Abstract
Glioblastoma is one of the most aggressive and fatal brain cancers due to the highly invasive nature of glioma cells. Microglia infiltrate most glioma tumors and, therefore, make up an important component of the glioma microenvironment. In the tumor environment, microglia release factors that lead to the degradation of the extracellular matrix and stimulate signaling pathways to promote glioma cell invasion. In the present study, we demonstrated that microglia can promote glioma migration through a mechanism independent of extracellular matrix degradation. Using western blot analysis, we found upregulation of proline rich tyrosine kinase 2 (Pyk2) protein phosphorylated at Tyr579/580 in glioma cells treated with microglia conditioned medium. This upregulation occurred in rodent C6 and GL261 as well as in human glioma cell lines with varying levels of invasiveness (U-87MG, A172, and HS683). siRNA knock-down of Pyk2 protein and pharmacological blockade by the Pyk2/focal-adhesion kinase (FAK) inhibitor PF-562,271 reversed the stimulatory effect of microglia on glioma migration in all cell lines. A lower concentration of PF-562,271 that selectively inhibits FAK, but not Pyk2, did not have any effect on glioma cell migration. Moreover, with the use of the CD11b-HSVTK microglia ablation mouse model we demonstrated that elimination of microglia in the implanted tumors (GL261 glioma cells were used for brain implantation) by the local in-tumor administration of Ganciclovir, significantly reduced the phosphorylation of Pyk2 at Tyr579/580 in implanted tumor cells. Taken together, these data indicate that microglial cells activate glioma cell migration/dispersal through the pro-migratory Pyk2 signaling pathway in glioma cells.
Introduction
Glioblastoma (GBM) is an extraordinarily aggressive type of brain cancer due to resistance to radiation and chemotherapy and the highly invasive nature of this tumor. A single GBM cell can invade throughout the brain and often produce secondary lesions at sites distant from the primary tumor [1], thus, reducing the efficacy of surgical resection [2, 3]. The tumor microenvironment has a critical role in tumor invasion and progression with microglia as a significant player. The amount of microglial infiltration of the tumor is associated with poor clinical prognosis in patients with high graded gliomas [4, 5, 6]. Accumulating evidence demonstrates a role for microglia in tumor growth [7, 8, 9, 10, 11, 12], but the molecular mechanisms through which tumor cells interact with their environment to regulate migration from primary tumor sites are not well investigated.
Microglial cells comprise up to 30% of GBM total tumor mass [13, 14], and therefore constitute a potentially important component of the microenvironment of these tumors. Microglial cells in gliomas undergo a morphological transformation and are capable of some innate immune responses such as phagocytosis and cytotoxicity. Paradoxically, glioma infiltrating microglia do not secrete some key cytokines such as IL-6, IL-1β and TNF-α [1, 15] that are critical to develop effective immune responses. In fact, it has been shown that tumor infiltrating microglia increase the infiltrative behavior of glioma cells increasing proteinase activity and degradation of the extracellular matrix in the tumor area [4, 5, 7, 8] as well as stimulate glioma cell proliferation and dispersal into surrounding healthy brain areas [5, 16, 17, 18]. Membrane type 1 metalloprotease (MT1-MMP), matrix metalloproteinase-2 (MMP2), cathepsin B, and urokinase receptor (uPAR) are overexpressed in gliomas and they are postulated to play central roles in breaking down the extracellular matrix in the tumor area and, thereby, creating pathways for tumor cells invasion [7, 8, 19, 20].
Proline-rich tyrosine kinase (Pyk2) is a member of the focal adhesion kinase (FAK) family. Pyk2 integrates signals from cell adhesion, growth factor, and G-protein-coupled receptors and has a key role in migration of specific cell types, particularly, in leukocytes and fibroblasts [21, 22]. Pyk2 plays an important role in cell motility and invasion [21, 22, 23, 24, 25], and Pyk2 expression is shown to occur frequently in human astrocytomas with a significant correlation between the grade of malignancy of astrocytomas and the expression of Pyk2 [26]. Inhibition of Pyk2 activity in glioma cells significantly reduced tumor invasion and increased survival in mice with glioma cell xenografts [27].
Involvement of microglia in Pyk2 signaling in glioma cells has never been published although Pyk2 has been identified as an important regulator of glioma cell migration. In this report, we have identified Pyk2 as a new intracellular signaling element mediating interactions between microglia and glioma cells which lead to activation of glioma cell migration. We hypothesize that microglia can stimulate glioma cell dispersal not just through degradation of the extracellular matrix, but also by directly activating intracellular signaling pathways in glioma cells.
To analyze the activation of Pyk2 in glioma cells in response to soluble factors released by microglia we investigated GL261 murine glioma cells, C6 rat glioma cells, and three different human glioma cell lines with varying levels of invasiveness. A172 glioblastoma cells are non-tumorigenic in anti-thymocyte serum treated NIH Swiss mice. U-87MG cells are tumorigenic and highly invasive glioblastoma, whereas HS683 are non-invasive non-tumorigenic low graded glioma [28, 29, 30]. Many signaling pathways may be affected in different types of glioma in response to soluble factors released by microglia. Using different glioma cell lines, we have identified a common and major signaling pathway that is exploited to increase glioma migration and invasiveness.
Materials and Methodology
Ethics Statement
All procedures involving rodents were conducted in accordance with the National Institutes of Health regulations concerning the use and care of experimental animals. All procedures involving animals were approved by Universidad Central del Caribe Institutional Animal Care and Use Committee. All efforts were made to minimize suffering.
Cell Culture
A172, U87, HS683 human glioma cell lines and C6 rat glioma cell line were obtained from ATCC (Manassas, VA). The GL261 glioma cell line derived from C57BL/6 mice was obtained from the NCI (Frederick, MD). Previously established CHME5 immortalized human fetal microglia [31] were used for experiments with human glioma cell lines. C57BL/6 mouse and Sprague Dawley rat primary microglial cultures were prepared in our lab and used for experiments with GL261 and C6 cells, respectively. All cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum, 0.2 mM glutamine and antibiotics (50 U/mL penicillin, 50 μG/ml streptomycin) and maintained in a humidified atmosphere of C02/air (5%/95%) at 37°C. The medium was exchanged with fresh culture medium about every 2–3 days.
Primary microglia cultures
Mouse and rat primary microglia cultures were obtained from cortical mixed glial cultures of 1–3 day old C57BL/6 mice and Sprague Dawley rats respectively as described by Markovic et al. 2005 [7]. Briefly, mixed glial cultures were prepared from neocortex of 1–3 day old rodents. Brains were removed after decapitation and the meninges stripped away to minimize fibroblast contamination. The forebrain cortices were dissociated using the stomacher blender method. The cell suspension was then allowed to filter by gravity through a #60 sieve and then through a #100 sieve. After centrifugation, the cells were suspended in DMEM (containing 30 mM glucose, 2 mM glutamine, 1 mM pyruvate and 10% fetal bovine serum, 100 units/ml of penicillin/streptomycin) and plated in 75 cm2 flasks at a density of 300,000 cells/cm2. The medium was exchanged with fresh culture medium about every 5 days. At confluence (about 12–14 days), microglial cells were collected by a mild shaking (1 h at 125 rpm). The medium was transferred to a new flask and incubated for 30 min before removal of non-adherent cells by changing the medium. The purity of the microglial culture was assessed by immunocytochemical detection of isolectin B4, a microglial marker [32].
Preparation of Conditioned Medium
For glioma/microglia co-culturing glioma and microglial cells were collected by treatment with trypsin 2X and seeded (1X105) in 60mm dishes in a ratio 2:1. Co-cultured cells were incubated in 2mL of DMEM with 10% FBS for 24 hours prior to obtaining activated microglia conditioned medium (AMCM) for the experiments. In addition, medium, conditioned from microglia (MCM) alone was used as well as medium conditioned from glioma cells pretreated with AMCM—glioma conditioned medium (GCM). Conditioned mediums were centrifuged to remove debris and dead cells and used for the experiments immediately.
Invasion/Migration Assays
Invasion assays were performed using modified Boyden chambers [33] coated with Matrigel. 8 μM pore transwells (Falcon HTS FluoroBlock Inserts, BD Biosciences, Bedford, MA, cat. # 351152) were pre-coated with 30 μL of BD MatrigelTM Matrix (BD Biosciences, Bedford, MA, cat. # 354263) for 2 hours at 37°C. Glioma cells (50,000 cells) were placed on top of the membrane in the upper chamber in 150 μL of DMEM and microglia (50,000 cells) were placed in the lower chamber in 500 μL of DMEM. The control group did not contain microglia. Invasion was allowed to proceed for 18 hours at 37°C, 5% CO2. In migration assays, Matrigel that mimics the extracellular matrix was not used. Migration assays were performed for 5 hours at 37°C, 5% CO2.
In both assays, migratory cells were fixed with 100% methanol and stained with 40 μg/μL Propidium iodide (PI). Images of the cells that migrated to the lower chamber were captured using an inverted microscope Olympus BX51WI (Olympus, Shinjuku, Tokyo, Japan), Qcolor 3 camera (Olympus) and Q-capture Pro software (Olympus) and the number of migrating and invading cells on the underside of the filter were counted. The mean of the total invading or migrating cells was determined from 3 independent experiments.
Cell Signaling Cascade Antibody Microarrays
Panorama Antibody Microarray—Cell Signaling Kits to total and phosphoproteins (Sigma Chemical Co., St. Louis, MO, cat. # CSAA1) were utilized and processed according to manufacturer’s instructions. Proteins used for array hybridization were extracted and labeled with biotin using the Full Moon Biosystems, Inc. (Sunnyvale, CA) Antibody Array Assay Kit (Cat. # KAS02) as per the manufacturer’s instructions. Antibody Microarrays were sent to Full Moon Biosystems, Inc. for measurements of the fluorescent signals.
Intracranial Implantation of Glioma Cells
All surgery was performed under isoflurane anesthesia, and all efforts were made to minimize suffering. GL261 glioma cells were implanted into the right cerebral hemisphere of 12–16 week old C57BL/6 mice. Implantation was performed according the protocol that we described earlier [34, 35]. Briefly, mice were anesthetized with isoflurane and a midline incision was made on the scalp. At stereotaxic coordinates of bregma, 2mm lateral, 1mm caudal and 3mm ventral a small burr hole (0.5mm diameter) was drilled on the skull. 1 μL of cell suspension (2X104 cells/μL in PBS) was delivered at a depth of 3 mm over 2 min. Sixteen days following injection, animals were anesthetized with pentobarbital (50 mg/kg) and transcardially perfused with PBS followed by 4% paraformaldehyde (PFA). Brains were removed and postfixed in 4% PFA/PBS for 24 h at 4°C, followed by 0.15M, 0.5M, and 0.8M sucrose at 4°C until fully dehydrated. Brains were then frozen-embedded in Cryo-M-Bed embedding compound (Bright Instrument, Huntingdon, England) and cut using a Vibratome UltraPro 5000 cryostat (American Instrument, Haverhill, MA).
Immunocytochemistry
Immunostaining was performed using the previously established protocol in our lab [19]. Frozen 25 μm coronal sections encompassing the entire tumor were generated from each mouse brain. The sections were blocked with 5% normal goat serum/5% normal horse serum (Vector lab., Burlingame, CA) in PBS containing 0.3% Triton X-100 and 0.05% Phenylhydrazine for 30 minutes and then incubated with monoclonal mouse anti-Pyk2 antibody, dilution 1:1000 (Cell Signaling; #3480S), polyclonal rabbit anti-Iba1 antibody (Wako; #019–19741) 1 μg/ml, and monoclonal GFAP-Cy3 antibody produced in mouse (Sigma, #C9205), dilution 1:500, in PBS-TAT (0.3% TritonX-100, 5% normal goat/5% normal horse serum, 1% sodium azide, 0.01% thimerosal) overnight at 4°C. The sections were incubated with corresponding secondary antibodies (AMCA anti-mouse IgG and fluorescein anti-rabbit IgG (Vector Lab., Burlingame, CA)) overnight and visualized using an Olympus Fluoview FV1000 confocal microscope with 10× objectives or 40× oil immersion objectives.
Western Blot Analysis
Glioma cells were seeded (1X105) in 60mm dishes and incubated in normal conditions 24 hours prior experiments. The cells were then treated for 2 hours with medium conditioned from glioma/microglia co-culturing (AMCM). Control glioma cells were treated with medium conditioned from microglia or glioma cells alone (MCM or GCM respectively). After 2 hours cells were lysed and clarified cell lysates were separated on 10% SDS-PAGE gels. The proteins were transferred to a PVDF membrane and probed with rabbit polyclonal anti-phospho-Pyk2(Tyr 579/580) primary antibody (Invitrogen; #44636G), dilution 1:1000, followed by anti-rabbit conjugated immunoglobulins (Sigma). Final detection was performed with enhanced chemiluminescence methodology (SuperSignal West Dura Extended Duration Substrate; Pierce, Rockford, IL) and the intensity of the signal was measured using a gel documentation system (Versa Doc Model 1000, Bio Rad). In all cases, intensity of the chemiluminescence signal was corrected for minor changes in protein content after densitometry analysis of the India ink stained membrane.
Model of local microglial ablation
A model of local microglial ablation using CD11b-HSVTK transgenic mice expressing the herpes simplex thymidine kinase (HSVTK) protein in microglia and macrophages [9, 36] was employed. When these animals are exposed to ganciclovir (GCV), the cells that express HSVTK are eliminated. CD11b-HSVTK (+/-) male mice (gift of Dr. Tsirka, Stonybrook University) were bred with C57BL/6 males. Offspring were genotyped by PCR using primers 5′ -GACTTCCGTGGCTTCTTGCTGC-3′ and 5′ -GTGCTGGCATTACAGGCGTGAG-3′. GL261 glioma cells were implanted into the right cerebral hemisphere of 12–16 week old CD11b-HSVTK (+/-) mice. C57BL/6 mice were used as controls. Tumors were allowed to grow for ten days and then mini-osmotic pumps (Alzet, DURECT, model 2004) were installed for local administration of GCV (Calbiochem, Billerica, MA, USA) to the tumor. Animals were anesthetized and a 3mm brain infusion cannula connected to pump was set up at the previous tumor implantation site using brain infusion kit (Alzet, DURECT). The pumps were placed subcutaneously on the mouse back. The drug was infused at 0.25 μL/h, 1 mg/mL over 7 days. Pumps with normal saline solution were used as a control.
Percoll purification of glioma cells from tumor tissue
Tumors were removed from the mouse brains, dissected to 1–2 mm pieces with a razor blade, and homogenized using a non-enzymatic cell dissociation solution (Sigma-Aldrich, St. Louis, MO, USA). Glioma cells were purified from the homogenized tissue using Percoll (Sigma-Aldrich, St. Louis, MO, USA) gradients of 30%, 37% and 70%. Following this procedure the glioma fraction was collected from the top and the distinct white ring of microglia cells was collected at the interphase of 37% and 70% Percoll levels. Both glioma and microglia fractions were used for further analysis. Purification efficiency was tested by western blot analysis using mouse monoclonal primary antibodies that detect Iba1, in dilution 1:200 (#1022–5 Lot: GR40934-12 Abcam, Cambridge, MA, USA).
PF-562,271 inhibition of Pyk2
PF-562,271 (MedKoo Biosciences, #202228) is a potent, ATP-competitive, reversible inhibitor of FAK and Pyk2 catalytic activity with IC(50) of 1.5 and 14 nmol/L, respectively [37]. In the present study, it was used at concentrations 5nM and 16nM to block FAK alone and FAK and Pyk2 together in glioma cells. A 1mM PF-562,271 stock solution was prepared by dilution in water.
RNA Interference by Small Double-stranded RNAs
Glioma cells were transfected with siRNA targeting Pyk2 (Qiagen, cat. # SI02225321) using HiPerfect Transfection reagent according to the manufacturer’s instructions (Qiagen) and as we previously described [38]. Briefly, 100μL of serum free medium containing 2μL of 20 nM Pyk2 siRNA and 20μL of HiPerfect were prepared and incubated for 30 minutes at room temperature. This complex was then added to 5.0X104 glioma cells containing 1.9mL of cell culture medium in a drop-wise fashion and the plate gently swirled to evenly distribute the transfection complex. In addition, mock transfections were performed and used as control where 100μL of serum free medium containing 20μL of HiPerfect without siRNA was added to the cells. Based on preliminary time course experiments (data not shown), a time point of 3 days after transfection was used. Efficiency of Pyk2 knock-down was determined using western blot.
Statistical Analysis
Results are expressed as mean ± standard deviation (SD). Statistical probability was calculated using GraphPad software. Unpaired t-tests or one-way ANOVA tests followed by the Tukey’s post-hoc test were used to determine significance between groups. P-values of less than 0.05 were considered as significant.
Results
Factors released from microglia stimulate migration and invasion of glioma cells
In order to separate the microglial effects on extracellular matrix degradation and on increasing the migratory ability of glioma cells, we performed migration and invasion assays for glioma cells. The data revealed that in the presence of microglia both glioma cell migration and invasion were significantly increased for all investigated glioma cell lines (Fig 1), including HS683 low graded glioma that has low basic level of invasiveness (Fig 1A). The data in the literature indicate that microglia residing in the tumor promote glioma invasion through release of a wide specter of proteases and degradation of the extracellular matrix and this is supported by our finding that microglia increase glioma cell invasion through Matrigel. Furthermore, our data demonstrate that microglia stimulate glioma cell migration without the presence of Matrigel suggesting that microglia promote glioma invasiveness not just by degrading the extracellular matrix, but also by direct activation of glioma cell mobility.
Fig 1. Factors released from microglia increase glioma invasiveness and migration.
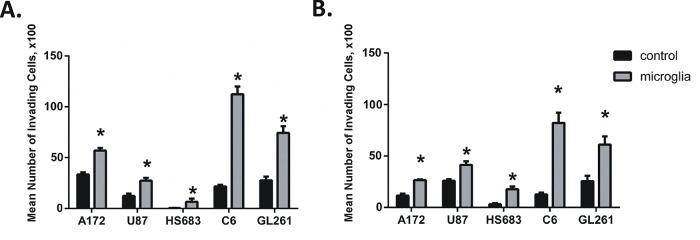
Invasion (A) and migration (B) assays were performed. Number of invading and migrating glioma cells without (control) and with microglia in the lower compartment. Results are presented as mean ± S.D. with significant differences from controls (*) shown (p < 0.05). Unpaired t-tests were used to determine significance between groups.
Factors released from microglia activate the Pyk2 pathway in glioma cells
We used a Panorama Antibody Microarray to survey potential intracellular signaling pathways that might be involved in microglial activated glioma cell migration. This microarray has 112 antibodies spotted and allows the relative levels of key proteins to be measured with a special emphasis on cell signaling proteins known to be involved with apoptosis, cell cycle, cytoskeleton, signal transduction and nuclear proteins. Our data indicate that 24 hours exposure of C6 glioma cells to microglia conditioned medium (MCM) leads to alteration of the expression levels of multiple signaling proteins in C6 cells. For further investigation we selected signaling proteins known to be involved in regulation of invasion and migration. Data are presented in Table 1. We determined that soluble factors released from microglia and contained in MCM up-regulate the expression of Epidermal Growth Factor Receptor (EGFR), Phospholipase C gamma 1 (PLCγ1) proteins, and most markedly, it significantly increased the phosphorylation levels of Pyk2 at Tyr 579/580 (Table 1), thereby stimulating the Pyk2 signaling pathway in glioma cells. All these data indicate that Pyk2 could carry out the signaling initiated by soluble factors released by microglia in order to regulate glioma cell migration. To extend and confirm our finding, we evaluated the localization and activity of Pyk2 in human and rodent glioma cell lines using immunocytochemical and western blot analysis.
Table 1. List of selected proteins that are altered in C6 glioma cells after treatment with microglia conditioned medium determined using Panorama Antibody Array System.
| Protein | Control/MCM,mean ± SEM | N | P value | P<0.05 |
|---|---|---|---|---|
| EGFR | 2.333±0.400 | 4 | 0.0072 | yes |
| PLA2 | 1.855±0.160 | 4 | 0.0004 | yes |
| PLCγ1 | 7.700±2.954 | 3 | 0.0209 | yes |
| Pyk2 | 1.370±0.151 | 6 | 0.0543 | no |
| pPyk2 (579/580) | 4.932±1.663 | 5 | 0.0457 | yes |
Only proteins that belong to signaling pathways related to migration were selected. The level of proteins was measured as a relative density of fluorescent signal. N indicates the number of repeated experiments.
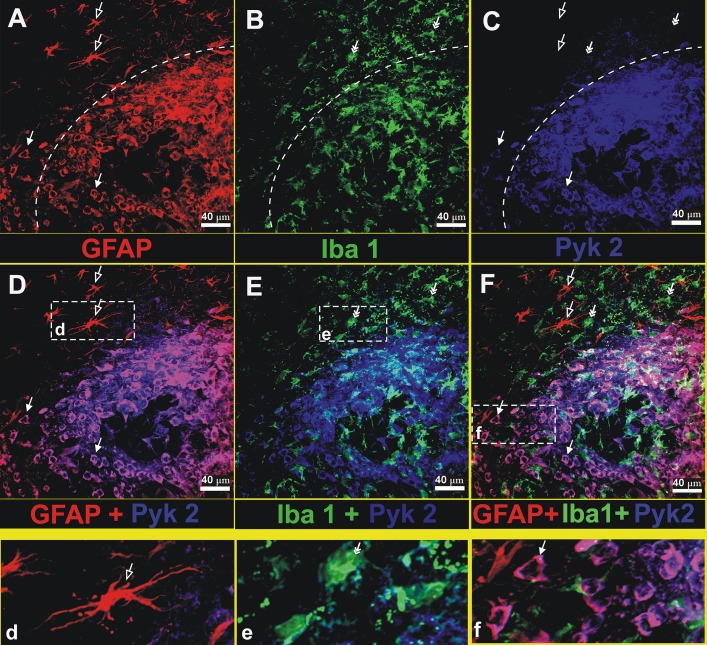
To detect the cellular localization of Pyk2 within a brain tumor, we utilized a murine glioma model. Fig 2 shows immunocytochemical analysis of Pyk2, glial fibrillary acidic protein (GFAP, a marker of glioma cells and astrocytes) and Iba1 (a marker of microglial cells) in brain slices obtained from tumor bearing C57BL/6 mice. Brain sections were prepared 16 days after the implantation procedure. Since glioma cells are much larger in size and do not have long branched processes characteristic for astrocytes, glioma cells and astrocytes can be easily distinguished from each other (Fig 2A, 2d and 2f), as we described earlier [35]. The merged image demonstrates that glioma cells express abundant levels of Pyk2 (Fig 2D and 2F) in the tumor core as well as at the invasion area (Fig 2f), whereas astrocytes and microglia do not show a marked amount of Pyk2 (Fig 2D, 2E and 2F). Although there have been previous reports indicating that Pyk2 is present in microglia [39, 40], it is weakly detected in microglia in the present study because the imaging settings were optimized to visualize the large quantities of Pyk2 present in glioma cells.
Fig 2. Pyk2 is mostly detected in glioma cells rather than in other cell types in mouse brain.
Immunohistochemistry was performed on C57BL/6 mice brain sections containing the tumor area. GL261 glioma cells were implanted into the brains of C57BL/6 mice and grown for 16 days. Photographs show the tumor and surrounding healthy tissue. The dash line outlines the border of tumor. Anti-GFAP antibody was used to detect glioma cells and astrocytes (red, panel A), anti-Iba 1 antibody was used to detect microglial cells (green, panel B) and Pyk2 detection is presented in blue (panel C). The merged images of anti-GFAP and anti-Pyk2, of anti-Iba1 and anti-Pyk2, and of all antibodies together can be seen in merged image boxes D, E, F correspondingly. Insert panels d, e, and f represent enlarged images of astrocytes, microglia, and invading glioma cells. Solid arrows indicate glioma cells, frame arrows indicate astrocytes, and double headed arrows indicate microglia. Scale bar: 40 μm.
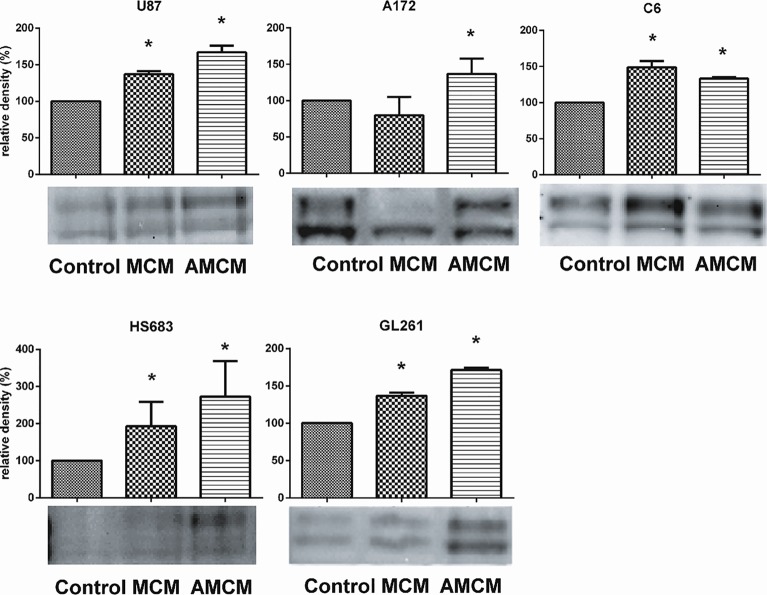
To determine if Pyk2 signaling in glioma cells is activated by soluble factors released from microglia, we detected the levels of Pyk2 phosphorylated at Tyr579/580 using western blot. Fig 3 demonstrates the relative levels of phosphorylated Pyk2 in control glioma cells, glioma cells treated with medium conditioned from microglia (MCM) or medium conditioned from microglia that was first co-cultured with glioma (AMCM) to simulate the cross-talk that generally occurs in the tumor microenvironment. Microglia change the pattern of cytokines and other soluble factors released when they are in close vicinity with glioma cells [7, 8, 15, 16]. For this reason, we investigated the effect of soluble factors released from microglia that never received any signaling from glioma cells (MCM) and the effect of soluble factors released by microglia previously interacting with glioma cells (AMCM). Two hours treatment with conditioned mediums were used since we found this treatment duration as an optimum in order to obtain full value effect on Pyk2 phosphorylation in glioma cells. The longer treatment provided steady effect without any future dynamic. The data indicate that all investigated glioma cell lines represent different levels of basic Pyk2 phosphorylation (Fig 3, S1 Fig) with the A172 demonstrating the highest and the HS682 the lowest pPyk2 phosphorylation (the pPyk2579/580 was almost undetected by western blot analysis in untreated HS683 compare to other cell lines). Significant up regulation of Pyk2 phosphorylation was detected in C6, HS683, U87 and GL261 glioma cells 2 hours after treatment with MCM. But even more prominent effect on Pyk2 phosphorylation was found out in all investigated cell lines after treatment with AMCM, with the strongest effect in glioma cells with the low basic level of Pyk2 phosphorylation: U87 and HS683 (70% and 150% up regulation correspondingly), compare to glioma cells with high basic level of Pyk2 phosphorylation: A172 and C6 (40% up regulation). The stronger effect of AMCM compared to MCM suggests that microglial cells change the composition of released soluble factors when they interact with glioma cells and this leads to additional activation of Pyk2 signaling in glioma cells.
Fig 3. Factors released from microglia upregulate phosphorylation of Pyk2 in glioma cells.
Western blot analysis of pPyk2 (Tyr 579/580) protein in glioma cell lines. The signal is detected at the area corresponding to molecular weight of 116 kDa. The graph shows the density of protein in MCM and AMCM treatments relative to control. Two bands in pPyk2 (Tyr 579/580) detection identify phosphorylation in one or both sites. Intensity of the chemiluminescence signal was corrected for minor changes in protein content after densitometry analysis of the India ink stained membrane. The India Ink stained membranes are provided in S1 Fig. The “relative density” axis for HS683 cells is shown in a higher grid scale compare to other cell lines due to higher relative up regulation of Pyk2 phosphorylation in this cell line. Results are presented as mean ± S.D. with significant differences from control (*) (p < 0.05). One-way ANOVA followed by the Tukey’s multiple comparison test was used to determine significance between MCM or AMCM groups compared to control. 5 repeated experiments (N = 5) for each cell line were used for statistical analysis.
In order to exclude possible paracrine effects of glioma cells in response to microglial soluble factors, an additional treatment with glioma conditioned medium (GCM) was used for glioma cells. For this purpose glioma cells were treated with medium, conditioned from other glioma cells pretreated with AMCM. Western blotting did not reveal any quantitative difference in Pyk2 phosphorylation in control and GCM treated cells, indicating that paracrine stimulation does not make significant contribution to microglial activation of Pyk2 in glioma cells.
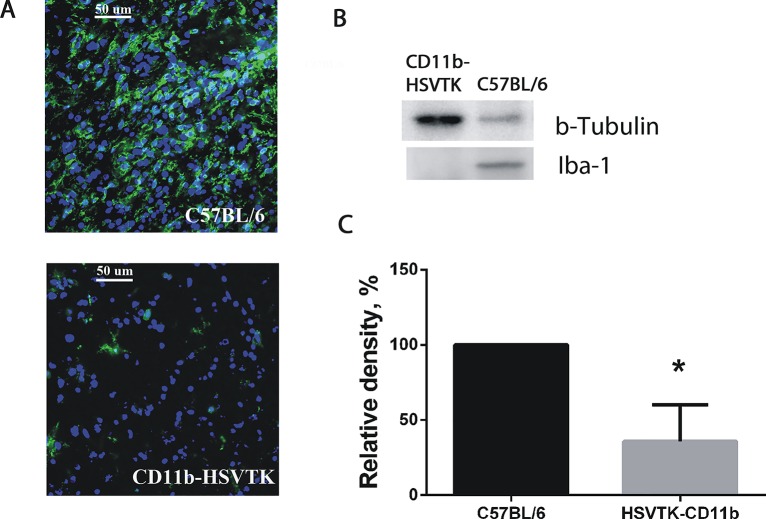
To further evaluate the participation of microglia in activation of the Pyk2 pathway in glioma cells we used a murine model of local microglia ablation. Our preliminary investigation with use of western blot and immunohistochemical staining did not reveal any difference in microglial infiltration of implanted tumors in CD11b-HSVTK and C57BL/6mice without administration of GCV. In order to assess the effectiveness of microglia ablation in tumor site, animals were sacrificed after 7 days of GCV administration and frozen brain sections were prepared followed by immunocytochemical detection of Iba-1. The images of tumors presented in Fig 4A demonstrate that microglial cells were significantly reduced in CD11b-HSVTK mice compared to C57BL/6 animals where microglial cells were still present. For further evaluation of the efficacy of microglia ablation in CD11b-HSVTK mice, quantitative analysis of Iba-1 by western blot was performed in C57BL/6 and CD11b-HSVTK mice brain tumor samples (Fig 4B and 4C). Results showed a significant reduction in the expression of Iba-1 in CD11b-HSVTK mice, confirming the substantial reduction of brain microglia in CD11b-HSVTK as compared to wild type animals.
Fig 4. Microglia ablation in brain tumors using the CD11b-HSVTK/GCV system.
(A) Immunocytochemical detection of microglia in tumors developed in C57BL/6 and CD11b-HSVTK mice brains after local GCV administration. The tumors were generated by intracranial implantation of GL261 glioma cells. GCV was delivered to the tumor area through mini-osmotic pumps. Image shows the significant reduction of microglia in tumors developed in CD11b-HSVTK compared to C57BL/6 mice. Anti-Iba 1 antibody was used to detect microglial cells (green) and DAPI was used to detect all cell nuclei (blue). (B) Western blot detection of Iba-1 in tumors extracted from C57BL/6 and CD11b-HSVTK mice brains after the treatment with GCV. (C) The graph shows corresponding levels of Iba-1 protein expression in C57BL/6 and CD11b-HSVTK mice brain tumors after GCV administration determined by western blot. Mean ± S.E and significant difference from control (*) are shown (p < 0.05).
In order to evaluate if Pyk2 phosphorylation in glioma cells depends on the grade of microglial infiltration of the tumor we performed western blot analysis of Pyk2(Tyr 579/580) in glioma cells extracted from tumors of C57BL/6 and CD11b-HSVTK mice after GCV administration. Taking into account that Pyk2 can also be expressed in other cell types that might be present in tumor, such as microglia [39, 41], we purified glioma cells extracted from tumor tissue using Percoll gradients. The efficacy of glioma cell purification is presented in S2 Fig. Western blotting revealed the significantly low level of Pyk2 phosphorylation in glioma cells in CD11b-HSVTK mice compared to C57BL/6 after GCV administration (Fig 5). These results match closely with our in vitro data indicating that microglia is involved in the regulation of the Pyk2 signaling in glioma cells.
Fig 5. Local microglia ablation in tumor area reduces phosphorylation of Pyk2 in implanted GL261 glioma tumors in CD11b-HSVTK transgenic mice.
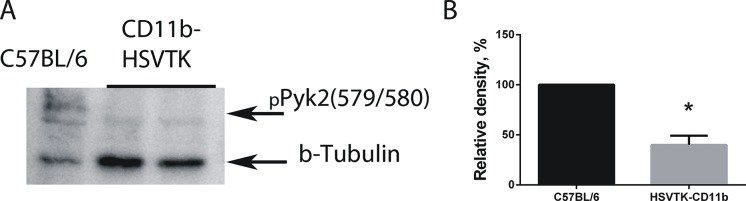
(A) Western blot detection of pPyk2 (Tyr 579/580) protein in glioma cells extracted from C57BL/6 and HSVTK-CD11b mice brains after treatment with GCV. Glioma cells were purified using Percoll gradients. (B) The graph shows the quantification of corresponding levels of pPyk2(Tyr 579/580) detected by western blot. Mean ± S.E and significant difference from control (*) is shown (p < 0.05).
Pyk2 pathway is involved in microglial activated glioma cell migration
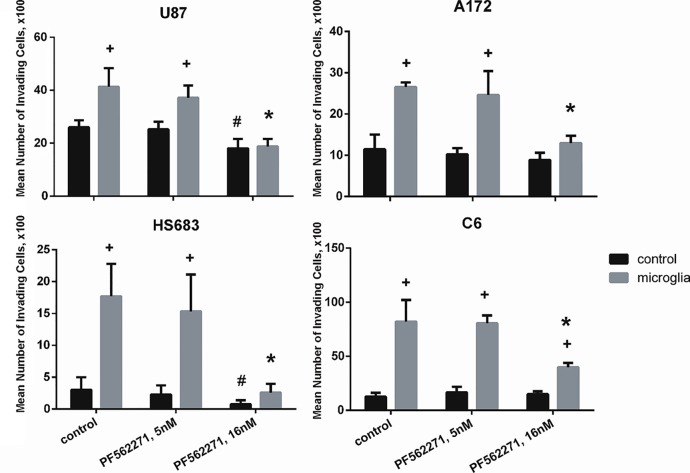
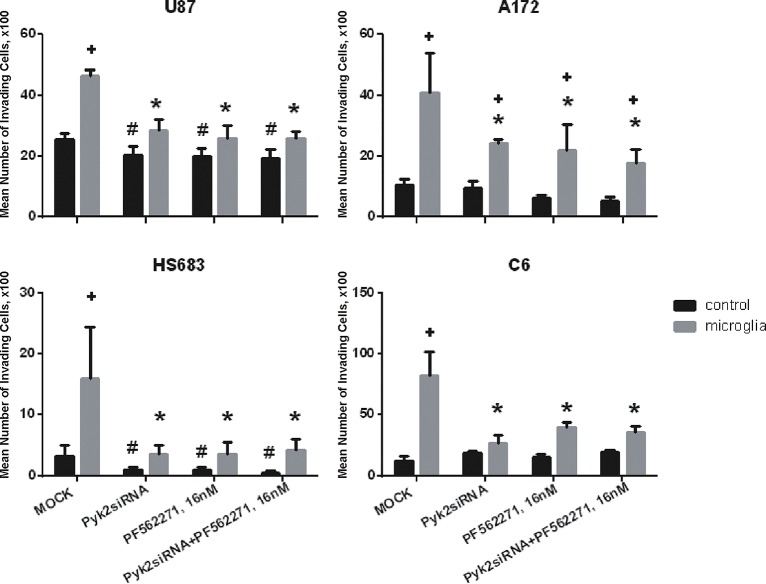
Our data indicate that microglia stimulate migration of glioma cells and that treatment of glioma cells with AMCM increases their levels of phosphorylated Pyk2. To assess the involvement of microglia on activation of Pyk2 signaling in glioma cells and on glioma cell mobility, we performed migration assays for glioma cells in the presence and absence of the Pyk2/FAK inhibitor, PF-562,271. PF-562,271 readily passes the blood-brain barrier and selectively blocks FAK at 5 nM and blocks both FAK and Pyk2 at 16 nM [37], S3 and S4 Figs Therefore, we investigated both FAK and FAK/Pyk2 selective doses of PF-562,271. PF-562,271 was given two hours prior to and during the assay. The lower (FAK selective) concentration of PF-562,271 (5 nM) had no effect on glioma cell migration whether microglia were present or not (Fig 6) indicating that FAK was not involved in glioma cell migration. The higher concentration of PF-562,271 (16 nM) effectively eliminated the effect of microglia on glioma cell migration in all investigated glioma cell lines and also reduced the basal migration rates of U87 and HS683 glioma cells in the absence of microglia (Fig 6). Pyk 2 blockade (16 nM PF-562,271) did not alter the basal migration rates of A172 and C6 cells, but significantly reduced migration of these cells when stimulated by microglia (Fig 6).
Fig 6. FAK is not involved in microglial stimulation of migration in glioma cells.
Data obtained from migration assays for glioma cells. Cells were treated with 5nM (concentration that effectively blocks FAK) and 16nM (concentration that effectively blocks Pyk2) of PF-562,271. Due to different migration abilities in all presented cell lines the Y-axis scales are adjusted for each cell line in order to demonstrate the absolute numbers of migrating cells. Results are presented as mean ± S.D. with significant differences from control in each group (+), from Mock without microglia on the bottom (#), or from Mock with microglia on the bottom (*) (p < 0.05). One-way ANOVA followed by the Tukey’s multiple comparison test was used to determine significance between groups.
As an independent measure of microglial activation of Pyk2 signaling in glioma cells and its role in glioma cell migration, we used siRNA targeting Pyk2 to selectively knock-down Pyk2 in glioma cells. Three days after transfection with siRNA, levels of Pyk2 were reduced by 60% in glioma cells (S5 Fig). The results obtained using siRNA knock-down of Pyk2 in glioma cells were identical to those obtained using 16 nM PF-562,271 to block Pyk2 (Fig 7). In addition, simultaneous knock-down of Pyk2 using siRNA together with application of 16nM PF-562,271 did not produce further reduction of migration induced by microglia compared to Pyk2 knock-down alone. Taken together the experiments shown in Figs 6 and 7 indicate that Pyk2, but not FAK, is the major signaling pathway involved in microglial stimulation of glioma cell migration.
Fig 7. siRNA knock-down and/or pharmacological blockade of Pyk2 by PF-562,271 eliminates the stimulatory effect of microglia on glioma cell migration.
Data obtained from standard migration assays for control glioma cells and cells transfected with siRNA against Pyk2 with and without additional application of PF-562,271 in the presence and absence of microglia in the lower compartment. Due to different migration abilities in all presented cell lines the Y-axis scales are adjusted for each cell line in order to demonstrate the absolute numbers of migrating cells. Results are presented as mean ± S.D. with significant difference from control in each group (+), from Mock without microglia on the bottom (#), or from Mock with microglia on the bottom (*) (p < 0.05). One-way ANOVA followed by the Tukey’s multiple comparison test was used to determine significance between groups.
Discussion
A large number of studies have described a supportive role of microglia and brain macrophages in tumors [5, 18, 42, 43, 44]. The natural defense mechanisms provided by microglia do not function properly within glioblastomas. Instead, the tumor cells control microglia to support tumor growth, invasion, angiogenesis, and survival [7, 8, 20, 45, 46, 47]. Growing evidence suggests that microglia and macrophages provide the main source of tumor-promoting molecules, such as matrix metalloproteinases, cathepsins and other endopeptidases that are involved in extracellular matrix degradation and are considered to be the key enzymes involved in glioma invasion [7, 8, 45, 48, 49, 50, 51, 52]. Our results reveal that microglia can promote glioma cell dispersal into surrounding areas through another mechanism that directly stimulates the mobility of glioma cells.
Here we demonstrate that glioma cells expressing a large amount of Pyk2 (A172, C6, GL261) also have strong migration and invasion abilities. Contrary, HS683 that has low basic level of Pyk2 phosphorylation also has low migration and invasion capacity. These data allow making a conclusion that the high basic level of Pyk2 phosphorylation in gliomas correlate with high level of invasiveness.
On the other hand the up regulation of Pyk2 phosphorylation at Tyr 579/580 in response to factors released by microglia was identified in all investigated glioma cell lines. At the same time microglia stimulate invasiveness and migration in glioma cells even in non-invasive HS683 low graded glioma. The pharmacological blockade with PF-562,271 and siRNA knockdown of Pyk2 eliminate the simulative effect of microglia on glioma cell migration and indicate that in the presence of microglia, Pyk2 participates as a regulator of migration stimulated by microglia in all investigated glioma cell lines.
In the tumor microenvironment, glioma cells are found in close interaction with tumor infiltrating microglia and macrophages (Fig 2), and consequently provide the stimulus to permanently activate the Pyk2 pathway and subsequently, glioma cell migration (Fig 1). This conclusion is supported by our data with use of the HSVTK/GCV system that allows us to model a tumor microenvironment deficient in microglia cells in vivo (Fig 5). The Pyk2 pathway is poorly activated in microglia ablated tumors and may explain the less invasive nature of the tumor in these animals, demonstrated in earlier reports [9]. We propose that activation of the Pyk2 pathway in gliomas as a result of glioma-microglial interactions allows aggressive dispersal and invasion of glioma cells into surrounding brain tissues. Additional investigation of Pyk2 expression and phosphorylation levels in different gliomas in correlation with the grade of microglial infiltration and tumor invasiveness are necessary.
Moreover, the contribution of Pyk2 in the regulation of glioma cell migration may vary in different glioma cell lines. Pyk2 blockade reduce the basal migration rates of U87 and HS683 glioma cells, but not A172, GL261, and C6 cells in the absence of microglia. These data indicate that Pyk2 has a key role in the basic migratory activity of U87 and HS683, but not A172, GL261, and C6 cells. These might suggest that in addition to Pyk2 other pathways may be involved in the regulation of basal rates of migration in gliomas such as Insulin-like growth factor binding protein 3 (IGFBP-3) that has been implicated in the pathogenesis of gliomas and was shown to be involved in proliferation and the invasive capacity of glioma cells [53]. Other studies demonstrated that Culllin1 (Cul1) is increased significantly in malignant brain tumors, and that silencing of Cul1 in glioma cells inhibited the cell migration and invasion abilities as well as down-regulated MMP-2 and MMP-9 expression that also greatly contribute to the reduced cell invasion and migration abilities [54]. Golgi phosphoprotein 3 (GOLPH3) is also found to be upregulated in gliomas and involved in glioma cell migration and invasion via the mammalian target of rapamycin (mTOR)-Y-box binding protein-1 (YB1) pathway [55]. Furthermore, in has been shown recently that aquaporins (in particular, AQP1) can be involved in migration of some tumor cells. AQP drives water influx, affect the organization of the cytoskeleton through Lin7/β-catenin, facilitating lamellipodia extension and cell migration [56]. These signaling proteins, in addition to Pyk2, might contribute to the regulation of basal migration rates in different gliomas.
FAK, a close relative of Pyk2 that is involved in migration in different tissues, has also been described to be expressed in gliomas. Reports about FAK participation in glioma cell migration are contradictive. Lipinski [25] demonstrated that FAK does not stimulate glioma cell migration, while other reports indicate that it does [57, 58]. Our data obtained by use of pharmacological blockade of FAK demonstrated that FAK is not involved in migration in all investigated cultured glioma cell lines regardless of the presence of microglia.
Our results using antibody microarrays suggest that factors released from microglia upregulate PLCγ1 and EGFR protein levels in C6 glioma cells. A recent publication demonstrated that microglia stimulate migration of glioma cells through EGFR [59]. Since PLCγ1 controls two Pyk2 activation pathways (elevation of [Ca2+]i and PKC phosphorylation) [60, 61] and PLCγ1 binds to the EGFR resulting in activation of PLCγ1-mediated downstream signaling [62, 63], PLCγ1 and EGFR may be upstream regulators of the Pyk2 pathway. PLCγ1 activation is linked to increased invasion of gliomas [64] making PLCγ1 a likely candidate linking the cell surface receptor (activated by microglia) to the Pyk2 pathway. EGFR is an activator of PLCγ1 [65] and EGFR gene amplification is one of the most frequent alterations occurring in glioblastoma [66] and associated with profuse tumor cell invasion [63, 67, 68, 69, 70]. All these together indicate that PLCγ1 and EGFR might be involved in enhancing migration of glioma cells exposed to microglia and could potentially be acting as upstream regulators of the Pyk2 pathway. Further research must be done in order to build the possible pathway involved in the activation of the Pyk2 protein in glioma cells after their interaction with microglia. It is critical to state that EGFR may not be the only receptor involved in the activation of migration of glioma cells. Other receptors such as AT1 and AT2 Angiotensin II receptors [71] have been associated with poor prognosis in human astrocytomas and are involved in the regulation of genes essential for glioma progression.
Conclusions
In summary, glioma and microglial cells are involved in a reciprocal interaction in the tumor microenvironment where they modulate the functions and abilities of each other in order to promote tumor progression and invasion. In this interaction microglial cells release soluble factors which activate Pyk2 intracellular signaling in glioma cells and thereby, promote migration of these cells.
Supporting Information
The figure is given in support of Fig 3 and represents membranes probed with antibodies against pPyk2(579/580) and corresponding India Ink staining used as a loading control for densitometry analysis for A172, U87, HS683, C6, Gl261 glioma cell lines.
(TIF)
GL261 glioma cells were implanted into the brains of C57BL/6 mice. In 16 days the half of tumors were removed and used for glioma cells purification with further western blot analysis. The other half of tumors were preceded directly for western blot analysis without glioma cells purification step. Mouse monoclonal primary antibodies that detect Iba1, in dilution 1:200 (#1022–5 Lot: GR40934-12 Abcam, Cambridge, MA, USA), followed by anti-mouse conjugated immunoglobulins (Cell Signaling) were used.
(TIF)
Rabbit polyclonal anti-phospho-Pyk2(Tyr 579/580) primary antibody (Invitrogen; #44636G) dilution 1:1000, were used, followed by anti-rabbit conjugated immunoglobulins (Sigma).
(TIF)
Anti-pFAK(576/577) primary antibody were used (Cell Signaling Technology, #93305), dilution 1:1000, followed by anti-rabbit conjugated immunoglobulins (Sigma).
(TIF)
Monoclonal mouse anti-Pyk2 antibody were used (Cell Signaling; #3480S), dilution 1:1000, followed by anti-mouse conjugated immunoglobulins (Cell Signaling).
(TIF)
Acknowledgments
The authors wish to thank Miguel Mendez González, Paola López Pieraldi and Natalia Skachkova for technical assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
LYK 5SC2GM102040, National Institute of Health, http://projectreporter.nih.gov/reporter_searchresults.cfm; YVK 5SC2GM095410, National Institute of Health, http://projectreporter.nih.gov/reporter_searchresults.cfm; SNS 5R01NS065201, National Institute of Health, http://projectreporter.nih.gov/reporter_searchresults.cfm; LAC R25GM110513, National Institute of Health, http://projectreporter.nih.gov/reporter_searchresults.cfm; MJE 8G12MD007583, National Institute of Health, http://projectreporter.nih.gov/project_info_description.cfm?aid=8495125&icde=23354349&ddparam=&ddvalue=&ddsub=&cr=1&csb=default&cs=ASC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Laws ER Jr, Goldberg WJ, Bernsteins JJ. Migration of human malignant astrocytoma cells in the mammalian brain: Scherer revisited. Int J Dev Neurosci. 1993; 11: 691–7. [DOI] [PubMed] [Google Scholar]
- 2. Hingtgen S, Figueiredo JL, Farrar C, Duebgen M, Martinez-Quintanilla J, Bhere D, at al. Real-time multi-modality imaging of glioblastoma tumor resection and recurrence. J Neurooncol. 2013; 111: 153–61. 10.1007/s11060-012-1008-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gilbert MR. Recurrent glioblastoma: a fresh look at current therapies and emerging novel approaches. Semin Oncol. 2011; 38: Suppl 4:S21–33. 10.1053/j.seminoncol.2011.09.008 [DOI] [PubMed] [Google Scholar]
- 4. Platten M, Kretz A, Naumann U, Aulwurm S, Egashira K, Isenmann S, et al. Monocyte chemoattractant protein-1 increases microglial infiltration and aggressiveness of gliomas. Ann Neurol. 2003; 54: 388–92. [DOI] [PubMed] [Google Scholar]
- 5. Graeber MB, Scheithauer BW, Kreutzberg GW. Microglia in brain tumors. Glia. 2002; 40: 252–9. [DOI] [PubMed] [Google Scholar]
- 6. Strik HM, Stoll M, Meyermann R. Immune cell infiltration of intrinsic and metastatic intracranial tumours. Anticancer Res. 2004; 24: 37–42. [PubMed] [Google Scholar]
- 7. Markovic DS, Glass R, Synowitz M, Rooijen Nv, Kettenmann H. Microglia stimulate the invasiveness of glioma cells by increasing the activity of matalloprotease-2. J Neuropathol. Exp. Neurol. 2005; 64: 754–762. [DOI] [PubMed] [Google Scholar]
- 8. Markovic DS, Vinnakota K, Chirasani S, Synowitz M, Raguet H, Stock K, et al. Gliomas induce and exploit microglial MT1-MMPexpression for tumor expansion. Proc. Natl. Acad. Sci. 2009; 106: 12530–12535. 10.1073/pnas.0804273106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhai H, Heppner FL, Tsirka SE. Microglia/Macrophages Promote Glioma Progression. Glia. 2011; 59: 472–485. 10.1002/glia.21117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Denny WA. Prodrugs for Gene-Directed Enzyme-Prodrug Therapy (Suicide Gene Therapy). J Biomed Biotechnol. 2003; 2003: 48–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang YX, Lu Y, Liu TJ, Yang J, Chen Y, Fang YW. Using HSV-TK/GCV suicide gene therapy to inhibit lens epithelial cell proliferation for treatment of posterior capsular opacification. Mol Vis. 2011; 17: 291–9. [PMC free article] [PubMed] [Google Scholar]
- 12. Jacobs VL, Liu Y, De Leo JA. Propentofylline targets TROY, a novel microglial signaling pathway. PLoS One. 2012; 7: e37955 10.1371/journal.pone.0037955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roggendorf W, Strupp S, Paulus W. Distribution and characterization of microglia/macrophages in human brain tumours. Acta Neuropathol. 1996; 92: 288–293. [DOI] [PubMed] [Google Scholar]
- 14. Badie B, Schartner J. Role of microglia in glioma biology. Microsc. Res. Tech. 2001; 54: 106–113. [DOI] [PubMed] [Google Scholar]
- 15. Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro. Oncol. 2006; 8: 261–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sliwa M, Markovic D, Gabrusiewicz K, Synowitz M, Glass R, Zawadzka M, et al. The invasion promoting effect of microglia on glioblastoma cells is inhibited by cyclosporin A. Brain. 2007; 130: 476–489. [DOI] [PubMed] [Google Scholar]
- 17. Bettinger I, Thanos S, Paulus W. Microglia promote glioma migration. Acta Neuropathol. 2002; 103: 351–355. [DOI] [PubMed] [Google Scholar]
- 18. Li W, Graeber MB. The molecular profile of microglia under the influence of glioma. Neuro Oncol. 2012; 14: 958–78. 10.1093/neuonc/nos116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eaton MJ, Skatchkov SN, Brune A. SURI and Kir6.1 subunits of K(ATP)-channels are co-localized in retinal glial (Müller) cells. Neuroreport. 2002; 13: 57–60. [DOI] [PubMed] [Google Scholar]
- 20. Watters JJ., Schartner JM, Badie B. Microglia function in brain tumors. J. Neurosci. Res. 2005; 81: 447–455. [DOI] [PubMed] [Google Scholar]
- 21. Dunty JM, Schaller MD. The N termini of focal adhesion kinase family members regulate substrate phosphorylation, localization, and cell morphology. J Biol Chem. 2002; 277: 45644–54. [DOI] [PubMed] [Google Scholar]
- 22. Riggs D, Yang Z, Kloss J. The Pyk2 FERM regulates Pyk2 complex formation and phosphorylation. Cell Signal. 2011; 23: 288–96. 10.1016/j.cellsig.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Loftus JC, Yang Z, Tran NL, Kloss J, Viso C, Berens ME, et al. The Pyk2 FERM domain as a target to inhibit glioma migration. Mol Cancer Ther. 2009; 8: 1505–14. 10.1158/1535-7163.MCT-08-1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paulino VM, Yang Z, Kloss J, Ennis MJ, Armstrong BA, Loftus JC, et al. TROY (TNFRSF19) is overexpressed in advanced glial tumors and promotes glioblastoma cell invasion via Pyk2-Rac1 signaling. Mol Cancer Res. 2010; 8: 1558–67. 10.1158/1541-7786.MCR-10-0334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lipinski CA, Tran NL, Menashi E, Rohl C, Kloss J, Bay RC, et al. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005; 7: 435–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gutenberg A, Brūck W, Buchfelder M. Expression of tyrosine kinases FAK and Pyk2 in 331 human astrocytomas. Acta Neuropathol. 2004; 108: 224–30. [DOI] [PubMed] [Google Scholar]
- 27. Lipinski CA, Tran NL, Viso C, Kloss J, Yang Z, Berens ME, et al. Extended survival of Pyk2 or FAK deficient orthotopic glioma xenografts. J Neurooncol. 2008; 90: 181–9. 10.1007/s11060-008-9656-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, et al. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973; 51: 1417–23. [DOI] [PubMed] [Google Scholar]
- 29. Fogh J, Fogh JM, Orfeo T. One hundred and twenty-seven cultured human tumor cell lines producing tumors in nude mice. J. Natl. Cancer Inst. 1977; 59: 221–226. [DOI] [PubMed] [Google Scholar]
- 30. Gershwin ME, Ikeda RM, Kawakami TG, Owens RB. Immunobiology of heterotransplanted human tumors in nude mice. J. Natl. Cancer Inst. 1977; 58: 1455–1463. [DOI] [PubMed] [Google Scholar]
- 31. Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. 1995;195:105–108. [DOI] [PubMed] [Google Scholar]
- 32. Streit WJ and Kreutzberg GW. Lectin binding by resting and reactive microglia. J Neurocytol. 1987; 16: 249–60. [DOI] [PubMed] [Google Scholar]
- 33. Baugher PJ, Krishnamoorthy L, Price JE, Dharmawardhane SF. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phonotype of human breast cancer cells. Breast Cancer Res. 2005; 7: R965–R974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008; 198: 98–105. 10.1016/j.jneuroim.2008.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kucheryavykh LY, Kucheryavykh YV, Rolón-Reyes K, Skatchkov SN, Eaton MJ, Cubano LA, et al. Visualization of implanted GL261 glioma cells in living mouse brain slices using fluorescent 4-(4-(dimethylamino)-styryl)-N-methylpyridinium iodide (ASP+). Biotechniques, 2012; 0: 1–4. 10.2144/000113926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hövelmeyer N, et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005; 11: 146–52. [DOI] [PubMed] [Google Scholar]
- 37. Roberts WG, Ung E, Whalen P, Cooper B, Hulford C, Autry C, et al. Antitumor activity and pharmacology of a selective focal adhesion kinase inhibitor, PF-562,271. Cancer Res. 2008; 68: 1935–44. 10.1158/0008-5472.CAN-07-5155 [DOI] [PubMed] [Google Scholar]
- 38. Kucheryavykh YV, Kucheryavykh LY, Nichols CG, Maldonado HM, Baksi K, Reichenbach A, et al. Downregulation of Kir4.1 Inward Rectifying Potassium Channel Subunit Expression by RNAi Impairs Potassium Transfer and Glutamate Uptake by Cultured Cortical Astrocytes. Glia, 2007; 55: 274–281. [DOI] [PubMed] [Google Scholar]
- 39. Tian D, Litvak V, Lev S. Cerebral ischemia and seizures induce tyrosine phosphorylation of PYK2 in neurons and microglial cells. J Neurosci. 2002; 20: 6478–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Combs CK, Johnson DE, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of beta-amyloid and prion proteins. J Neurosci. 1999; 19: 928–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rolls A, Avidan H, Cahalon L, Schori H, Bakalash S, Litvak V, et al. A disaccharide derived from chondroitin sulphate proteoglycan promotes central nervous system repair in rats and mice. Eur J Neurosci. 2004; 20:1973–83. [DOI] [PubMed] [Google Scholar]
- 42. Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010; 119: 89–105. 10.1007/s00401-009-0622-0 [DOI] [PubMed] [Google Scholar]
- 43. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007; 10: 1387–94. [DOI] [PubMed] [Google Scholar]
- 44. Gabrusiewicz K, Ellert-Miklaszewska A, Lipko M, Sielska M, Frankowska M, Kaminska B. Characteristics of the alternative phenotype of microglia/macrophages and its modulation in experimental gliomas. PLoS One. 2011; 6: e23902 10.1371/journal.pone.0023902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat. Rev. Cancer. 2003; 3: 489–501. [DOI] [PubMed] [Google Scholar]
- 46. Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001; 52: 401–10. [DOI] [PubMed] [Google Scholar]
- 47. Arnold T, Betsholtz C. The importance of microglia in the development of the vasculature in the central nervous system. Vasc Cell. 2013; 5: 4 10.1186/2045-824X-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H. (2011) The brain tumor microenvironment. Glia. 2011; 59: 1169–1180. 10.1002/glia.21136 [DOI] [PubMed] [Google Scholar]
- 49. Sloane BF, Rozhin J, Robinson D, Honn KV. Role for cathepsin B and cystatins in tumor growth and progression. Biol. Chem. Hoppe Seyler. 1990; 371: Suppl,193–198. [PubMed] [Google Scholar]
- 50. Rempel SA, Rosenblum ML, Mikkelsen T, Yan PS, Ellis KD, Golembieski WA, et al. Cathepsin B expression and localization in glioma progression and invasion. Cancer Res. 1994; 54: 6027–6031. [PubMed] [Google Scholar]
- 51. Mai J, Sameni M, Mikkelsen T, Sloane BF. Degradation of extracellular matrix protein tenascin-C by cathepsin B: an interaction involved in the progression of gliomas. Biol. Chem. 2002; 383: 1407–1413. [DOI] [PubMed] [Google Scholar]
- 52. Flannery T, Gibson D, Mirakhur M, McQuaid S, Greenan C, Trimble A, et al. The Clinical Significance of Cathepsin S Expression in Human Astrocytomas. Am. J. Path. 2003; 163: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thota B, Arimappamagan A, Kandavel T, Shastry AH, Pandey P, Chandramouli BA, et al. STAT-1 expression is regulated by IGFBP-3 in malignant glioma cells and is a strong predictor of poor survival in patients with glioblastoma. J Neurosurg. 2014; 121: 374–83. 10.3171/2014.4.JNS131198 [DOI] [PubMed] [Google Scholar]
- 54. Fan YC, Zhu YS, Mei PJ, Sun SG, Zhang H, Chen HF, et al. Cullin1 regulates proliferation, migration and invasion of glioma cells. Med Oncol. 2014; 31: 227 10.1007/s12032-014-0227-x [DOI] [PubMed] [Google Scholar]
- 55. Zhang X, Ding Z, Mo J, Sang B, Shi Q, Hu J, et al. GOLPH3 promotes glioblastoma cell migration and invasion via the mTOR-YB1 pathway in vitro. Mol. Carcinog. 2014. August 23 10.1002/mc.22197 [DOI] [PubMed] [Google Scholar]
- 56. La Porta C. AQP1 is not only a water channel: It contributes to cell migration through Lin7/beta-catenin. Cell Adh Migr. 2010. Apr-Jun;4(2):204–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yang M, Li Y, Chilukuri K, Brady OA, Boulos MI, Kappes JC, et al. L1 stimulation of human glioma cell motility correlates with FAK activation. J Neurooncol. 2011; 105: 27–44. 10.1007/s11060-011-0557-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lindemann C, Hackmann O, Delic S, Schmidt N, Reifenberger G, Riemenschneider MJ. SOCS3 promoter methylation is mutually exclusive to EGFR amplification in gliomas and promotes glioma cell invasion through STAT3 and FAK activation. Acta Neuropathol. 2011; 122: 241–51. 10.1007/s00401-011-0832-0 [DOI] [PubMed] [Google Scholar]
- 59. Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, et al. Microglial stimulation of glioblastoma invasion involves EGFR and CSF-1R signaling. Mol Med. 2012; 18: 519–27. 10.2119/molmed.2011.00217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lysechko TL, Cheung SM, Ostergaard HL. Regulation of the tyrosine kinase Pyk2 by calcium is through production of reactive oxygen species in cytotoxic T lymphocytes. J Biol Chem. 2010; 285: 31174–84. 10.1074/jbc.M110.118265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hodges RR, Horikawa Y, Rios JD, Shatos MA, Dartt DA. Effect of protein kinase C and Ca(2+) on p42/p44 MAPK, Pyk2, and Src activation in rat conjunctival goblet cells. Exp Eye Res. 2007; 85: 836–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Emlet DR, Moscatello DK, Ludlow LB, Wong AJ. Subsets of epidermal growth factor receptors during activation and endocytosis. J Biol Chem. 1997; 272: 4079–86. [DOI] [PubMed] [Google Scholar]
- 63. Yamaoka T, Frey MR, Dise RS, Bernard JK, Polk DB. Specific epidermal growth factor receptor autophosphorylation sites promote mouse colon epithelial cell chemotaxis and restitution. Am J Physiol Gastrointest Liver Physiol. 2011; 301: G368–76. 10.1152/ajpgi.00327.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Phillips-Mason PJ, Kaur H, Burden-Gulley SM, Craig SE, Brady-Kalnay SM. Identification of Phospholipase C gamma 1 as a Protein Tyrosine Phosphatase mu Substrate that regulates cell migration. J Cell Biochem. 2011; 112: 39–48. 10.1002/jcb.22710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krall JA, Beyer EM, MacBeath G. High- and low-affinity epidermal growth factor receptor-ligand interactions activate distinct signaling pathways. PLoS One. 2011; 6: e15945 10.1371/journal.pone.0015945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, et al. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985; 313: 144–7. [DOI] [PubMed] [Google Scholar]
- 67. Roth P, Weller M. Challenges to targeting epidermal growth factor receptor in glioblastoma: escape mechanisms and combinatorial treatment strategies. Neruo Oncol. 2014; October;16 Suppl 8:viii14–9. 10.1093/neuonc/nou222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hu J, Muller KA, Furnari FB, Cavenee WK, VandenBerg SR, Gonias SL. Neutralizing the EGF receptor in glioblastoma cells stimulates cell migration by activating uPAR-initiated cell signaling. Oncogene. 2014; October 27 10.1038/onc.2014336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zahonero C, Sanchez-Gómez P. EGFR-dependent mechanisms in glioblastoma: towards a better therapeutic strategy. Cell Mol Life Sci. 2014; 71: 3465–88. 10.1007/s00018-014-1608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guillamo JS, de Boüard S, Valable S, Marteau L, Leuraud P, Marie Y, et al. Molecular mechanisms underlying effects of epidermal growth factor receptor inhibition on invasion, proliferation, and angiogenesis in experimental glioma. Clin Cancer Res. 2009; 15: 3697–704. 10.1158/1078-0432.CCR-08-2042 [DOI] [PubMed] [Google Scholar]
- 71. Azevedo H, Fujita A, Bando SY, lamashita P, Moreira-Filho CA. Transcriptional network reveals that AT1 and AT2 Angiotensis II receptors are both involved in the regulation of genes essential for glioma progression. PloS One. 2014. November 3 10.1371/journal.pone.0110934 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The figure is given in support of Fig 3 and represents membranes probed with antibodies against pPyk2(579/580) and corresponding India Ink staining used as a loading control for densitometry analysis for A172, U87, HS683, C6, Gl261 glioma cell lines.
(TIF)
GL261 glioma cells were implanted into the brains of C57BL/6 mice. In 16 days the half of tumors were removed and used for glioma cells purification with further western blot analysis. The other half of tumors were preceded directly for western blot analysis without glioma cells purification step. Mouse monoclonal primary antibodies that detect Iba1, in dilution 1:200 (#1022–5 Lot: GR40934-12 Abcam, Cambridge, MA, USA), followed by anti-mouse conjugated immunoglobulins (Cell Signaling) were used.
(TIF)
Rabbit polyclonal anti-phospho-Pyk2(Tyr 579/580) primary antibody (Invitrogen; #44636G) dilution 1:1000, were used, followed by anti-rabbit conjugated immunoglobulins (Sigma).
(TIF)
Anti-pFAK(576/577) primary antibody were used (Cell Signaling Technology, #93305), dilution 1:1000, followed by anti-rabbit conjugated immunoglobulins (Sigma).
(TIF)
Monoclonal mouse anti-Pyk2 antibody were used (Cell Signaling; #3480S), dilution 1:1000, followed by anti-mouse conjugated immunoglobulins (Cell Signaling).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.