Abstract
Rationale: Asthma clinical guidelines suggest written asthma action plans are essential for improving self-management and outcomes.
Objectives: To assess the efficacy of written instructions in the form of a written asthma action plan provided by subspecialist physicians as part of usual asthma care during office visits.
Methods: A total of 407 children and adults with persistent asthma receiving first-time care in pulmonary and allergy practices at 4 urban medical centers were randomized to receive either written instructions (n = 204) or no written instructions other than prescriptions (n = 203) from physicians.
Measurements and Main Results: Using written asthma action plan forms as a vehicle for providing self-management instructions did not have a significant effect on any of the primary outcomes: (1) asthma symptom frequency, (2) emergency visits, or (3) asthma quality of life from baseline to 12-month follow-up. Both groups showed similar and significant reductions in asthma symptom frequency (daytime symptoms [P < 0.0001], nocturnal symptoms [P < 0.0001], β-agonist use [P < 0.0001]). There was also a significant reduction in emergency visits for the intervention (P < 0.0001) and control (P < 0.0006) groups. There was significant improvement in asthma quality-of-life scores for adults (P < 0.0001) and pediatric caregivers (P < 0.0001).
Conclusions: Our results suggest that using a written asthma action plan form as a vehicle for providing asthma management instructions to patients with persistent asthma who are receiving subspecialty care for the first time confers no added benefit beyond subspecialty-based medical care and education for asthma.
Clinical trial registered with www.clinicaltrials.gov (NCT 00149461).
Keywords: asthma, action plans, physicians, minority, self-management
At a Glance Commentary
Scientific Knowledge on the Subject
Most asthma guidelines suggest that instructions in the form of a written asthma action plan are an essential tool that helps patients recognize and respond appropriately to changes in asthma status. However, the independent contribution of written instructions on improving asthma outcomes is unknown.
What This Study Adds to the Field
The goal of this study was to determine the efficacy of written instructions in the form of a written asthma action plan versus no written instructions when provided by subspecialist physicians. Our results suggest that using a written asthma action plan form as a vehicle for providing asthma management instructions to patients with persistent asthma who are receiving subspecialty care for the first time provides no additional benefit beyond subspecialty-based medical care and education, particularly with respect to reducing asthma symptoms, β-agonist use, days with activity limitations, emergency department visits, and increasing asthma-related quality of life.
One of the cornerstones of asthma self-management is the written asthma action plan (WAAP), which provides instructions to help guide patient self-management interventions. Most asthma guidelines suggest that a WAAP developed in partnership with patients is an essential tool that helps patients respond appropriately to changes in asthma status (1–3). However, the most recent Expert Panel Report-3 of the National Asthma Education and Prevention Program (NAEPP) acknowledges that the recommendation to “provide all patients with a written asthma action plan that includes instructions for daily management and recognizing and handling worsening asthma” (1) is based on a limited body of evidence with few randomized controlled trials that involve substantial numbers of participants (1). Therefore the independent contribution of WAAPs to improving asthma outcomes remains unknown (4).
The goal of our study was to determine the efficacy of written instructions in the form of a WAAP versus no written instructions when provided by a subspecialist physician as part of usual asthma care during an office visit. For the purposes of this study, we defined usual care as the following: (1) asthma education was provided during the visit with the physician; (2) medications, particularly inhaled corticosteroids, were not standardized before or after randomization; and (3) the schedule for follow-up visits was not predetermined by the researchers. Follow-up visits were scheduled at the discretion of each physician. Note that in discussing WAAPs, we are referring to the WAAP form only, not the therapeutic recommendations of the physicians. Because many patients obtain a WAAP along with self-management instructions directly from their subspecialist physicians, we sought to determine whether there is any additional benefit to having instructions written on an action plan form. Some of the results of this study have been previously reported in the form of abstracts (5–8).
Methods
Participants
We conducted a prospective, randomized parallel-group controlled trial of children and adults aged 5–80 years with a physician diagnosis of persistent asthma (as defined by the NAEPP guidelines) (1). Participants had been referred to either a pulmonologist or allergist at one of four medical centers in New York City from 2006 to 2009. Within these four hospitals, participants were recruited from seven subspecialty clinics, five of which served predominantly minority patient populations that are publicly ensured. They were new patients to the practices, had never been seen by a subspecialist physician for asthma care, and had never received a WAAP. Participants were excluded if they were diagnosed with a comorbid condition affecting lung health. We set these criteria to eliminate participants in whom the diagnosis of asthma had not been confirmed or whose asthma was so mild or intermittent that little intervention was needed, or whose underlying health condition would confound the relationship of interest.
Subspecialists rather than primary care physicians were selected for the trial because subspecialist care has been shown to be less variable and more consistent with guidelines (9, 10), thus controlling for quality of care. After written informed consent and assent were obtained, eligible participants completed a baseline interview. The protocol was approved by the Columbia University Medical Center (New York, NY) Institutional Review Board.
Study Design
Computer-generated random number sequence was used for randomization. The allocation was concealed in sealed numbered envelopes. Double blinding was not possible; therefore we used a randomized block, mixed-effects factorial design with intervention group status as a fixed factor, physicians as a random factor, and participants nested within physicians. Blocks were of variable sizes to eliminate predictability. Half of the participants enrolled visiting each of the physicians were assigned to the WAAP group and half to the control group. Using this strategy, between-physician variability was minimized.
Intervention Procedures
Participant medical records were color coded to alert the physician to the enrollment status. The intervention group had a blank WAAP form inserted into their charts and the control group had no WAAP form, but a sticker was applied to the outside of the chart to remind physicians to provide their usual instructions without giving any written materials other than prescriptions.
After the visit, all participants completed an exit interview during which they were asked about their ease of communication, important messages the physician stressed, and problems they anticipated in following the physician’s instructions at home. To ensure fidelity to treatment groups, all participants were asked to show all written materials they received from the physician. After the initial visit, participants were interviewed via telephone by staff from the New England Research Institute (Watertown, MA) every 3 months during the 12-month follow-up period.
Development of the Written Asthma Action Plan Form
We developed and pilot tested a WAAP form for this study. We started with a standard color-coded (green, yellow, and red) plan and reformatted it to read from left to right instead of top to bottom to ensure easy readability. We incorporated specific, personalized clinical indicators based on symptoms and/or peak flow rates and action points designed to trigger an intervention by participants. We received input from physicians to ensure ease of use. We pilot tested the form with patients at various levels of health literacy measured using the Test of Functional Health Literacy in Adults–Short Version (S-TOFHLA) (11) to ensure that the WAAP form was easily understood and communicated the asthma messages and preventive strategies intended by the physician. We incorporated feedback from patients and physicians into the final WAAP form and produced it in English and Spanish (Figure 1). All physicians used this standardized WAAP form.
Figure 1.

Written asthma action plan form.
Training for Physicians
Interactive training sessions for physicians focused on using the WAAP form in a standardized way. We asked physicians to point to the instructions on the form as they were reviewing them to help connect the written and verbal instructions and to confirm that their messages were clearly understood using the “teach-back” method (12). Physicians were asked to confirm participant written comprehension using the “read-back” method (12, 13).
Outcome Measures
Primary outcomes included (1) asthma symptom frequency, (2) emergency visits, and (3) asthma quality of life (QOL). Using a 2-week recall period, asthma symptom frequency was measured in three ways: (a) the average number of days with asthma symptoms, (b) the average number of nights with symptoms, and (c) the average number of days of short-acting bronchodilator use. Emergency department (ED) visits for asthma were assessed using 3-month recall. Asthma QOL was measured at 6- and 12-month follow-ups using the Juniper Mini Asthma QOL Questionnaire (MiniAQLQ) (14) for adult participants and the Pediatric Asthma Caregivers QOL Questionnaire (PACQLQ) (15). Responses on the MiniAQLQ and PACQLQ were rated on a seven-point Likert scale (all the time, totally limited, or very, very worried/concerned = 1, none of the time, not at all limited, or not worried/concerned = 7). Higher scores indicate better QOL. Secondary outcomes, assessed every 3 months, included number of hospitalizations and days with activity limitation.
We also examined participant use of the WAAP by inquiring about the following: (1) did the participant refer to the WAAP during the 3-month interval; (2) if so, what circumstances prompted them; and (3) what information were they seeking and were they able to find it. Retention of the WAAP was assessed during the 12-month telephone interview. Participants were asked to read a specific line on the plan to demonstrate that they had the WAAP.
Potential mediating variables were considered including racial/ethnic group, age, sex, and health literacy level. We compared asthma morbidity and QOL between minority and white participants on the basis of treatment group status. We also assessed differences in WAAP use by functional health literacy levels.
Statistical Analysis
All analyses were conducted according to treatment group assignment. We compared differences in the outcomes between groups adjusted for their baseline values. Poisson regression analysis was conducted to compare asthma symptom frequency and the number of ED visits between the two groups, adjusting for baseline levels. Logistic regression models were fit to examine differences between treatment groups in whether ED visits occurred, adjusting for baseline levels. To compare QOL scores between groups controlling for baseline level, linear regression analysis was conducted. The paired t test was used to test the change in QOL scores over time within each group. Trend tests were performed for a linear trend in health literacy across educational levels. Chi-squared tests or Fisher exact tests were used to compare (1) characteristics such as sex, race, insurance status, education, and household income; and (2) participant recall of the asthma education messages they received between the two treatment groups. The study was powered to detect a difference between the two groups of 0.5 symptom days per 2-week period, the minimally clinically significant difference with a two-sided test with α = 0.05 (80% power) and 0.5 units in the quality of life scores, the minimally clinically significant difference, with α set at 0.05. P values were two-sided, and P ≤ 0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.2 (SAS Institute, Inc., Cary, NC).
Results
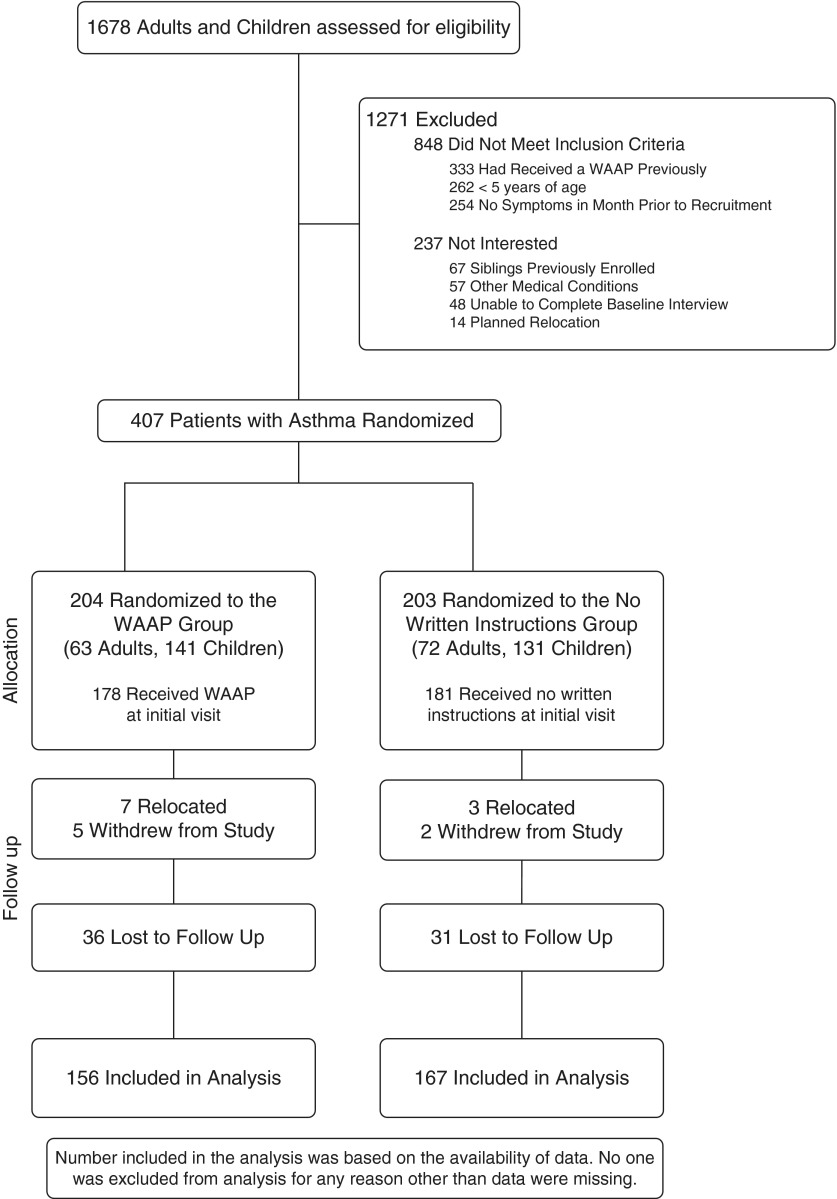
A total of 1,678 participants were screened for eligibility; 407 were randomized between December 2006 and May 2008. The sample included 135 adults (33%) and 272 children (67%). Of the 407 participants, 323 (79%) completed the study and were included in the analysis (Figure 2). Those lost to follow-up did not differ significantly from those who completed the study regarding age, race/ethnicity, insurance status, or any of the primary outcomes at baseline. Each participant was treated by 1 of 54 subspecialist physicians (16).
Figure 2.
Participant flow for written asthma action plan (WAAP) study.
Participants and Baseline Characteristics
Most participants (56%) were Latino (predominantly Dominican American) and 29% were African American. Most were publicly ensured and had a household annual income less than 200% federal poverty threshold (Table 1). Participant characteristics were similar across both groups with the exception of significantly more females in the intervention group.
Table 1.
Baseline Characteristics of Randomized Participants
| WAAP Group (n = 204) | Control Group (n = 203) | P Value | |
|---|---|---|---|
| Demographic characteristics, n (%) | |||
| Race/ethnicity | |||
| Latino | 114 (56) | 114 (56) | NS |
| African American | 53 (26) | 63 (31) | NS |
| White | 27 (13) | 24 (12) | NS |
| Other | 10 (5) | 2 (1) | NS |
| Females | 188 (92) | 175 (86) | 0.05 |
| Insurance | |||
| Medicaid | 112 (55) | 110 (54) | NS |
| Medicare | 6 (3) | 8 (4) | NS |
| Commercial | 70 (34) | 75 (37) | NS |
| Other | 16 (8) | 10 (5) | NS |
| Educational level | |||
| Less than high school | 20 (10) | 16 (8) | NS |
| High school | 84 (41) | 86 (42) | NS |
| Vocational/trade/college | 82 (40) | 83 (41) | NS |
| Graduate education | 18 (9) | 18 (9) | NS |
| Household annual income | |||
| <200% federal poverty threshold | 159 (78) | 150 (74) | NS |
| Baseline asthma characteristics, mean ± SD | |||
| Asthma symptom frequency (d), 2-wk period before enrollment | |||
| Daytime asthma symptoms | 7.4 ± 4.6 | 7.7 ± 4.7 | NS |
| Nocturnal asthma symptoms | 5.9 ± 4.7 | 6.5 ± 5 | NS |
| Days with activity limitation, 2-wk period before enrollment | 3.6 ± 4.5 | 3.9 ± 4.7 | NS |
| Emergency department visits, previous 12 mo | 3.3 ± 5.9 | 2.5 ± 3.9 | NS |
| Hospitalizations | |||
| Lifetime admissions | 6.6 ± 20.4 | 5.5 ± 14.0 | NS |
| Previous 12 mo | 0.67 ± 1.6 | 0.57 ± 1.7 | |
| Duration of asthma, yr | |||
| Children | 6.6 ± 4 | 6.2 ± 4 | NS |
| Adults | 28 ± 18 | 30 ± 18 | NS |
Definition of abbreviations: NS = not significant; WAAP = written asthma action plan.
Characteristics regarding asthma morbidity were comparable between both groups at baseline (Table 1). There were also no statistically significant differences in medication use at baseline (Table 2).
Table 2.
Baseline Medication Use during the 30 Days before Study Enrollment
| Medication | WAAP Group (n = 200) [n (%)] | Control Group (n = 201) [n (%)] | P Value |
|---|---|---|---|
| Short-acting β-agonists | 198 (99) | 197 (98) | 0.72 |
| LABAs | 0 (0) | 2 (1) | 0.59 |
| ICS | 116 (58) | 101 (50) | 0.06 |
| Systemic corticosteroids | 100 (50) | 84 (42) | 0.12 |
| Leukotriene antagonists | 120 (60) | 114 (57) | 0.64 |
| Combination LABA + ICS | 62 (31) | 72 (36) | 0.38 |
Definition of abbreviations: ICS = inhaled corticosteroids; LABA = long-acting β-agonist; WAAP = written asthma action plan.
Primary Outcomes
Asthma symptom frequency
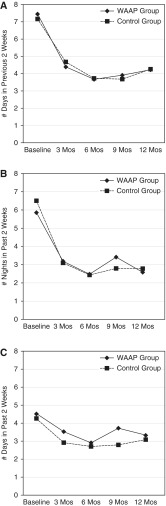
After adjusting for baseline asthma symptom frequency, our results showed that having a WAAP was not significantly associated with a reduction in days with symptoms (relative risk [RR], 1.01; 95% confidence interval [CI], 0.8–1.3; P = 0.94), nocturnal symptoms (RR, 0.98; 95% CI, 0.7–1.3; P = 0.9), or β-agonist use (RR, 1.00; 95% CI, 0.8–1.2; P = 0.97) from baseline to 12 months. Overall, both groups showed a significant reduction in all three variables for asthma symptom frequency (P < 0.0001 for all three variables) (Figures 3A–3C).
Figure 3.
(A) Daytime asthma symptom frequency (baseline through 12 mo); shown are the reductions in daytime symptoms for each group (P < 0.0001). (B) Nocturnal asthma symptom frequency (baseline through 12 mo); shown are the reductions in nocturnal symptoms for each group (P < 0.0001). (C) Frequency of β-agonist use (baseline through 12 mo); shown are the reductions in β-agonist use for each group (P < 0.0001). WAAP = written asthma action plan.
Emergency department visits for asthma
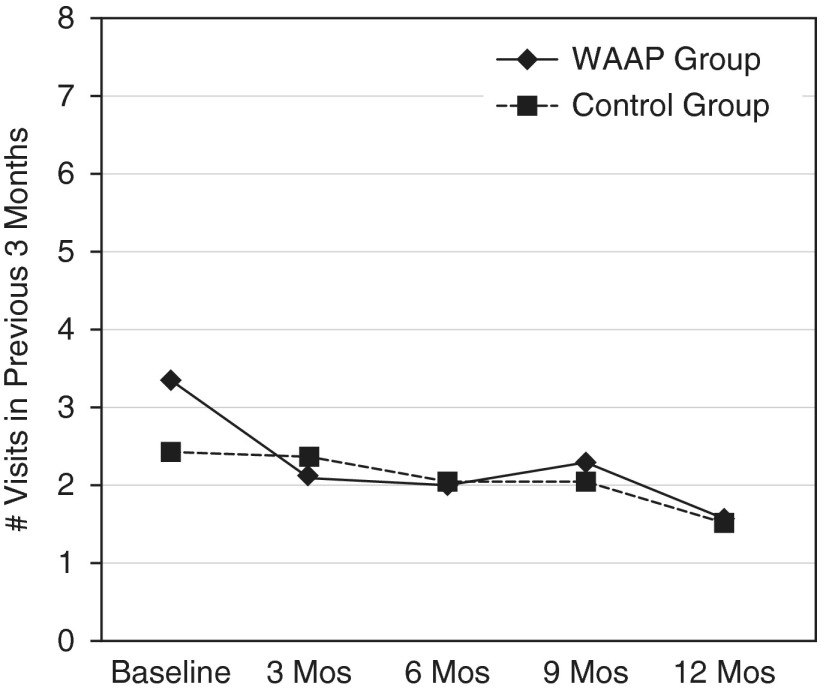
At baseline, there was no significant difference in ED visits, with the WAAP group reporting a mean of 3.3 (±5.9) ED visits in the 12 months before enrollment and 2.5 (±3.9) ED visits for the control group (P = 0.61). At 12 months, there were no between-group differences; however, reductions were significant for both groups: WAAP group, 1.6 ± 3 ED visits (P < 0.0001); and control group, 1.5 ± 3 ED visits (P = 0.0006) (Figure 4).
Figure 4.
Emergency department visits for asthma (baseline through 12 mo). Shown are the reductions in emergency department visits for the written asthma action plan (WAAP) group (P < 0.0001) and for the control group (P = 0.0006).
Asthma QOL.
There was a significant increase in mean total scores and in the two subscale scores (activity limitation and emotional function) from baseline to 12-month follow-up for the PACQOL scale (P < 0.0001). However, no between-group differences were noted at any time. Scores on the MiniAQLQ also significant increased from baseline to 12 months (P < 0.0001). There were no significant between-group differences (Table 3).
Table 3.
Asthma Quality of Life Scores: Baseline through 12 Months
| Intervention Group | Control Group | |
|---|---|---|
| PACQLQ | ||
| Baseline | 4.85 ± 1.4 (1.5–7) | 4.83 ± 1.3 (1.2–7) |
| 6 mo | 5.92 ± 1.3 (1.9–7) | 5.99 ± 1.2 (2–7) |
| 12 mo* | 6.24 ± 0.93 (2.3–7) | 6.28 ± 0.96 (1.7–7) |
| MiniAQLQ | ||
| Baseline | 3.49 ± 1.2 (1.4–6.6) | 3.64 ± 1.5 (1–6.8) |
| 6 mo | 4.46 ± 1.7 (1–7) | 4.61 ± 1.5 (1–7) |
| 12 mo† | 4.52 ± 1.5 (1.7–7) | 4.55 ± 1.5 (1–7) |
Definition of abbreviations: MiniAQLQ = Mini Asthma QOL (Quality of Life) Questionnaire; PACQLQ = Pediatric Asthma Caregivers QOL Questionnaire.
Data are mean ± SD (range).
PACQLQ scores from baseline to 12-month follow-up for both groups, P < 0.0001.
MiniAQLQ scores from baseline to 12-month follow-up for both groups, P < 0.0001.
Secondary Outcomes
Hospitalizations
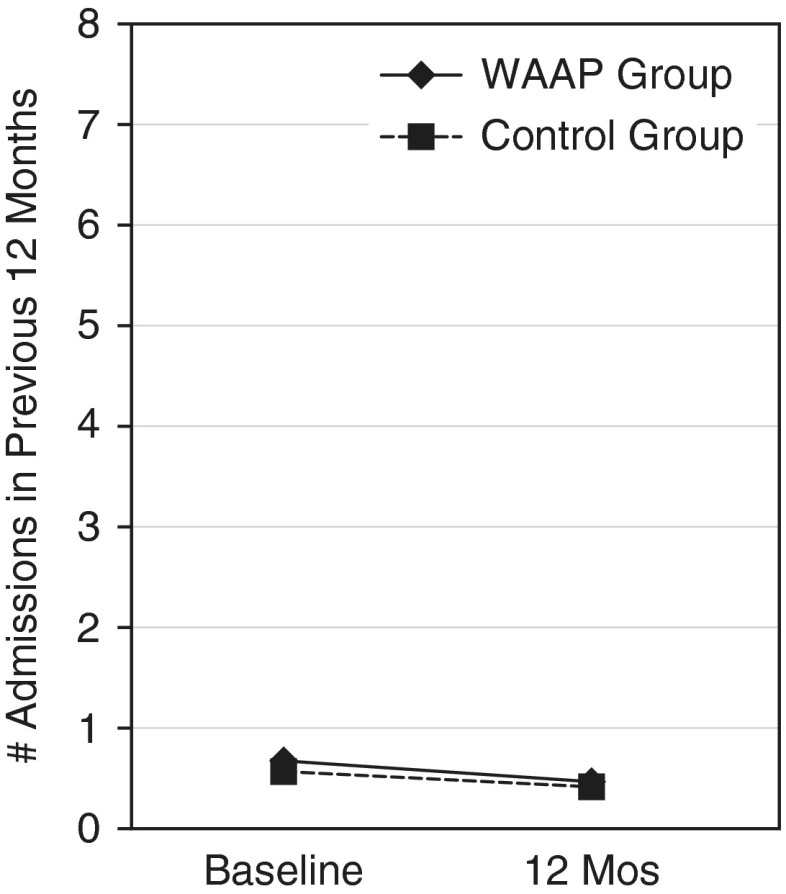
There were no significant between-group differences in asthma hospitalizations at baseline, and the reduction in admissions was not significant for either group at the 12-month follow-up period. The WAAP and control groups reported 0.7 and 0.6 hospitalizations in the previous 12 months at baseline, respectively, and 0.5 and 0.4 at the 12-month follow-up (Figure 5).
Figure 5.
Hospitalizations (baseline through 12 mo). Shown are the reductions in hospitalizations for asthma for each group (P = 0.9).
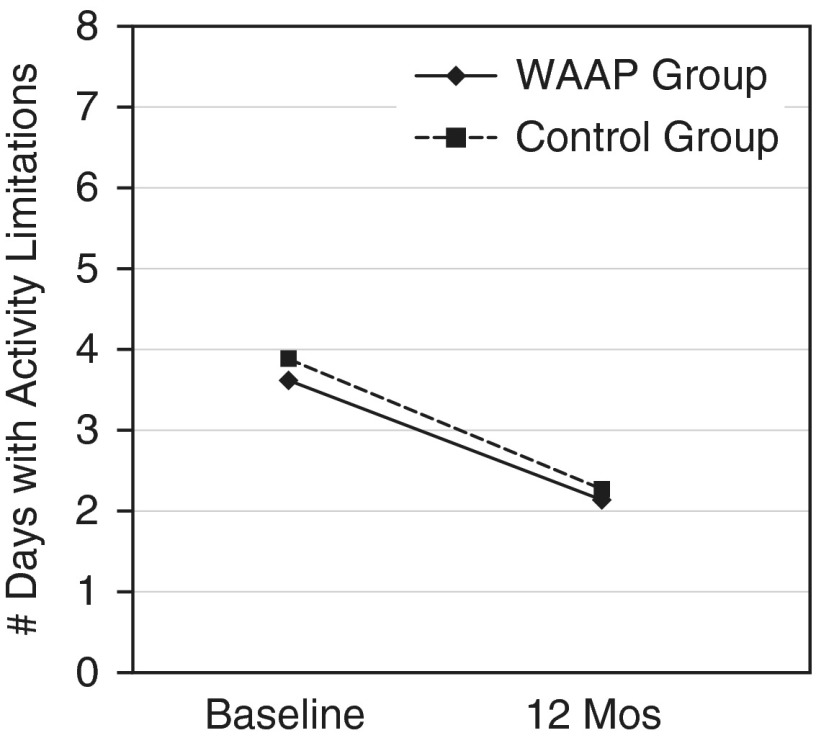
Days with activity limitation
Days with activity limitation declined significantly for both groups, from 3.6 to 2.1 days (WAAP group; P < 0.0005) and from 3.9 to 2.3 days (control subjects; P < 0.0001), but there were no significant between-group differences (Figure 6).
Figure 6.
Days with activity limitations (baseline through 12 mo). Reduction in days with activity limitation due to asthma for written asthma action plan (WAAP) group, P < 0.0005; for control group, P < 0.0001.
Other Subgroup Comparisons
Retention and use of the WAAP
Ninety-eight percent of intervention group participants possessed their WAAP at 12-month follow-up; 79% reported using the WAAP in the previous 12 months and 98% of those found the information they needed on the form. The most common reasons for referring to the WAAP included looking for (1) the correct dose of medicine (30%), (2) correct timing for taking medicine (19%), (3) instructions for responding to worsening asthma symptoms (14%), (4) correct medicine to take (6%), and (5) instructions for responding to improving asthma status (2%). The remainder reported using the WAAP for refreshing their memories or looking for peak flow levels; 10% could not recall what information they were seeking on the plan.
Physician fidelity to the protocol
We found that 87% of the intervention group received a completed WAAP and 89% of control participants received no written instructions other than prescriptions at the initial visit. We analyzed our primary outcomes with and without participants who did and did not receive WAAPs as intended and found no significant differences between the two groups.
Role of the WAAP in patient education
At the end of the initial visit, we used open-ended questioning to assess the asthma education messages that doctors stressed during the visit. WAAP participants spontaneously recounted significantly more educational messages than did their control group counterparts (Table 4).
Table 4.
Participant Report of Most Important Asthma Education Messages Stressed by Physician
| Message | Intervention Group (%) | Control Group (%) | P Value |
|---|---|---|---|
| Take controller everyday | 64 | 50 | 0.002 |
| Take reliever when symptoms present | 42 | 32 | 0.025 |
| When to call EMS/go to ED | 28 | 15 | 0.001 |
| When to call doctor | 25 | 13 | 0.001 |
| What to do during asthma exacerbation | 21 | 12 | 0.016 |
| When to start OCS | 13 | 7 | 0.029 |
| Trigger avoidance | 10 | 11 | 0.77 |
| Keep follow-up appointments | 5 | 4 | 0.63 |
| Take reliever before exercise | 4 | 7 | 0.215 |
Definition of abbreviations: ED = emergency department; EMS = emergency medical services; OCS = oral corticosteroids.
Exploratory Analyses
Racial/ethnic differences
There were no significant differences in outcomes between minority and white participants from baseline to 12 months for any of the primary or secondary outcomes.
Functional health literacy levels
Eighty-five percent of participants had adequate functional health literacy, 8% marginal, and 7% inadequate. There was no significant difference in literacy levels between treatment groups. Although asthma symptom frequency was not significantly related to literacy levels, we found at baseline that participants with higher educational levels classified their asthma as less severe than did participants with lower educational achievement (ρ = 0.01). Participants with adequate functional health literacy skills in both groups reported that it was significantly easier for them to understand the doctor’s instructions (ρ < 0.0001). Despite this finding, there was no difference among the three literacy groups regarding the problems they anticipated in following the physician’s instructions for managing asthma at home (ρ = 0.5).
Discussion
Our results showed that there was no independent association between WAAP use and (1) asthma symptom frequency, (2) ED visits, (3) asthma QOL, (4) hospitalizations, or (5) days with activity limitation after controlling for age, sex, race/ethnicity, household income, and health literacy. This suggests no additional benefit of the WAAP form beyond subspecialist-based medical care and education. Participants in both groups had significant and similar improvements in all outcomes from baseline to 12 months.
So why did we fail to show an independent effect of WAAPs on reducing asthma morbidity and improving QOL? Is it because of the characteristics of our study design? Or is it because WAAPs really do not help improve asthma outcomes? Regarding our study design, we included only subspecialist physicians to ensure a more uniform approach to management, but this choice may have affected our outcomes in four important ways. First, we tested the intervention in participants who were seeing subspecialists for the first time and, as a result, study enrollment coincided with the initiation of subspecialty care, possibly blunting the effect of the WAAP on outcomes. Second, studies have shown subspecialists are more likely to follow clinical guidelines compared with primary care physicians (16–18). As a result, they are more likely to incorporate WAAPs as part of usual care (16). We postulate that physicians who regularly use WAAPs are likely to have developed their own asthma educational and self-management techniques that they use when giving WAAPs to patients. We believe physicians in our study provided asthma education to all participants whether or not they received a WAAP form and that the verbal instructions they provided were as effective as the written instructions. Effective asthma education may have obscured any additional effect that the WAAP might have had on asthma outcomes. Third, studies show that subspecialist physicians are more likely to prescribe inhaled and systemic corticosteroids and manage asthma exacerbations in the outpatient setting compared with primary care physicians (13, 16, 17, 19–21). These practices probably account for the marked reduction in asthma morbidity, thereby increasing asthma QOL, in participants in both study arms. Fourth, participant behaviors and motivation may have played an important role in the outcomes and need further study. Patients who access subspecialty care may be different in important ways that could have influenced our outcomes. If recent asthma exacerbations were the reason participants were referred to subspecialists, they may be more motivated to change behaviors (22, 23) and more likely to adhere to recommendations (22) whether or not they had been given a WAAP. Patients who successfully accessed subspecialty care may have a higher level of personal organization that enabled them to follow through with appointment-keeping (17). In addition, they may have more resources (e.g., financial, social support, etc.), all of which could have contributed to improved outcomes.
To address the effect of WAAPs on asthma outcomes we surveyed the literature. Three studies comparing WAAPs versus no plan showed positive results; however, these studies have significant limitations. Cowie and colleagues studied 150 patients with asthma with an urgent visit for asthma in the preceding 12 months (24). Participants received individualized asthma education from a nurse clinician before randomization to the no action plan group, peak flow–based plan group, or symptom-based action plan group. The results showed that a peak flow–based WAAP significantly reduced ED visits compared with a symptom-based plan or no plan 6 months after enrollment (24).
A trial involving 68 children with moderate persistent asthma demonstrated significant reductions in asthma morbidity among those who received a WAAP compared with no plan (25). All received asthma education from a physician and social scientist and were placed on a moderate dose of inhaled corticosteroids before randomization. The outcomes were assessed at 4 months.
A trial conducted with 219 children with asthma treated in the ED demonstrated that the WAAP group was more likely to adhere to inhaled corticosteroids, fill oral corticosteroid prescriptions, and report better asthma control at 28 days postdischarge (26).
The first two trials involved small numbers of participants, and all participants in both studies (24, 25) received asthma instructions from someone other than their treating physician and not as part of their usual care. All three aforementioned studies had short follow-up periods: 6 months, 4 months, and 28 days, respectively. Our study design has the advantage of being adequately powered to test the efficacy of a WAAP form itself versus no plan, and our follow-up period of 12 months ensured adequate time to detect differences in primary outcomes.
Other trials, including several meta-analyses, examining the benefits of WAAPs have been inconclusive (27–30). A Cochrane review examining WAAP use, medication adherence, and asthma outcomes had too few trials with insufficient numbers of participants to assess the contribution of WAAPs (31). A systematic review of nine randomized controlled trials had similar results (4). There was insufficient evidence to demonstrate that WAAPs had an independent effect on hospitalizations, ED visits, symptom control, or lung function. Our study is the first to demonstrate the lack of an independent effect of WAAPs when added to usual care in subspecialty care. However, this conclusion cannot be extrapolated to other settings, particularly primary care, where more studies are needed to answer this important question.
An unexpected finding was the lack of disparity in asthma outcomes between African American and Latino participants and their white counterparts. Although there were substantially fewer white participants in the analysis, all participants experienced similar improvements in asthma symptoms and quality of life. We believe the subspecialists optimized treatment and provided effective asthma education to participants resulting in closure of the outcomes disparity. Our results suggest that subspecialty care for minority and economically disadvantaged patients can reduce the asthma burden in this highly vulnerable population.
There are limitations to our study. First, this study involved only subspecialist physicians. Our findings may not apply to asthma care in primary care settings where physicians typically have less time to devote to asthma education and have fewer asthma-focused visits. We cannot generalize our findings to all patients with asthma because our sample was largely minority, low income, and residing in a large U.S. city. Moreover, our study population does not represent the majority of allergy or pulmonary practices. Because studies show that minority and poor populations are less likely to receive asthma subspecialty care compared with their white counterparts, despite having more frequent daily symptoms, exacerbations, emergency visits, and hospitalizations (32–34), there may be important participant characteristics that resulted in our sample being referred for subspecialty care and/or having improved outcomes that we did not examine. In addition, all data were collected by participant report, which can be affected by their recall.
Conclusions
Using a WAAP form as a vehicle to give asthma management instructions to patients with persistent asthma who are receiving subspecialty care for asthma for the first time does not improve asthma outcomes when compared with verbal instructions given by a pulmonologist or allergist. Receiving subspecialty care for asthma may be a key factor in reducing asthma morbidity and improving quality of life for high-risk, urban, minority populations. Further studies are needed to clarify whether there is a benefit of using WAAP forms in primary care settings where most patients with asthma are treated.
Acknowledgments
Acknowledgment
The authors gratefully acknowledge the diligence and meticulous effort of the study coordinator, Belkis Merete-Roa, M.D., and research assistants Maria L. Forero-Aranguren, Altagracia Moronta, M.D., and Nereida Lewin, who made the conduct of this study possible. The authors thank Mercedes Blanco, who designed the WAAP form used in this study, and Christina Zarcadoolas, Ph.D., the sociolinguist who helped design and pilot test the WAAP form with patients at various literacy levels. The authors express appreciation to the New England Research Institute for its stewardship of the follow-up data collection over 12 months, and to Howard Andrews and the Data Management Center at Columbia University for oversight of the research data.
Footnotes
Supported by the NIH/NHLBI (grant R01HL73955) and the National Center for Advancing Translational Sciences, NIH (UL1 TR000040), formerly the National Center for Research Resources (UL1 RR024156). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or NHLBI.
Author Contributions: B.J.S. and D.E. were centrally involved in the study’s design, conduct, and interpretation of the data. B.J.S. wrote the first draft of the manuscript. All authors made substantial contributions to the conception and design of the study, acquisition of data, or analysis and interpretation of data. They participated in drafting and revising this manuscript, take full responsibility for its content, and all authors, with the exception of R.B.M. (who died before the final draft was completed), have approved the version submitted for review. There were no substantive changes made to the manuscript after R.B.M.’s death. B.J.S. and D.E. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analyses.
Originally Published in Press as DOI: 10.1164/rccm.201407-1338OC on April 13, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.National Asthma Education Prevention Program. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [Published erratum appears in J Allergy Clin Immunol 2008;121:1330.] [DOI] [PubMed] [Google Scholar]
- 2.Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O’Byrne P, Pedersen SE, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–178. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 3.Lougheed MD, Lemière C, Dell SD, Ducharme FM, Fitzgerald JM, Leigh R, Licskai C, Rowe BH, Bowie D, Becker A, et al. Canadian Thoracic Society Asthma Committee. Canadian Thoracic Society Asthma Management Continuum—2010 Consensus Summary for children six years of age and over, and adults. Can Respir J. 2010;17:15–24. doi: 10.1155/2010/827281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lefevre F, Piper M, Weiss K, Mark D, Clark N, Aronson N. Do written action plans improve patient outcomes in asthma? An evidence-based analysis. J Fam Pract. 2002;51:842–848. [PubMed] [Google Scholar]
- 5.Sheares BJ, Vazquez TL, Evans D. Assessing physician barriers to the use of written treatment plans in asthma [abstract] Proc Am Thoracic Soc. 2005;2:A906. [Google Scholar]
- 6.Sheares BJ, Vazquez TL, Evans D. Impact of health literacy on written treatment plan use in asthma [abstract] Proc Am Thoracic Soc. 2006;3:A270. [Google Scholar]
- 7.Sheares BJ, Vazquez TL, Evans D. How the use of written treatment plans in asthma influences patients’ self-management behaviors [abstract] Proc Am Thoracic Soc. 2006;3:A528. [Google Scholar]
- 8.Sheares BJ, Evans D. Do patients of specialist physicians really benefit from the use of a written asthma action plan? [abstract] Am J Respir Crit Care Med. 2013;187:A6012. [Google Scholar]
- 9.Janson S, Weiss K. A national survey of asthma knowledge and practices among specialists and primary care physicians. J Asthma. 2004;41:343–348. doi: 10.1081/jas-120026093. [DOI] [PubMed] [Google Scholar]
- 10.Vollmer WM, O’Hollaren M, Ettinger KM, Stibolt T, Wilkins J, Buist AS, Linton KL, Osborne ML. Specialty differences in the management of asthma: a cross-sectional assessment of allergists’ patients and generalists’ patients in a large HMO. Arch Intern Med. 1997;157:1201–1208. [PubMed] [Google Scholar]
- 11.Parker RM, Baker DW, Williams MV, Nurss JR. The Test of Functional Health Literacy in Adults: a new instrument for measuring patients’ literacy skills. J Gen Intern Med. 1995;10:537–541. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 12.Kripalani S, Bengtzen R, Henderson LE, Jacobson TA. Clinical research in low-literacy populations: using teach-back to assess comprehension of informed consent and privacy information. IRB. 2008;30:13–19. [PubMed] [Google Scholar]
- 13.Schillinger D, Piette J, Grumbach K, Wang F, Wilson C, Daher C, Leong-Grotz K, Castro C, Bindman AB. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med. 2003;163:83–90. doi: 10.1001/archinte.163.1.83. [DOI] [PubMed] [Google Scholar]
- 14.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32–38. doi: 10.1034/j.1399-3003.1999.14a08.x. [DOI] [PubMed] [Google Scholar]
- 15.Juniper EF, Guyatt GH, Feeny DH, Ferrie PJ, Griffith LE, Townsend M. Measuring quality of life in the parents of children with asthma. Qual Life Res. 1996;5:27–34. doi: 10.1007/BF00435966. [DOI] [PubMed] [Google Scholar]
- 16.Sheares BJ, Du Y, Vazquez TL, Mellins RB, Evans D. Use of written treatment plans for asthma by specialist physicians. Pediatr Pulmonol. 2007;42:348–356. doi: 10.1002/ppul.20586. [DOI] [PubMed] [Google Scholar]
- 17.Diette GB, Skinner EA, Nguyen TT, Markson L, Clark BD, Wu AW. Comparison of quality of care by specialist and generalist physicians as usual source of asthma care for children. Pediatrics. 2001;108:432–437. doi: 10.1542/peds.108.2.432. [DOI] [PubMed] [Google Scholar]
- 18.Patel MR, Valerio MA, Sanders G, Thomas LJ, Clark NM. Asthma action plans and patient satisfaction among women with asthma. Chest. 2012;142:1143–1149. doi: 10.1378/chest.11-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Backer V, Nepper-Christensen S, Nolte H. Quality of care in patients with asthma and rhinitis treated by respiratory specialists and primary care physicians: a 3-year randomized and prospective follow-up study. Ann Allergy Asthma Immunol. 2006;97:490–496. doi: 10.1016/S1081-1206(10)60940-4. [DOI] [PubMed] [Google Scholar]
- 20.Ducharme FM, Morin J, Davis GM, Gingras J, Noya FJ. High physician adherence to phenotype-specific asthma guidelines, but large variability in phenotype assessment in children. Curr Med Res Opin. 2012;28:1561–1570. doi: 10.1185/03007995.2012.716031. [DOI] [PubMed] [Google Scholar]
- 21.Erickson S, Tolstykh I, Selby JV, Mendoza G, Iribarren C, Eisner MD. The impact of allergy and pulmonary specialist care on emergency asthma utilization in a large managed care organization. Health Serv Res. 2005;40:1443–1465. doi: 10.1111/j.1475-6773.2005.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chambers CV, Markson L, Diamond JJ, Lasch L, Berger M. Health beliefs and compliance with inhaled corticosteroids by asthmatic patients in primary care practices. Respir Med. 1999;93:88–94. doi: 10.1016/s0954-6111(99)90296-2. [DOI] [PubMed] [Google Scholar]
- 23.Greaves CJ, Eiser C, Seamark D, Halpin DM. Attack context: an important mediator of the relationship between psychological status and asthma outcomes. Thorax. 2002;57:217–221. doi: 10.1136/thorax.57.3.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cowie RL, Revitt SG, Underwood MF, Field SK. The effect of a peak flow–based action plan in the prevention of exacerbations of asthma. Chest. 1997;112:1534–1538. doi: 10.1378/chest.112.6.1534. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal SK, Singh M, Mathew JL, Malhi P. Efficacy of an individualized written home-management plan in the control of moderate persistent asthma: a randomized, controlled trial. Acta Paediatr. 2005;94:1742–1746. doi: 10.1111/j.1651-2227.2005.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 26.Ducharme FM, Zemek RL, Chalut D, McGillivray D, Noya FJ, Resendes S, Khomenko L, Rouleau R, Zhang X. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. Am J Respir Crit Care Med. 2011;183:195–203. doi: 10.1164/rccm.201001-0115OC. [DOI] [PubMed] [Google Scholar]
- 27.Grampian Asthma Study of Integrated Care (GRASSIC) Effectiveness of routine self monitoring of peak flow in patients with asthma. BMJ. 1994;308:564–567. [PMC free article] [PubMed] [Google Scholar]
- 28.Coté J, Cartier A, Robichaud P, Boutin H, Malo JL, Rouleau M, Fillion A, Lavallée M, Krusky M, Boulet LP. Influence on asthma morbidity of asthma education programs based on self-management plans following treatment optimization. Am J Respir Crit Care Med. 1997;155:1509–1514. doi: 10.1164/ajrccm.155.5.9154850. [DOI] [PubMed] [Google Scholar]
- 29.Jones KP, Mullee MA, Middleton M, Chapman E, Holgate ST British Thoracic Society Research Committee. Peak flow based asthma self-management: a randomised controlled study in general practice. Thorax. 1995;50:851–857. doi: 10.1136/thx.50.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Palen J, Klein JJ, Zielhuis GA, van Herwaarden CL, Seydel ER. Behavioural effect of self-treatment guidelines in a self-management program for adults with asthma. Patient Educ Couns. 2001;43:161–169. doi: 10.1016/s0738-3991(00)00155-5. [DOI] [PubMed] [Google Scholar]
- 31.Toelle BG, Ram FS. Written individualised management plans for asthma in children and adults. Cochrane Database Syst Rev. 2004;2:CD002171. doi: 10.1002/14651858.CD002171.pub2. [DOI] [PubMed] [Google Scholar]
- 32.Flores G, Snowden-Bridon C, Torres S, Perez R, Walter T, Brotanek J, Lin H, Tomany-Korman S. Urban minority children with asthma: substantial morbidity, compromised quality and access to specialists, and the importance of poverty and specialty care. J Asthma. 2009;46:392–398. doi: 10.1080/02770900802712971. [DOI] [PubMed] [Google Scholar]
- 33.Ortega AN, Gergen PJ, Paltiel AD, Bauchner H, Belanger KD, Leaderer BP. Impact of site of care, race, and Hispanic ethnicity on medication use for childhood asthma. Pediatrics. 2002;109:E1. doi: 10.1542/peds.109.1.e1. [DOI] [PubMed] [Google Scholar]
- 34.Stingone JA, Claudio L. Disparities in the use of urgent health care services among asthmatic children. Ann Allergy Asthma Immunol. 2006;97:244–250. doi: 10.1016/S1081-1206(10)60021-X. [DOI] [PubMed] [Google Scholar]