Abstract
Sink/source relationships, regulating the mobilization of stored carbohydrates from the vegetative tissues to the grains, are of key importance for grain filling and grain yield. We used different inhibitors of plant hormone action to assess their effects on grain yield and on the expression of hormone-associated genes. Among the tested chemicals, 2-indol-3-yl-4-oxo-4-phenylbutanoic acid (PEO-IAA; antagonist of auxin receptor), nordihydroguaiaretic acid (NDGA; abscisic acid (ABA) biosynthesis inhibitor), and 2-aminoisobutyric acid (AIB; ethylene biosynthesis inhibitor) improved grain yield in a concentration dependent manner. These effects were also dependent on the plant developmental stage. NDGA and AIB treatments induced an increase in photosynthesis in flag leaves concomitant to the increments of starch content in flag leaves and grains. NDGA inhibited the expression of ABA-responsive gene, but did not significantly decrease ABA content. Instead, NDGA significantly decreased jasmonic acid and jasmonic acid-isoleucine. Our results support the notion that the specific inhibition of jasmonic acid and ethylene biosynthesis resulted in grain yield increase in rice.
Introduction
Rice is one of the most important food crops worldwide, and an increased interest in developing high grain-yielding cultivars have led to the development of new varieties using conventional breeding programs [1]. Grain filling and consequently Grain Yield (GY) are dependent on plant source/sink relationships, where the carbohydrates stored during pre-anthesis are mobilized from the vegetative tissues to the grains. Several genes associated with GY improvement have been identified by QTL analysis [2–5]. Most of the genes identified have been functionally associated with sink strengthening and only in the case of the gene THOUSAND-GRAIN WEIGHT 6 (TGW6), a source reinforcement effect has been described [4]. The mechanisms mediating source strengthening that result in sink improvement are still largely unknown.
Plant hormones and their interactions have been shown to play pivotal roles on plant growth and development [6–8]. For instance, ABA plays important roles regulating seed maturation and seed dormancy counteracting with gibberellic acid (GA), brassinosteroids (BRs) and ethylene [9]. Also, ABA stimulates Ethylene production to let leaves abscise [10]. Cytokinins (CK) are well known key regulators of plant growth controlling shoot meristematic activity and cell differentiation in the root meristem [11, 12]. On the other hand, auxins are regulated temporally and spatially to control developmental processes such as organogenesis, embryogenesis and tropism [13]. The antagonistic interaction between auxins and CK is determinant of root and shoot cell differentiation and division, controlling growth and morphogenesis [14]. Interestingly, an induction of ABA synthesis driven by jasmonic acid (JA) has also been proposed [15]. Jasmonic acid (JA) has been mainly related to plant defense responses although a role in development independent of the pathogenesis-related response has also been described [16]. The constitutive synthesis of methyl jasmonate (MeJA) resulted in increased contents of ABA leading to GY penalties in rice. Thus, plant hormones act through a complex network that involves the interaction with other hormones under the fine tune control of master regulators (such as transcription factors) to regulate plant growth, development and more specifically, sink and source relationships [7, 8]. To differentiate specific plant hormone mechanisms, the use of reporters of hormone-specific responsive genes was proposed [17]. Also, the identification of plant hormone-responsive rice genes will be useful to detect and analyze the specific signaling pathways related to each plant hormone [18].
Several hormone inhibitors can improve plant growth only when applied during the late reproductive phase (post-anthesis stage). For instance, paclobutrazol (PBZ), a gibberellic acid (GA) biosynthesis inhibitor that primarily targets the ent-kaurene oxidase [19, 20], was applied two weeks after rice panicle initiation, an improvement in GY was achieved [21]. The application of the antagonists of ethylene receptors, such as 1-methylcyclopropane (1-MCP) or 3-cyclopropyr-1-enyl-propanoic acid sodium salt (CPAS), resulted in increased GY in wheat and soybean [22, 23]. NDGA has been used as ABA biosynthesis inhibitor to prevent fruit maturation in tomato [24]. Also, the application of aminoethoxyvinylglycine (AVG) decreases ethylene production to reduce pre-harvest fruit drop in apple ([25] and references therein).
Here, we tested the effects of chemicals that inhibit the biosynthesis, perception, or signaling of plant hormones, and characterize their effects on GY in rice. Among the tested chemicals, we observed that those that inhibit the biosynthesis of GA, BR or auxin negatively affected GY, while the use of inhibitors affecting auxin perception, JA and ethylene biosynthesis improved GY.
Materials and Methods
Plant material and growth conditions
Rice seeds (Oryza sativa L. ssp. japonica cv. Kitaake) were germinated on moist paper for 1 week (28°C in the dark). Seedlings were transplanted into 8 L pots (2 plants per pot), using soil harvested in California rice field (capay series, 38°32′23.93′′N,121°48′30.81′′W, shredded and steamed for 1.5 h to eradicate soil pathogens). Greenhouse conditions were 12 h, 30°C (day) / 12 h, 20°C (night). Plants were fertilized using 50% N:P:K (20:10:20) (Peters professional) and 50% ammonium sulphate (VIKING SHIP). Total nitrogen added was 0.8 g/pot, every 2 weeks until panicle initiation.
Chemical treatment
The application of plant hormone inhibitors was done by spraying the aerial part of the rice plants using different concentrations of the chemicals (S1 Table) at two developmental stages: pre-anthesis (just before heading stage) and/or post-anthesis (2 weeks after flowering, during grain filling stage). All spraying solutions contained 0.1% dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and 0.05% Tween20 (Sigma-Aldrich, St. Louis, MO) to allow chemical penetration into the plant tissue. Untreated control (UTC) plants were sprayed using the same solution without plant hormone inhibitors.
Gas exchange measurements
Rates of CO2 assimilation were determined in flag leaves of rice plants under same developmental stage using the portable gas exchange system LI-COR 6400–40 (Li-COR Inc. Lincoln, NE, USA). The leaf cuvette was set at photosynthetic photon flux density (PPFD) of 1,500 μmol.m-2.s-1, 50–60% relative humidity and 29°C of block temperature. Photosynthesis activity and stomata conductance were determined after 2, 9 and 16 days after spray and respiration was estimated by using the equation previously described [26].
Quantitative PCR analysis (qPCR)
For gene expression analysis, total RNA was extracted from the flag leaves using RNeasyMini kit (Qiagen, Valencia, CA). The quality of RNA was determined using Nanodrop ND-1000. First strand cDNA was synthesized from 1 μg of total RNA using QuantiTect Reverse Transcription kit (Qiagen). Quantitative PCR was performed on the StepOnePlus (Applied Biosystems, Foster City, CA), using SYBR GREEN. The 2−ΔΔCT method [27] was used to normalize and calibrate transcript values relative to the endogenous rice transcription elongation factor (TEF) gene. Six biological replicates were used for the expression analysis. The primer sets used for amplifying different target genes are shown in S5 Table.
Starch and sugar quantification
Flag leaves and immature grains were sampled 2 days after spray at pre-anthesis stage or post-anthesis stage, and immediately frozen in liquid-N. Mature grains were harvested at the end of the experiment. The frozen samples and mature grains were freeze-dried, and 10 mg of tissue powder was used for the soluble sugar extraction as previously described [28]. Separation of sugars was performed with water as a mobile phase flowing at 0.6 ml min-1 using an Aminex HPX-87C column (300 mm × 7.8 mm; Bio Rad Laboratories, Hercules, CA, USA) which was preceded by a micro-guard cartridge (Carbo-C, pH range 5–9, 30 mm × 4.6 mm; Bio Rad Laboratories, Hercules, CA, USA) and maintained at 80°C. 10 μl extract was injected by an auto-sampler and sugars were detected using a refractive index detector (Agilent G1362A) with Agilent HPLC 1100 series. Starch was quantified as previously described [8].
Quantification of plant hormones
Plant hormones were quantified using an Agilent 1200 series rapid resolution liquid chromatography and 6420 Triple Quadrupole LCMS system (Agilent). Chromatography was performed on a ZORBAX Eclipse XDB-C18 column (1.8 μm, 2.1 × 50 mm). d2-GA1, d2-GA4, d5-tZ, d3-DHZ, d6-iP, d5-tZR, and d6-iPR obtained from OlChemim, d6-ABA obtained from Icon Isotopes, d2-IAA and d6-SA obtained from Sigma-Aldrich, d2-JA obtained from Tokyo Kasei, and synthesized 13C6-JA-Ile [29] were used as internal standards. Two days after spray of NDGA 0.12 mM at pre-anthesis stage, flag leaves were collected in the morning and immediately frozen in liquid N2. The frozen samples (about 200 mg in fresh weight) were freeze-dried, pulverized with 5-mm zirconia beads by tissue lyzer (QIAGEN), and then extracted in 10 volume of extraction solvent (80% acetonitrile with 1% acetic acid) containing internal standards for 1 h. Extracts were centrifuged at 4°C,14,000 x g, 10 min, and the supernatant was collected. This procedure was repeated once without internal standards, and acetonitrile was removed in a Speed Vac (Thermo Fisher). Acidic water extracts were loaded onto an Oasis HLB extraction cartridge (30 mg, 1mL; Waters) and washed with 1 mL of water containing 1% acetic acid to segregate high polar impurities. Plant hormones were eluted with 2 mL of 80% acetonitrile containing 1% acetic acid, and the methanol in this eluent was removed in a Speed Vac. Acidic water extracts were loaded onto an Oasis MCX extraction cartridge (30 mg, 1 mL). After washing with 1 mL of water containing 1% acetic acid, acidic and neutral compounds were eluted with 2 mL of acetonitrile (AN fraction). After washing with water containing 5% (v/v) ammonia solution (28% as NH3), basic compounds were eluted with 2 mL of 60% (v/v) acetonitrile containing 5% (v/v) ammonia solution (28% as NH3). After drying these basic fractions, 30 μL of water containing 1% acetic acid was added and the basic hormones (tZ and iP) in these fractions were analyzed by LC-MS/MS. Subfractions (2.5%) of the AN fractions were collected and prepared as basic fractions to analyze for SA. The remaining 97.5% of the AN fractions were concentrated to acidic water in the Speed Vac to remove acetonitrile and loaded onto Oasis WAX extraction cartridges (30 mg, 1 mL). After washing with 1mL of water containing 1% acetic acid, neutral compounds were eluted with 2 mL of acetonitrile, and acidic compounds were eluted with 2 mL of 80% acetonitrile containing 1% acetic acid. After concentrating these acidic fractions to dryness, 30 μL of water containing 1% acetic acid was added and acidic hormones (IAA, ABA, JA, JA-Ile, GA1, and GA4) in these fractions were analyzed. LC conditions and parameters for LC-ESI-MS/MS analysis are described in S2 and S3 Tables.
Statistical analysis
The JMP (ver.8.0) statistical package (SAS Institute, Cary, NC) was used for statistical analyses. Three-way ANOVA (level of significance P <0.05) was used to test the effect of chemicals on phenotype. LSMeans t-test was used to compare means by treatment at a probability level of 5%. Levels of significance are represented by asterisks as follows: * indicating significance at P ≤ 0.05. The experiments were based on randomized complete block design, using four–six replicates.
Results
Effects of plant hormone inhibitors on plant biomass
Eight different hormone inhibitors (S1 Table) and three different concentrations of each inhibitor were used to study the effects of hormone regulation on GY in rice. The inhibitor concentrations used were based on the effective concentrations previously described [30–37]
Plants treated at pre-anthesis with the highest and intermediate concentrations of all the hormone inhibitors tested, and the lowest concentrations of 2-aminoisobutyric acid (AIB), silver nitrate (AgNO3), and 2-indol-3-yl-4-oxo-4-phenylbutanoic acid (PEO-IAA), induced a reduction in shoot dry weight (SDW) and/or GY (Table 1). Highest concentrations of all tested chemicals decreased plant height and number of tillers (S1 Fig), and increased white aborted seeds (S2 Fig). The lowest concentration of nordihydroguaiaretic acid (NDGA) significantly improved SDW and GY, as compared to the untreated control (UTC) plants (Table 1 and S4 Table).
Table 1. Effects of plant hormone inhibitors applied at pre-anthesis on the shoot dry weight and grain yield.
| Primary target for inhibition | Chemicals | mM | Shoot dry weight (g plant-1) | Grain yield (g plant-1) |
|---|---|---|---|---|
| - | UTC | - | 17.0 ± 0.7 | 28.1±0.5 |
| ABA synthesis | NDGA | 0.12 | 20.8 ± 1.1 * | 33.9 ± 2.0 * |
| 0.6 | 13.7 ± 0.4 * | 23.5 ± 0.7 * | ||
| 3 | 13.4 ± 0.5 * | 23.5 ± 0.7 * | ||
| GA synthesis | Paclobutrazol | 0.016 | 13.9 ± 0.7 * | 25.3 ± 1.8 |
| 0.08 | 13.2 ± 0.9 * | 22.5 ± 0.8 * | ||
| 0.4 | 11.9 ± 0.7 * | 21.3 ± 1.5 * | ||
| BR synthesis | Imazalil | 0.04 | 14.3 ± 1.3 | 24.3 ± 2.4 |
| 0.2 | 13.8 ± 0.9 * | 22.8 ± 1.2 * | ||
| 1 | 13.4 ± 0.5 * | 19.7 ± 1.2 * | ||
| BR synthesis | Propiconazole | 0.04 | 15.1 ± 1.0 | 26.9 ± 2.1 |
| 0.2 | 13.5 ± 0.7 * | 23.9 ± 1.1 * | ||
| 1 | 13.4 ± 0.7 * | 25.5 ± 1.9 | ||
| Auxin synthesis | L-AOPP | 0.04 | 14.1 ± 1.4 | 23.8 ± 2.5 |
| 0.2 | 13.8 ± 0.5 * | 22.9 ± 0.7 * | ||
| 1 | 12.5 ± 1.3 * | 23.3 ± 1.8 * | ||
| Auxin perception | PEO-IAA | 0.04 | 15.2 ± 0.5 * | 26.4 ± 1.8 |
| 0.2 | 13.6 ± 0.7 * | 26.1 ± 2.1 | ||
| 1 | 13.7 ± 0.8 * | 22.6 ± 0.8 * | ||
| Ethylene synthesis | AIB | 0.2 | 14.0 ± 0.8 * | 24.2 ± 2.0 |
| 1 | 13.8 ± 0.7 * | 23.1 ± 1.7 * | ||
| 5 | 14.7 ± 1.4 | 22.3 ± 2.2 * | ||
| Ethylene perception | AgNO3 | 0.12 | 14.3 ± 0.8 * | 23.8 ± 0.8 * |
| 0.6 | 14.2 ± 0.6 * | 21.8 ± 1.8 * | ||
| 3 | 12.7 ± 0.7 * | 13.6 ± 0.8 * |
Values are Mean ± SE (n = 6).
* indicates significance at P ≤ 0.05.
When the hormone inhibitors were applied at both pre- and post-anthesis stages, they induced a decrease in SDW and/or GY with exception of PEO-IAA, AIB and NDGA (Table 2). Similar to plants treated with chemicals at only pre-anthesis, highest or intermediate concentrations of all tested chemicals increased white aborted seeds (S3 Fig). It has been shown that the loss of indole-3-acetic acid (IAA)-glucose hydrolase function, mediating the formation of active-auxin from conjugated-auxin, improved grain yield [4]. Consistent with this work, when PEO-IAA was applied at pre- and post-anthesis stages, the lowest concentration significantly improved GY (Table 2). When the lowest concentration of AIB was applied at the pre- and post-anthesis stages, no changes in SDW or GY were observed. Nonetheless, significant improvements on SDW and GY were seen when AIB was applied exclusively at the post-anthesis stage (S4 Table).
Table 2. Effects of plant hormone inhibitors applied at both pre-anthesis and post-anthesis on the biomass production and grain yield.
| Primary target for inhibition | Chemicals | mM | Shoot dry weight (g plant-1) | Grain yield (g plant-1) |
|---|---|---|---|---|
| - | UTC | 17.8 ± 1.4 | 25.7 ± 0.3 | |
| ABA synthesis | NDGA | 0.12 | 18.1 ± 1.4 | 30.5 ± 1.8 * |
| 0.6 | 13.8 ± 0.9 * | 21.9 ± 1.4 * | ||
| 3 | 14.0 ± 0.2 * | 23.1 ± 0.9 * | ||
| GA synthesis | Paclobutrazol | 0.016 | 14.3 ± 1.2 | 24.8 ± 1.8 |
| 0.08 | 14.5 ± 0.4 * | 25.2 ± 0.9 | ||
| 0.4 | 13.5 ± 0.9 * | 22.1 ± 0.9 * | ||
| BR synthesis | Imazalil | 0.04 | 14.4 ± 0.8 * | 25.3 ± 2 |
| 0.2 | 14.1 ± 0.9 * | 22.3 ± 1.4 * | ||
| 1 | 13.5 z± 0.8 * | 22.0 ± 1.9 | ||
| BR synthesis | Propiconazole | 0.04 | 14.8 ± 0.5 * | 25.8 ± 1.4 |
| 0.2 | 15.2 ± 1.5 | 26.7 ± 1.3 | ||
| 1 | 13.7 ± 0.7 * | 22.2 ± 1.9 | ||
| Auxin synthesis | L-AOPP | 0.04 | 15.3 ± 1.3 | 21.9 ± 1.4 * |
| 0.2 | 14.2 ± 1.0 * | 23.1 ± 1.8 * | ||
| 1 | 13.9 ± 1.0 * | 23.6 ± 2.1 | ||
| Auxin perception | PEO-IAA | 0.04 | 17.1 ± 1.4 | 29.9 ± 1.9 * |
| 0.2 | 14.2 ± 1.0 | 23.1 ± 1.8 | ||
| 1 | 14.7 ± 0.6 | 22.6 ± 1.2 * | ||
| Ethylene synthesis | AIB | 0.2 | 15.7 ± 0.4 | 26.4 ± 1.4 |
| 1 | 14.8 ± 0.8 * | 24.4 ± 0.9 | ||
| 5 | 15.1 ± 1.0 * | 24.7 ± 1.1 | ||
| Ethylene perception | AgNO3 | 0.12 | 13.4 ± 0.5 * | 21.4 ±0.7 |
| 0.6 | 15.5 ± 0.8 | 21.5 ± 0.9 * | ||
| 3 | 13.7 ± 0.6 * | 12.7 ± 0.8 * |
Values are Mean ± SE (n = 6).
* indicates significance at P ≤ 0.05.
Changes in gene expression associated with alterations in hormone contents and/or signaling
Changes in hormone homeostasis in plants influence many aspects of plant growth and development [7, 38, 39]. To determine the action of the different inhibitors used in this work, we evaluated the changes in the expression of genes associated with the biosynthesis, perception or signaling of each hormone as they were affected by the chemical treatments.
Because of the extensive crosstalk between the hormone-dependent signaling pathways, it is difficult to ascribe specific inhibitory roles to the different compounds used. The expression of hormone-specific marker genes [18] was assessed for each treatment. For each chemical, we selected and evaluated the ABA-specific responsive genes Rice b asic-region leucine- zip per 23 (OsbZIP23) and L ate- e mbryogenesis a bundant 3 (LEA3) [18, 40, 41], the GA responsive gene Gibberellin 20 oxidase 2 (GA20ox2) [2, 8], the BR responsive Dw ar f4 (DWF4) and the B rassinosteroid u p-regulated 1 (BU1) [42–44], the auxin-specific responsive genes Rice i ndole-3- a cetic a cid 4 (OsIAA4) and LOC_Os11g32110 [18, 45], and the ethylene-specific responsive genes Rice ethylene receptor 2 (OsETR2) and Rice e thylene r esponse s ensor 1 (OsERS1) [18, 46]. The qRT-PCR data showed that the expression of OsbZIP23 and LEA3 were down-regulated with increasing NDGA concentrations (Table 3). It has been previously shown that the expression of the GA-associated gene GA20ox2 was inhibited by exogenous GA application and up-regulated by the GA inhibitors uniconazole or paclobutrazol [2]. Here, the expression of GA20ox2 was up-regulated with increasing NDGA concentrations, while the expression of OsIAA4 decreased (Table 3). Taken together, the results suggest that the lowest NDGA concentrations preferentially inhibited ABA signaling-related transcripts.
Table 3. Changes in the relative expression of rice hormone-responsive genes after inhibitor application.
| ABA responsive genes | GA responsive gene | BR responsive genes | Auxin responsive genes | Ethylene responsive genes | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemicals | mM | OsbZIP23 | LEA3 | GA20ox2 | DWF4 | BU1 | OsIAA4 | LOC_Os11g32110 | OsETR2 | OsERS1 |
| NDGA | 0.12 | 0.9 ± 0.07 | 0.8 ± 0.09 * | 1.3 ± 0.08 | 1.2 ± 0.10 | - | 0.9 ± 0.20 | - | 1.0 ± 0.10 | - |
| 0.6 | 0.7 ± 0.05 * | 0.8 ± 0.07 * | 1.7 ± 0.25 * | 1.2 ± 0.13 | - | 0.9 ± 0.12 | - | 0.9 ± 0.14 | - | |
| 3 | 0.4 ± 0.04 * | 0.6 ± 0.09 * | 1.8 ± 0.35 * | 1.0 ± 0.25 | - | 0.6 ± 0.04 * | - | 0.7 ± 0.10 * | - | |
| Paclobutrazol | 0.016 | 0.8 ± 0.2 | - | 1.2 ± 0.15 | 0.8 ± 0.10 | - | 0.8 ± 0.10 | - | 1.0 ± 0.07 | - |
| 0.08 | 0.9 ± 0.03 | - | 1.2 ± 0.15 | 0.8 ± 0.10 | - | 1.1 ± 0.20 | - | 1.0 ± 0.03 | - | |
| 0.4 | 0.9 ± 0.07 | - | 1.6 ± 0.07 * | 0.8 ± 0.20 | - | 0.8 ± 0.10 | - | 0.9 ± 0.08 | - | |
| Imazalil | 0.04 | 0.8 ± 0.14 | - | 0.9 ± 0.08 | 1.5 ± 0.10 * | 0.9 ± 0.20 | 1.1 ± 0.13 | - | 0.9 ± 0.11 | - |
| 0.2 | 0.8 ± 0.03 | - | 0.9 ± 0.08 | 1.5 ± 0.07 * | 0.6 ± 0.03 * | 1.1 ± 0.26 | - | 1.0 ± 0.08 | - | |
| 1 | 0.7 ± 0.04 * | - | 2.5 ± 0.25 * | 1.4 ± 0.23 | 0.9 ± 0.05 | 2.0 ± 0.39 * | - | 1.0 ± 0.18 | - | |
| Propiconazole | 0.04 | 0.8 ± 0.03 | - | 1.6 ± 0.33 * | 0.8 ± 0.17 | 0.5 ± 0.12 * | 1.0 ± 0.06 | - | 0.9 ± 0.25 | - |
| 0.2 | 0.8 ± 0.09 | - | 1.2 ± 0.26 | 0.9 ± 0.08 | 1.1 ± 0.20 | 1.0 ± 0.08 | - | 0.9 ± 0.06 | - | |
| 1 | 0.7 ± 0.17 * | - | 1.8 ± 0.20 * | 1.0 ± 0.08 | 0.6 ± 0.11 * | 1.2 ± 0.11 | - | 1.0 ± 0.11 | - | |
| L-AOPP | 0.04 | 0.5 ± 0.03 * | - | 1.0 ± 0.08 | 0.7 ± 0.07 * | - | 0.7 ± 0.09 * | 0.7 ± 0.11 * | 1.1 ± 0.13 | - |
| 0.2 | 0.6 ± 0.15 * | - | 1.2 ± 0.12 | 0.7 ± 0.12 * | - | 0.7 ± 0.03 * | 0.5 ± 0.08 * | 1.2 ± 0.12 | - | |
| 1 | 0.6 ± 0.10 * | - | 1.8 ± 0.19 * | 0.7 ± 0.13 * | - | 0.9 ± 0.14 | 0.5 ± 0.06 * | 1.3 ± 0.28 | - | |
| PEO-IAA | 0.04 | 0.7 ± 0.05 * | - | 1.1 ± 0.13 | 0.8 ± 0.11 | - | 0.8 ± 0.08 | 0.7 ± 0.05 * | 1.1 ± 0.19 | - |
| 0.2 | 0.6 ± 0.08 * | - | 1.3 ± 0.33 | 0.8 ± 0.14 | - | 0.7 ± 0.06 * | 0.6 ± 0.07 * | 1.3 ± 0.16 | - | |
| 1 | 0.6 ± 0.04 * | - | 1.4 ± 0.27 | 0.7 ± 0.11 * | - | 0.9 ± 0.09 | 0.6 ± 0.10 * | 0.9 ± 0.15 | - | |
| AIB | 0.2 | 0.9 ± 0.13 | - | 1.8 ± 0.27 * | 1.1 ± 0.13 | - | 1.1 ± 0.27 | - | 0.8 ± 0.04 * | 0.7 ± 0.04 * |
| 1 | 1.0 ± 0.13 | - | 1.5 ± 0.07 * | 1.6 ± 0.16 * | - | 1.1 ± 0.27 | - | 0.8 ± 0.09 * | 0.9 ± 0.10 | |
| 5 | 1.0 ± 0.18 | - | 1.6 ± 0.16 * | 1.6 ± 0.31 * | - | 1.6 ± 0.25 * | - | 1.1 ± 0.19 | 0.9 ± 0.06 | |
| AgNO3 | 0.12 | 0.7 ± 0.07 * | - | 1.6 ± 0.21 * | 1.0 ± 0.06 | - | 1.3 ± 0.17 | - | 0.8 ± 0.08 * | 0.9 ± 0.05 |
| 0.6 | 1.0 ± 0.13 | - | 2.1 ± 0.16 * | 2.5 ± 0.49 * | - | 1.3 ± 0.05 | - | 0.9 ± 0.10 | 1.0 ± 0.04 | |
| 3 | 1.3 ± 0.19 | - | 4.6 ± 0.48 * | 2.8 ± 0.35 * | - | 1.4 ± 0.22 | - | 1.7 ± 0.08 * | 1.0 ± 0.17 | |
Values are Mean ± SE (n = 6). Relative gene expression values setting UTC as 1.
* indicates significance at P ≤ 0.05.
In the case of Paclobutrazol, a GA synthesis inhibitor, the expression levels of GA20ox2 [2], and other plant hormone responsive genes, as OsbZIP23 (ABA), DWF4 (BR), OsIAA4 (Auxin), and OsETR2 (Ethylene) were evaluated (Table 3). GA20ox2 expression was up-regulated by increasing Paclobutrazol concentrations, while the expression of OsbZIP23, DWF4, OsIAA4, and OsETR2 did not change significantly (Table 3).
The expression levels of the BR-responsive genes DWF4 and BU1 were evaluated following the application of BR inhibitors. In aerial parts, the up-regulation of BU1 [43] and the down-regulation of DWF4 [34, 44], following the application of BR, has been reported. DWF4 expression level was up-regulated when the lowest and intermediate concentrations of Imazalil were applied (Table 3), and the expression of BU1, which increased with BR, was down-regulated by intermediate Imazalil concentration and the lowest Propiconazole concentration (Table 3). The expression of other plant hormone responsive genes, including OsbZIP23, GA20ox2, OsIAA4, and OsETR2, were analyzed. Only when the highest concentration of Imazalil and Propiconazole, and the lowest concentration of Propiconazole were applied, decreased expression of OsbZIP23 and increased expression of GA20ox2 were observed (Table 3). Our results would suggest that a specific inhibition of BR synthesis-related transcripts is elicited with either low or intermediate concentrations of Imazalil.
The expression levels of OsIAA4 and LOC_Os11g32110, which were specifically increased by auxin [18, 45], and other plant hormone responsive genes were evaluated when the plants were treated with auxin inhibitors. PEO-IAA and L-AOPP reduced the expression of LOC_Os11g32110, while the expression of OsIAA4 decreased with intermediate concentrations of PEO-IAA and L-AOPP (Table 3). PEO-IAA and L-AOPP also induced the down-regulation of OsbZIP23 expression.
The effects of the ethylene inhibitors AIB and AgNO3 on the expression levels of OsETR2 and OsERS1, which were specifically induced by ethylene [18, 46], and other plant hormone responsive genes (OsbZIP23, GA20ox2, DWF4, and OsIAA4) were evaluated (Table 3). Low AIB concentrations induced a decrease in OsETR2 and OsERS1 expression, while the expression of GA20ox2 increased under both AIB and AgNO3 applications. The expression of DWF4 was up-regulated at intermediate and high AIB and AgNO3 (Table 3). Thus, low concentrations of AIB inhibited the expression of genes associated with ethylene biosynthesis, and also affected GA-associated genes.
NDGA and AIB improved photosynthesis and C assimilation
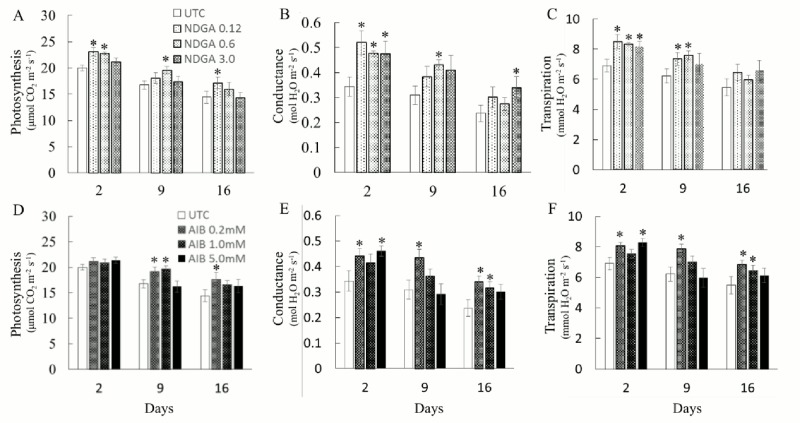
Photosynthesis and transpiration rates of rice flag leaves improved significantly 2 d after application of low NDGA concentration (Fig 1A and 1C). Higher transpiration and photosynthetic rates were maintained 9 and 16 d after treatment, respectively (Fig 1A and 1C). When NDGA was applied at the intermediate and higher concentrations, transpiration and conductance were significantly improved 2 d after application (Fig 1B and 1C). The low and intermediate AIB concentrations caused improvements on the photosynthetic and transpiration rates (Fig 1D and 1F). AIB treatments also improved photosynthesis and transpiration rate when applied at post-anthesis (S6 Fig). These results suggested that treatments with NDGA or AIB can increase the photosynthetic activity and leaf transpiration rates leading to increases of GY and SDW.
Fig 1. Effects of ABA and Ethylene biosynthesis inhibitors on photosynthesis, conductance, and transpiration rates at pre-anthesis.
Photosynthesis rate (A, D), Conductance (B, E), and Transpiration rate (C, F) of UTC and NDGA treated plants (A, B, C) or UTC and AIB treated plants (D, E, F) 2, 9, and 16 d after spray at pre-anthesis stage. Values are Mean ± SE (n = 6). * indicating significance at P ≤ 0.05.
Highest paclobutrazol concentrations, decreased photosynthesis, stomatal conductance, and transpiration rates in flag leaves (S4 Fig), while Imazaril and PEO-IAA had no effect on these parameters (S5 Fig).
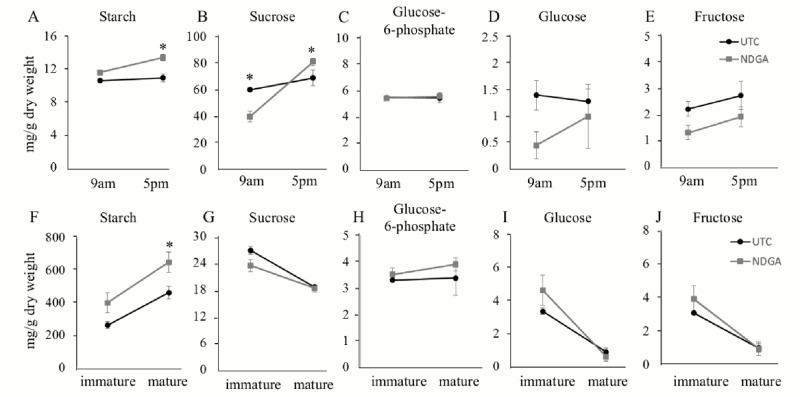
Photosynthetic activity was shown to be positively correlated with the carbohydrate contents of flag leaves and grains [28, 47, 48]. In order to assess the effects of hormone inhibition on photosynthesis and the implications in carbon metabolism, we analyzed carbohydrate contents in flag leaves and grains of rice after NDGA or AIB application. Flag leaf samples treated with 0.12 mM NDGA at pre-anthesis displayed significantly higher starch and sucrose contents at the end of the day, as compared to the untreated controls (UTC). (Fig 2A and 2B) On the other hand, when the leaf samples were collected in the early morning (before the transitory starch starts to accumulate in leaves [49, 50]), no differences in starch content were found between UTC and 0.12 mM NDGA-treated samples (Fig 2A). These results were well correlated with the higher photosynthetic rates shown by NDGA-treated plants at pre-anthesis (Fig 1A). Starch and Sucrose contents of flag leaves were higher at the end of the day compared to early morning contents, but the increase was markedly larger in NDGA-treated plants (Fig 2A and 2B). No differences in glucose-6-phosphate (Fig 2C) and fructose contents (Fig 2E) were seen between NDGA-treated and UTC plants during the day. While the glucose contents of UTC plants at the early morning were similar to that at the end of the day (Fig 2D), the glucose content of flag leaves from NDGA-treated plants was lower than that of UTC plants at the early morning and increased at the end of the day to UTC levels. Starch content in mature grains was higher than immature grains in both NDGA-treated and UTC plants (Fig 2F). Sucrose, glucose and fructose contents in immature grains of UTC and NDGA-treated plants were higher compared to those of mature seeds (Fig 2G, 2I and 2J), while the glucose-6-phosphate contents of immature and mature grains from both NDGA and UTC plants were similar (Fig 2H).
Fig 2. Starch and sugars contents in source and sink tissues treated with ABA biosynthesis inhibitor.
Starch (A,F), Sucrose (B,G), Glucose-6-phosphate (C,H), Glucose (D,I), Fructose (E,J) contents in flag leaves (A-E) and immature/mature grains (F-J) treated with UTC and NDGA at pre-anthesis stage. Values are Mean ± SE (n = 4). * indicates significance at P ≤ 0.05.
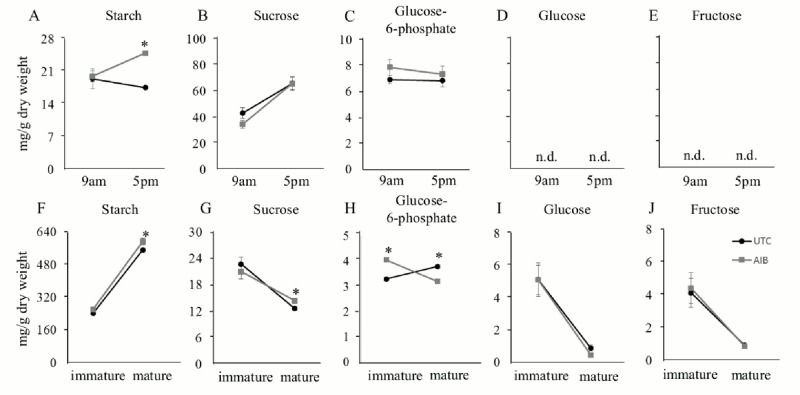
Flag leaves from plants treated with 0.2 mM AIB at post-anthesis displayed higher starch content than the UTC plants at the end of the day, while starch content in flag leaves were similar to those from UTC plants at the early morning (Fig 3A). Flag leaves sucrose contents increased during the day, but there were no differences between AIB-treated and UTC plants (Fig 3B). In flag leaves of both AIB-treated and UTC plants, glucose-6-phosphate contents remained unchanged during the day (Fig 3C). Glucose and fructose contents could not be detected in plants treated with the inhibitor or in UTC plants (Fig 3D and 3E), a results that is consistent with sugar remobilization processes to sinks (grains) occurring at the end of plant life cycle [51]. Starch contents in mature seeds from AIB-treated and UTC plants were higher than those found in immature seeds (Fig 3F), while the sucrose content from both AIB-treated and UTC plants were higher in immature seeds than in mature seeds. No large differences were seen between AIB-treated and UTC plants in starch or sucrose contents (Fig 3G). Glucose-6-phosphate contents in AIB treated rice was higher or lower compared to UTC rice in immature or mature grains, respectively (Fig 3H). Fructose and glucose contents were higher in immature grains when compared to the contents found in mature grains from both AIB-treated and UTC plants (Fig 3I and 3J). These results suggested that the increase in photosynthetic activity by the NDGA and AIB applications can lead to increases of starch and/or sucrose in flag leaves, resulting in the increment of the starch content in mature grains of the NDGA and AIB treated plants.
Fig 3. Starch and soluble sugars contents in source and sink tissues treated with Ethylene biosynthesis inhibitors.
Starch (A,F), Sucrose (B,G), Glucose-6-phosphate (C,H), Glucose (D,I), Fructose (E,J) contents in flag leaves (A-E) and immature/mature grains (F-J) treated with UTC and AIB at post-anthesis stage. Values are Mean ± SE (n = 4). * indicates significance at P ≤ 0.05; n.d. indicates not detected.
In order to confirm whether the lowest NDGA concentration (0.12mM) inhibited ABA synthesis specifically, we quantified the contents of ABA, indole-3-acetic acid (IAA), GA1, GA4, trans-Zeatin (tZ), 2-isopentenyladenosine (iP), jasmonic acid (JA), jasmonic acid-Isoleucine (JA-Ile), and salicylic acid (SA). JA and JA-Ile contents decreased significantly after the application of 0.12 mM NDGA (Table 4). Although the down-regulation of ABA-related transcripts was observed after the applicaton of 0.12 mM NDGA, ABA contents were not significantly reduced in the 0.12 mM NDGA-treated plants. Also, IAA, GAs, Cytokinins, and SA did not change significantly with 0.12mM NDGA treatment (Table 4). In the AIB-treated rice plants, no significant differences in plant hormone contents were found when compared to UTC rice (Table 4).
Table 4. Plant hormone contents in NDGA and AIB treated rice.
| Hormone content (ng/gDW) | UTC | NDGA | UTC | AIB |
|---|---|---|---|---|
| ABA | 117.5 ± 11.0 | 107.8 ± 11.6 | 90.0 ± 20 | 76.2 ± 12 |
| IAA | 21.3 ± 2.1 | 18.3 ± 1.6 | 36.2 ± 8.5 | 32.1 ± 6.5 |
| GA1 | 0.56 ± 0.34 | 0.9 ± 0.03 | n.d. | n.d. |
| GA4 | 0.4 ± 0.2 | 0.5 ± 0.06 | 0.8 ± 0.5 | 2.6 ± 1.1 |
| tZ | 1.4 ± 0.2 | 1.5 ± 0.1 | 0.5 ± 0.05 | 0.32 ± 0.06 |
| iP | 0.2 ± 0.02 | 0.18 ± 0.01 | 0.33 ± 0.02 | 0.42 ± 0.08 |
| JA | 74.6 ± 14.8 | 42.8 ± 6.2 * | 5.2 ± 0.5 | 5.3 ± 0.9 |
| JA-Ile | 30.2 ± 5.9 | 13.5 ± 2.4 * | 2.6 ± 0.5 | 1.7 ± 0.7 |
| SA (μg/gDW) | 34.7 ± 3.3 | 28.7 ± 1.1 | 20.2 ± 1.2 | 17.8 ± 1.2 |
0.12mM NDGA was applied at pre-anthesis and 0.2mM AIB was applied at post-anthesis. Values are Mean ± SE (n = 5 or 4). DW, dry weight.
* indicates significance at P ≤ 0.05. n.d. indicates not detected.
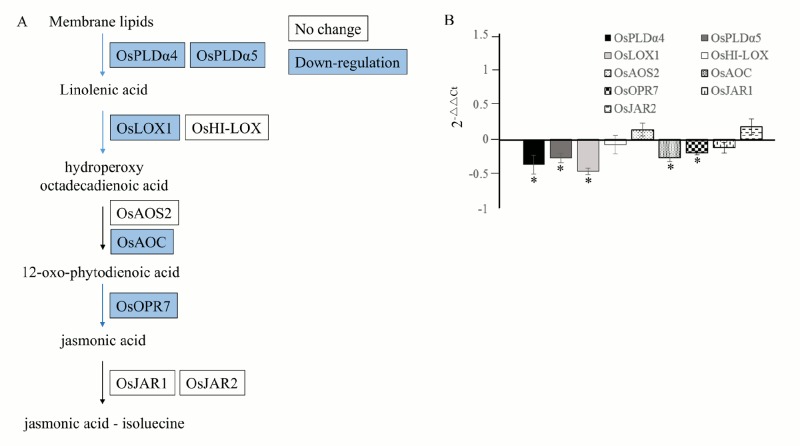
We also evaluated the effects of 0.12 mM NDGA on the expression of JA synthesis-related genes including, rice Phospholipase D alpha 4 (OsPLDα4) [LOC_Os06g40170], rice Phospholipase D alpha 5 (OsPLDα5) [LOC_Os06g40180], rice lipoxygenases (OsLOX1 [LOC_Os03g49380] and OsHI-LOX [LOC_Os08g39840]), rice allen oxide synthase (OsAOS2 [LOC_Os03g12500]), rice allen oxide cyclase (OsAOC [LOC_Os03g32314]), rice 12-oxophytodienoate reductase 7 (OsOPR7 [LOC_Os08g35740]), rice jasmonic acid resistance 1 and 2 (OsJAR1 [LOC_Os05g50890] and OsJAR2 [LOC_Os01g12160]) 2 d after treatment at pre-anthesis ([52] and references therein). The decreased expression of OsPLDα4, OsPLDα5, OsLOX1, OsAOC, and OsOPR7, following the NDGA treatment, suggested the NDGA-induced inhibition of the JA synthesis pathway (Fig 4). These results indicated that the repression of JA synthesis-related genes expressions might contribute to the reduction of JA and JA-Ile contents.
Fig 4. Effects of NDGA on the expression of JA synthesis-related genes.
Effects of NDGA on the expression of JA synthesis-related genes (OsPLDα4 [LOC_Os06g40170], OsPLDα5 [LOC_Os06g40180], OsLOX1 [LOC_Os03g49380], OsHI-LOX [LOC_Os08g39840], OsAOS2 [LOC_Os03g12500], OsAOC [LOC_Os03g32314], OsOPR7 [LOC_Os08g35740], OsJAR1 [LOC_Os05g50890] and OsJAR2 [LOC_Os01g12160]) 2 days after treatment at pre-anthesis stage. (A) Scheme of the main steps in the JA synthesis pathway indicating (in colored boxes) the genes that were differently expressed in NDGA treated plants with respect to UTC plants. White indicates genes not significantly different and Blue indicates genes with a significantly lower gene expression. (B) Relative gene expression values setting UTC as 0. Values are Mean ± SE (n = 6). * indicating significance at P ≤ 0.05.
Discussion
In this study we analyzed the effects of hormone biosynthesis/signaling inhibition at reproductive stage on grain yield and plant biomass in rice. The expression analysis of different hormone responsive genes allowed determining the inhibitor concentrations that down-regulate specific plant hormone pathways. Also, the correlation between the hormone inhibition and alterations in C assimilation, GY and plant biomass, could contribute to the targeting of pathways to improve cereal crops productivity.
Effects of hormone inhibition on plant biomass
Plant hormones play important roles in the regulation of plant growth and development and in the plant response(s) to changes in the environment [6, 7, 53].
GA is known to regulate plant growth and grain yield [54, 55]. Paclobutrazole and uniconazole were reported to inhibit GA biosynthesis based on the analysis of GA-associated phenotypes, GA content measurements (before and after treatments) and by the expression analysis of GA20ox2 [37, 56]. When high Paclobutrazol concentrations (0.28kg/ha = 28mg/m2) were applied at pre-anthesis in rice grown under field conditions, GY was decreased [21]. In the present study, Paclobutrazol treatments of 18.3mg/ m2 or 91.4mg/ m2 (S1 Table), caused a GY decrease of 20% and 24%, respectively (Table 1). Since, the expression of genes associated with other hormones remained unchanged, the Paclobutrazol-induced decrease in GY could be considered to be exclusively associated with its inhibitor effect on GA biosynthesis.
The overexpression of DWF4, a gene encoding a cytochrome P450 that mediates BR biosynthesis in plants, was shown to improve GY in rice transgenic plants grown in the field [57]. In Arabidopsis, Imazalil was reported to inhibit the growth of hypocotyls and roots in a concentration dependent manner through the regulation of DWF4 [33]. Our results showed that intermediate concentrations of Imazalil reduced BU1 expression [43] and up-regulated DWF4 expression, which has been shown to be down-regulated by increased BR levels [34, 42]. The effects of intermediate concentrations of Imazalil appeared to specifically affect BR biosynthesis, since the expression of other hormone-responsive genes remained unchanged (Table 3). BRs have been associated with important roles controlling plant biomass and GY [58–61]. Although it has been reported that mutations in DWF4 improved GY in rice [61], the ectopic overexpression of DWF4 resulted in the improvement of GY in Arabidopsis, rice and cotton [62]. Our results (Tables 1 and 3) indicated that the increase in DWF4 expression did not result in increased plant biomass or GY, thus highlighting the importance of developmental stage and tissue-specificity in BR regulation. Propiconazole has been shown to inhibit specifically BR synthesis, limiting the growth of Arabidopsis and Maize in a concentration dependent manner [34]. A significant decrease in shoot biomass occurred when Propiconazole was applied (Tables 1 and 2). Contrary to the results previously shown [34], GA20ox-2 expression was affected by propiconazole application and by high concentrations of imazalil (Table 3) suggesting a possible crosstalk between BR and GA biosynthesis in rice. Interestingly, a mechanism of crosstalk between BR and GA pathways has been identified ([63] and references therein).
In barley and Arabidopsis, a steeped auxin reduction by high temperature caused the abortion of pollen development and a decreased GY [64]. In rice, auxin deficiency resulted in severe growth retardation at the early vegetative stage [65]. To test the effects of auxin deficiency at the reproductive stage, we used L-AOPP (an inhibitor of Auxin biosynthesis [31]), and PEO-IAA (a TIR1 (Transport Inhibitor Response 1)-dependent auxin receptor inhibitor [66]). The low and intermediate concentration of L-AOPP inhibited auxin responses (Table 3), and decreased GY by 15% and 20%, respectively (Tables 1 and 2), suggesting that auxin deficiency at the reproductive stage has also a negative impact on growth. In rice, the over-expression of OsGH3-2, a gene encoding an auxin-conjugating enzyme, resulted in auxin deficiency leading to a significant down-regulation of ABA-related genes such as OsbZIP23 [67]. We observed that all L-AOPP and PEO-IAA concentrations reduced the expression levels of OsbZIP23 (Table 3), indicating a L-AOPP inhibition of auxin biosynthesis similar to that reported in OsGH3-2 overexpression lines [67]. Auxin binds to two types of receptors designated as AUXIN BINDING-PROTEIN 1 (ABP1), which regulates clathrin-dependent vesicle transport, and TRANSPORT INHIBITOR RESPONSE 1 (TIR1), that regulates auxin-dependent transcription [68–71]. PEO-IAA is an antagonist on TIR1 Auxin receptor [66]. Our results suggested that auxin-dependent transcription through TIR1 regulation, modified OsbZIP23 expression in rice.
Our results show that ethylene biosynthesis inhibitor increased GY and SDW in rice as is the case with ethylene perception inhibitors [22, 23]. The lowest concentration of AIB reduced the expression levels of OsETR2 and OsERS1, and induced GA20ox2 expression (Table 3). GA20ox2 is involved in GA biosynthesis and the loss of GA20ox2 function was associated with dwarf rice phenotypes and increased GY [72, 73]. On the other hand, ethylene regulates GA content and signaling, but this regulation is dependent on ethylene concentrations, the plant developmental stage and the environmental conditions [74, 75]. This complex regulation may explain the variation observed in the GA20ox2 expression levels among the different AIB concentrations used in this study.
NDGA inhibits the function of a lipoxygenase required for ABA biosynthesis [76, 77]. It was also shown that NDGA inhibited ABA biosynthesis in some fruits during fruit maturation period [24, 78], and in maize, soybean, and rice under drought stress conditions [30, 77, 79]. Nevertheless, effects of NDGA on GY have not yet been reported. Our results indicated that that lowest NDGA concentration did not affect the expression levels of any plant hormone-responsive genes other than LEA3, but resulted in a significant increase of rice GY (21%) (Tables 1 and 3). In contrast, the intermediate NDGA concentration decreased GY by 16%, reduced the expression levels of OsbZIP23 and LEA3, and promoted the expression level of GA20ox2 (Tables 1–3). It has been shown that ABA inhibits GA biosynthesis through the transcriptional regulation of GA biosynthesis-related genes including GA20ox during germination [38]. Based on this evidence, we could assume that the intermediate concentration of NDGA might reduce ABA content, causing the up-regulation of GA20ox2 at the reproductive stage in rice. The GA20ox2 up-regulation was comparable to the gene induction observed when the high concentration of Paclobutrazol treatment was used (Table 3).
Inhibition of jasmonic acid and ethylene biosynthesis improved photosynthesis promoting changes in C metabolism
In rice, the flag leaves are the main source of photoassimilates that will contribute rice grain filling [80, 81]. Thus, flag leaf photosynthesis can be considered one of the main metabolic pathways determining grain filling in grains and flag leaf photosynthetic activity is directly correlated with grain yield [82–84].
When applied at pre-anthesis stage, NDGA improved photosynthesis and transpiration rates (Fig 1A and 1C), and promoted transitory starch accumulation in flag leaves (Fig 2A). Transitory starch will be converted to sucrose in the leaves and subsequently translocated into the grains during grain filling, thus transitory starch content in leaves at pre-anthesis stage is critical to determine grain carbohydrate content [80, 85]. The increase in grain starch content observed after NDGA treatment was correlated with starch accumulation in flag leaves (Fig 2A and 2F). Gene expression analysis also revealed that ABA responsive genes were down-regulated (Table 3) but this reduction was not correlated with a significant decrease in ABA content (Table 4). Interestingly, 0.12mM NDGA significantly decreased JA and JA-Ile contents (Table 4), and the expression of some JA synthesis-related genes (Fig 4). OsPLDα4 and OsPLDα5 mediate the release of linolenic acid from membrane phospholipids in the chloroplast [86] and LOX oxidize linolenic acid to generate hydroperoxy octadecadienoic acid (HPODE) [87]. HPODE is then converted to 12-oxo-phytodienoic acid (OPDA) by OsAOS and OsAOC. OsOPR7 generates jasmonic acid from OPDA and OsJAR1 / OsJAR2 catalyze the final step to form bioactive JA and, JA-Ile [88]. Among of these genes, the expressions of OsPLDα4, OsPLDα5, OsLOX1, OsAOC, and OsOPR7 were significantly decreased by the 0.12mM NDGA application (Fig 4). The reduced expression of OsPLDα4 / OsPLDα5, and a mutation in OsAOC were correlated with the decrease of JA contents in rice, altering plant defense responses [86, 89]. Thus, it is possible that the decreased expression of OsPLDα4, OsPLDα5, and OsAOC results in JA and JA-Ile decreased content in rice leaves followed by NDGA application. Similar to ABA [90], the exogenous application of JA inhibited photosynthesis by promoting stomata closure [91, 92], resulting in grain yield penalties in rice and soybean [15, 92]. Thus, we can assume that the inhibition of JA synthesis might be responsible for the grain yield improvement observed after NDGA application.
Ethylene has been shown to inhibit photosynthesis in peanut, sunflower, and sweet potato by promoting stomata closure [93], and the inhibition of ethylene synthesis and/or perception improved GY under stressed conditions in rice [94]. Yet, the mechanism(s) by which ethylene inhibition acts in a time-dependent manner on photosynthesis regulation remains unclear. In order to assess the effect of ethylene regulation on grain yield and plant biomass, AIB was applied at two different time points during the reproductive stage (pre- and post-anthesis). When the lower AIB concentration was applied at pre-anthesis, no improvement of GY and a decreased SDW were observed (Table 1). However, when AIB was applied at post-anthesis, an improvement of GY (22%) and SDW (21%) was detected (S4 Table). Furthermore, AIB improved photosynthesis and transpiration (Fig 1D, 1F and S6 Fig), and these were well correlated with increased starch content in the flag leaves and mature grains in AIB-treated plants (Fig 3A and 3F). Ethylene inhibition seems to be beneficial for photosynthesis and C-metabolism in flag leaves. Nevertheless, ethylene play important roles during flower development, regulating the stimulation of gametophyte development and pollen tube growth [95, 96]. Ethylene inhibition at pre-anthesis also inhibits proper flower development in rice [97], thus the development-specific regulation of ethylene might be critical to determine GY during rice development.
Our data supports the notion that the inhibition of JA or ethylene at reproductive stage improved grain yield in rice. The specific inhibition of GA, BR or auxins, negatively affected GY and plant biomass while the inhibition of JA or ethylene resulted in higher GY and plant Biomass through the improvement of photosynthesis, C-assimilation and sugar mobilization to the sinks.
Supporting Information
Plant Phenotypes of UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), and AgNO3 (I) treated rice 9 days after spray at pre-anthesis. Each figure shows the plants sprayed with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 20 cm.
(TIF)
Grains of either UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), or AgNO3 (I) treated rice sprayed at pre-anthesis. Each figure shows the grains harvested from the rice treated with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 1.5 cm.
(TIF)
Grains of either UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), or AgNO3 (I) treated rice sprayed at both pre-anthesis and post-anthesis. Each figure shows the grains harvested from the rice treated with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 1.5 cm.
(TIF)
Photosynthesis (A), Conductance (B), and Transpiration rates (C) of UTC and Paclobutrazol treated plants 2, 9, and 16 days after treatment at pre-anthesis stage. Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(TIF)
Photosynthesis (A, D), Conductance (B, E), and Transpiration rates (C, F) of UTC and Imazalil treated plants (A, B, C) or UTC and PEO-IAA treated plants (D, E, F) 2, 9, and 16 days after treatment at pre-anthesis stage. Values are Mean ± SE (n = 6). No significant difference among treatments.
(TIF)
Photosynthesis (A), Conductance (B), and Transpiration rates (C) of UTC and AIB treated plants 2 and 9 days after treatment at post-anthesis stage. Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(TIF)
(XLSX)
(DOCX)
(DOCX)
Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(XLSX)
(XLSX)
Acknowledgments
We thank Dr. Gyunghoon Hong for measuring sugars. We also thank Matthew Wright and Arjun Natarajan for their technical assistance.
Data Availability
All relevant data are within the paper and its Supporting information files.
Funding Statement
This study was supported by a grant from the Will W. Lester Endowment of University of California. Sumitomo Chemical Co. Ltd. provided support in the form of salaries for an author (H.T.), but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1. Guimaraes EP. Rice breeding Cereals: Springer; 2009. p. 99–126. [Google Scholar]
- 2. Ashikari M, Sasaki A, Ueguchi-Tanaka M, Itoh H, Nishimura A, Datta S, et al. Loss-of-function of a rice gibberellin biosynthetic gene, GA20 oxidase (GA20ox-2), led to the rice 'green revolution'. Breed Sci. 2002;52(2):143–150. 10.1270/jsbbs.52.143 WOS:000176692800011. [DOI] [Google Scholar]
- 3. Fan C, Xing Y, Mao H, Lu T, Han B, Xu C, et al. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. TAG Theoretical and applied genetics Theoretische und angewandte Genetik. 2006;112(6):1164–1171. 10.1007/s00122-006-0218-1 . [DOI] [PubMed] [Google Scholar]
- 4. Ishimaru K, Hirotsu N, Madoka Y, Murakami N, Hara N, Onodera H, et al. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nature genetics. 2013;45(6):707–711. 10.1038/ng.2612 . [DOI] [PubMed] [Google Scholar]
- 5. Wang E, Wang J, Zhu X, Hao W, Wang L, Li Q, et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nature genetics. 2008;40(11):1370–1374. 10.1038/ng.220 . [DOI] [PubMed] [Google Scholar]
- 6. Gray WM. Hormonal Regulation of Plant Growth and Development. PLoS Biol. 2004;2(9):e311 10.1371/journal.pbio.0020311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Peleg Z, Blumwald E. Hormone balance and abiotic stress tolerance in crop plants. Curr Opin Plant Biol. 2011;14(3):290–295. 10.1016/j.pbi.2011.02.001 . [DOI] [PubMed] [Google Scholar]
- 8. Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E. Cytokinin‐mediated source/sink modifications improve drought tolerance and increase grain yield in rice under water‐stress. Plant Biotechnol J. 2011;9(7):747–758. 10.1111/j.1467-7652.2010.00584.x [DOI] [PubMed] [Google Scholar]
- 9. Kucera B, Cohn MA, Leubner-Metzger G. Plant hormone interactions during seed dormancy release and germination. Seed Science Research. 2005;15(04):281–307. 10.1079/SSR2005218 [DOI] [Google Scholar]
- 10. Cracker LE, Abeles FB. Abscission: role of abscisic Acid. Plant Physiol. 1969;44(8):1144–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rupp H-M, Frank M, Werner T, Strnad M, Schmülling T. Increased steady state mRNA levels of the STM and KNAT1 homeobox genes in cytokinin overproducing Arabidopsis thaliana indicate a role for cytokinins in the shoot apical meristem. The Plant Journal. 1999;18(5):557–563. 10.1046/j.1365-313X.1999.00472.x [DOI] [PubMed] [Google Scholar]
- 12. Kyozuka J. Control of shoot and root meristem function by cytokinin. Current Opinion in Plant Biology. 2007;10(5):442–446. 10.1016/j.pbi.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 13. Vanneste S, Friml J. Auxin: a trigger for change in plant development. Cell. 2009;136(6):1005–1016. 10.1016/j.cell.2009.03.001 . [DOI] [PubMed] [Google Scholar]
- 14. Su Y-H, Liu Y-B, Zhang X-S. Auxin—Cytokinin Interaction Regulates Meristem Development. Molecular plant. 2011;4(4):616–625. 10.1093/mp/ssr007 PMC3146736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim EH, Kim YS, Park SH, Koo YJ, Choi YD, Chung YY, et al. Methyl jasmonate reduces grain yield by mediating stress signals to alter spikelet development in rice. Plant Physiol. 2009;149(4):1751–1760. 10.1104/pp.108.134684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matschi S, Hake K, Herde M, Hause B, Romeis T. The Calcium-Dependent Protein Kinase CPK28 Regulates Development by Inducing Growth Phase-Specific, Spatially Restricted Alterations in Jasmonic Acid Levels Independent of Defense Responses in Arabidopsis. The Plant cell. 2015;27(3):591–606. 10.1105/tpc.15.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jaillais Y, Chory J. Unraveling the paradoxes of plant hormone signaling integration. Nature structural & molecular biology. 2010;17(6):642–645. 10.1038/nsmb0610-642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garg R, Tyagi AK, Jain M. Microarray analysis reveals overlapping and specific transcriptional responses to different plant hormones in rice. Plant Signal Behav. 2012;7(8):951–956. 10.4161/psb.20910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Graebe J. Gibberellin biosynthesis and control. Annual review of plant physiology. 1987;38(1):419–465. [Google Scholar]
- 20. Rademacher W, Fritsch H, Graebe J, Sauter H, Jung J. Tetcyclacis and triazole‐type plant growth retardants: Their influence on the biosynthesis of gibberellins and other metabolic processes. Pestic Sci. 1987;21(4):241–252. [Google Scholar]
- 21. Street J, Jordan J, Ebelhar M, Boykin D. Plant height and yield responses of rice to paclobutrazol. Agronomy Journal. 1986;78(2):288–291. [Google Scholar]
- 22. Hays DB, Do JH, Mason RE, Morgan G, Finlayson SA. Heat stress induced ethylene production in developing wheat grains induces kernel abortion and increased maturation in a susceptible cultivar. Plant Sci. 2007;172(6):1113–1123. [Google Scholar]
- 23. Huberman M, Riov J, Goldschmidt E, Apelbaum A, Goren R. The novel ethylene antagonist, 3-cyclopropyl-1-enyl-propanoic acid sodium salt (CPAS), increases grain yield in wheat by delaying leaf senescence. Plant Growth Regul. 2014;73(3):249–255. 10.1007/s10725-013-9885-5 WOS:000337035500005. [DOI] [Google Scholar]
- 24. Zhang M, Yuan B, Leng P. The role of ABA in triggering ethylene biosynthesis and ripening of tomato fruit. J Exp Bot. 2009;60(6):1579–1588. 10.1093/jxb/erp026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Silverman FP, Petracek PD, Noll MR, Warrior P. Aminoethoxyvinylglycine effects on late-season apple fruit maturation. Plant growth regulation. 2004;43(2):153–161. [Google Scholar]
- 26. Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149(1):78–90. 10.1007/BF00386231 . [DOI] [PubMed] [Google Scholar]
- 27. Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402–408. 10.1006/meth.2001.1262 WOS:000173949500003. [DOI] [PubMed] [Google Scholar]
- 28. Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E. Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol. 2013;163(4):1609–1622. 10.1104/pp.113.227702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jikumaru Y, Asami T, Seto H, Yoshida S, Yokoyama T, Obara N, et al. Preparation and biological activity of molecular probes to identify and analyze jasmonic acid-binding proteins. Bioscience, biotechnology, and biochemistry. 2004;68(7):1461–1466. . [DOI] [PubMed] [Google Scholar]
- 30. Ren H, Gao Z, Chen L, Wei K, Liu J, Fan Y, et al. Dynamic analysis of ABA accumulation in relation to the rate of ABA catabolism in maize tissues under water deficit. J Exp Bot. 2007;58(2):211–219. 10.1093/jxb/erl117 . [DOI] [PubMed] [Google Scholar]
- 31. Soeno K, Goda H, Ishii T, Ogura T, Tachikawa T, Sasaki E, et al. Auxin biosynthesis inhibitors, identified by a genomics-based approach, provide insights into auxin biosynthesis. Plant Cell Physiol. 2010;51(4):524–536. 10.1093/pcp/pcq032 [DOI] [PubMed] [Google Scholar]
- 32. Nishimura T, Nakano H, Hayashi K, Niwa C, Koshiba T. Differential downward stream of auxin synthesized at the tip has a key role in gravitropic curvature via TIR1/AFBs-mediated auxin signaling pathways. Plant Cell Physiol. 2009;50(11):1874–1885. 10.1093/pcp/pcp129 . [DOI] [PubMed] [Google Scholar]
- 33. Werbrouck S, Saibo N, Dhuyvetter H, De Schepper S, Van Der Straeten D, Debergh P. Physiological and morphological evidence of brassinosteroid‐biosynthesis inhibition by the fungicide imazalil. Physiol Plant. 2003;119(1):69–77. [Google Scholar]
- 34. Hartwig T, Corvalan C, Best NB, Budka JS, Zhu JY, Choe S, et al. Propiconazole is a specific and accessible brassinosteroid (BR) biosynthesis inhibitor for Arabidopsis and maize. PLoS One. 2012;7(5):e36625 10.1371/journal.pone.0036625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Satoh S, Esashi Y. α‐Aminoisabutyric acid, propyl gallate and cobalt ion and the mode of inhibition of ethylene production by cotyledonary segments of cocklebur seeds. Physiol Plant. 1983;57(4):521–526. [Google Scholar]
- 36. Yang S, Hoffman N. Ethylene biosynthesis and its regulation in higher plants. Annual review of plant physiology. 1984;35(1):155–189. [Google Scholar]
- 37. Lenton J, Appleford N, Temple-Smith K. Growth retardant activity of paclobutrazol enantiomers in wheat seedlings. Plant Growth Regul. 1994;15(3):281–291. [Google Scholar]
- 38. Pérez‐Flores L, Carrari F, Osuna‐Fernández R, Rodríguez M, Enciso S, Stanelloni R, et al. Expression analysis of a GA 20‐oxidase in embryos from two sorghum lines with contrasting dormancy: possible participation of this gene in the hormonal control of germination. J Exp Bot. 2003;54(390):2071–2079. [DOI] [PubMed] [Google Scholar]
- 39. Rzewuski G, Sauter M. Ethylene biosynthesis and signaling in rice. Plant Sci. 2008;175(1–2):32–42. 10.1016/j.plantsci.2008.01.012 WOS:000257257500006. 18650958 [DOI] [Google Scholar]
- 40. Xiang Y, Tang N, Du H, Ye H, Xiong L. Characterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. Plant Physiol. 2008;148(4):1938–1952. 10.1104/pp.108.128199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu G, Gao C, Zheng X, Han B. Identification of OsbZIP72 as a positive regulator of ABA response and drought tolerance in rice. Planta. 2009;229(3):605–615. 10.1007/s00425-008-0857-3 . [DOI] [PubMed] [Google Scholar]
- 42. Chung Y, Choe S. The Regulation of Brassinosteroid Biosynthesis in Arabidopsis. Critical reviews in plant sciences. 2013;32(6):396–410. 10.1080/07352689.2013.797856 WOS:000321285700003. [DOI] [Google Scholar]
- 43. Tanaka A, Nakagawa H, Tomita C, Shimatani Z, Ohtake M, Nomura T, et al. BRASSINOSTEROID UPREGULATED1, encoding a helix-loop-helix protein, is a novel gene involved in brassinosteroid signaling and controls bending of the lamina joint in rice. Plant Physiol. 2009;151(2):669–680. 10.1104/pp.109.140806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yoshimitsu Y, Tanaka K, Fukuda W, Asami T, Yoshida S, Hayashi K, et al. Transcription of DWARF4 plays a crucial role in auxin-regulated root elongation in addition to brassinosteroid homeostasis in Arabidopsis thaliana. PLoS One. 2011;6(8):e23851 10.1371/journal.pone.0023851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jain M, Tyagi A, Khurana J. Genome-wide analysis, evolutionary expansion, and expression of early auxin-responsive SAUR gene family in rice (Oryza sativa). Genomics. 2006;88(3):360–371. 10.1016/j.ygeno.2006.04.008 WOS:000240371500012. [DOI] [PubMed] [Google Scholar]
- 46. Yau CP, Wang L, Yu M, Zee SY, Yip WK. Differential expression of three genes encoding an ethylene receptor in rice during development, and in response to indole-3-acetic acid and silver ions. J Exp Bot. 2004;55(397):547–556. 10.1093/jxb/erh055 . [DOI] [PubMed] [Google Scholar]
- 47. Thorne JH, Koller HR. Influence of assimilate demand on photosynthesis, diffusive resistances, translocation, and carbohydrate levels of soybean leaves. Plant Physiol. 1974;54(2):201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nakano H, Makino A, Mae T. Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant Cell Physiol. 1995;36(4):653–659. [Google Scholar]
- 49. Graf A, Schlereth A, Stitt M, Smith AM. Circadian control of carbohydrate availability for growth in Arabidopsis plants at night. Proc Natl Acad Sci U S A. 2010;107(20):9458–9463. 10.1073/pnas.0914299107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lu Y, Gehan JP, Sharkey TD. Daylength and circadian effects on starch degradation and maltose metabolism. Plant Physiol. 2005;138(4):2280–2291. 10.1104/pp.105.061903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mi G, Tang L, Zhang F, Zhang J. Carbohydrate storage and utilization during grain filling as regulated by nitrogen application in two wheat cultivars. J Plant Nutr. 2002;25(2):213–229. 10.1081/PLN-100108831 WOS:000173880200001. [DOI] [Google Scholar]
- 52. Lyons R, Manners JM, Kazan K. Jasmonate biosynthesis and signaling in monocots: a comparative overview. Plant cell reports. 2013;32(6):815–827. 10.1007/s00299-013-1400-y . [DOI] [PubMed] [Google Scholar]
- 53. Depuydt S, Hardtke Christian S. Hormone Signalling Crosstalk in Plant Growth Regulation. Current Biology. 2011;21(9):R365–R373. 10.1016/j.cub.2011.03.013. 10.1016/j.cub.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 54. Peng J, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, et al. 'Green revolution' genes encode mutant gibberellin response modulators. Nature. 1999;400(6741):256–261. 10.1038/22307 . [DOI] [PubMed] [Google Scholar]
- 55. Rademacher W. Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Annu Rev Plant Physiol Plant Mol Biol. 2000;51:501–531. 10.1146/annurev.arplant.51.1.501 . [DOI] [PubMed] [Google Scholar]
- 56. Toyomasu T, Kawaide H, Sekimoto H, Numers C, Phillips A, Hedden P, et al. Cloning and characterization of a cDNA encoding gibberellin 20‐oxidase from rice (Oryza sativa) seedlings. Physiol Plant. 1997;99(1):111–118. [Google Scholar]
- 57. Wu CY, Trieu A, Radhakrishnan P, Kwok SF, Harris S, Zhang K, et al. Brassinosteroids regulate grain filling in rice. Plant Cell. 2008;20(8):2130–2145. 10.1105/tpc.107.055087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Morinaka Y, Sakamoto T, Inukai Y, Agetsuma M, Kitano H, Ashikari M, et al. Morphological alteration caused by brassinosteroid insensitivity increases the biomass and grain production of rice. Plant Physiol. 2006;141(3):924–931. 10.1104/pp.106.077081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sairam R. Effects of homobrassinolide application on plant metabolism and grain yield under irrigated and moisture-stress conditions of two wheat varieties. Plant Growth Regul. 1994;14(2):173–181. [Google Scholar]
- 60. Salas Fernandez M, Becraft P, Yin Y, Lübberstedt T. From dwarves to giants? Plant height manipulation for biomass yield. Trends in plant science. 2009;14(8):454–461. 10.1016/j.tplants.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 61. Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nature biotechnology. 2006;24(1):105–109. 10.1038/nbt1173 . [DOI] [PubMed] [Google Scholar]
- 62. Divi UK, Krishna P. Brassinosteroid: a biotechnological target for enhancing crop yield and stress tolerance. New Biotechnology. 2009;26(3–4):131–136. 10.1016/j.nbt.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 63. Guo H, Li L, Aluru M, Aluru S, Yin Y. Mechanisms and networks for brassinosteroid regulated gene expression. Curr Opin Plant Biol. 2013;16(5):545–553. 10.1016/j.pbi.2013.08.002 . [DOI] [PubMed] [Google Scholar]
- 64. Sakata T, Oshino T, Miura S, Tomabechi M, Tsunaga Y, Higashitani N, et al. Auxins reverse plant male sterility caused by high temperatures. Proc Natl Acad Sci U S A. 2010;107(19):8569–8574. 10.1073/pnas.1000869107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yamamoto Y, Kamiya N, Morinaka Y, Matsuoka M, Sazuka T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007;143(3):1362–1371. 10.1104/pp.106.091561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hayashi K, Neve J, Hirose M, Kuboki A, Shimada Y, Kepinski S, et al. Rational design of an auxin antagonist of the SCF(TIR1) auxin receptor complex. ACS Chem Biol. 2012;7(3):590–598. 10.1021/cb200404c . [DOI] [PubMed] [Google Scholar]
- 67. Du H, Wu N, Fu J, Wang S, Li X, Xiao J, et al. A GH3 family member, OsGH3-2, modulates auxin and abscisic acid levels and differentially affects drought and cold tolerance in rice. J Exp Bot. 2012;63(18):6467–6480. 10.1093/jxb/ers300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen X, Naramoto S, Robert S, Tejos R, Lofke C, Lin D, et al. ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Current biology: CB. 2012;22(14):1326–1332. 10.1016/j.cub.2012.05.020 . [DOI] [PubMed] [Google Scholar]
- 69. Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, et al. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell. 2010;143(1):111–121. 10.1016/j.cell.2010.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):441–445. 10.1038/nature03543 . [DOI] [PubMed] [Google Scholar]
- 71. Kepinski S, Leyser O. The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature. 2005;435(7041):446–451. 10.1038/nature03542 . [DOI] [PubMed] [Google Scholar]
- 72. Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, et al. Green revolution: a mutant gibberellin-synthesis gene in rice. Nature. 2002;416(6882):701–702. 10.1038/416701a . [DOI] [PubMed] [Google Scholar]
- 73. Spielmeyer W, Ellis MH, Chandler PM. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proceedings of the National Academy of Sciences. 2002;99(13):9043–9048. 10.1073/pnas.132266399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Weiss D, Ori N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007;144(3):1240–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rijnders J, Yang Y, Kamiya Y, Takahashi N, Barendse G, Blom C, et al. Ethylene enhances gibberellin levels and petiole sensitivity in flooding-tolerant Rumex palustris but not in flooding-intolerant R. acetosa. Planta. 1997;203(1):20–25. [Google Scholar]
- 76. Han SY, Kitahata N, Sekimata K, Saito T, Kobayashi M, Nakashima K, et al. A novel inhibitor of 9-cis-epoxycarotenoid dioxygenase in abscisic acid biosynthesis in higher plants. Plant Physiol. 2004;135(3):1574–1582. 10.1104/pp.104.039511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Creelman RA, Bell E, Mullet JE. Involvement of a lipoxygenase-like enzyme in abscisic Acid biosynthesis. Plant Physiol. 1992;99(3):1258–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang M, Leng P, Zhang G, Li X. Cloning and functional analysis of 9-cis-epoxycarotenoid dioxygenase (NCED) genes encoding a key enzyme during abscisic acid biosynthesis from peach and grape fruits. J Plant Physiol. 2009;166(12):1241–1252. 10.1016/j.jplph.2009.01.013 [DOI] [PubMed] [Google Scholar]
- 79. Ye N, Zhu G, Liu Y, Li Y, Zhang J. ABA controls H2O2 accumulation through the induction of OsCATB in rice leaves under water stress. Plant Cell Physiol. 2011;52(4):689–698. 10.1093/pcp/pcr028 [DOI] [PubMed] [Google Scholar]
- 80. Gladun I, Karpov E. Production and partitioning of assimilates between the panicle and vegetative organs of rice after flowering. Russ J Plant Physiol. 1993;40(5):629–633. [Google Scholar]
- 81. Abdalla basyouni Abou-khalifa A, Misra A, Salem A-A. Effect of leaf cutting on physiological traits and yield of two rice cultivars. Afr J Plant Sci. 2008;2(12):147–150. [Google Scholar]
- 82. Biswal A, Kohli A. Cereal flag leaf adaptations for grain yield under drought: knowledge status and gaps. Molecular Breeding. 2013;31(4):749–766. [Google Scholar]
- 83. Adachi S, Nakae T, Uchida M, Soda K, Takai T, Oi T, et al. The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J Exp Bot. 2013;64(4):1061–1072. 10.1093/jxb/ers382 . [DOI] [PubMed] [Google Scholar]
- 84. Hirasawa T, Ozawa S, Taylaran R, Ookawa T. Varietal Differences in Photosynthetic Rates in Rice Plants, with Special Reference to the Nitrogen Content of Leaves. Plant Prod Sci. 2010;13(1):53–57. WOS:000273867700008. [Google Scholar]
- 85. Perez CM, Palmiano EP, Baun LC, Juliano BO. Starch metabolism in the leaf sheaths and culm of rice. Plant Physiol. 1971;47(3):404–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qi J, Zhou G, Yang L, Erb M, Lu Y, Sun X, et al. The chloroplast-localized phospholipases D alpha4 and alpha5 regulate herbivore-induced direct and indirect defenses in rice. Plant Physiol. 2011;157(4):1987–1999. 10.1104/pp.111.183749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wang R, Shen W, Liu L, Jiang L, Liu Y, Su N, et al. A novel lipoxygenase gene from developing rice seeds confers dual position specificity and responds to wounding and insect attack. Plant molecular biology. 2008;66(4):401–414. 10.1007/s11103-007-9278-0 . [DOI] [PubMed] [Google Scholar]
- 88. Wakuta S, Suzuki E, Saburi W, Matsuura H, Nabeta K, Imai R, et al. OsJAR1 and OsJAR2 are jasmonyl-L-isoleucine synthases involved in wound- and pathogen-induced jasmonic acid signalling. Biochemical and biophysical research communications. 2011;409(4):634–639. 10.1016/j.bbrc.2011.05.055 . [DOI] [PubMed] [Google Scholar]
- 89. Riemann M, Haga K, Shimizu T, Okada K, Ando S, Mochizuki S, et al. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. The Plant journal: for cell and molecular biology. 2013;74(2):226–238. 10.1111/tpj.12115 . [DOI] [PubMed] [Google Scholar]
- 90. Liang J, Zhang J, Wong MH. Can stomatal closure caused by xylem ABA explain the inhibition of leaf photosynthesis under soil drying? Photosynth Res. 1997;51(2):149–159. 10.1023/A:1005797410190 WOS:A1997XG77600007. [DOI] [Google Scholar]
- 91. Wang SY. Methyl Jasmonate Reduces Water Stress in Strawberry. J Plant Growth Regul. 1999;18(3):127–134. . [DOI] [PubMed] [Google Scholar]
- 92. Beltrano J, Ronco MG, Montaldi ER, Carbone A. Senescence of flag leaves and ears of wheat hastened by methyl jasmonate. Journal of Plant Growth Regulation. 1998;17(1):53–57. 10.1007/Pl00007012 WOS:000073737500009. [DOI] [Google Scholar]
- 93. Pallas JE, Kays SJ. Inhibition of photosynthesis by ethylene-a stomatal effect. Plant Physiol. 1982;70(2):598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yang J, Zhang J, Ye Y, Wang Z, Zhu Q, Liu L. Involvement of abscisic acid and ethylene in the responses of rice grains to water stress during filling. Plant, cell & environment. 2004;27(8):1055–1064. [Google Scholar]
- 95. Holden MJ, Marty JA, Singh-Cundy A. Pollination-induced ethylene promotes the early phase of pollen tube growth in Petunia inflata. J Plant Physiol. 2003;160(3):261–269. . [DOI] [PubMed] [Google Scholar]
- 96. Zhang XS, O'Neill SD. Ovary and Gametophyte Development Are Coordinately Regulated by Auxin and Ethylene following Pollination. Plant Cell. 1993;5(4):403–418. 10.1105/tpc.5.4.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wuriyanghan H, Zhang B, Cao WH, Ma B, Lei G, Liu YF, et al. The ethylene receptor ETR2 delays floral transition and affects starch accumulation in rice. Plant Cell. 2009;21(5):1473–1494. 10.1105/tpc.108.065391 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plant Phenotypes of UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), and AgNO3 (I) treated rice 9 days after spray at pre-anthesis. Each figure shows the plants sprayed with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 20 cm.
(TIF)
Grains of either UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), or AgNO3 (I) treated rice sprayed at pre-anthesis. Each figure shows the grains harvested from the rice treated with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 1.5 cm.
(TIF)
Grains of either UTC (A), NDGA (B), Paclobutrazol (C), Imazalil (D), Propiconazole (E), L-AOPP (F), PEO-IAA (G), AIB (H), or AgNO3 (I) treated rice sprayed at both pre-anthesis and post-anthesis. Each figure shows the grains harvested from the rice treated with either the lowest (L), intermediate (M), or the highest (H) concentration (mM) of each chemical treatment. Bar = 1.5 cm.
(TIF)
Photosynthesis (A), Conductance (B), and Transpiration rates (C) of UTC and Paclobutrazol treated plants 2, 9, and 16 days after treatment at pre-anthesis stage. Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(TIF)
Photosynthesis (A, D), Conductance (B, E), and Transpiration rates (C, F) of UTC and Imazalil treated plants (A, B, C) or UTC and PEO-IAA treated plants (D, E, F) 2, 9, and 16 days after treatment at pre-anthesis stage. Values are Mean ± SE (n = 6). No significant difference among treatments.
(TIF)
Photosynthesis (A), Conductance (B), and Transpiration rates (C) of UTC and AIB treated plants 2 and 9 days after treatment at post-anthesis stage. Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(TIF)
(XLSX)
(DOCX)
(DOCX)
Values are Mean ± SE (n = 6). * indicates significance at P ≤ 0.05.
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting information files.