Abstract
Galangin and myricetin are flavonoids isolated from vegetables and fruits which exhibit anti-proliferative activity in human cancer cells. In this study, their anti-angiogenic effects were investigated with in vitro (HUVEC) and in vivo (CAM) models, which showed that galangin and myricetin inhibited angiogenesis induced by OVCAR-3 cells. The molecular mechanisms through which galangin and myricetin suppress angiogenesis were also studied. It was observed that galangin and myricetin inhibited secretion of the key angiogenesis mediator vascular endothelial growth factor (VEGF) and decreased levels of p-Akt, p-70S6K and hypoxia-inducible factor-1α (HIF-1α) proteins in A2780/CP70 and OVCAR-3 cells. Transient transfection experiments showed that galangin and myricetin inhibited secretion of VEGF by the Akt/p70S6K/ HIF-1α pathway. Moreover, a novel pathway, p21/HIF-1α/VEGF, was found to be involved in the inhibitory effect of myricetin on angiogenesis in OVCAR-3 cells. These data suggest that galangin and myricetin might serve as potential anti-angiogenic agents in the prevention of ovarian cancers dependent on new blood vessel networks.
Keywords: Galangin, Myricetin, p21, Angiogenesis, Ovarian cancer
1. Introduction
Recent studies have focused on the anti-cancer activity of flavonoids isolated from plants (Park et al., 2014; Zhang et al., 2014; Zivkovic et al., 2014). Flavonoids are natural polyphenols present in many different foods, especially fruits and vegetables. Previous studies have suggested that flavonoids display anticancer characteristics and might be able to decrease cancer risk through the mechanisms of preventing oxidation and inflammation, diminishing angiogenesis and cell proliferation, and inducing apoptosis (Gates et al., 2009; Kandasamy & Ashokkumar, 2013; Li et al., 2009; Li, Wang, Guo, Zhao, & Ho, 2014; Ma et al., 2014; Yu et al., 2014). Galangin and myricetin are members of the flavonol subclass of flavonoids with antioxidant activities. Galangin was isolated from the galangal rhizome and propolis. Myricetin is more common than galangin and can be found in fruits, vegetables, nuts, berries, tea and red wine (Basli et al., 2012; Ross & Kasum, 2002).
Angiogenesis is a physiologic process through which new blood vessels form from pre-existing vessels (Birbrair et al., 2014). It is also the fundamental step in the transition of tumours, including ovarian tumours, from a benign state to a malignant one. Angiogenesis is typically quiescent in normal adult tissues (Bertl, Bartsch, & Gerhauser, 2006). A microtumour growing beyond 1 to 2 mm2 faces a limited nutrition and oxygen supply and requires an intact blood system to support its own growth and shed metabolites (Bertl, Bartsch, & Gerhauser, 2006). In contrast, tumour-free adults have no need for angiogenesis in normal situations (Fotsis et al., 1993; Glade-Bender, Kandel, & Yamashiro, 2003). Anti-angiogenesis has been reported to be feasible for treating human cancers and has become one of the most promising strategies in the chemoprevention and treatment of cancers. These early-stage tiny tumours cannot be successfully diagnosed but their continued development demands a new blood vessel network (Sanchez-Munoz, Perez-Ruiz, Mendiola, Alba, & Gonzalez-Martin, 2009). Previous studies have reported that galangin and myricetin decrease angiogenesis in human umbilical vein endothelial cells (HUVECs) (Kim, Liu, Guo, & Meydani, 2006). Myricetin also inhibited angiogenesis in a SKH-1 hairless mouse skin tumourigenesis model induced by UVB (Jung et al., 2010).
Vascular endothelial growth factor (VEGF) plays a central role in the mediation of tumour vascular development and maintenance (Hefler et al., 2007). Therefore, anti-VEGF therapies are important in the treatment of cancers. The VEGF gene is directly regulated by hypoxia-inducible factor 1α (HIF-1α), a heterodimeric basic helix-loop-helix protein (Forsythe et al., 1996). Stabilization and up-regulation of HIF-1α promotes the expression of VEGF by binding to HIF-responsive elements (HREs) in promoters. Ribosomal protein S6 kinase (p70S6K), a serine/threonine kinase that acts downstream of the PI3K/Akt pathway, controls angiogenesis through regulating HIF-1α and VEGF proteins (Bian, Shi, Meng, Jiang, Liu, & Jiang, 2010). Akt is a mediator of VEGF that enhances pathological angiogenesis and tumour growth associated with matrix abnormalities in skin and blood vessels (Chen et al., 2005; Somanath, Razorenova, Chen, & Byzova, 2006). P21, known as a regulator of cell cycle progression, also negatively regulates VEGF protein in some cancer cells including OVCAR-3 cells (Farhang, Goossens, & Haigh, 2013; Luo, Rankin, Juliano, Jiang, & Chen, 2012).
In this study, the effects of galangin and myricetin on reducing angiogenesis were examined in two platinum-resistant ovarian cancer cell lines: A2780/CP70 and OVCAR-3. The mechanisms involved in the effects of galangin and myricetin on angiogenesis were also investigated.
2. Materials and methods
2.1. Cell culture and reagents
Human ovarian cancer cell lines, OVCAR-3 and A2780/CP70, were kindly provided by Dr. B. Jiang, Department of Microbiology, Immunology, and Cell Biology, West Virginia University, Morgantown, WV, USA. Cells were maintained in RPMI 1640 medium (Sigma, St. Louis, MO, USA) supplemented with 10% US-qualified fetal bovine serum (Invitrogen, Grand Island, NY, USA). HUVEC cells were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and cultured in a vascular cell basal medium (ATCC) supplemented with Endothelial Cell Growth Kit-VEGF (ATCC). All cells were maintained in a humidified incubator with 5% CO2 at 37 °C. Galangin (purity: 98%) was purchased from Shanghai yuanye Bio-Technology (Shanghai, China). Myricetin (purity: 97%) was purchased from J & K Chemical Technology (Beijing, China). Galangin and myricetin were dissolved in dimethyl sulphoxide (DMSO) to make stock solutions of 100 mM. The primary antibodies against phospho-Akt (Ser473) (p-Akt), total-Akt (t-Akt), phospho-ribosomal protein S6 kinase (p-p70S6K), total-p70S6K (t-p70S6K), and p21 were purchased from Cell Signaling Technology, Inc. (Dancers, MA, USA). The primary antibodies against p53, NFκB (p50), PTEN and GAPDH were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). HIF-1α, mAkt, p70S6K plasmids and VEGF, HIF-1α reporter were purchased from Addgene (Cambridge, MA, USA).
2.2. Cell proliferation
Cell growth inhibition was determined with a “CellTiter 96 AQueous One Solution Cell Proliferation Assay” kit from Promega (Madison, WI, USA). The cells were seeded into 96-well plates at a density of 2×104/well and incubated at 37 °C. Cells were allowed to attach to the bottom overnight and then treated with different concentrations of galangin/myricetin. Control cells received an equal volume of DMSO only. After 24 hours, the 100 μL CellTiter 96 AQueous One Solution Reagent dilute solution (80 μL PBS + 20μL CellTiter 96 AQueous One Solution Reagent) were added to each well. Cells were then incubated at 37 °C for another 1 h and measured at 490 nm. Cell viability was expressed as a percentage of the control from three independent experiments.
2.3. ELISA for VEGF
The levels of VEGF in cell culture supernatants were analyzed by a Quantikine Human VEGF Immunoassay Kit (R&D Systems, Minneapolis, MN, USA). A2780/CP70 and OVCAR-3 cells (2×104/well) were seeded into 96-well plates and incubated overnight. Subsequently, the cells were treated with galangin/myricetin or DMSO for 24 h in serum free medium. Culture supernatants were collected. The amounts of VEGF were measured following the manufacturer's instructions, normalized to total protein levels, and expressed as a percentage of the untreated control.
2.4. In-vitro angiogenesis assay
OVCAR-3 cancer cells were seeded into 96-well plates at 2×104/well and incubated overnight before treatment with different concentrations of galangin/myricetin for 24 h. The conditioned medium was collected. Growth factor reduced Matrigels (BD Biosciences, San Jose, CA, USA) were added into 96-well plates at 50 μL/well and incubated at 37 °C for 30 min to gel. HUVEC cells were harvested in vascular cell basal medium and seeded into Matrigel beds at a concentration of 1.5×104/90 μL medium. Afterwards, 10 μL of collected conditioned medium were added to each well and then incubated at 37 °C for 6 h. Each well was photographed under a microscope. Each picture of 1388×1040 pixels was further divided into 6 rectangular areas by gridlines to obtain the tube length using the NIH ImageJ software. Angiogenesis was evaluated by normalizing tube length to that of the control.
2.5. In-vivo angiogenesis assay
Specific pathogen-free fertile chicken eggs (Charles River Laboratories, North Franklin, CT, USA) were incubated at 37.5 °C and slowly turned by an automatic egg turner (G.Q.F. Manufacturing Company, Savannah, GA, USA). The OVCAR-3 cells (1.2×106 cells in a 20 μL FBS-free medium) were mixed with 80 μL of Matrigel (BD Bioscience), supplemented with different concentrations of galangin/myricetin, pre-gelled on an autoclaved silicone mat for 30 min, and implanted into the chorioallantoic membrane (CAM) of the 9-day-old chicken embryo. After incubating another 5 days, tumour implants and blood vessels were photographed and counted for branching blood vessels by three investigators blinded to the treatment. Angiogenesis was evaluated by normalizing the number of branching vessels to that of control CAM.
2.6. Western blot
Ovarian cancer cells (106) were seeded in 60-mm dishes and incubated overnight before treatment with galangin/myricetin or DMSO for 24 h. The cells were washed with PBS, lysed in 100 μL mammalian protein extraction reagent including 1 μL Halt Protease, 1 μL phosphatase inhibitor and 2 μL eathylenediaminetetraacetic acid (EDTA) (M-PER, Pierce, Rockford, IL, USA), as per manufacturer's instructions. Total protein levels were assayed with a BCA Protein Assay Kit (Pierce). Cell lysates were separated by 10% SDS-PAGE and blotted onto a nitrocellulose membrane with a Mini-Protean 3 System (Bio-Rad, Hercules, CA, USA). The membranes were blocked in 5% nonfat milk in Tris-buffer saline containing 0.1% Tween-20 for 1 h at room temperature. The membranes were incubated with the appropriate dilutions of the primary antibodies and secondary antibodies. After washing with TBST, the antigen-antibody complex was visualized with the SuperSignal West Dura Extended Duration Substrate (Pierce). Protein bands were quantitated with NIH ImageJ software, normalized by corresponding GAPDH for analysis.
2.7. Transfection with small interfering RNA (siRNA)
OVCAR-3 cells were seeded in 60-mm dishes at 5 × 105/dish and incubated overnight before transfection with p21 siRNA or control siRNA (Santa Cruz Biotechnology) using jetPRIME™ DNA and siRNA Transfection Reagent (VWR International, Radnor, PA, USA) according to the manufacturer's protocol. After 24 hours, cells were treated with myricetin or DMSO. Cell lysates were collected for Western blot to test p70S6K, Akt, and HIF-lα proteins.
2.8. Plasmid transfection and luciferase assay
OVCAR-3 cells were seeded in 96-well plates at 10,000 cells/well and incubated overnight. The OVCAR-3 cells were transfected with Akt, p70S6K/HIF-lα, or SR-α (as vehicle) plasmids, and HIF-1α/VEGF luciferase reporter using jetPRIME™ DNA and siRNA Transfection Reagent (VWR International) according to the manufacturer's protocol. Four hours after transfection, the mediums were removed and followed by a 24 h treatment with galangin/myricetin. The cells were harvested and analyzed for luciferase activities with ONE-Glo Luciferase Assay System (Promega) and detected by Lumat LB9507 (Berthold Technologies, Bad Wildbad, Germany). Total protein levels were analyzed with a BCA Protein Assay Kit (Pierce), and the activities of the reporter were normalized by corresponding total protein levels for statistical analysis.
2.9. Small interfering RNA (siRNA) transfection luciferase assay
OVCAR-3 ovarian cancer cells (5000 cells/well) were seeded in 96-well plates and incubated overnight. The cells were transfected with siRNA p21 or control siRNA, and then transfected with HIF-1α/VEGF luciferase reporter for 24 hours using jetPRIME™ DNA and siRNA Transfection Reagent (VWR International) according to the manufacturer's protocol. The mediums were removed and followed by a 24 h treatment with galangin/myricetin. The cells were harvested and analyzed for luciferase activities with ONE-Glo Luciferase Assay System (Promega) and detected by Lumat LB9507 (Berthold Technologies). Total protein levels were analyzed with a BCA Protein Assay Kit (Pierce), and the activities of the reporter were normalized by corresponding total protein levels for statistical analysis.
2.10. Statistical analysis
All experiments were performed at least three times independently. Results were expressed as mean ± standard error of mean (SEM) using Microsoft Excel (2007). Statistical assessment was carried out with the program system of SPSS (Version 18.0 for Windows). The results were analyzed using one-way analysis of variance (ANOVA) and post hoc test (2-sided Dunnett's test) to test both overall differences and specific differences between each treatment and control. A p value of less than 0.05 was considered significant.
3. Results
3.1. Effect of galangin and myricetin on ovarian cancer cells viability
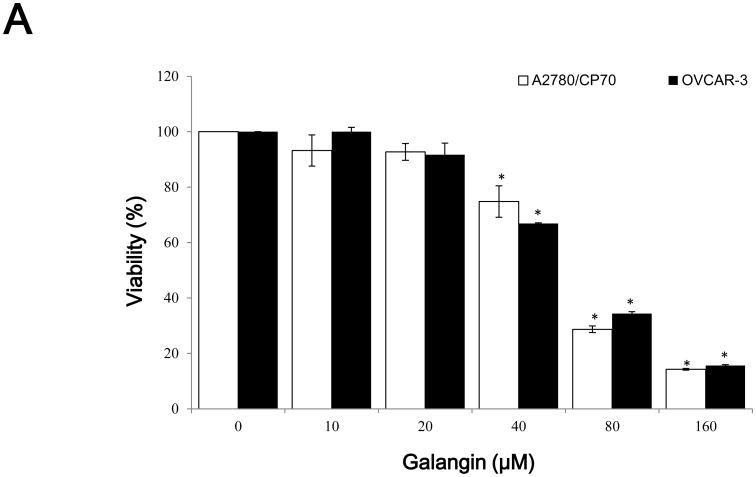
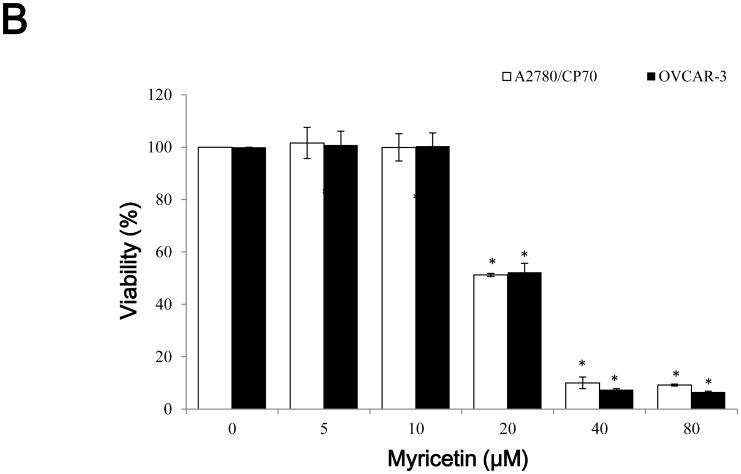
CellTiter 96 AQueous One Solution Cell Proliferation Assay was performed after treatment of ovarian cancer cells with galangin and myricetin to investigate their effects on the viability of A2780/CP70 and OVCAR-3 cells. It showed that galangin had little effect on A2780/CP70 and OVCAR-3 cell viability at concentrations of 20 μM galangin and below. Cell viability was decreased from 74.4 and 66.8% at a concentration of 40 μM galangin to 14.2 and 15.6% at a concentration of 160 μM galangin in A2780/CP70 and OVCAR-3 cells. (p<0.05) (Figure 1A). Myricetin had more cytotoxicity to the ovarian cancer cells than galangin. Compared with controls (DMSO), cell viability with myricetin treatment (5-80 μM) for 24 h ranged from 101.6 and 100.8% to 9.1 and 6.6% (p<0.05) for A2780/CP70 and OVCAR-3 cells, respectively (Figure 1B).
Fig. 1. The cytotoxic effects of galangin and myricetin on ovarian cancer cells A2780/CP70 and OVCAR-3.
(A) Galangin decreased cell viability in ovarian cancer cells. (B) Myricetin decreased cell viability in ovarian cancer cells. Cells (2×104 /well) were seeded in 96-well plates, incubated overnight, and then treated with galangin or myricetin for 24 h. Cell viability was determined by CellTiter 96 AQueous One Solution Cell Proliferation Assay and expressed as percentages of control. *p < 0.05 as compared to control.
3.2. Galangin and myricetin reduce VEGF secreted by A2780/CP70 and OVCAR-3 cells
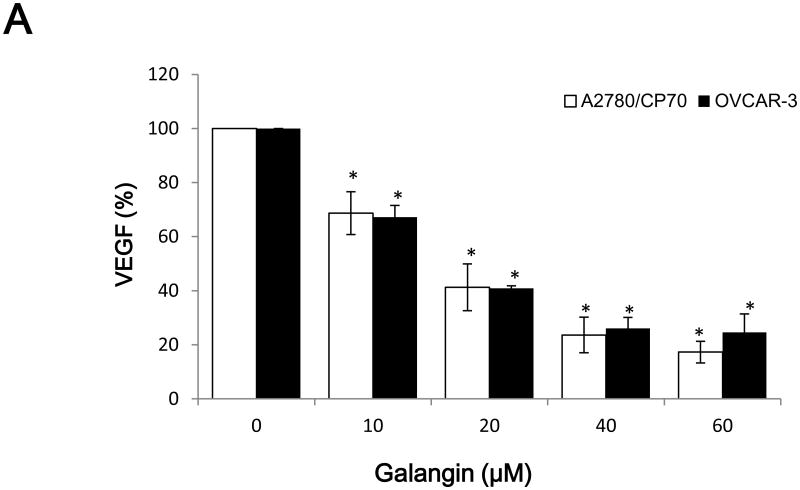
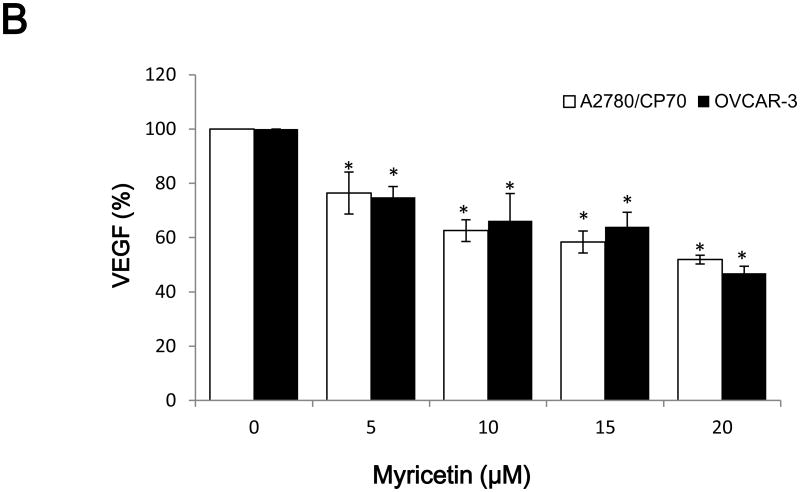
Vascular endothelial growth factor (VEGF), an important growth factor that is involved in tumour vascular development and maintenance (Duyndam et al., 2002), was investigated. As shown in Figure 2A, VEGF levels were 68.7%, 17.3% (p<0.05) in A2780/CP70 cell and 67.2%, 24.6% (p<0.05) in OVCAR-3 cell inhibited by 10 and 60 μM galangin treatment, respectively. Similarly, Figure 2B shows that VEGF levels were 74.4 and 51.9% (p<0.05) in A2780/CP70 cell and 74.9 and 46.9% (p<0.05) in OVCAR-3 cell inhibited by 5 and 20 μM myricetin treatment.
Fig. 2. The inhibitory effects of galangin and myricetin on VEGF secretion.
(A) Galangin inhibited secretion of VEGF by A2780/CP70 and OVCAR-3 cells. (B) Myricetin inhibited secretion of VEGF by A2780/CP70 and OVCAR-3 cells. The human ovarian cancer cell line A2780/CP70 and OVCAR-3 were treated with varying concentrations of galaing and myricetin for 24 hours. Subsequently, the VEGF levels in the supernatant were measured by ELISA. *p < 0.05 as compared to control.
3.3. Galangin and myricetin inhibit in vitro angiogenesis induced by OVCAR-3 cells
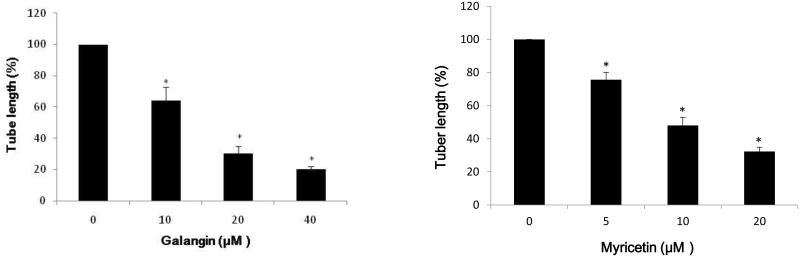
The effective inhibition of VEGF secretion by galangin and myricetin was expected to inhibit angiogenesis in vitro. Therefore, in vitro tube formation by HUVEC cells induced by the culture medium of ovarian cancer cells treated with different concentrations of galangin and myricetin was tested. It was found that culture media conditioned by ovarian cancer cells OVCAR-3 promoted in vitro angiogenesis and a well-established network formation of HUVEC cells (Figure 3). HUVEC networks, however, were truncated after galangin and myricetin treatment. A significant reduction in tube length was observed at a concentration of 10 μM galangin and 5 μM myricetin when compared to the corresponding controls (p<0.05) (Figure 3).
Fig. 3. Galangin and myricetin inhibited in vitro (HUVEC) angiogenesis induced by OVCAR-3 cells.
OVCAR-3 cells were seeded (2×104), treated with vary concentrations of galangin or myricetin for 24 hours, and then the media were collected. HUVEC cells were harvested, counted and seeded onto the gelled Matrigel beds. The collected conditioned cell culture media were added and the cells were incubated for 6 hours.
3.4. Galangin and myricetin inhibit in vivo angiogenesis induced by OVCAR-3 cells
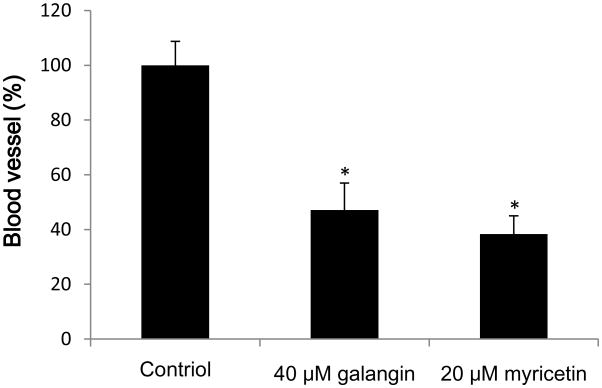
This experiment was performed to test the hypothesis that effective inhibition of VEGF secretion by galangin and myricetin treatment can be translated into anti-angiogenic effects in vivo. Chicken chorioallantoic membrane (CAM) assay was used to detect their effects on angiogenesis induced by ovarian cancer cells. As shown in Figure 4, galangin (40 μM) and myricetin (20 μM) significantly reduced formation of blood vessels which were induced by OVCAR-3 cells in CAM (p<0.05).
Fig. 4. Galangin and myricetin inhibited in vivo (CAM) angiogenesis induced by OVCAR-3 cells.
Angiogenesis was evaluated by normalizing number of branching vessels to that of control CAM. The data are represented as mean ± SEM (n=6 eggs/group). *p < 0.05 as compared to control.
3.5. The pathway of anti-angiogenesis in ovarian cancer cells treated with galangin and myricetin
An investigation into which proteins are involved in the expression of VEGF in A2780/CP70 and OVCAR-3 cells was conducted. We detected the levels of HIF-1α, PTEN, NFκB (p50), p-Akt (Ser473), t-Akt, p-p70S6K and t-p70S6K proteins in A2780/CP70 and OVCAR-3 cells. Figure 5A and 5B show that galangin and myricetin strongly down-regulated levels of HIF-1α, inhibited phosphorylation of Akt and p70S6K, but had no effect on the expression of PTEN and NFκB (p50) in A2780/CP70 and OVCAR-3 cells.
Fig. 5. Galangin and myricetin inhibited angiogenesis in ovarian cancer cells were involved in Akt- and p70S6K-dependent pathways.
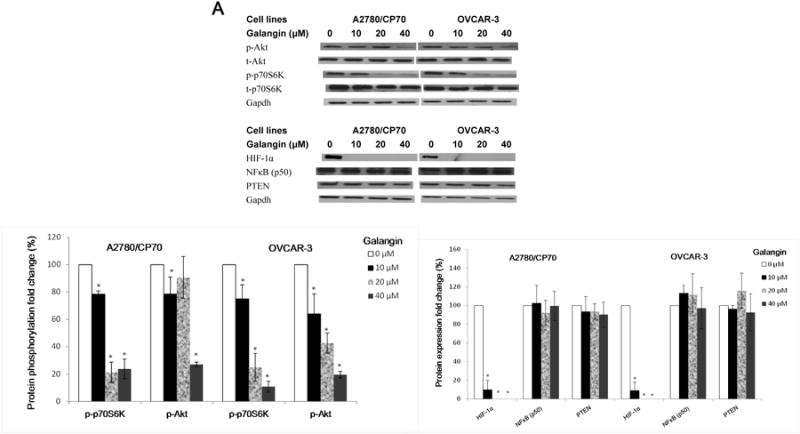
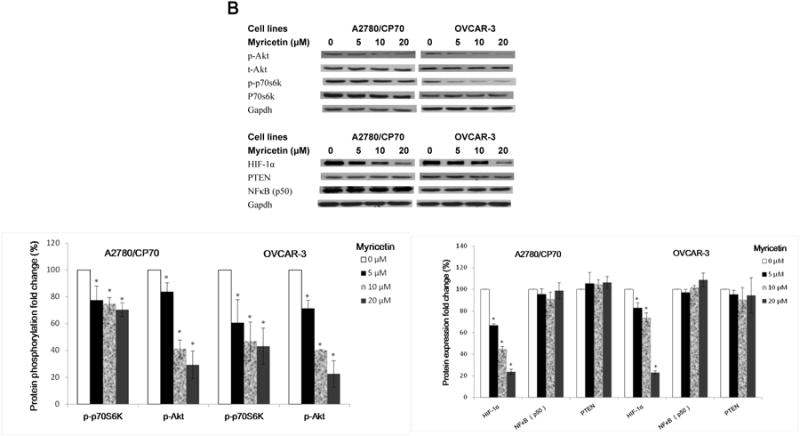
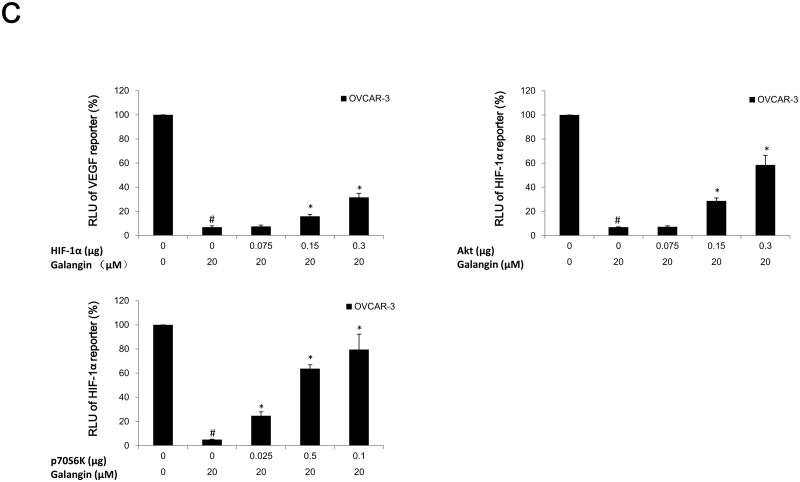
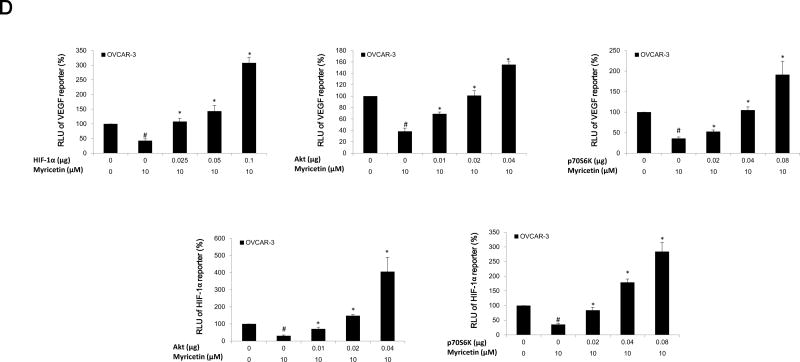
(A) Galangin decreased the levels of HIF-1 α, phosphor-Akt, phosphor-P70S6K and had no effect on NFκB and PTEN in A2780/CP70 and OVCAR-3. (B) Myricetin decreased the levels of HIF-1α, phosphor-Akt, phosphor-p70S6K and had no effect on NFκB and PTEN in A2780/CP70 and OVCAR-3. Protein bands were normalized by corresponding GAPDH bands (p-Akt/p-p70S6K were normalized by t-Akt/t-p70S6K), and expressed as percentages of control. *p < 0.05 as compared to control. (C) Overexpression of HIF-1α and Akt (p70S6K) protein attenuated the inhibitory effect of galangin on VEGF and HIF-1α transcriptional activation respectively. (D) Overexpression of HIF-1α, Akt and p70S6K protein reversed the inhibitory effect of myricetin on VEGF and HIF-1 α transcriptional activation. OVCAR-3 cancer cells were seeded in 96-well plate and incubated overnight. The cells were then transfected with VEGF (HIF-1α) luciferase reporter, HIF-1α (Akt, p70S6K) or SR-α plasmids for 4 hours, followed by 24 hours treatment with or without galangin or myricetin. The cells were harvested and analyzed for luciferase and total protein levels, and the levels of VEGF and HIF-1α reporters were normalized by corresponding total protein levels. #p < 0.05 as compared to control; *p < 0.05 as compared to galangin (myricetin)-treated control. (RLU: relative luminescence units)
HIF-1α is one of the key factors for the regulation of VEGF expression. Transfected plasmid HIF-1α concentration-dependently attenuated galangin's inhibitory effects on expression of VEGF in OVCAR-3 cells (Figure 5C). Next, OVCAR-3 cells were transfected with the HIF-1α reporter together with p70S6K and Akt plasmids. Galangin treatment significantly inhibited HIF-1α transcriptional activity; however, this inhibition was significantly attenuated by overexpression of p70S6K and Akt proteins (Figure 5C). Similarly, myricetin also significantly inhibited HIF-1α and VEGF transcriptional activities in OVCAR-3 cells. Concentration-dependent overexpression of p70S6K and Akt proteins significantly reversed this inhibition (Figure 5D). These results indicate that galangin and myricetin inhibit angiogenesis in OVCAR-3 cells, at least partly, through decreasing levels of HIF-1α, Akt and p70S6K.
3.6. Role of p21 on the myricetin-inhibited angiogenesis in OVCAR-3 cells
P21, as a regulator of cell cycle progression, has been reported to inhibit VEGF expression in OVCAR-3 cells (Luo, Rankin, Juliano, Jiang, & Chen, 2012). Therefore, some proteins which are associated with p21 were examined to clarify whether myricetin inhibits viability in OVCAR-3 cells via the p21 protein (Figure 6A). It was found that myricetin up-regulated levels of the p21 and p53 proteins and down-regulated levels of the oncogene cmyc protein.
Fig.6. Myricetin inhibited angiogenesis in OVCAR-3 cells with a p21-dependent pathway.
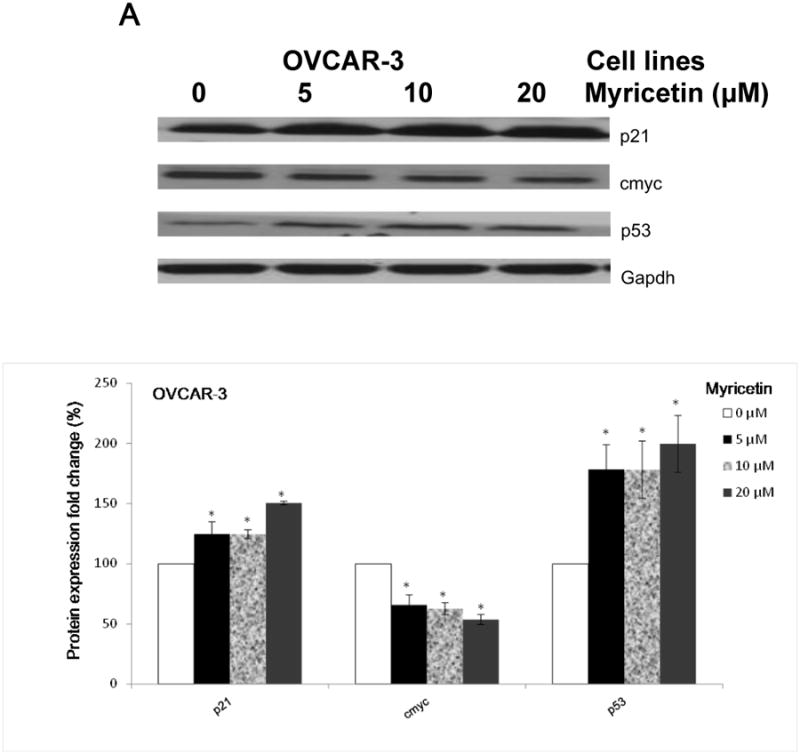
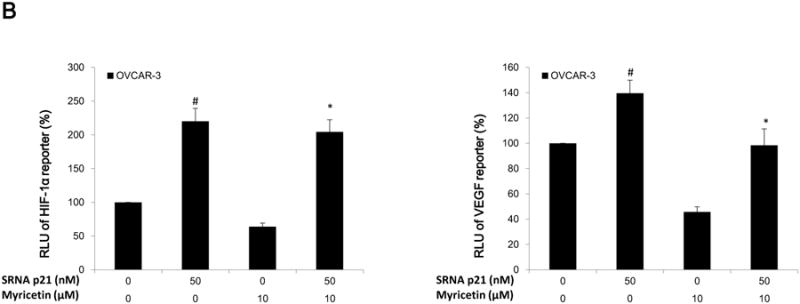
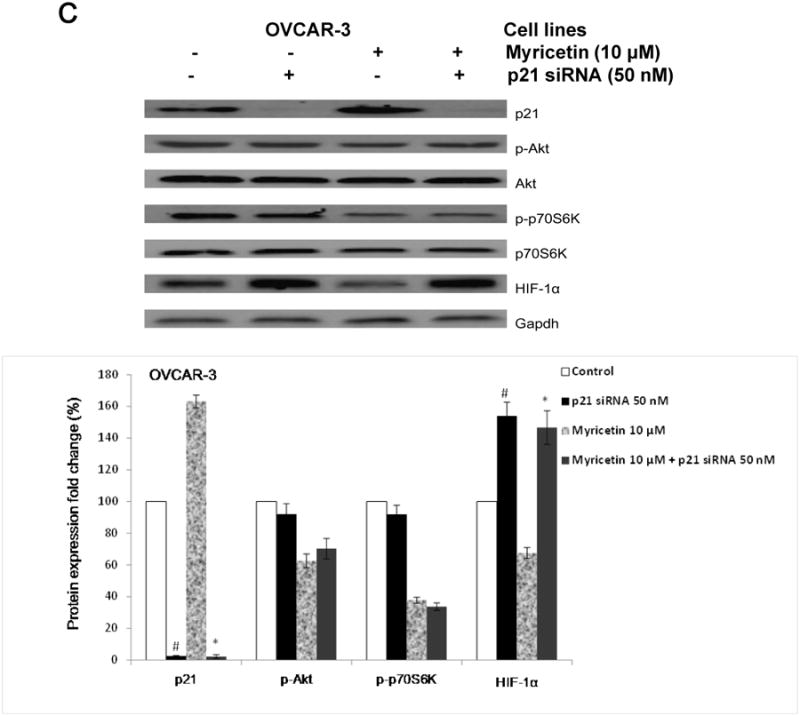
(A) Myricetin decreased the levels of cmyc and increased expression of p21 and p53 in OVCAR-3. Protein bands were normalized by corresponding GAPDH bands, and expressed as percentages of control. *p < 0.05 as compared to control. (B) Knockdown of p21 resulted in reversing inhibitory effect of myricetin on VEGF and HIF-1α transcriptional activation. OVCAR-3 cells were transfected with VEGF (HIF-1α) luciferase reporter with p21 siRNA or control siRNA for 24 hours, followed by 24 hours treatment with or without myricetin. The cells were harvested and analyzed for luciferase and total protein levels, and the levels of VEGF and HIF-1α reporters were normalized by corresponding total protein levels. (C) Knockdown p21 resulted in abrogation of myricetin-decreased levels of HIF-1α but not p-Akt and p-p70S6K protein. OVCAR-3 cells were seeded in 60-mm dishes, incubated overnight, and then transfected with p21 siRNA or control siRNA. After 24 hours, cells were treated with myricetin or DMSO. Cell lysates were collected for Western blot. Protein bands were normalized by corresponding GAPDH bands, and expressed as percentages of control. #p < 0.05 as compared to control; *p < 0.05 as compared to myricetin-treated control.
To determine the role of p21 on the anti-angiogenesis effect of myricetin, OVCAR-3 cells were transfected with HIF-1α and VEGF-promoter with p21 siRNA (50 nM). Figure 6B shows knockdown of p21 by specific siRNA (50 nM) resulted in the abrogation of myricetin-inhibited HIF-1α and VEGF transcriptional activities. As the western blot result shows, transfection with p21 siRNA increased the levels of HIF-1α protein in OVCAR-3 cells treated with and without myricetin. From these results, it was inferred that p21/HIF-1α/VEGF is one of the pathways involved with myricetin-inhibited angiogenesis in OVCAR-3 cells. However, the results from western blot showed that knockdown of p21 didn't abrogate the effect of myricetin on the levels of p-Akt and p-p70S6K proteins in OVCAR-3 cells (Figure 6C).
4. Discussion
While early diagnosis of ovarian cancer is still difficult, chemoprevention with dietary compounds might be a good strategy for the vast majority of healthy women who are at risk of developing ovarian cancers. Previous studies have reported that galangin and myricetin inhibited cell growth in several cancer cells, such as hepatoma, pancreatic, esophageal, melanoma, gastric, and colon carcinoma cells (Kim, Jeon, & Nam, 2012; Kim, Ha, Yoon, & Lee, 2014; Phillips, Sangwan, Borja-Cacho, Dudeja, Vickers, & Saluja, 2011; Zang et al., 2014; Zhang et al., 2010; Zhang, Lan, Huang, & Hua, 2013; Zhang, Chen et al., 2013). However, their effects on ovarian cancer cells are currently unknown. Galangin and myricetin, which have similar structure (same parent structure, ring A and ring C), are members of flavonol. However, compared to galangin, myricetin has 3 adjacent hydroxyl groups in ring B that results in different chemical properties such as polarity. The difference of structural characteristics between galangin and myricetin may lead to different efficacy in inhibiting the development of ovarian cancers. In this research, myricetin have more cytotoxicity to ovarian cancer cell lines A2780/CP70 and OVCAR-3 than galangin. It is in accordance with previous work which exhibited the inhibitory effects of flavonoids on leukemia cell line HL-60 as follows: myricetin > quercetin (2 adjacent hydroxyl groups in ring B) > galangin. The ortho-substituting hydroxyl group in ring B is associated with enhanced inhibitory effects of flavonol on the cancer cells proliferation (Chang, Mi, Gu, Yuan, Ling, & Lin, 2007).
Tumours need an intact blood system to supply nutritients and oxygen and shed metabolites (Bertl, Bartsch, & Gerhauser, 2006). Angiogenesis is a physiologic process through which new blood vessels form from pre-existing vessels (Birbrair et al., 2014). It is a necessary condition for sustained tumour growth and plays a central role in the development and progression of cancers. Ovarian tumours are richly vascularized, and the degree of neovascularization and angiogenesis is associated with biological aggressiveness (Alvarez, Krigman, Whitaker, Dodge, & Rodriguez, 1999). In this research, galangin and myricetin effectively inhibited formation of blood vessel networks induced by ovarian cancer cells. In the CAM model, galangin and myricetin treatment significantly shrunk blood vessel counts, confirming their potency in countering angiogenesis.
Vascular endothelial growth factor (VEGF) plays a central role in the mediation of tumour vascular development and maintenance. It is connected to various steps of ovarian carcinogenesis (Hefler et al., 2007). The VEGF gene is a direct target of hypoxia-inducible factor 1α (HIF-1α), a heterodimeric basic helix-loop-helix protein (Forsythe et al., 1996). Stabilization and up-regulation of HIF-1α promotes the expression of VEGF by binding to HIF-responsive elements (HREs) in promoters that contain the sequence NCGTG. Previous studies show that another flavonoid compound with similar chemical structure, kaempferol, inhibited angiogenesis in ovarian cancer cells through down-regulating HIF-1α expression (Jiang & Liu, 2008; Luo, Rankin, Liu, Daddysman, Jiang, & Chen, 2009). This is consistent with the result obtained here, indicating that galangin's and myricetin's inhibitory effects on angiogenesis in ovarian cancer cells can be traced back to suppression of HIF-1α expression. Combined with our previous work (Luo, Rankin, Liu, Daddysman, Jiang, & Chen, 2009), we found that galangin, myricetin and keampferol all significantly inhibited HIF-1α protein in A2780/CP70 and OVCAR-3 cells at a low concentration (< 10 μM). As members of flavonol, the three compounds have exactly similar chemical structure except ring B which is substituted with different amounts of hydroxyl group. We speculate that the parent structure of flavonol is related with the high inhibitory effects on the HIF-1α expression in A2780/CP70 and OVCAR-3 cells.
A previous study performed in OVCAR-3 cells showed that p70S6K had an effect on angiogenesis via regulating HIF-1α and VEGF proteins (Bian, Shi, Meng, Jiang, Liu, & Jiang, 2010). Akt has also been involved in angiogenesis and tumour development. In several in vivo studies, Akt, known as a mediator of VEGF, enhanced pathological angiogenesis and tumour growth associated with matrix abnormalities in skin and blood vessels (Chen et al., 2005; Somanath, Razorenova, Chen, & Byzova, 2006). Previous works indicate that HIF-1α and p70S6K proteins are also regulated by Akt (Blancher, Moore, Robertson, & Harris, 2001; Chung, Grammer, Lemon, Kazlauskas, & Blenis, 1994). In this present work, VEGF, HIF-1α, p-Akt and p-p70S6K proteins were all decreased after treatment with galangin and myricetin in A2780/CP70 and OVCAR-3 cells. Overexpression of HIF-1α protein reversed galangin's and myricetin's inhibitory effects on VEGF transcription in OVCAR-3 cells. Meanwhile overexpression of both p70S6K and Akt proteins in the presence of galangin and myricetin neutralized their inhibitory effects on HIF-1α transcription. We also found that the effect of myricetin on VEGF was neutralized by overexpression of either p70S6K or Akt proteins. These results confirm speculation that galangin and myricetin inhibit angiogenesis in OVCAR-3 cells, at least partly, through the Akt/p70S6K/HIF-1α/VEGF pathway. Previous works found that myricetin had effects on the Akt/p70S6K pathway in HepG2 and SKH-1 cells (Jung et al., 2010; Zhang, Chen et al., 2013) and galangin inhibits the phosphorylation of Akt in HepG2 cells (Zhang, Li et al., 2013). This agrees with the results obtained in this study. NFκB, a direct modulator of HIF-1α expression (van Uden, Kenneth, & Rocha, 2008), is associated with angiogenesis (Luo, Rankin, Juliano, Jiang, & Chen, 2012). It is regulated by Akt at the expression, activation, and translocation levels. It was reported that myricetin inhibited IkappaB kinase (IKK) activity and NFκB levels in ECV304 cells (Tsai, Liang, Lin-Shiau, & Lin, 1999). However, in the present work, galangin and myricetin had no effect on the expression of NFκB protein in A2780/CP70 and OVCAR-3 cells. PTEN, which acts as a tumour suppressor gene through the action of its phosphatase protein product such as p-Akt, sensitized human ovarian cancer cells to cisplatin-induced apoptosis and inhibited angiogenesis (Takei et al., 2008). Therefore, an investigation was made to determine whether galangin and myricetin inhibit the phosphorylation of Akt through promoting PTEN expression. However, no effect on the expression of PTEN was found.
P21, known as cyclin-dependent kinase inhibitor 1 or CDK-interacting protein 1, is a regulator of cell cycle progression. Some previous works indicate that p21 is a negative regulator of VEGF protein in cancer cells including OVCAR-3 cells (Farhang, Goossens, & Haigh, 2013; Luo, Rankin, Juliano, Jiang, & Chen, 2012). The effect of p21 protein on the myricetin-inhibited angiogenesis in OVCAR-3 cells was determined in this study. The result agrees with previous works in that p21 negatively regulated transcription of VEGF protein in OVCAR-3 cells. It was also found that p21 is a negative regulator of HIF-α at the transcriptional level, but has no effect on the phosphorylation of p-Akt and p-p70S6K. These results indicate that an Akt and p70S6K-independent pathway of p21/HIF-1α/VEGF, at least partly, was one of the pathways involved in myricetin-inhibited angiogenesis in OVCAR-3 cells. It is a new pathway, different from previous works that show HIF-1α as a regulator of p21 expression (Cho et al., 2008; He et al., 2013).
It is clear that p21 is tightly controlled by the tumour suppressor protein p53 (Gartel & Radhakrishnan, 2005). p53 is a multifunctional tumour suppressor that regulates transcription, DNA repair, cell cycle arrest, genomic instability, differentiation, senescence, apoptosis, angiogenesis and glucose metabolism (Darcy et al., 2008). Another study indicates that p53 inhibits angiogenesis through suppressing expression of VEGF (Farhang et al., 2013). HIF-1α, a direct regulator of VEGF expression, has been reported to be degraded by accumulation of p53 (Chen, Li, Luo, & Gu, 2003). Moreover, p53 has been shown to suppress transcriptional activity of HIF-1α (Ravi et al., 2000). Cmyc, a multifunctional nuclear protein, plays a role in several cell processes such as cell cycle progression, apoptosis, and cellular transformation, and it is regulated by the p53 protein (Ho, Ma, Mao, & Benchimol, 2005). Our previous study indicates that cmyc regulates VEGF expression via a p21-dependent pathway (Luo, Rankin, Juliano, Jiang, & Chen, 2012). In this current research, p53/cmyc/p21/HIF-1α/VEGF might be a pathway associated with myricetin-inhibited angiogenesis in OVCAR-3 cells. The two pathways associated with myricetin-inhibited VEGF secretion by OVCAR-3 cells are both involved in HIF-1α protein expression, indicating that HIF-1α is the key protein on the regulation of VEGF secretion by OVCAR-3 cells which is inhibited by myricetin.
5. Conclusion
This research indicates that galangin and myricetin are good candidates for the chemoprevention of ovarian cancer in women. In vitro (HUVEC) and in vivo (CAM) models demonstrate that galangin and myricetin inhibit angiogenesis induced by ovarian cancer cell lines. In OVCAR-3, the inhibitory effects of galangin and myricetin on angiogenesis, at least partly, are involved in the Akt/p70S6K/HIF-1α/VEGF pathway. It was also found that myricetin inhibits angiogenesis in OVCAR-3 cells through a new pathway: p21/HIF-1α/VEGF. As an in vitro model, cell cultures cannot take absorption, distribution, metabolism, and excretion of galangin or myricetin into consideration. Further studies in animal models and human trials are needed to further determine the efficacy of this natural compound as an agent for chemoprevention of ovarian cancer.
Two dietary compounds inhibited in vitro (HUVEC) angiogenesis.
Two dietary compounds inhibited in vivo (CAM) angiogenesis.
The effects on the secretion of VEGF is involved in Akt/p70S6K/HIF-1α pathway.
Angiogenesis inhibition is through p21/ HIF-1α/VEGF pathway.
Acknowledgments
We thank Dr. Kathy Brundage from the Flow Cytometry Core at West Virgina University for providing technical help on apoptosis and cell cycle analysis, and Yu Fu for giving critical review of the manuscript. This research was supported by a West Virginia Experimental Program to Stimulate Competitive Research grant and NIH grants (P20RR016477 and P20GM103434) from the National Institutes of Health awarded to the West Virginia IDeA Network of Biomedical Research Excellence. This project was also supported by the Chinese National Key Technologies R&D Program of 12th Five-year Plan (2012BAD31B06).
References
- Alvarez AA, Krigman HR, Whitaker RS, Dodge RK, Rodriguez GC. The prognostic significance of angiogenesis in epithelial ovarian carcinoma. Clinical Cancer Research. 1999;5(3):587–591. [PubMed] [Google Scholar]
- Basli A, Soulet S, Chaher N, Merillon JM, Chibane M, Monti JP, Richard T. Wine polyphenols: potential agents in neuroprotection. Oxidative Medicine and Cellular Longevity. 2012;2012:805762. doi: 10.1155/2012/805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5(3):575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- Bian CX, Shi Z, Meng Q, Jiang Y, Liu LZ, Jiang BH. P70S6K 1 regulation of angiogenesis through VEGF and HIF-1alpha expression. Biochem Biophys Res Commun. 2010;398(3):395–399. doi: 10.1016/j.bbrc.2010.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, Delbono O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol. 2014;307(1):C25–38. doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1alpha, HIF-2alpha, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61(19):7349–7355. [PubMed] [Google Scholar]
- Chang H, Mi MT, Gu YY, Yuan JL, Ling WH, Lin H. Effects of flavonoids with different structures on proliferation of leukemia cell line HL-60. Ai Zheng. 2007;26(12):1309–1314. [PubMed] [Google Scholar]
- Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. Journal of Biological Chemistry. 2003;278(16):13595–13598. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Bae JM, Chun YS, Chung JH, Jeon YK, Kim IS, Kim MS, Park JW. HIF-1alpha controls keratinocyte proliferation by up-regulating p21(WAF1/Cip1) Biochimica Et Biophysica Acta. 2008;1783(2):323–333. doi: 10.1016/j.bbamcr.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Chung J, Grammer TC, Lemon KP, Kazlauskas A, Blenis J. PDGF- and insulin-dependent pp70S6k activation mediated by phosphatidylinositol-3-OH kinase. Nature. 1994;370(6484):71–75. doi: 10.1038/370071a0. [DOI] [PubMed] [Google Scholar]
- Darcy KM, Brady WE, McBroom JW, Bell JG, Young RC, McGuire WP, Linnoila RI, Hendricks D, Bonome T, Farley JH Gynecologic Oncology G. Associations between p53 overexpression and multiple measures of clinical outcome in high-risk, early stage or suboptimally-resected, advanced stage epithelial ovarian cancers A Gynecologic Oncology Group study. Gynecol Oncol. 2008;111(3):487–495. doi: 10.1016/j.ygyno.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duyndam MC, Hilhorst MC, Schluper HM, Verheul HM, van Diest PJ, Kraal G, Pinedo HM, Boven E. Vascular endothelial growth factor-165 overexpression stimulates angiogenesis and induces cyst formation and macrophage infiltration in human ovarian cancer xenografts. Am J Pathol. 2002;160(2):537–548. doi: 10.1016/s0002-9440(10)64873-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang GM, Goossens S, Haigh JJ. The p53 family and VEGF regulation: “It's complicated”. Cell Cycle. 2013;12(9):1331–1332. doi: 10.4161/cc.24579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhang GM, Goossens S, Nittner D, Bisteau X, Bartunkova S, Zwolinska A, Hulpiau P, Haigh K, Haenebalcke L, Drogat B, Jochemsen A, Roger PP, Marine JC, Haigh JJ. p53 promotes VEGF expression and angiogenesis in the absence of an intact p21-Rb pathway. Cell Death Differ. 2013;20(7):888–897. doi: 10.1038/cdd.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotsis T, Pepper M, Adlercreutz H, Fleischmann G, Hase T, Montesano R, Schweigerer L. Genistein, a dietary-derived inhibitor of in vitro angiogenesis. Proc Natl Acad Sci U S A. 1993;90(7):2690–2694. doi: 10.1073/pnas.90.7.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65(10):3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- Gates MA, Vitonis AF, Tworoger SS, Rosner B, Titus-Ernstoff L, Hankinson SE, Cramer DW. Flavonoid intake and ovarian cancer risk in a population-based case-control study. International Journal of Cancer. 2009;124(8):1918–1925. doi: 10.1002/ijc.24151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glade-Bender J, Kandel JJ, Yamashiro DJ. VEGF blocking therapy in the treatment of cancer. Expert Opin Biol Ther. 2003;3(2):263–276. doi: 10.1517/14712598.3.2.263. [DOI] [PubMed] [Google Scholar]
- He M, Wang QY, Yin QQ, Tang J, Lu Y, Zhou CX, Duan CW, Hong DL, Tanaka T, Chen GQ, Zhao Q. HIF-1alpha downregulates miR-17/20a directly targeting p21 and STAT3: a role in myeloid leukemic cell differentiation. Cell Death Differ. 2013;20(3):408–418. doi: 10.1038/cdd.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefler LA, Mustea A, Konsgen D, Concin N, Tanner B, Strick R, Heinze G, Grimm C, Schuster E, Tempfer C, Reinthaller A, Zeillinger R. Vascular endothelial growth factor gene polymorphisms are associated with prognosis in ovarian cancer. Clinical Cancer Research. 2007;13(3):898–901. doi: 10.1158/1078-0432.CCR-06-1008. [DOI] [PubMed] [Google Scholar]
- Ho JS, Ma W, Mao DY, Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol Cell Biol. 2005;25(17):7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang BH, Liu LZ. AKT signaling in regulating angiogenesis. Curr Cancer Drug Targets. 2008;8(1):19–26. doi: 10.2174/156800908783497122. [DOI] [PubMed] [Google Scholar]
- Jung SK, Lee KW, Byun S, Lee EJ, Kim JE, Bode AM, Dong Z, Lee HJ. Myricetin inhibits UVB-induced angiogenesis by regulating PI-3 kinase in vivo. Carcinogenesis. 2010;31(5):911–917. doi: 10.1093/carcin/bgp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandasamy N, Ashokkumar N. Myricetin modulates streptozotocin-cadmium induced oxidative stress in long term experimental diabetic nephrotoxic rats. Journal of Functional Foods. 2013;5(3):1466–1477. [Google Scholar]
- Kim DA, Jeon YK, Nam MJ. Galangin induces apoptosis in gastric cancer cells via regulation of ubiquitin carboxy-terminal hydrolase isozyme L1 and glutathione S-transferase P. Food and Chemical Toxicology. 2012;50(3-4):684–688. doi: 10.1016/j.fct.2011.11.039. [DOI] [PubMed] [Google Scholar]
- Kim JD, Liu L, Guo W, Meydani M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. Journal of Nutritional Biochemistry. 2006;17(3):165–176. doi: 10.1016/j.jnutbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Kim ME, Ha TK, Yoon JH, Lee JS. Myricetin induces cell death of human colon cancer cells via BAX/BCL2-dependent pathway. Anticancer Research. 2014;34(2):701–706. [PubMed] [Google Scholar]
- Li S, Pan MH, Lo CY, Tan D, Wang Y, Shahidi F, Ho CT. Chemistry and health effects of polymethoxyflavones and hydroxylated polymethoxyflavones. Journal of Functional Foods. 2009;1(1):2–12. [Google Scholar]
- Li S, Wang H, Guo L, Zhao H, Ho CT. Chemistry and bioactivity of nobiletin and its metabolites. Journal of Functional Foods. 2014;6:2–10. [Google Scholar]
- Luo H, Rankin GO, Juliano N, Jiang BH, Chen YC. Kaempferol inhibits VEGF expression and in vitro angiogenesis through a novel ERK-NFkappaB-cMyc-p21 pathway. Food Chem. 2012;130(2):321–328. doi: 10.1016/j.foodchem.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Rankin GO, Liu L, Daddysman MK, Jiang BH, Chen YC. Kaempferol inhibits angiogenesis and VEGF expression through both HIF dependent and independent pathways in human ovarian cancer cells. Nutrition and Cancer-an International Journal. 2009;61(4):554–563. doi: 10.1080/01635580802666281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Lai CS, Chung CH, Yang JM, Hsu KC, Chen CY, Chung TS, Li S, Ho CT, Pan MH. 5-Demethyltangeretin is more potent than tangeretin in inhibiting dimethylbenz(a)anthracene (DMBA)/12-O-tetradecanoylphorbol-13-acetate (TPA)-induced skin tumorigenesis. Journal of Functional Foods. 2014;11:528–537. [Google Scholar]
- Park KI, Park HS, Kim MK, Hong GE, Nagappan A, Lee HJ, Yumnam S, Lee WS, Won CK, Shin SC, Kim GS. Flavonoids identified from Korean Citrus aurantium L. inhibit Non-Small Cell Lung Cancer growth in vivo and in vitro. Journal of Functional Foods. 2014;7:287–297. [Google Scholar]
- Phillips PA, Sangwan V, Borja-Cacho D, Dudeja V, Vickers SM, Saluja AK. Myricetin induces pancreatic cancer cell death via the induction of apoptosis and inhibition of the phosphatidylinositol 3-kinase (PI3K) signaling pathway. Cancer Letters. 2011;308(2):181–188. doi: 10.1016/j.canlet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi R, Mookerjee B, Bhujwalla ZM, Sutter CH, Artemov D, Zeng Q, Dillehay LE, Madan A, Semenza GL, Bedi A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000;14(1):34–44. [PMC free article] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Sanchez-Munoz A, Perez-Ruiz E, Mendiola FC, Alba CE, Gonzalez-Martin A. Current status of anti-angiogenic agents in the treatment of ovarian carcinoma. Clin Transl Oncol. 2009;11(9):589–595. doi: 10.1007/s12094-009-0409-8. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Razorenova OV, Chen J, Byzova TV. Akt1 in endothelial cell and angiogenesis. Cell Cycle. 2006;5(5):512–518. doi: 10.4161/cc.5.5.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Saga Y, Mizukami H, Takayama T, Ohwada M, Ozawa K, Suzuki M. Overexpression of PTEN in ovarian cancer cells suppresses i.p. dissemination and extends survival in mice. Mol Cancer Ther. 2008;7(3):704–711. doi: 10.1158/1535-7163.MCT-06-0724. [DOI] [PubMed] [Google Scholar]
- Tsai SH, Liang YC, Lin-Shiau SY, Lin JK. Suppression of TNFalpha-mediated NFkappaB activity by myricetin and other flavonoids through downregulating the activity of IKK in ECV304 cells. J Cell Biochem. 1999;74(4):606–615. [PubMed] [Google Scholar]
- van Uden P, Kenneth NS, Rocha S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochemical Journal. 2008;412(3):477–484. doi: 10.1042/BJ20080476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu EA, Kim GS, Jeong SW, Park S, Lee SJ, Kim JH, Lee WS, Bark KM, Jin JS, Shin SC. Flavonoid profile and biological activity of Korean citrus varieties (II): Pyunkyul (Citrus tangerina Hort. ex Tanaka) and overall contribution of its flavonoids to antioxidant effect. Journal of Functional Foods. 2014;6:637–642. [Google Scholar]
- Zang W, Wang T, Wang Y, Li M, Xuan X, Ma Y, Du Y, Liu K, Dong Z, Zhao G. Myricetin exerts anti-proliferative, anti-invasive, and pro-apoptotic effects on esophageal carcinoma EC9706 and KYSE30 cells via RSK2. Tumour Biol. 2014;35(12):12583–12592. doi: 10.1007/s13277-014-2579-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Li N, Wu J, Su L, Chen X, Lin B, Luo H. Galangin inhibits proliferation of HepG2 cells by activating AMPK via increasing the AMP/TAN ratio in a LKB1-independent manner. European Journal of Pharmacology. 2013;718(1-3):235–244. doi: 10.1016/j.ejphar.2013.08.026. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Luo H, Wu J, Lan LB, Fan DH, Zhu KD, Chen XY, Wen M, Liu HM. Galangin induces apoptosis of hepatocellular carcinoma cells via the mitochondrial pathway. World J Gastroenterol. 2010;16(27):3377–3384. doi: 10.3748/wjg.v16.i27.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JK, Wu YP, Zhao XY, Luo FL, Li X, Zhu H, Sun CD, Chen KS. Chemopreventive effect of flavonoids from Ougan (Citrus reticulata cv. Suavissima) fruit against cancer cell proliferation and migration. Journal of Functional Foods. 2014;10:511–519. [Google Scholar]
- Zhang W, Lan Y, Huang Q, Hua Z. Galangin induces B16F10 melanoma cell apoptosis via mitochondrial pathway and sustained activation of p38 MAPK. Cytotechnology. 2013;65(3):447–455. doi: 10.1007/s10616-012-9499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XH, Chen SY, Tang L, Shen YZ, Luo L, Xu CW, Liu Q, Li D. Myricetin induces apoptosis in HepG2 cells through Akt/p70S6K/bad signaling and mitochondrial apoptotic pathway. Anticancer Agents Med Chem. 2013;13(10):1575–1581. doi: 10.2174/1871520613666131125123059. [DOI] [PubMed] [Google Scholar]
- Zivkovic J, Barreira JCM, Stojkovic D, Cebovic T, Santos-Buelga C, Maksimovic Z, Ferreira ICFR. Phenolic profile, antibacterial, antimutagenic and antitumour evaluation of Veronica urticifolia Jacq. Journal of Functional Foods. 2014;9:192–201. [Google Scholar]