connected to a cannula. The cannula was inserted just anterior to primary visual cortex (2.5-mm lateral and 3-mm posterior to bregma).
Arc mRNA induction and in situ hybridization
Arc mRNA was induced by placing mice overnight in total darkness (16 to 18 hours), followed by bright illumination for 30 min to permit vision through the open eye before euthanasia via isoflurane anesthesia and decapitation (8). A digoxigenin-labeled Arcantisense mRNA probe was used for colorimetric in situ hybridizations performed on brain sections (8, 33). Images were acquired via brightfield microscopy and analyzed using the Line Scan function of NeuroLens software to measure the width of the Arc mRNA hybridization signal ipsilateral to the open (nondeprived) eye along L4 of the visual cortex, at the 3 to 4 border (fig. S1). Multiple sections were scanned and averaged per animal (for example, Fig. 2).
VEP recordings
Animals were anesthetized with urethane (0.6 to 1.2 g/kg; Sigma) and chlorprothixene (5 mg/kg; Sigma), and at incisions with lidocaine (2%, Sparhawk Laboratories), and then the scalp was exposed and the minipump was cannula removed. After a craniotomy centered over V1, a glass pipette filled with ACSF (artificial cerebrospinal fluid) was inserted to record local field potentials at a depth of 450 to 600 mm. Responses to sinusoidal grating stimuli were averaged over stimulus blocks, and a peak response amplitude within a 500-ms window after stimulus onset was determined. Visual acuity was estimated by finding the x-intercept of a semilogarithmic regression of response amplitudes across different spatial frequencies (11, 29).
Statistical analyses
All statistical analyses were performed with Prism software (Graphpad). When only two groups were involved, two-sample t tests were used, with Welch’s correction for unequal variances applied where appropriate. Data for which a normal distribution could not be assumed were analyzed with Mann-Whitney U tests. In cases where both treatment/genotype and visual manipulation or hemisphere were varied, a two-way ANOVA was conducted, with Tukey post hoc tests for individual pairs of columns.
Supplementary Material
Acknowledgments
We thank N. Sotelo-Kury, P. Kemper, and C. Chechelski for logistics and mouse breeding, and G. Vidal for microscopy advice and training. We thank B.-Q. Zhuang and L. Hsieh-Wilson at the California Institute of Technology for the sPirB plasmid, and J. Schaffer for illustrating Fig. 2A. We also thank L. Luo, E. Knudsen, and T. Clandinin for helpful feedback. Funding: This project was supported by NIH grants EY02858 and MH07166, the Mathers Charitable Foundation, and the Rosenberg Family Foundation to C.J.S.; NIH grant EY018861 to Y.D.; National Science Foundation Graduate Research Fellowships to D.N.B. and J.D.A.; and National Defense Science and Engineering Fellowship to J.D.A. R.W.S. received a Bio-X Summer Undergraduate Research Fellowship.
C.J.S. and J.S. are inventors on U.S. Patent application 12/087799 assigned to the President and Fellows of Harvard College on Compositions and methods for enhancing neuronal plasticity and regeneration.
Footnotes
Author contributions: D.N.B. and C.J.S. proposed and outlined the experimental plan. D.N.B. performed all experiments involving tamoxifen-induced deletion; D.N.B. and R.W.S. performed minipump implantation surgeries and subsequent analysis; J.D.A., R.W.S., and M.D. performed and analyzed the studies of CamKIIa-Cre; PirB flox/flox visual cortex. S.Z. performed the VEP recordings and analysis, and Y.D. supervised that collaboration. H.L. performed whole-cell recordings of mEPSCs in L5 neurons. M.D. characterized a new batch of the PirB antibody used here and made substantial intellectual contributions to the project. J.S. created the floxed PirB and PirB−/− mouse in the Shatz laboratory. D.N.B. and C.J.S. wrote the manuscript and reviewed all data collection and analysis.
Competing interests: The other authors declare that they have no competing interests.
www.sciencetranslationalmedicine.org/cgi/content/full/6/258/258ra140/DC1
Materials and Methods
Fig. S1. Example line scan measurements of Arc mRNA in situ hybridization signal in visual cortex induced by stimulation of the ipsilateral eye.
Fig. S2. Plasticity indices for genetic or pharmacological disruption of PirB function.
Fig. S3. Characterization of sPirB minipump infusion area and effect on OD plasticity.
Fig. S4. Effect of minipump infusions of sPirB or BSA on dendritic spines by cells and by spine type.
REFERENCES AND NOTES
- 1.Hubel DH, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J. Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen EI. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 3.Levelt CN, Hübener M. Critical-period plasticity in the visual cortex. Annu. Rev. Neurosci. 2012;35:309–330. doi: 10.1146/annurev-neuro-061010-113813. [DOI] [PubMed] [Google Scholar]
- 4.Pizzorusso T, Medini P, Landi S, Baldini S, Berardi N, Maffei L. Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl. Acad. Sci. U.S.A. 2006;103:8517–8522. doi: 10.1073/pnas.0602657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi DM. Visual processing in amblyopia: Human studies. Strabismus. 2006;14:11–19. doi: 10.1080/09273970500536243. [DOI] [PubMed] [Google Scholar]
- 6.Kanonidou E. Amblyopia: A mini review of the literature. Int. Ophthalmol. 2011;31:249–256. doi: 10.1007/s10792-011-9434-z. [DOI] [PubMed] [Google Scholar]
- 7.Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J. Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat. Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Stryker MP. Distinctive features of adult ocular dominance plasticity. J. Neurosci. 2008;28:10278–10286. doi: 10.1523/JNEUROSCI.2451-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehmann K, Löwel S. Age-dependent ocular dominance plasticity in adult mice. PLOS One. 2008;3:e3120. doi: 10.1371/journal.pone.0003120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawtell NB, Frenkel MY, Philpot BD, Nakazawa K, Tonegawa S, Bear MF. NMDA receptor-dependent ocular dominance plasticity in adult visual cortex. Neuron. 2003;38:977–985. doi: 10.1016/s0896-6273(03)00323-4. [DOI] [PubMed] [Google Scholar]
- 12.He HY, Ray B, Dennis K, Quinlan EM. Experience-dependent recovery of vision following chronic deprivation amblyopia. Nat. Neurosci. 2007;10:1134–1136. doi: 10.1038/nn1965. [DOI] [PubMed] [Google Scholar]
- 13.Kang E, Durand S, LeBlanc JJ, Hensch TK, Chen C, Fagiolini M. Visual acuity development and plasticity in the absence of sensory experience. J. Neurosci. 2013;33:17789–17796. doi: 10.1523/JNEUROSCI.1500-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Syken J, Grandpre T, Kanold PO, Shatz CJ. PirB restricts ocular-dominance plasticity in visual cortex. Science. 2006;313:1795–1800. doi: 10.1126/science.1128232. [DOI] [PubMed] [Google Scholar]
- 15.Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGee AW, Yang Y, Fischer QS, Daw NW, Strittmatter SM. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morishita H, Miwa JM, Heintz N, Hensch TK. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science. 2010;330:1238–1240. doi: 10.1126/science.1195320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marik SA, Olsen O, Tessier-Lavigne M, Gilbert CD. Death receptor 6 regulates adult experience-dependent cortical plasticity. J. Neurosci. 2013;33:14998–15003. doi: 10.1523/JNEUROSCI.2398-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. doi: 10.1126/science.1161151. [DOI] [PubMed] [Google Scholar]
- 20.Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Shatz CJ. Human LilrB2 is a b-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer’s model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maeda A, Kurosaki M, Ono M, Takai T, Kurosaki T. Requirement of SH2-containing protein tyrosine phosphatases SHP-1 and SHP-2 for paired immunoglobulin-like receptor B (PIR-B)–mediated inhibitory signal. J. Exp. Med. 1998;187:1355–1360. doi: 10.1084/jem.187.8.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamura A, Kobayashi E, Takai T. Exacerbated graft-versus-host disease in Pirb−/− mice. Nat. Immunol. 2004;5:623–629. doi: 10.1038/ni1074. [DOI] [PubMed] [Google Scholar]
- 23.Adelson JD, Barreto GE, Xu L, Kim T, Brott BK, Ouyang YB, Naserke T, Djurisic M, Xiong X, Shatz CJ, Giffard RG. Neuroprotection from stroke in the absence of MHCI or PirB. Neuron. 2012;73:1100–1107. doi: 10.1016/j.neuron.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djurisic M, Vidal GS, Mann M, Aharon A, Kim T, Ferrao Santos A, Zuo Y, Hübener M, Shatz CJ. PirB regulates a structural substrate for cortical plasticity. Proc. Natl. Acad. Sci. U.S.A. 2013;110:20771–20776. doi: 10.1073/pnas.1321092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hensch TK, Fagiolini M, Mataga N, Stryker MP, Baekkeskov S, Kash SF. Local GABA circuit control of experience-dependent plasticity in developing visual cortex. Science. 1998;282:1504–1508. doi: 10.1126/science.282.5393.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanold PO, Kim YA, GrandPre T, Shatz CJ. Co-regulation of ocular dominance plasticity and NMDA receptor subunit expression in glutamic acid decarboxylase-65 knock-out mice. J. Physiol. 2009;587:2857–2867. doi: 10.1113/jphysiol.2009.171215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porciatti V, Pizzorusso T, Maffei L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Res. 1999;39:3071–3081. doi: 10.1016/s0042-6989(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 30.Schuett S, Bonhoeffer T, Hübener M. Mapping retinotopic structure in mouse visual cortex with optical imaging. J. Neurosci. 2002;22:6549–6559. doi: 10.1523/JNEUROSCI.22-15-06549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tohmi M, Kitaura H, Komagata S, Kudoh M, Shibuki K. Enduring critical period plasticity visualized by transcranial flavoprotein imaging in mouse primary visual cortex. J. Neurosci. 2006;26:11775–11785. doi: 10.1523/JNEUROSCI.1643-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tohmi M, Takahashi K, Kubota Y, Hishida R, Shibuki K. Transcranial flavoprotein fluorescence imaging of mouse cortical activity and plasticity. J. Neurochem. 2009;109(Suppl. 1):3–9. doi: 10.1111/j.1471-4159.2009.05926.x. [DOI] [PubMed] [Google Scholar]
- 33.William CM, Andermann ML, Goldey GJ, Roumis DK, Reid RC, Shatz CJ, Albers MW, Frosch MP, Hyman BT. Synaptic plasticity defect following visual deprivation in Alzheimer’s disease model transgenic mice. J. Neurosci. 2012;32:8004–8011. doi: 10.1523/JNEUROSCI.5369-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsien JZ, Chen DF, Gerber D, Tom C, Mercer EH, Anderson DJ, Mayford M, Kandel ER, Tonegawa S. Subregion- and cell type–restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- 35.Ramanan N, Shen Y, Sarsfield S, Lemberger T, Schütz G, Linden DJ, Ginty DD. SRF mediates activity-induced gene expression and synaptic plasticity but not neuronal viability. Nat. Neurosci. 2005;8:759–767. doi: 10.1038/nn1462. [DOI] [PubMed] [Google Scholar]
- 36.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, Lein ES, Zeng H. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis S, Gale NW, Aldrich TH, Maisonpierre PC, Lhotak V, Pawson T, Goldfarb M, Yancopoulos GD. Ligands for EPH-related receptor tyrosine kinases that require membrane attachment or clustering for activity. Science. 1994;266:816–819. doi: 10.1126/science.7973638. [DOI] [PubMed] [Google Scholar]
- 38.Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- 39.Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsushita H, Endo S, Kobayashi E, Sakamoto Y, Kobayashi K, Kitaguchi K, Kuroki K, Söderhäll A, Maenaka K, Nakamura A, Strittmatter SM, Takai T. Differential but competitive binding of Nogo protein and class I major histocompatibility complex (MHCI) to the PIR-B ectodomain provides an inhibition of cells. J. Biol. Chem. 2011;286:25739–25747. doi: 10.1074/jbc.M110.157859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Prior experience enhances plasticity in adult visual cortex. Nat. Neurosci. 2006;9:127–132. doi: 10.1038/nn1610. [DOI] [PubMed] [Google Scholar]
- 42.Knudsen EI, Zheng W, DeBello WM. Traces of learning in the auditory localization pathway. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11815–11820. doi: 10.1073/pnas.97.22.11815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Linkenhoker BA, von der Ohe CG, Knudsen EI. Anatomical traces of juvenile learning in the auditory system of adult barn owls. Nat. Neurosci. 2005;8:93–98. doi: 10.1038/nn1367. [DOI] [PubMed] [Google Scholar]
- 44.Hofer SB, Mrsic-Flogel TD, Bonhoeffer T, Hübener M. Experience leaves a lasting structural trace in cortical circuits. Nature. 2009;457:313–317. doi: 10.1038/nature07487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR. Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron. 2000;28:41–51. doi: 10.1016/s0896-6273(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 46.Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: Implications for the maturation of synaptic physiology and long-term potentiation. J. Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitchell DE, Duffy KR. The case from animal studies for balanced binocular treatment strategies for human amblyopia. Ophthalmic Physiol. Opt. 2014;34:129–145. doi: 10.1111/opo.12122. [DOI] [PubMed] [Google Scholar]
- 48.Sengpiel F. Experimental models of amblyopia: Insights for prevention and treatment. Strabismus. 2011;19:87–90. doi: 10.3109/09273972.2011.600419. [DOI] [PubMed] [Google Scholar]
- 49.Montey KL, Quinlan EM. Recovery from chronic monocular deprivation following reactivation of thalamocortical plasticity by dark exposure. Nat. Commun. 2011;2:317. doi: 10.1038/ncomms1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beurdeley M, Spatazza J, Lee HH, Sugiyama S, Bernard C, Di Nardo AA, Hensch TK, Prochiantz A. Otx2 binding to perineuronal nets persistently regulates plasticity in the mature visual cortex. J. Neurosci. 2012;32:9429–9437. doi: 10.1523/JNEUROSCI.0394-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akbik FV, Bhagat SM, Patel PR, Cafferty WB, Strittmatter SM. Anatomical plasticity of adult brain is titrated by Nogo Receptor 1. Neuron. 2013;77:859–866. doi: 10.1016/j.neuron.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zemmar A, Weinmann O, Kellner Y, Yu X, Vicente R, Gullo M, Kasper H, Lussi K, Ristic Z, Luft AR, Rioult-Pedotti M, Zuo Y, Zagrebelsky M, Schwab ME. Neutralization of Nogo-A enhances synaptic plasticity in the rodent motor cortex and improves motor learning in vivo. J. Neurosci. 2014;34:8685–8698. doi: 10.1523/JNEUROSCI.3817-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Montey KL, Eaton NC, Quinlan EM. Repetitive visual stimulation enhances recovery from severe amblyopia. Learn. Mem. 2013;20:311–317. doi: 10.1101/lm.030361.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsien JZ. Behavioral genetics: Subregion- and cell type–restricted gene knockout in mouse brain. Pathol. Biol. (Paris) 1998;46:699–700. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.