Abstract
Rationale: Females are predisposed to pulmonary arterial hypertension (PAH); evidence suggests that serotonin, mutations in the bone morphogenetic protein receptor (BMPR) II gene, and estrogens influence development of PAH. The 5-hydroxytryptamine 1B receptor (5-HT1BR) mediates human pulmonary artery smooth muscle cell (hPASMC) proliferation.
Objectives: We aimed to determine whether selected microRNAs (miRNAs) expressed in PASMCs are influenced by sex, BMPR-II mutations, and estrogens, and contribute to PASMC proliferation in PAH.
Methods: Expression levels of miRNAs targeting genes related to PAH, estrogen, and serotonin were determined by quantitative RT-PCR in hPASMCs and mouse PASMCs harboring a heterozygous mutation in BMPR-II (BMPR-IIR899X+/− PASMCs). miRNA-96 targets 5-HT1BR and was selected for further investigation. miRNA target validation was confirmed by luciferase reporter assay. Precursor miRNA-96 was transfected into hPASMCs to examine effects on proliferation and 5-HT1BR expression. The effect of a miRNA-96 mimic on the development of hypoxic pulmonary hypertension in mice was also assessed.
Measurements and Main Results: miRNA-96 expression was reduced in BMPR-IIR899X+/− PASMCs from female mice and hPASMCs from female patients with PAH; this was associated with increased 5-HT1BR expression and serotonin-mediated proliferation. 5-HT1BR was validated as a target for miRNA-96. Transfection of precursor miRNA-96 into hPASMCs reduced 5-HT1BR expression and inhibited serotonin-induced proliferation. Restoration of miRNA-96 expression in pulmonary arteries in vivo via administration of an miRNA-96 mimic reduced the development of hypoxia-induced pulmonary hypertension in the mouse.
Conclusions: Increased 5-HT1BR expression may be a consequence of decreased miRNA-96 expression in female patient PASMCs, and this may contribute to the development of PAH.
Keywords: microRNA, pulmonary hypertension, estrogen, serotonin, animal models
At a Glance Commentary
Scientific Knowledge on the Subject
Females develop pulmonary arterial hypertension (PAH) more frequently than males, and loss-of-function bone morphogenetic protein receptor (BMPR) II mutations underlie heritable PAH. Genes can be regulated by microRNAs (miRNAs), but it is unknown if sex affects the activity of these.
What This Study Adds to the Field
Our research shows that, in female pulmonary artery smooth muscle cells (PASMCs) from BMPR-II mutant mice and patients, there is down-regulation of miRNA-96 associated with concomitant up-regulation of the 5-hydroxytryptamine 1B receptor, a direct miRNA-96 target. We show that this contributes to a proliferative phenotype in female human PASMCs and that an miRNA-96 mimic prevents the development of pulmonary hypertension in a model of PAH. These findings suggest that down-regulation of miRNA-96 and associated increases in serotonin-induced proliferation may contribute to the pathology of PAH, especially in females.
Mutations within the bone morphogenetic protein receptor (BMPR) II gene are associated with approximately 70% of heritable pulmonary arterial hypertension (hPAH) cases and roughly 10–40% of idiopathic PAH (1). Loss of function of BMPR-II results in a decrease in BMPR-II signaling, translating into aberrant proliferation of pulmonary artery smooth muscle cells (PASMCs) (2). Restoration of the BMPR-II signaling pathway represents a novel therapeutic strategy (3). We recently demonstrated, in the hypoxic mouse and Sugen 5,416/hypoxic rat models, that the therapeutic effect of anastrozole, an inhibitor of estrogen synthesis, was only observed in females, and was related to restoration of BMPR-II signaling (4). BMPR-II mutations exhibit a relatively low frequency of disease penetrance (20–30%), suggesting that other factors also contribute to the development of PAH.
Epidemiological studies report a greater incidence of PAH in females; the female-to-male ratio ranges from 3:1 to 4:1 (5). Hence, female sex hormones, primarily estrogens, may play a causative role in the development of PAH. 17β-estradiol, the main circulating estrogen, increases proliferation of human PASMCs (hPASMCs), and anomalous synthesis and metabolism of estrogen have been associated with the disease (4, 6, 7). Within the promoter region of the BMPR-II gene, there is an estrogen-response element that may play a role in the suppression of BMPR-II receptor expression (8).
Evidence suggests that serotonin is involved in the pathogenesis of PAH. Endothelial expression of tryptophan hydroxylase (TPH)-1, the rate-limiting enzyme in the synthesis of serotonin, is increased in remodeled pulmonary arteries from patients with idiopathic PAH (9). In addition, TPH-1 knockout mice are protected from hypoxia-induced pulmonary hypertension (PH) (10, 11). We have shown that only female mice develop PH via a serotonin-dependent mechanism—for example, mice overexpressing the serotonin transporter (SERT), calcium-binding protein S100A4/mts1–overexpressing mice, and mice dosed with dexfenfluramine (6, 12, 13). This PH phenotype is estrogen dependent, and suggests that female sex and estrogen influence the development of PH in these serotonin-dependent models. Serotonin is a potent mitogen and vasoconstrictor in the pulmonary vasculature acting through the SERT and 5-hydroxytryptamine1B receptor (5-HT1BR) (14, 15). The expression of TPH-1, SERT, and 5-HT1BR in hPASMCs is increased by 17β-estradiol (6).
MicroRNAs (miRNAs) are noncoding RNA sequences that have the ability to direct expression of genes in a post-transcriptional manner through either degradation of target messenger RNA (mRNA) or silencing of mRNA translation. Several miRNAs have recently been shown to play a role in PAH pathophysiology (16). Literature suggests that sex hormones (i.e., estrogens) have the ability to regulate the expression of miRNAs (17), which led us to speculate whether sex can also influence expression of miRNAs. Thus, in the present study, we investigated whether sex and/or BMPR-II expression affects miRNA expression within PASMCs and hence the expression of genes that may influence the development of PAH.
Methods
PASMCs
PASMCs derived from knockin mice harboring a heterozygous mutation in BMPR-II (R899X) and littermate wild-type (WT) control mice were prepared as described previously (18) and in the online supplement. These mice develop spontaneous PH (19). Human PASMCs were prepared as described previously (6) and in the online supplement.
Cell Proliferation Assay
To assess proliferation, PASMCs were counted using the hemocytometer approach, as described previously (6).
hPASMC Transfection with miRNA Mimic
hPASMCs were transfected with either 1 nM precursor (pre-) miRNA-96 (PM10422; Ambion, Paisley, UK) or pre-miRNA–negative control (AM17110 #1; Ambion), as described in the online supplement. For expression analysis, each condition was performed in biological replicates to allow for RNA and protein harvest 48 hours and 72 hours post-transfection, respectively. For proliferation analysis, PASMCs were then quiesced in 0.2% fetal bovine serum and the proliferation protocol followed as described in the online supplement.
Taqman Quantitative PCR Analysis of miRNAs and mRNAs
Total RNA extraction and Taqman quantitative RT-PCR were conducted as described previously (16, 20) and in the online supplement.
Western Blot Analysis of Protein
Protein extraction and Western blot analysis was performed as described previously (7, 16) and in the online supplement.
Pulmonary Arterial Remodeling
Remodeled pulmonary arteries were investigated by elastin-picrosirius red staining as described previously (16, 20) and in the online supplement.
Luicferase Reporter Assay
The psi-CHECK-2 dual luciferase reporter vector (Promega, Southampton, UK) was used for the reporter assay as described in the online supplement. Luciferase activity was measured using the Dual-Glo Luciferase Assay System (Promega) and luminescence detection performed via LUMIstar OPTIMA microplate reader (BMG Labtech, Ortenberg, Germany).
Chronic Hypoxia
Female WT C57/Bl (Charles River Laboratories, Ormiston, UK) mice aged 2 months were exposed to 14 days of hypobaric hypoxia (10% O2, 550 mbar), as described previously (21). Mice maintained in normoxic conditions (21% O2, 1,000 mbar) were studied as controls.
Administration of miRNA-96 Mimic
To assess whether miRNA-96 was involved in the pathology of PH, the miRNA-96 mimic (no. MC10422; Applied Biosystems, Paisley, UK) was administered intravenously via the tail vein once per week for the 2 weeks of hypoxic exposure. See the online supplement for further details.
Anastrozole-treated Mice
Lungs from mice treated with anastrozole (3 mg/kg) or vehicle (1% carboxymethylcellulose) via subcutaneous injection for 14 days (4) were studied to examine the influence of endogenous estrogen depletion on miRNA-96 regulation.
In Vivo Assessment of PH
Measurements of right ventricular systolic pressure (RVSP), right ventricular hypertrophy (RVH), and pulmonary vascular remodeling were performed as described previously (4, 7) and in the online supplement.
In Situ Hybridization
Localization of miRNA-96 within pulmonary arteries was performed by in situ hybridization as described previously (22) and in the online supplement.
Statistical Analysis
Values are expressed as mean (±SEM). A t test or one-way ANOVA followed by Tukey’s post hoc test was performed to evaluate the statistical significance between all groups, where appropriate. A probability level of P less than 0.05 was defined as being statistically significant.
Results
Proliferation and miRNA expression in BMPR-IIR899X+/− PASMCs
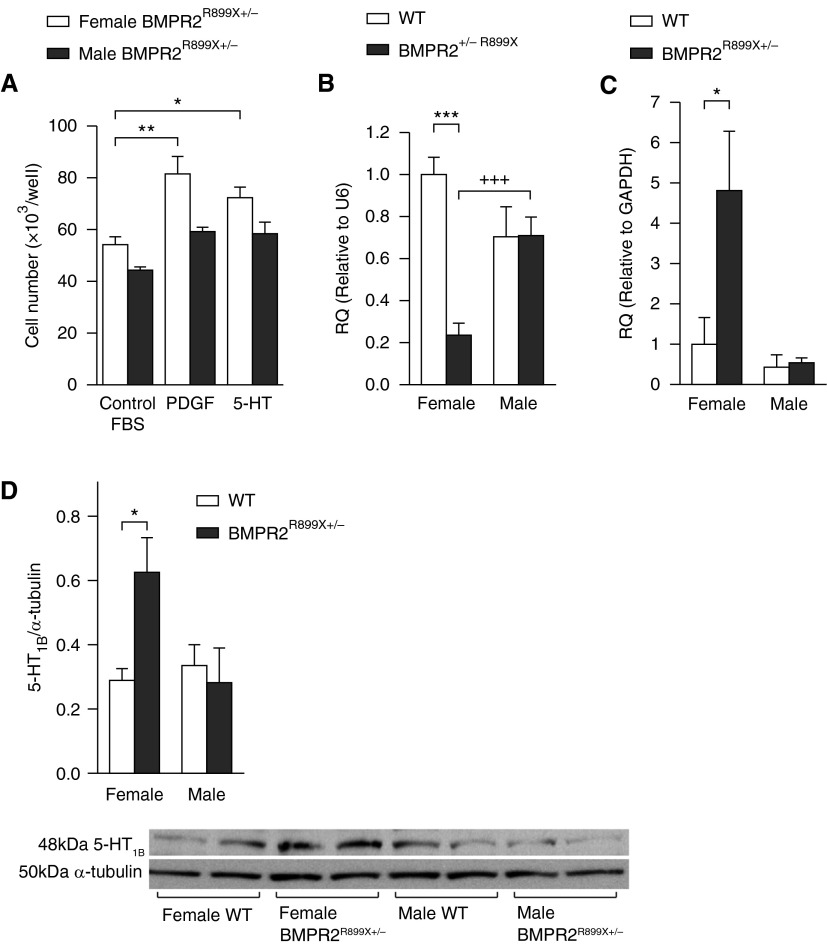
Female BMPR-IIR899X+/− PASMCs were more proliferative to platelet-derived growth factor (PDGF) and serotonin compared with male BMPR-IIR899X+/− PASMCs (Figure 1A). We examined miRNAs that are associated with PAH, hypoxia, serotonin, and estrogen systems in the female and male BMPR-IIR899X+/− PASMCs. We examined miRNA-503 (targets, e.g., fibroblast growth factor 2), miRNA-145 (targets krueppel-like factor 5 [23]), and miRNA-155 (targets, e.g., hypoxia-inducible factor α) (24). We also studied miRNAs targeting estrogen receptor α (ERα) (miRNA-22 and miRNA-206 [25]), CYP1B1 (miRNA-27b [26]), 5-HT1BR (miRNA-96 [27]), or SERT (miRNA-16 [28]).
Figure 1.
Characteristics of pulmonary artery smooth muscle cells (PASMCs) in female and male bone morphogenetic protein receptor (BMPR) 2R899X+/− mice (BMPR-IIR899X+/− PASMCs) and wild-type (WT) controls. (A) Proliferative responses after 72 hours to 10% fetal bovine serum (FBS), platelet-derived growth factor (PDGF), and serotonin (5-HT) in male and female BMPR-IIR899X+/− PASMCs (n = 3, in triplicate). (B) Expression of microRNA-96 in male and female BMPR-IIR899X+/− PASMCs and WT control cells (n = 8, all isolates repeated three times). (C and D) The expression of 5-HT 1B receptor (5-HT1BR) messenger RNA (C) and protein (D) in male and female BMPR-IIR899X+/− PASMCs and WT control cells (n = 6, all isolates repeated three times) and representative immunoblot of 5-HT1BR protein expression in male and female BMPR-IIR899X+/− PASMCs and WT control cells. Data displayed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, +++P < 0.001, determined by one-way ANOVA with Tukey’s post hoc test. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; RQ = relative quantity; U6 = small nuclear RNA U6.
There was no significant difference between the expression of miRNAs 16, 22, and 27b in male or female BMPR-IIR899X+/− PASMCs versus WT cells (see Figure E1 in the online supplement). miRNA-145 and miRNA-206 were significantly increased in both female and male BMPR-IIR899X+/− PASMCs compared with WT. miRNA-155 was down-regulated in female BMPR-IIR899X+/− PASMCs compared with WT, and up-regulated in male BMPR-IIR899X+/− PASMCs compared with WT. There was reduced expression of miRNA-503 in female BMPR-IIR899X+/− PASMCs compared with WT (Figure E1).
Expression of miRNA-96 was reduced by approximately 80% in female BMPR-IIR899X+/− PASMCs compared with their WT controls; expression was equal in male BMPR-IIR899X+/− PASMCs compared with their WT controls (Figure 1B). In WT PASMCs, there was no significant difference in miRNA-96 expression between female and male. In the BMPR-IIR899X+/− PASMCs, there was decreased expression of miRNA-96 in females compared with males. In silico analysis (miRwalk; available from http://www.umm.uni-heidelberg.de/apps/zmf/mirwalk/) suggested that miRNA-96 may target the 5-HT1BR. Therefore, we analyzed the expression of the 5-HT1BR in female and male BMPR- IIR899X+/− PASMCs. 5-HT1BR expression was only increased in female BMPR-IIR899X+/− PASMCs (Figures 1C and 1D), accompanied by a decrease in miRNA-96 expression.
Our rationale for focusing our studies on miRNA-96 was that this is the only known miRNA to target 5-HT1BR (27); 5-HT1BR mediates pathogenic effects of serotonin in PAH (14, 21). In addition, a role for miRNA-96 in PAH has not previously been described, hence this novel, sex-specific miRNA-96/5-HT1BR axis was worthy of further investigation.
miRNA-96 Expression in hPASMCs
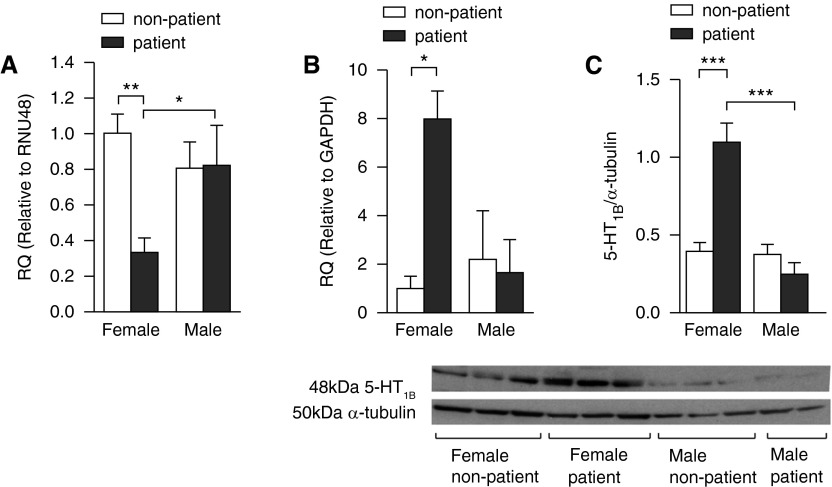
miRNA-96 was also down-regulated in PASMCs from female patients with PAH compared with control patients without PAH (Figure 2A). Consistent with findings from the BMPR-IIR899X+/− PASMCs, there was no change in male patient PASMCs (Figure 2A). Notably, 5-HT1BR expression was only increased in female patients with PAH (Figures 2B and 2C).
Figure 2.
Expression of microRNA (miRNA)-96 and 5-hydroxytryptamine 1B receptor (5-HT1BR) in human pulmonary artery smooth muscle cells (PASMCs). (A) Expression of miRNA-96 in human PASMCs from female and male patients with pulmonary arterial hypertension (PAH) and control patients without PAH (n = 4–6, all isolates repeated three times). The expression of 5-HT1BR messenger RNA (B) and protein (C) in human PASMCs from female and male patients with PAH and control patients without PAH (n = 4–6, all isolates repeated three times) and representative immunoblot of 5-HT1BR protein expression in human PASMCs from female and male patients and nonpatient control subjects. Data displayed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, determined by one-way ANOVA with Tukey’s post hoc test. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; RNU48 = small nuclear RNA 48; RQ = relative quantity.
Validation of 5-HT1BR as a Direct Target of miRNA-96
To assess transfection conditions in HeLa cells, we performed quantitative RT-PCR analysis to examine the mature form of miRNA-96 after pre–miRNA-96 and pre-miRNA–negative control transfections. A significant increase in miRNA-96 expression in the pre–miRNA-96 transfected cells was observed compared with control, with no increase observed in the pre-miRNA–negative control group (Figure 3A). Luciferase reporter assay confirmed a miRNA-96 binding site in the 3′ untranslated region (UTR) of 5-HT1BR mRNA as pre–miRNA-96 was able to significantly reduce luciferase activity at a concentration of 25 nM, with pre-miRNA–negative control showing no significant reduction in luciferase activity of the 5-HT1BR construct (Figure 3B). Importantly, pre–miRNA-96 had no effect on reducing luciferase activity of the psi-CHECK-2 control vector and of the mutated 5-HT1BR construct (Figure 3B).
Figure 3.
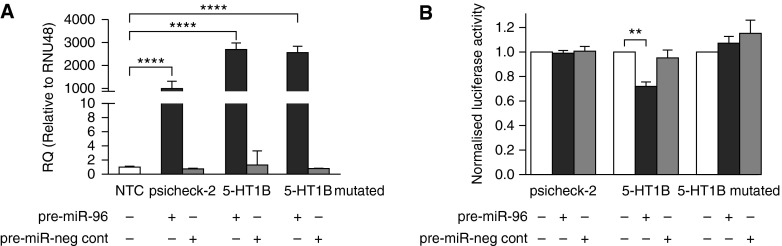
Validation of microRNA (miRNA-96) gene target 5-hydroxytryptamine 1B receptor (5-HT1BR). (A) The effect of precursor (pre-) miRNA-96 transfection on the expression of miRNA-96 (n = 3). (B) The effect of pre–miRNA-96 on the luciferase activity of the psi-CHECK-2 control construct, the 5-HT1BR construct, and mutated 5-HT1BR construct (n = 3, all isolates repeated three times). Data displayed as mean ± SEM. **P < 0.01, ****P < 0.0001 determined by one-way ANOVA with Tukey’s post hoc test. miR = miRNA; neg cont = negative control; NTC = nontransfection control; RNU48 = small nuclear RNA 48; RQ = relative quantity.
miRNA-96 and hPASMC Proliferation
The proliferative response to serotonin after 72 hours was only observed in female patient PASMCs, and was inhibited by the 5-HT1BR–selective antagonist, SB224289 (Figures 4A–4D). The selective 5-HT1BR agonist, CP94253, induced proliferation only in female patient PASMCs, and this was inhibited by SB224289 (Figures 4A–4D). Direct overexpression of miRNA-96 (Figure 5A) did not affect 5-HT1BR mRNA expression (Figure 5B), but did significantly decrease endogenous 5-HT1BR protein expression (Figure 5C). Pre–miRNA-96 abolished serotonin-induced proliferation (Figure 5D). Moreover, the effect of pre–miRNA-96 was comparable to the effects of the 5-HT1BR antagonist, SB224289 (Figure 5D). These results suggest that miRNA-96 expression can regulate 5-HT1BR–mediated proliferation in female patient PASMCs.
Figure 4.
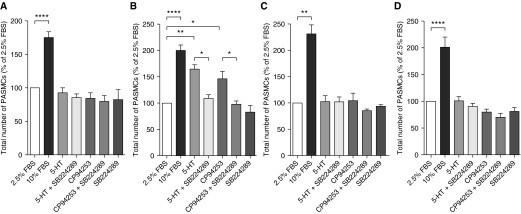
The effect of serotonin (5-HT) on proliferation in human pulmonary artery smooth muscle cell (PASMCs). The proliferative response to serotonin in PASMCs from female control patient without pulmonary arterial hypertension (PAH) (A), female patient with PAH (B), male control patient without PAH (C), and male patient with PAH (D) human (n = 3, in triplicate). SB224289 is the selective 5-HT 1B receptor (5-HT1BR) antagonist, and CP94253 is the selective 5-HT1BR agonist. Data displayed as percentage of 2.5% fetal bovine serum (FBS) negative control. *P < 0.05, **P < 0.01, ****P < 0.0001, determined by one-way ANOVA with Tukey’s post hoc test.
Figure 5.
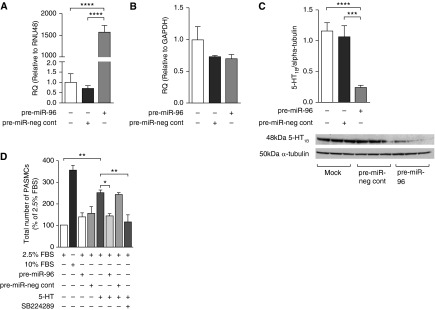
The effect of over-expressing microRNA (miRNA)-96 in human pulmonary artery smooth muscle cells (PASMCs) from female patients. The effect of pre–miRNA-96 transfection in human PASMCs from female patients on miRNA-96 expression (A), 5-hydroxytryptamine 1B receptor (5-HT1BR) messenger RNA (B), and 5-HT1BR protein (C) (n = 3, all isolates repeated three times). (D) The effect of overexpression of miRNA-96 on 5-HT–induced proliferation in human PASMCs from female patients with pulmonary arterial hypertension (n = 3, all isolates repeated three times). Data displayed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, determined by one-way ANOVA with Tukey’s post hoc test. FBS = fetal bovine serum; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; miR = miRNA; neg cont = negative control; RNU48 = small nuclear RNA 48; RQ = relative quantity.
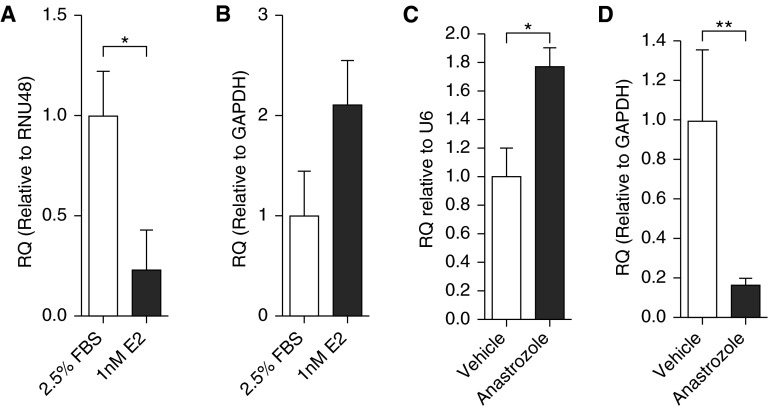
The Influence of Estrogen on miRNA-96
17β-estradiol decreased miRNA-96 expression (Figure 6A), but did not significantly affect 5-HT1BR mRNA expression (Figure 6B). To determine if endogenous 17β-estradiol also influences miRNA-96 expression, we assessed miRNA-96 and 5-HT1BR protein expression in lung from female and male mice that had been dosed with an aromatase inhibitor, anastrozole. These mice have depleted circulating and local lung synthesis of estrogen, and elevated BMPR-II signaling (4). miRNA-96 was elevated in the lungs from the estrogen-depleted female mice (Figure 6C), and this was accompanied by a decrease in 5-HT1BR mRNA expression (Figure 6D). No changes in miRNA-96 or 5-HT1BR mRNA expression were observed within male lung tissue (Figure E2).
Figure 6.
The effect of 17β-estradiol (E2) on microRNA (miRNA)-96 and 5-hydroxytryptamine 1B receptor (5-HT1BR) messenger RNA (mRNA) expression. The effect of E2 stimulation on miRNA-96 expression (A) and 5-HT1BR mRNA expression (B) in human pulmonary artery smooth muscle cells (n = 6, all isolates repeated three times). The effect of depleting endogenous E2 levels by inhibiting aromatase on miRNA-96 expression (C) and 5-HT1BR mRNA expression (D) in whole female mouse lung homogenate (n = 5, all isolates repeated three times). Data displayed as mean ± SEM. *P < 0.05, **P < 0.01, determined by one-way ANOVA with Tukey’s post hoc test. FBS = fetal bovine serum; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; RNU48 = small nuclear RNA 48; RQ = relative quantity; U6 = small nuclear RNA U6.
In Vivo Effect of a miRNA-96 Mimic in the Hypoxic Mouse
We determined if a miRNA-96 mimic would prevent the development of hypoxia-induced PH (associated with decreased BMPR-II signaling [4, 29]) and if any therapeutic effect was related to 5-HT1BR expression. First, we confirmed that MaxSuppressor In Vivo RNA-LANCEr II (Bioo Scientific, Austin, TX) delivered miRNA-96 mimic to the pulmonary arteries and, specifically, their medial layer. Figure 7A demonstrates that miRNA-96 expression was increased in the pulmonary arteries after administration of the miRNA-96 mimic. From in situ hybridization, we report that the miRNA-96 expression is localized within the smooth muscle medial layer of the pulmonary artery (Figure 7B). The miRNA-96 mimic reduced hypoxia-induced increases in RVSP (Figure 7C) and RVH (Figure 7D), and inhibited pulmonary vascular remodeling (Figures 7E and 7F). Importantly, the miRNA-96 mimic had no effect under normoxic conditions (Figures 7C–7F), and did not affect mean systemic arterial pressure or heart rate among experimental groups (Figure E3). We confirmed that the hypoxic mouse demonstrates decreased BMPR-II mRNA expression (Figure 8A). miRNA-96 expression was decreased by hypoxia and restored after the administration of the miRNA-96 mimic (Figure 8B). 5-HT1BR protein expression was markedly increased in the hypoxic lung, and the miRNA-96 mimic returned 5-HT1BR expression to normoxic levels (Figure 8C).
Figure 7.
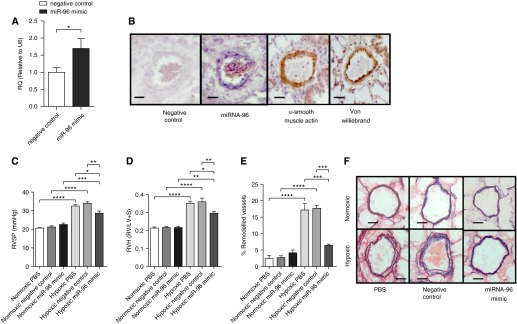
The effect of a microRNA (miRNA)-96 mimic on hypoxia-induced pulmonary hypertension in female mice. (A) The effect of a 30-μg dose (intravenous) of miRNA-96 (miR-96) mimic on miRNA-96 expression in pulmonary artery (n = 5). (B) Representative images of miRNA-96 in situ hybridization in pulmonary arteries from hypoxic mice (scale bars = 40 μm). Effects of a miRNA-96 mimic 30-μg dose on (C) right ventricular systolic pressure (RVSP; n = 9–10), (D) right ventricular hypertrophy (RVH; n = 10), and (E) percentage of remodeled pulmonary arteries (n = 6) in normoxic and hypoxic female mice. (F) Representative images of pulmonary arteries (Elastin Van Giesen stain; scale bars = 50 μm). Data displayed as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, determined by unpaired t test and one-way ANOVA with Tukey’s post hoc test. LV+S = left ventricle plus septum; RQ = relative quantity; RV = right ventricle; U6 = small nuclear RNA U6.
Figure 8.
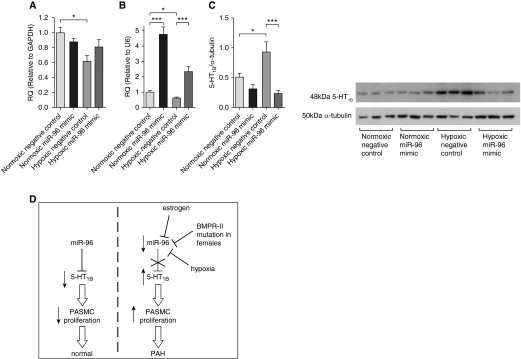
The effect of a microRNA (miRNA)-96 mimic on miRNA, messenger RNA, and protein level. (A) The effect of hypoxia and miRNA-96 mimic on bone morphogenetic protein receptor (BMPR) II expression (n = 6), (B) miRNA-96 expression (n = 6), and (C) 5-hydroxytryptamine 1B receptor (5-HT1BR) protein expression (n = 6) in whole-lung homogenate. (D) Schematic outlining proposed pathway: miRNA-96 normally down-regulates 5-HT1BR, which decreases serotonin-induced proliferation of pulmonary artery smooth muscle cells (PASMCs). Estrogen, hypoxia, and BMPR-II deficiency in females can decrease miRNA-96 expression, causing an increase in 5-HT1BR expression, which increases serotonin-induced proliferation, and this may contribute to the pathobiology of pulmonary arterial hypertension (PAH) in females. Data displayed as mean ± SEM. *P < 0.05, ***P < 0.001, determined by one-way ANOVA with Tukey’s post hoc test. GAPDH = glyceraldehyde 3-phosphate dehydrogenase; miR-96 = miRNA-96; RQ = relative quantity; U6 = small nuclear RNA U6.
Discussion
Sexual dimorphism of miRNA expression has been recognized within both physiological and pathological processes (30, 31). However, this is the first study to observe sex differences in miRNA expression with regard to PAH. Through in vitro cell culture models and in vivo animal studies, we present novel data demonstrating how miRNA-96, under the potential influence of estrogen, plays a role in the development of PH in a sex-dependent manner by regulating 5-HT1BR expression and hence serotonin-induced proliferation.
We have previously demonstrated that female mouse lung displays decreased BMPR-II and inhibitor of DNA binding 1 (Id1) expression compared with male lung (4). We have also demonstrated that, as a consequence of mutation in BMPR-II, there is unopposed p38 mitogen-activated protein kinase/extracellular signal–regulated kinase (ERK) signaling and increased proliferation in hPASMCs (2). PDGF induced proliferation only in female non-PAH hPASMCs, and we attribute this to decreased BMPR-II signaling in these cells (32). These observations are consistent with our observation that the female BMPR-IIR899X+/− PASMCs proliferate to a greater extent to PDGF and serotonin compared with male BMPR-IIR899X+/− PASMCs. We have previously shown that administration of serotonin unmasks a PH phenotype in BMPR-II+/− mice (26). This is consistent with our novel observation that there is an increase in the proliferative response to serotonin and 5-HT1BR expression in female BMPR-IIR899X+/− PASMCs, associated with a decrease in miRNA-96 expression.
To examine if these observations translated to human tissue, we demonstrated that serotonin-induced proliferation of hPASMCs from female patients was mediated by 5-HT1BR, as the effect was replicated by a 5-HT1BR agonist and abolished by a 5-HT1BR–selective antagonist. These results are consistent with previous studies showing the importance of the 5-HT1BR–mediating hPASMC proliferation (21, 23). miRNA-96 expression was decreased in PASMCs of female patients with PAH correlating with an increase in 5-HT1BR expression. This is consistent with our observations in female mouse BMPR-IIR899X+/− PASMCs, where miRNA-96 expression is decreased and 5-HT1BR expression is increased compared with male mouse BMPR-IIR899X+/− PASMCs, where there was no differential effect.
Interestingly, proliferation to serotonin in hPASMCs was only observed in female patient PASMCs, despite low expression levels of the 5-HT1BR in male patient and control PASMCs. 5-HT1BR is Gi linked, and Gi-linked responses are regulated by synergistic influences, such that a threshold for activation is required (33, 34). Indeed, activity and/or expression of 5-HT1BR can be increased synergistically by Gq-linked receptor stimulation (14, 35–37), estrogen (6), coactivation of SERT (14, 38, 39), and phospho-ERK (40, 41). Estrogen synthesis is increased in female hPASMCs (4), providing a stimulus for overexpression of SERT and 5-HT1BR (6), and SERT expression is increased in patient PASMCs (42). In addition, pERK2 expression is elevated in female hPASMCs and further enhanced in PASMCs from patients with PAH (2, 41). In addition, there is increased expression of Gq-linked receptors (e.g., PDGF, endothelin-1 [43]) in PASMCs from patients with PAH. Hence, female hPASMCs from patients with PAH are influenced by a unique combination of synergins (SERT, 5-HT1BR, Gq stimulation, estrogen) that can facilitate 5-HT1BR–mediated responses.
In silico bioinformatic analysis suggests that 5-HT1BR is a putative target of miRNA-96. In addition, a polymorphism in the 3′UTR of the 5-HT1BR mRNA disrupts binding of miRNA-96 (27). We validated that 5-HT1BR was a target of miRNA-96 through luciferase reporter assay. In addition, we demonstrated that direct overexpression of miRNA-96 (via transfection of pre–miRNA-96) in hPASMCs could decrease expression of target 5-HT1BR protein in in vitro cell culture. This acute overexpression of the miRNA-96 had no effect on 5-HT1BR mRNA, suggesting that, after 48 hours, the miRNA-96 is binding to the 3′UTR of 5-HT1BR mRNA, silencing translation to protein without actual degradation of the mRNA.
We demonstrated that direct overexpression of miRNA-96 ablated the ability of serotonin to induce proliferation of PASMCs of female patients with PAH, which accompanied a decrease in 5-HT1BR expression. Thus, within the PASMCs of female patients with PAH, down-regulation of miRNA-96 may lead to overexpression of 5-HT1BR, which subsequently increased the capacity of serotonin to induce proliferation. This is the first report that miRNA-96 expression influences distal hPASMC proliferation, and that manipulation of miRNA-96 can influence proliferation through targeting the 5-HT1BR. It is distal PASMCs that contribute to pulmonary vascular remodeling, but it is of interest that down-regulation of miRNA-96 may mediate an increase in BMP-4 signaling in proximal PASMCs, which demonstrate a distinct phenotype and are less involved in the pathobiology associated with PAH.
Little is currently known about the role that miRNA-96 plays in vascular physiology and pathology. Aberrant expression of miRNA-96 has been observed in cancer biology, where it has been described as being either oncogenic (44) or antioncogenic (45), and miRNA-96 has previously been associated with breast cancer (46). Breast cancer is a disease strongly influenced by estrogen, and this suggests that there could be a link between estrogen and miRNA-96 expression. Indeed, estrogen can alter miRNA expression through three mechanisms: first, through estrogen response elements in the promoter element of the primary miRNA (pri-miRNA) gene (47); second, through transcription of estrogen-related genes (e.g., c-MYC, which can, in turn, interact with transcription of pri-miRNA gene) (48); and, third, through interaction with miRNA biogenesis, as there is an ERα binding site in the promoter region of the Dicer gene (17). We have previously demonstrated that estrogen increases the protein expression of 5-HT1BR in hPASMCs (8). It was therefore of interest to investigate if estrogen can influence 5-HT1BR expression through modulating epigenetic control. Stimulation of hPASMCs with estrogen decreased the expression of miRNA-96, suggesting that estrogen could influence 5-HT1BR expression via regulation of miRNA-96. This is consistent with bioinformatic analysis showing the primary miRNA-96 gene harbors an estrogen response element in the promoter region (49). It is equally possible that estrogen could influence miRNA-96 expression indirectly though reduction in BMPR-II signaling (8), as SMADs are multifunctional proteins that modify gene expression themselves, transcriptionally through DNA binding and post-transcriptionally through pri-miRNA binding and regulation of miRNA processing. For example, it has been reported that the expression of miRNA-21 is influenced by BMP signaling. Here, SMAD proteins interact with DROSHA to increase the biogenic processing of pri-miRNA-21 into pre–miRNA-21 and finally into mature miRNA-21 (50). It is also known that pre-miRNAs may compete with their mature miRNA, and hence serve as post-transcriptional regulators of miRNA activity (51). Regulation of gene activity by miRNAs may therefore be affected by changes in BMPR-II/Smad signaling; decreased BMPR-II signaling by estrogen may facilitate the gene-silencing effects of miRNA-96 indirectly via decreased pre-miRNA processing.
Previous studies have shown that exogenously administered estrogen can actually protect against PH in male mice (52). We recently demonstrated expression of aromatase (the estrogen-synthesizing enzyme) in human, rat, and mouse pulmonary artery smooth muscle, and that the expression of aromatase was greatest in females (4). In addition, we showed that inhibition of endogenous aromatase by anastrozole increases lung BMPR-II expression and can prevent and reverse PH in the hypoxic mouse and rat Sugen 5,416/hypoxic model, but only in females (6). Anastrozole depletes all endogenous estrogen, both circulating (4) and vascular. This suggests that endogenous estrogen plays a role in the development of PH in female mice and rats via restoration of BMPR-II signaling, but not in the males, and that the combination of circulating estrogen and local endogenous synthesis of estrogen in pulmonary arteries drives a PH phenotype in females. Lungs from mice treated with anastrozole demonstrated a significantly higher expression of miRNA-96 compared with control lung. This increase in miRNA-96 was associated with a decrease in 5-HT1BR expression, and was only observed in lungs from female mice, and not males. Collectively, our results suggest that endogenous estrogen regulates BMPR-II and miRNA-96 expression only in female lung. This supports the hypothesis that sex plays an important role in the regulation of miRNAs and BMPR-II. Indeed, we have recently demonstrated that hPASMCs from females without PAH have reduced BMPR-II signaling compared with male hPASMCs, and this contributes to increased proliferative responses in the female hPASMCs (32). As PAH is more frequently presented in women, and endogenous estrogens may influence this, it also raises the question as to whether or not female and male patients with PAH may require different therapeutics. This concept has previously been addressed with regard to current PAH treatments, where it was observed that women responded better to endothelin-1 receptor antagonists compared with men (53).
To investigate if restoration of miRNA-96 expression in vivo can protect against PH via 5-HT1BR, we performed an in vivo study to examine the effects of a miRNA-96 mimic. BMPR-II expression is reduced in the hypoxic mouse, the monocrotaline rat, and the Sugen/hypoxic rat (4, 54, 55). Here, we used the hypoxic mouse model of PH, which produces a robust disease phenotype, including decreased BMPR-II signaling. We confirmed that BMPR-II mRNA expression was reduced in the lungs of the hypoxic mice. We report that 5-HT1BR protein expression is markedly elevated in the lungs from hypoxic mice, whereas miRNA-96 expression is reduced. In situ analysis demonstrated that the miRNA-96 expression is primarily in the medial smooth muscle cell layer of the pulmonary arteries. We showed that intravenous administration of the miRNA-96 mimic delivered the miRNA-96 to the pulmonary arteries, and also that the miRNA-96 mimic reduced hypoxia-induced increases in RVSP and RVH and prevented pulmonary vascular remodeling. This was associated with an increase in lung miRNA-96 expression and a decrease in 5-HT1BR expression. This substantiates our hypothesis that PH is associated with increased 5-HT1BR–mediated remodeling/proliferation under the control of miRNA-96. The miRNA-96 mimic had no effect on mean systemic arterial pressure or heart rate, suggesting that this therapeutic strategy could be pulmonary selective.
In summary, the present work suggests that estrogen and BMPR-II deficiency can decrease miRNA-96 expression in PASMCs, causing an increase in 5-HT1BR expression, and this may influence the pathobiology of PAH in females. In addition, an miRNA-96 mimic can reduce lung 5-HT1BR expression in vivo and can prevent the development of experimental PH (see Figure 8D for summary). Further study into the potential of an miRNA-96 mimic as a novel therapeutic strategy in PAH is warranted.
Footnotes
Supported by a Medical Research Council Ph.D. studentship (E.W.) and British Heart Foundation (BHF) program grant RG/11/7/28916; infrastructure support was provided by the Cambridge National Institute for Health Research Biomedical Research Centre; A.H.B. is supported by a BHF Chair of Translational Cardiovascular Sciences.
Author Contributions: Involvement in the conception, hypothesis delineation, and design of the study—E.W., A.H.B., N.W.M., and M.R.M.; acquisition of the data or the analysis and interpretation of such information—E.W., K.M.M., X.D.Y., L. Loughlin, H.S., M.N., L. Long, N.W.M., A.H.B., and M.R.M.; writing the article or substantial involvement in its revision prior to submission—E.W. and M.R.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201412-2148OC on April 14, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Machado RD, Aldred MA, James V, Harrison RE, Patel B, Schwalbe EC, Gruenig E, Janssen B, Koehler R, Seeger W, et al. Mutations of the TGF-beta type II receptor BMPR2 in pulmonary arterial hypertension. Hum Mutat. 2006;27:121–132. doi: 10.1002/humu.20285. [DOI] [PubMed] [Google Scholar]
- 2.Yang X, Long L, Southwood M, Rudarakanchana N, Upton PD, Jeffery TK, Atkinson C, Chen H, Trembath RC, Morrell NW. Dysfunctional Smad signaling contributes to abnormal smooth muscle cell proliferation in familial pulmonary arterial hypertension. Circ Res. 2005;96:1053–1063. doi: 10.1161/01.RES.0000166926.54293.68. [DOI] [PubMed] [Google Scholar]
- 3.Spiekerkoetter E, Tian X, Cai J, Hopper RK, Sudheendra D, Li CG, El-Bizri N, Sawada H, Haghighat R, Chan R, et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J Clin Invest. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, et al. Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:456–467. doi: 10.1164/rccm.201403-0483OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGoon MD, Benza RL, Escribano-Subias P, Jiang X, Miller DP, Peacock AJ, Pepke-Zaba J, Pulido T, Rich S, Rosenkranz S, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol. 2013;62(suppl25):D51–D59. doi: 10.1016/j.jacc.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 6.White K, Dempsie Y, Nilsen M, Wright AF, Loughlin L, MacLean MR. The serotonin transporter, gender, and 17β oestradiol in the development of pulmonary arterial hypertension. Cardiovasc Res. 2011;90:373–382. doi: 10.1093/cvr/cvq408. [DOI] [PubMed] [Google Scholar]
- 7.White K, Johansen AK, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure JD, et al. Activity of the estrogen-metabolizing enzyme cytochrome P450 1B1 influences the development of pulmonary arterial hypertension. Circulation. 2012;126:1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927. [DOI] [PubMed] [Google Scholar]
- 8.Austin ED, Hamid R, Hemnes AR, Loyd JE, Blackwell T, Yu C, Phillips Iii JA, Gaddipati R, Gladson S, Gu E, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3:6. doi: 10.1186/2042-6410-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, et al. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: critical role for serotonin-induced smooth muscle hyperplasia. Circulation. 2006;113:1857–1864. doi: 10.1161/CIRCULATIONAHA.105.591321. [DOI] [PubMed] [Google Scholar]
- 10.Abid S, Houssaini A, Chevarin C, Marcos E, Tissot CM, Gary-Bobo G, Wan F, Mouraret N, Amsellem V, Dubois-Randé JL, et al. Inhibition of gut- and lung-derived serotonin attenuates pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol. 2012;303:L500–L508. doi: 10.1152/ajplung.00049.2012. [DOI] [PubMed] [Google Scholar]
- 11.Morecroft I, Dempsie Y, Bader M, Walther DJ, Kotnik K, Loughlin L, Nilsen M, MacLean MR. Effect of tryptophan hydroxylase 1 deficiency on the development of hypoxia-induced pulmonary hypertension. Hypertension. 2007;49:232–236. doi: 10.1161/01.HYP.0000252210.58849.78. [DOI] [PubMed] [Google Scholar]
- 12.Dempsie Y, MacRitchie NA, White K, Morecroft I, Wright AF, Nilsen M, Loughlin L, Mair KM, MacLean MR. Dexfenfluramine and the oestrogen-metabolizing enzyme CYP1B1 in the development of pulmonary arterial hypertension. Cardiovasc Res. 2013;99:24–34. doi: 10.1093/cvr/cvt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dempsie Y, Nilsen M, White K, Mair KM, Loughlin L, Ambartsumian N, Rabinovitch M, Maclean MR. Development of pulmonary arterial hypertension in mice over-expressing S100A4/Mts1 is specific to females. Respir Res. 2011;12:159. doi: 10.1186/1465-9921-12-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lawrie A, Spiekerkoetter E, Martinez EC, Ambartsumian N, Sheward WJ, MacLean MR, Harmar AJ, Schmidt AM, Lukanidin E, Rabinovitch M. Interdependent serotonin transporter and receptor pathways regulate S100A4/Mts1, a gene associated with pulmonary vascular disease. Circ Res. 2005;97:227–235. doi: 10.1161/01.RES.0000176025.57706.1e. [DOI] [PubMed] [Google Scholar]
- 15.Morecroft I, Heeley RP, Prentice HM, Kirk A, MacLean MR. 5-hydroxytryptamine receptors mediating contraction in human small muscular pulmonary arteries: importance of the 5-HT1B receptor. Br J Pharmacol. 1999;128:730–734. doi: 10.1038/sj.bjp.0702841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caruso P, MacLean MR, Khanin R, McClure J, Soon E, Southgate M, MacDonald RA, Greig JA, Robertson KE, Masson R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol. 2010;30:716–723. doi: 10.1161/ATVBAHA.109.202028. [DOI] [PubMed] [Google Scholar]
- 17.Bhat-Nakshatri P, Wang G, Collins NR, Thomson MJ, Geistlinger TR, Carroll JS, Brown M, Hammond S, Srour EF, Liu Y, et al. Estradiol-regulated microRNAs control estradiol response in breast cancer cells. Nucleic Acids Res. 2009;37:4850–4861. doi: 10.1093/nar/gkp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long L, MacLean MR, Jeffery TK, Morecroft I, Yang X, Rudarakanchana N, Southwood M, James V, Trembath RC, Morrell NW. Serotonin increases susceptibility to pulmonary hypertension in BMPR2-deficient mice. Circ Res. 2006;98:818–827. doi: 10.1161/01.RES.0000215809.47923.fd. [DOI] [PubMed] [Google Scholar]
- 19.Long L, Yang X, Morrell NW, Southwood M. BMPR2 R899X knock-in mice developed age-related pulmonary hypertension [abstract] Thorax. 2011;66:A46. [Google Scholar]
- 20.White K, Loughlin L, Maqbool Z, Nilsen M, McClure J, Dempsie Y, Baker AH, MacLean MR. Serotonin transporter, sex, and hypoxia: microarray analysis in the pulmonary arteries of mice identifies genes with relevance to human PAH. Physiol Genomics. 2011;43:417–437. doi: 10.1152/physiolgenomics.00249.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keegan A, Morecroft I, Smillie D, Hicks MN, MacLean MR. Contribution of the 5-HT(1B) receptor to hypoxia-induced pulmonary hypertension: converging evidence using 5-HT(1B)-receptor knockout mice and the 5-HT(1B/1D)-receptor antagonist GR127935. Circ Res. 2001;89:1231–1239. doi: 10.1161/hh2401.100426. [DOI] [PubMed] [Google Scholar]
- 22.Pena JTG, Sohn-Lee C, Rouhanifard SH, Ludwig J, Hafner M, Mihailovic A, Lim C, Holoch D, Berninger P, Zavolan M, et al. miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nat Methods. 2009;6:139–141. doi: 10.1038/nmeth.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, et al. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse and patient samples. Circ Res. 2012;111:290–300. doi: 10.1161/CIRCRESAHA.112.267591. [DOI] [PubMed] [Google Scholar]
- 24.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, et al. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31:4087–4096. doi: 10.1128/MCB.01276-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshimoto N, Toyama T, Takahashi S, Sugiura H, Endo Y, Iwasa M, Fujii Y, Yamashita H. Distinct expressions of microRNAs that directly target estrogen receptor α in human breast cancer. Breast Cancer Res Treat. 2011;130:331–339. doi: 10.1007/s10549-011-1672-2. [DOI] [PubMed] [Google Scholar]
- 26.Tsuchiya Y, Nakajima M, Takagi S, Taniya T, Yokoi T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006;66:9090–9098. doi: 10.1158/0008-5472.CAN-06-1403. [DOI] [PubMed] [Google Scholar]
- 27.Jensen KP, Covault J, Conner TS, Tennen H, Kranzler HR, Furneaux HM. A common polymorphism in serotonin receptor 1B mRNA moderates regulation by miR-96 and associates with aggressive human behaviors. Mol Psychiatry. 2009;14:381–389. doi: 10.1038/mp.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 29.Upton PD, Morrell NW. The transforming growth factor-β–bone morphogenetic protein type signalling pathway in pulmonary vascular homeostasis and disease. Exp Physiol. 2013;98:1262–1266. doi: 10.1113/expphysiol.2012.069104. [DOI] [PubMed] [Google Scholar]
- 30.Ciaudo C, Servant N, Cognat V, Sarazin A, Kieffer E, Viville S, Colot V, Barillot E, Heard E, Voinnet O. Highly dynamic and sex-specific expression of microRNAs during early ES cell differentiation. PLoS Genet. 2009;5:e1000620. doi: 10.1371/journal.pgen.1000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mujahid S, Logvinenko T, Volpe MV, Nielsen HC. miRNA regulated pathways in late stage murine lung development. BMC Dev Biol. 2013;13:13. doi: 10.1186/1471-213X-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mair KM, Yang XD, Long L, White K, Wallace E, Ewart MA, Docherty CK, Morrell NW, MacLean MR. Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2015;191:693–703. doi: 10.1164/rccm.201410-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dickenson JM, Hill SJ. Synergistic interactions between human transfected adenosine A1 receptors and endogenous cholecystokinin receptors in CHO cells. Eur J Pharmacol. 1996;302:141–151. doi: 10.1016/0014-2999(96)00039-8. [DOI] [PubMed] [Google Scholar]
- 34.Dickenson JM, Hill SJ. Human 5-HT1B receptor stimulated inositol phospholipid hydrolysis in CHO cells: synergy with Gq-coupled receptors. Eur J Pharmacol. 1998;348:279–285. doi: 10.1016/s0014-2999(98)00148-4. [DOI] [PubMed] [Google Scholar]
- 35.MacLean MR. Pulmonary hypertension, anorexigens and 5-HT: pharmacological synergism in action? Trends Pharmacol Sci. 1999;20:490–495. doi: 10.1016/s0165-6147(99)01389-9. [DOI] [PubMed] [Google Scholar]
- 36.MacLean MR, Morecroft I. Increased contractile response to 5-hydroxytryptamine1-receptor stimulation in pulmonary arteries from chronic hypoxic rats: role of pharmacological synergy. Br J Pharmacol. 2001;134:614–620. doi: 10.1038/sj.bjp.0704273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sweeney G, Templeton A, Clayton RA, Baird M, Sheridan S, Johnston ED, MacLean MR. Contractile responses to sumatriptan in isolated bovine pulmonary artery rings: relationship to tone and cyclic nucleotide levels. J Cardiovasc Pharmacol. 1995;26:751–760. doi: 10.1097/00005344-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Morecroft I, Loughlin L, Nilsen M, Colston J, Dempsie Y, Sheward J, Harmar A, MacLean MR. Functional interactions between 5-hydroxytryptamine receptors and the serotonin transporter in pulmonary arteries. J Pharmacol Exp Ther. 2005;313:539–548. doi: 10.1124/jpet.104.081182. [DOI] [PubMed] [Google Scholar]
- 39.Morecroft I, Pang L, Baranowska M, Nilsen M, Loughlin L, Dempsie Y, Millet C, MacLean MR. In vivo effects of a combined 5-HT1B receptor/SERT antagonist in experimental pulmonary hypertension. Cardiovasc Res. 2010;85:593–603. doi: 10.1093/cvr/cvp306. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Suzuki YJ, Day RM, Fanburg BL. Rho kinase–induced nuclear translocation of ERK1/ERK2 in smooth muscle cell mitogenesis caused by serotonin. Circ Res. 2004;95:579–586. doi: 10.1161/01.RES.0000141428.53262.a4. [DOI] [PubMed] [Google Scholar]
- 41.Mair KM, MacLean MR, Morecroft I, Dempsie Y, Palmer TM. Novel interactions between the 5-HT transporter, 5-HT1B receptors and Rho kinase in vivo and in pulmonary fibroblasts. Br J Pharmacol. 2008;155:606–616. doi: 10.1038/bjp.2008.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcos E, Fadel E, Sanchez O, Humbert M, Dartevelle P, Simonneau G, Hamon M, Adnot S, Eddahibi S. Serotonin-induced smooth muscle hyperplasia in various forms of human pulmonary hypertension. Circ Res. 2004;94:1263–1270. doi: 10.1161/01.RES.0000126847.27660.69. [DOI] [PubMed] [Google Scholar]
- 43.Davie N, Haleen SJ, Upton PD, Polak JM, Yacoub MH, Morrell NW, Wharton J. ET(A) and ET(B) receptors modulate the proliferation of human pulmonary artery smooth muscle cells. Am J Respir Crit Care Med. 2002;165:398–405. doi: 10.1164/ajrccm.165.3.2104059. [DOI] [PubMed] [Google Scholar]
- 44.Haflidadóttir BS, Larne O, Martin M, Persson M, Edsjö A, Bjartell A, Ceder Y. Upregulation of miR-96 enhances cellular proliferation of prostate cancer cells through FOXO1. PLoS ONE. 2013;8:e72400. doi: 10.1371/journal.pone.0072400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W, Liu J, Yu J, Chen J. miRNA-96 suppresses KRAS and functions as a tumor suppressor gene in pancreatic cancer. Cancer Res. 2010;70:6015–6025. doi: 10.1158/0008-5472.CAN-09-4531. [DOI] [PubMed] [Google Scholar]
- 46.Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu C, Li J, Wang X, Song L. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS ONE. 2010;5:e15797. doi: 10.1371/journal.pone.0015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Di Leva G, Gasparini P, Piovan C, Ngankeu A, Garofalo M, Taccioli C, Iorio MV, Li M, Volinia S, Alder H, et al. MicroRNA cluster 221-222 and estrogen receptor alpha interactions in breast cancer. J Natl Cancer Inst. 2010;102:706–721. doi: 10.1093/jnci/djq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, Barton G, Jiao LR, Wait R, Waxman J, et al. The estrogen receptor-alpha–induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA. 2009;106:15732–15737. doi: 10.1073/pnas.0906947106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matys V, Fricke E, Geffers R, Gössling E, Haubrock M, Hehl R, Hornischer K, Karas D, Kel AE, Kel-Margoulis OV, et al. TRANSFAC: transcriptional regulation, from patterns to profiles. Nucleic Acids Res. 2003;31:374–378. doi: 10.1093/nar/gkg108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roy-Chaudhuri B, Valdmanis PN, Zhang Y, Wang Q, Luo QJ, Kay MA. Regulation of microRNA-mediated gene silencing by microRNA precursors. Nat Struct Mol Biol. 2014;21:825–832. doi: 10.1038/nsmb.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, et al. 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, Kawut SM, Halpern SD. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest. 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long L, Crosby A, Yang X, Southwood M, Upton PD, Kim DK, Morrell NW. Altered bone morphogenetic protein and transforming growth factor-beta signaling in rat models of pulmonary hypertension: potential for activin receptor–like kinase-5 inhibition in prevention and progression of disease. Circulation. 2009;119:566–576. doi: 10.1161/CIRCULATIONAHA.108.821504. [DOI] [PubMed] [Google Scholar]
- 55.Meloche J, Courchesne A, Barrier M, Carter S, Bisserier M, Paulin R, Lauzon-Joset JF, Breuils-Bonnet S, Tremblay É, Biardel S, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc. 2013;2:e005157. doi: 10.1161/JAHA.112.005157. [DOI] [PMC free article] [PubMed] [Google Scholar]