Abstract
Background
Few studies have examined the contribution of treatment on the mortality of dementia based on a population-based study.
Objective
To investigate the effects of anti-dementia and nootropic treatments on the mortality of dementia using a population-based cohort study.
Methods
12,193 incident dementia patients were found from 2000 to 2010. Their data were compared with 12,193 age- and sex-matched non-dementia controls that were randomly selected from the same database. Dementia was classified into vascular (VaD) and degenerative dementia. Mortality incidence and hazard ratios (HRs) were calculated.
Results
The median survival time was 3.39 years (95% confidence interval [CI]: 2.88–3.79) for VaD without medication, 6.62 years (95% CI: 6.24–7.21) for VaD with nootropics, 3.01 years (95% CI: 2.85–3.21) for degenerative dementia without medication, 8.11 years (95% CI: 6.30–8.55) for degenerative dementia with anti-dementia medication, 6.00 years (95% CI: 5.73–6.17) for degenerative dementia with nootropics, and 9.03 years (95% CI: 8.02–9.87) for degenerative dementia with both anti-dementia and nootropic medications. Compared to the non-dementia group, the HRs among individuals with degenerative dementia were 2.69 (95% CI: 2.55–2.83) without medication, 1.46 (95% CI: 1.39–1.54) with nootropics, 1.05 (95% CI: 0.82–1.34) with anti-dementia medication, and 0.92 (95% CI: 0.80–1.05) with both nootropic and anti-dementia medications. VaD with nootropics had a lower mortality (HR: 1.25, 95% CI: 1.15–1.37) than VaD without medication (HR: 2.46, 95% CI: 2.22–2.72).
Conclusion
Pharmacological treatments have beneficial effects for patients with dementia in prolonging their survival.
Introduction
Around 24.3 million people suffer from dementia worldwide, and this number is expected to increase to 81.1 million by 2040 [1]. This increase in the prevalence of dementia raises questions about survival rates in patients with dementia. Accurate estimates of prognosis are critical for making decisions about treatment. This is also an important public health issue and provides information for policy makers and health care providers.
People with dementia reportedly have decreased survival compared to those without [2–7]. Clinical experience and empirical data suggest that dementia is a leading cause of death and shortens the lifespan of elderly patients [8]. However, limited information exists about the disease course and outcomes. Therefore, strategies to delay the progression of dementia and mortality are currently being investigated.
The efficacies of anti-dementia drugs, cholinesterase inhibitors, and memantine have been demonstrated in randomized controlled trials [9, 10]. Long-term observational controlled studies show that persistent anti-dementia drug treatment is associated with a slower decline in cognition, daily function, and global severity [11]. Nootropics are known as cognition enhancers that improve mental function and can be used to treat a range of conditions including memory and concentration problems [12–14]. However, limited information regarding the contribution of anti-dementia and nootropic treatments on the mortality of patients with dementia.
Under the health insurance policy in Taiwan, patients are reimbursed for anti-dementia medication, i.e., donepezil, rivastigmine, galantamine, or memantine, only if they are undergoing treatment for Alzheimer disease (AD) but not vascular dementia (VaD), while all dementia patients are reimbursed for nootropics. Nootropics and/or anti-dementia treatments were further compared in the degenerative dementia group, whereas for the VaD group, only nootropics were analyzed. Using the National Health Insurance Research database, we conducted a nationwide population-based cohort study to investigate the effects of long-term therapeutic effects of anti-dementia and nootropic medications on the mortality of patients with dementia between 2000 and 2010.
Materials and Methods
Data sources
The National Health Insurance (NHI) is a mandatory universal health insurance program offering comprehensive medical care coverage to all Taiwanese residents. Of the residents in Taiwan, 96% have joined the NHI program since 1996. The NHI sample files, which are constructed and managed by the National Health Research Institute (NHRI), consist of comprehensive utilization and enrollment information for a randomly selected sample of 1,000,000 NHI beneficiaries, representing approximately 5% of all enrollees in Taiwan in 2000. A multistage, stratified, systematic sampling design was used. There were no statistically significant differences in age or gender between the sample groups and all enrollees. The comprehensive health care data include the enrollment files; the claims data for outpatients, inpatients, and emergency room use; and the registry for drug prescriptions.
Incident cases of dementia
We conducted a retrospective cohort study from January 1, 2000 to December 31, 2010, based on the 1,000,000 NHI sample files. All patients aged 65 years or older with incident dementia were included in our study.
We enrolled the incident cases of dementia by choosing those free of dementia diagnoses in 1999 before being enrolled, and the onset date of dementia defined according to the first appearance of the code in the claim data. A total of 12,193 patients with dementia were included in this study. Owing to the strict regulation regarding reimbursement of anti-dementia medication and complexity of the reimbursement procedure in Taiwan, a limited number of dementia patients received reimbursement for anti-dementia medications. In our study, AD patients were defined according to the International Classification of Diseases, Ninth Revision (ICD-9: 331.0), or depending on whether they ever received reimbursement for anti-dementia medication. Only 1,793 patients (14.7% of all dementia patients) were identified as having AD. Most patients (7,607, 62.4% of all dementia patients) with dementia were diagnosed with senile dementia (ICD-9: 290.0, 290.1, 290.2, 290.3). To present this valuable information regarding dementia patients, we chose to group these patients with non-vascular dementia under degenerative dementia. All patients with dementia were classified into VaD (ICD-9: 290.4) and degenerative dementia (i.e., all other dementia cases except VaD, i.e., ICD-9: 290.0, 290.1, 290.2, 290.3, 294.1, 294.2, 331.0, 331.1, and 331.2) in this study. We included the cases where the subjects were diagnosed with dementia at least three times at an outpatient clinic, or at least one time at admission.
Control group
Subjects without dementia records were used as the control group. One control was selected to match each dementia patient by random sampling stratified for age and sex from the database within the same observational period. A total of 12,193 subjects served as a comparison group.
Outcome variables
All study subjects were followed from the index date to withdrawal from insurance (mostly due to deaths at this age group), or December 31, 2010, which ever date came first. Subjects with the latter two conditions were considered censored in the analysis.
Covariates
Baseline covariates included socioeconomic status (low, medium, high), urbanity (urban, suburban, rural), and the Charlson comorbidity index (CCI). Urbanity was included to minimize the potential confounding effects of differential accessibility to and availability of medical care. Since the NHI program is financed by wage-based premiums for people with clearly-defined monthly wages and by fixed premiums for people without clearly-defined monthly wages, the socioeconomic status was classified into three categories according to each patient’s own insurance wage [15]. The CCI, which is a weighted summary measure of clinically important concomitant diseases adapted for use with ICD-9-CM-coded administrative data, was used to control for comorbidities [16]. To calculate the index, we retrieved the underlying diseases from each study subject’s inpatient claims from 2000 to 2010.
Anti-dementia and nootropic medications
We defined anti-dementia and nootropic medications according to the Anatomical Therapeutic Chemical Classification System. The usage of nootropics, including piracetam, ginkgo folium, dihydroergocristine, and dihydroergotamine, was recorded in the claim data. There were no strict reimbursement regulations for nootropic prescriptions among patients with dementia.
However, to apply for any anti-dementia medication, i.e., donepezil, rivastigmine, galantamine, or memantine, in-charge neurologists or psychiatrists are requested to provide information including at least one report of computed tomography, magnetic resonance imaging, or Hachinski ischemic score, and a thorough laboratory examination report and detailed medical summary are needed for confirming the diagnosis of AD. The patient’s scores on the Mini-Mental State Examination (MMSE) or Clinical Dementia Rating (CDR) are used to indicate their current level of cognitive function. The diagnosis of AD and approval of these anti-dementia medications are reviewed and confirmed by a board-certified neurologist or psychiatrist. Only patients with MMSE scores of 10–26 or CDR scores of 1–2 are allowed to be reimbursed for cholinesterase inhibitors, while patients with MMSE scores of 10–14 or CDR scores of 2 are allowed to be reimbursed for memantine. The patients’ conditions must be re-evaluated every year after the initial prescription, and they are asked to discontinue the use of these medications if their MMSE scores decline by more than 2 points, or if the CDR scores deteriorate. Donepezil and rivastigmine have been covered by health insurance since October 1, 2001, galantamine since January 1, 2003, and memantine since June 1, 2006. Rivastigmine has been covered by health insurance for the treatment of Parkinson’s disease with dementia since 2010.
Statistical analysis
The demographic information of patients with or without dementia was compared using χ2 tests for categorical variables and t-tests for continuous variables. The mortality incidence was calculated by dividing the number of individuals who died by person-years at risk during the follow up. Survival curves and median survival times were constructed based on the Kaplan-Meier methods. Comparisons of survival rates were performed using log rank tests. The log-log survival curve was used to test the proportional assumption. The plots of log-log survival curves were not parallel, and the linear association with the log of time suggested the appropriateness of a parametric survival model with Weibull distribution. Two models were used to estimate the hazard ratios (HRs), with the basic model adjusted for age and sex, and the full model adjusted for age, sex, socioeconomic status, urbanity, and comorbidity.
We used SAS 9.1 (SAS Institute Inc., Cary, NC, USA) to link the data, and Stata 12 (Stata Corporation, College Station, TX, USA) to perform the statistical analyses.
Ethical Approval
Insurance reimbursement claims adopted in this study were from Taiwan’s NHIRDs, which is available for research purposes. All information that would allow a specific individual patient to be identified was encrypted. The confidentiality of the data abides by the data regulations of the National Health Insurance Administration, Ministry of Health and Welfare, Taiwan. The study was in accordance with the Helsinki Declaration and was approved by the NHRI and the Institutional Review Board of Taipei City Hospital (IRB: TCHIRB-1021224-E).
Results
The baseline characteristics of the individuals who participated in the study are presented in Table 1. A total of 24,386 patients (12,193 dementia cases and 12,193 non-dementia cases) were included and followed for an average of 4.56 years (93,481 person-years at risk with 9,548 deaths). The mean age for patients with incident dementia was 79.1 years (standard deviation [SD]: 7.1). The individual matching process between patients with dementia and control subjects resulted in comparable distributions of age and sex.
Table 1. Baseline characteristics of the study samples.
| Variables | Dementia cases | Non-dementia cases | P | ||
|---|---|---|---|---|---|
| (n = 12,193) | % | (n = 12,193) | % | ||
| Age (mean ± SD) | 79.1 ± 7.1 | 79.1 ± 6.9 | 0.55 | ||
| 65–69 | 1,194 | 9.79 | 1,194 | 9.79 | 1.00 |
| 70–74 | 2,145 | 17.59 | 2,145 | 17.59 | |
| 75–79 | 3,006 | 24.65 | 3,006 | 24.65 | |
| 80–84 | 3,057 | 25.07 | 3,057 | 25.07 | |
| ≥85 | 2,791 | 22.89 | 2,791 | 22.89 | |
| Sex | |||||
| Male | 5,934 | 48.67 | 5,934 | 48.67 | 1.00 |
| Female | 6,259 | 51.33 | 6,259 | 51.33 | |
| Socioeconomic status | |||||
| Low | 9,177 | 75.26 | 9,282 | 76.13 | 0.29 |
| Medium | 1,449 | 11.88 | 1,391 | 11.41 | |
| High | 1,567 | 12.85 | 1,520 | 12.47 | |
| Urbanity | |||||
| Urban | 6,288 | 51.57 | 6,174 | 50.64 | 0.35 |
| Suburban | 3,988 | 32.71 | 4,063 | 33.33 | |
| Rural | 1,917 | 15.72 | 1,955 | 16.04 | |
| Charlson comorbidity index (mean ± SD) | 1.93 ± 2.36 | 1.40 ± 2.33 | <0.001 | ||
| Myocardial infarction | 450 | 3.69 | 427 | 3.50 | 0.43 |
| Congestive heart failure | 1,913 | 15.69 | 1,359 | 11.15 | <0.001 |
| Peripheral vascular disease | 356 | 2.92 | 232 | 1.90 | <0.001 |
| Cerebrovascular disease | 2,326 | 19.08 | 1,207 | 9.90 | <0.001 |
| Dementia | 2,415 | 19.81 | 0 | <0.001 | |
| Chronic pulmonary disease | 2,566 | 21.04 | 1,627 | 13.34 | <0.001 |
| Rheumatologic disease | 61 | 0.50 | 55 | 0.45 | 0.58 |
| Peptic ulcer disease | 1,841 | 15.10 | 1,318 | 10.81 | <0.001 |
| Mild liver disease | 499 | 4.09 | 428 | 3.51 | 0.02 |
| Diabetes (mild to moderate) | 2,671 | 21.91 | 1,666 | 13.66 | <0.001 |
| Diabetes with chronic complications | 1,118 | 9.17 | 601 | 4.93 | <0.001 |
| Hemiplegia or paraplegia | 406 | 3.33 | 189 | 1.55 | <0.001 |
| Renal disease | 871 | 7.14 | 621 | 5.09 | <0.001 |
| Any malignancy | 1,139 | 9.34 | 1,404 | 11.51 | <0.001 |
| Moderate or severe liver disease | 134 | 1.10 | 115 | 0.94 | 0.23 |
| Metastatic solid tumor | 354 | 2.90 | 558 | 4.58 | <0.001 |
| AIDS | 0 | 0 | |||
SD: standard deviation; AIDS: acquired immune deficiency syndrome
There were no differences in socioeconomic status or urbanity between the dementia and non-dementia groups. The average CCI score of the non-dementia group was 1.40 (SD: 2.33), while the average CCI score of the dementia group was 1.93 (SD: 2.36), with significantly higher incidences of congestive heart failure, peripheral vascular disease, cerebrovascular disease, chronic pulmonary diseases, peptic ulcer diseases, mild liver disease, diabetes, hemiplegia or paraplegia, and renal disease, but lower incidences of malignancy and metastatic solid tumors.
Patients with VaD were slightly younger in age than those with degenerative dementia (78.2 ± 6.7 for VaD, and 79.3 ± 7.1 for degenerative dementia, P < 0.001), and had a higher CCI (2.44 ± 2.55 for VaD, and 1.83 ± 2.30 for degenerative dementia, P < 0.001) (Table 2). Compared to female patients, male patients had a higher ratio of VaD, but a lower ratio of degenerative dementia (54.6% vs. 47.4%, P < 0.001).
Table 2. Crude mortality incidence rate ratios for the dementia and non-dementia group.
| Variables | Dementia | No dementia | ||
|---|---|---|---|---|
| Total | Vascular dementia | Degenerative dementia | ||
| (n = 12,193) | (n = 2,142, 17.6%) | (n = 10,051, 82.4%) | (n = 12,193) | |
| Age (mean ± SD) | 79.1 ± 7.1 | 78.2 ± 6.7 | 79.3 ± 7.1 | 79.1 ± 6.9 |
| Male: Female | 5,934:6,259 | 1169:973 | 4765:5286 | 5,934:6,259 |
| Charlson comorbidity index (CCI) | 1.93 ± 2.36 | 2.44 ± 2.55 | 1.83 ± 2.30 | 1.40 ± 2.33 |
| CCI except dementia | 1.74 ± 2.25 | 2.13 ± 2.40 | 1.65 ± 2.21 | 1.40 ± 2.33 |
| Median follow-up, years (IQR) | 2.72 (1.20–4.94) | 3.03 (1.47–5.35) | 2.66 (1.16–4.86) | 3.93 (1.95–6.00) |
| Person-years at risk | 40,788 | 7,755 | 33,033 | 52,693 |
| Death | 5,688 | 1,008 | 4,680 | 3,860 |
| Mortality incidence (/1000 py) | 139 | 130 | 142 | 73 |
| Crude IRR (ref: non-dementia) (95% CI) | 1.90 (1.83–1.98) | 1.77 (1.65–1.90) | 1.93 (1.85–2.02) | |
| Median survival, year (95% CI) | ||||
| Total | 5.07 (4.91–5.25) | 5.50 (5.05–5.94) | 4.99 (4.83–5.18) | 9.23 (8.90–9.44) |
| Male | 4.36 (4.14–4.58) | 4.88 (4.42–5.40) | 4.22 (3.98–4.44) | 8.55 (8.02–8.96) |
| Female | 5.89 (5.65–6.13) | 6.31 (5.66–6.95) | 5.77 (5.54–6.08) | 9.84 (9.39–10.89) |
| 65–69 years | 9.97 (9.22–10.32) | 7.74 (6.81–8,91) | 10.25 (9.97–—) | 11.00 (11.00–—-) |
| 70–74 years | 7.55 (7.07–8.28) | 7.64 (6.44–8.36) | 7.55 (7.03–8.41) | >11 |
| 75–79 years | 5.89 (5.48–6.26) | 6.27 (5.43–7.11) | 5.79 (5.41–6.16) | 10.78 (10.17–—) |
| 80–84 years | 4.38 (4.14–4.67) | 4.01 (3.53–4.73) | 4.47 (4.20–4.79) | 7.29 (6.89–7.60) |
| ≥85 years | 2.90 (2.76–3.11) | 3.92 (3.21–4.51) | 2.80 (2.64–2.98) | 4.51 (4.25–4.78) |
SD: standard deviation; CCI: Charlson comorbidity index; IQR: interquartile range; py: person-years; IRR: incidence rate ratio; CI: confidence interval
Incidence rate ratio (IRR) of mortality for degenerative dementia and VaD
At the end of the study, 46.6% of the people with dementia died versus 31.7% of the people without dementia. The crude mortality incidence ratios and median survival stratified by sex and age among the dementia and non-dementia groups are shown in Table 2. Compared to the non-dementia group, the crude IRR of mortality among patients with dementia was 1.90 (95% confidence interval [CI]: 1.83–1.98). For patients with VaD the IRR was 1.77 (95% CI: 1.65–1.90), while the IRR for patients with degenerative dementia was 1.93 (95% CI: 1.85–2.02). Female patients had longer survival times than male patients, regardless of whether they were in the VaD, degenerative dementia, or non-dementia group. Compared to degenerative dementia, VaD had a shorter median survival time at ages 65–69 (7.74 years [95% CI: 6.81–8.91] for VaD vs. 10.25 years [95% CI: 9.97–-] for degenerative dementia), and the risk reversed in patients aged 85 years or over, where degenerative dementia had a shorter survival time (3.92 years [95% CI: 3.21–4.51] for VaD vs. 2.80 years [95% CI: 2.64–2.98] for degenerative dementia).
IRR of mortality for dementia with and without medication
The crude mortality incidence ratios with detailed classifications of patients with VaD and degenerative dementia according to their usage of medications for dementia are presented in Table 3. Among subjects with degenerative dementia, those using nootropic and/or anti-dementia medications were younger than those who were not using medication. Compared to the non-dementia group, the IRRs of mortality among patients with degenerative dementia were 3.07 (95% CI: 2.91–3.23) for patients not using medication, 1.56 (95% CI: 1.48–1.65) for those using nootropics, 1.02 (95% CI: 0.79–1.31) for those using anti-dementia medication, and 0.80 (95% CI: 0.69–0.91) for those using both nootropic and anti-dementia medications. In patients with VaD, those using nootropics were younger than those who were not using this medication. The patients with VaD who were using nootropics had lower mortality (IRR: 1.41, 95% CI: 1.29–1.54) than patients with VaD who were not using this medication (IRR: 2.82, 95% CI: 2.54–3.12).
Table 3. Crude mortality incidence rate ratio among the vascular dementia and non-vascular dementia groups.
| Variables | Vascular dementia (n = 2,142) | Degenerative dementia (n = 10,051) | ||||
|---|---|---|---|---|---|---|
| No medication | Nootropics | No medication | Nootropics | Anti-dementia medication | Both medications | |
| (n = 750, 35.0%)) | (n = 1,392, 65.0%) | (n = 4,130, 41.1%) | (n = 4,850, 48.3%) | (n = 240, 2.4%) | (n = 831, 8.3%) | |
| Age (mean ± SD) | 78.8 ± 6.8 | 77.9 ± 6.7 | 80.5 ± 7.4 | 78.8 ± 6.9 | 78.4 ± 6.3 | 76.7 ± 6.2 |
| Charlson comorbidity index (CCI) | 2.29 ± 2.49 | 2.52 ± 2.58 | 1.77 .77.58 | 1.93 .93.58 | 1.38 ±.38 5 | 1.60 ±.60 5 |
| CCI except dementia | 2.01 ± 2.34 | 2.20 ± 2.42 | 1.62 .62424 | 1.76 .76424 | 1.14 ±.1495 | 1.30 ±.30 5 |
| Prescription cumulative days (IQR) | ||||||
| Nootropics | 0 | 210 (63–577) | 0 | 140 (44–420) | 0.00 | 175 (58–511) |
| Anti-dementia medication | 0 | 0 | 0 | 0 | 308 (90–739) | 336 (126–805) |
| Median follow-up, years (IQR) | 2.04 (0.86–3.80) | 3.66 (1.91–6.03) | 1.79 (0.68–3.62) | 3.25 (1.57–5.41) | 3.04 (1.50–5.11) | 4.13 (2.35–6.37) |
| Person-years at risk | 2,009 | 5,746 | 10,333 | 18,118 | 853 | 3729 |
| Death | 415 | 593 | 2,321 | 2,077 | 64 | 218 |
| Mortality incidence (/1000 py) | 207 | 103 | 225 | 115 | 75 | 58 |
| Crude IRR (ref: non-dementia) (95% CI) | 2.82 (2.54–3.12) | 1.41 (1.29–1.54) | 3.07 (2.91–3.23) | 1.56 (1.48–1.65) | 1.02 (0.79–1.31) | 0.80 (0.69–0.91) |
SD: standard deviation; IQR: interquartile range; py: person-year; IRR: incidence rate ratio; CI: confidence interval
Survival time for degenerative dementia and VaD
The Kaplan-Meier analysis demonstrated a reduction in the survival probability associated with the different dementia groups compared to the non-dementia group. The median survival times were 9.23 years (95% CI: 8.90–9.44) for the non-dementia group, 5.50 years (95% CI: 5.05–5.94) for VaD, and 4.99 years (95% CI: 4.83–5.18) for degenerative dementia (Fig 1).
Fig 1. Kaplan-Meier survival estimates.
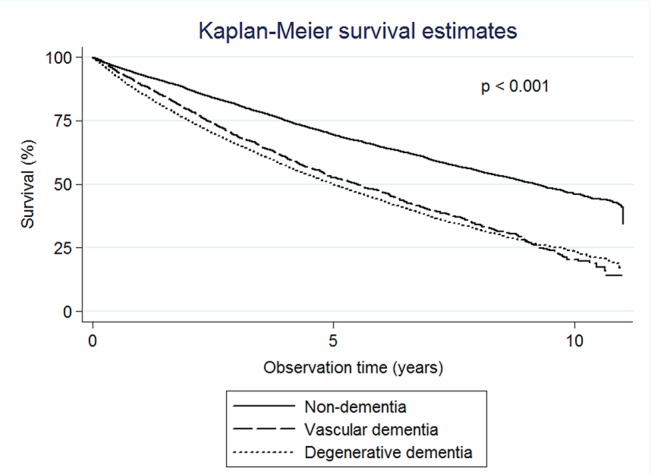
The median survival times were 9.23 years for the non-dementia group, 5.50 years for vascular dementia, and 4.99 years for degenerative dementia.
Survival time for dementia with and without medication
The median survival times were 3.01 years (95% CI: 2.85–3.21) for degenerative dementia without medications, 8.11 years (95% CI: 6.30–8.55) for degenerative dementia with anti-dementia medications, 6.00 years (95% CI: 5.73–6.17) for degenerative dementia with nootropics, and 9.03 years (95% CI: 8.02–9.87) for degenerative dementia with both nootropic and anti-dementia medications (Fig 2). The median survival times were 3.39 years (95% CI: 2.88–3.79) for VaD without medications, and 6.62 years (95% CI: 6.24–7.21) for VaD with nootropics (Fig 3).
Fig 2. Kaplan-Meier survival estimates.
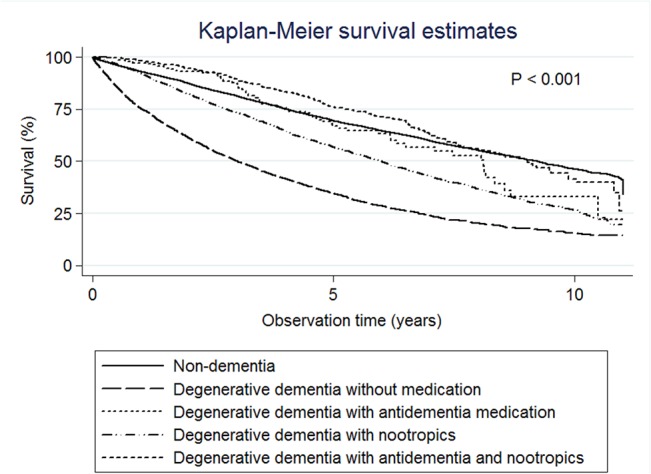
The median survival times were 9.23 years for the non-dementia group, 3.01 years for degenerative dementia without medications, 8.11 years for degenerative dementia with anti-dementia medications, 6.00 years for degenerative dementia with nootropics, and 9.03 years for degenerative dementia with both nootropic and anti-dementia medications.
Fig 3. Kaplan-Meier survival estimates.
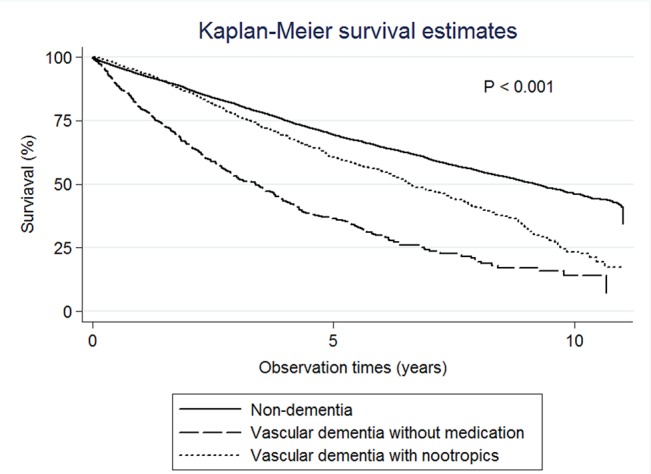
The median survival times were 9.23 years for the non-dementia group, 3.39 years for vascular dementia without medications, and 6.62 years for vascular dementia with nootropics.
HRs for dementia with and without medications
The results of the survival models are shown in Table 4. The HRs were attenuated slightly when adjusted from the basic model to the full model. In the full model, the HRs of degenerative dementia were 2.69 (95% CI: 2.55–2.83) for those not using medications, 1.46 (95% CI: 1.39–1.54) for those using nootropics, 1.05 for (95% CI: 0.82–1.34) for those using anti-dementia medications, and 0.92 (95% CI: 0.80–1.05) for those using both nootropic and anti-dementia medications. The VaD cases that used nootropics had a lower mortality (HR: 1.25, 95% CI: 1.15–1.37) than those who were not using any medications (HR: 2.46, 95% CI: 2.22–2.72).
Table 4. Hazard ratios in the vascular dementia and degenerative dementia groups.
| Variables | Basic model | Full model | ||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Age (increased 1 year) | 1.08 | 1.07–1.08 | 1.08 | 1.07–1.08 |
| Male (ref: female) | 1.36 | 1.30–1.41 | 1.29 | 1.24–1.34 |
| Vascular dementia (ref: non-dementia) | ||||
| No medication | 2.90 | 2.62–3.21 | 2.46 | 2.22–2.72 |
| Nootropics | 1.50 | 1.37–1.63 | 1.25 | 1.15–1.37 |
| Degenerative dementia (ref: non-dementia) | ||||
| No medication | 2.91 | 2.76–3.6 | 2.69 | 2.55–2.83 |
| Nootropics | 1.61 | 1.53–1.70 | 1.46 | 1.39–1.54 |
| Anti-dementia medication | 1.06 | 0.83–1.36 | 1.05 | 0.82–1.34 |
| Both medications | 0.96 | 0.84–1.10 | 0.92 | 0.80–1.05 |
| Socioeconomic status (ref: low) | ||||
| Medium | 0.99 | 0.92–1.06 | ||
| High | 0.98 | 0.92–1.04 | ||
| Urbanity (ref: urban) | ||||
| Suburban | 1.10 | 1.05–1.15 | ||
| Rural | 1.19 | 1.13–1.27 | ||
| Charlson comorbidity index (increased 1) | 1.14 | 1.13–1.14 | ||
Discussion
Our study is unique because it utilized the general population, which is more representative of actual cases. Additionally, we used incident cases of dementia to evaluate the effects of anti-dementia and nootropic medications on patient survival.
Anti-dementia medications were introduced in the mid-1990s to treat AD [17]. Persistent anti-dementia medications are associated with a slower decline in cognition, even in patients with advanced diseases [11, 18, 19]. These mediations also significantly altered the treatment history by extending the time to nursing home admission [20, 21]. However, the effects of anti-dementia medications on longevity remain controversial [22]. Some studies found no relationship between anti-dementia medications and survival [23–25], while others reported a better survival rate for those using such medications [26, 27]. Nootropics are drugs used to treat cognition deficits in patients with AD, schizophrenia, stroke, and attention deficit hyperactivity disorder as well as aging patients and have also been use to improved cognitive impairment in patients with VaD. [13, 14, 28] The beneficial effects of nootropics on dementia are reportedly inconsistent and unreliable [13, 14]. Although anti-dementia drugs such as donepezil and memantine are used in clinical practice for treating VaD, they are not approved by the Food and Drug Administration of Taiwan and are not reimbursed for in the insurance policy. Therefore, anti-dementia agents are rarely used in Taiwan and were not analyzed in our study. In this population-based study, we found that nootropics alone have beneficial effects on mortality in patients with degenerative dementia and VaD. Although some studies have reported that anti-dementia medications do not prolong survival in patients with AD [11, 23–25], we observed beneficial effects of anti-dementia medication on the survival of patients with degenerative dementia, a finding also observed consistently in some other studies [26, 27, 29–31]. In patients with degenerative dementia who received only anti-dementia medication or a combination of anti-dementia and nootropic medications, the mortality risk reduced to that in patients without dementia. Besides, the patients who received the combined medication tended to showed greater advantages, which reflects the synergistic effect of anti-dementia and nootropics on dementia. Piracetam, a nootropic, has been shown to have a neuroprotective effect when used during coronary bypass surgery [32], and ginko biloba showed efficacy comparable to that of anti-dementia medication in the treatment of Alzheimer’s type dementia [33]. A recent study indicated a considerable overlap between cerebrovascular disease and AD and suggests that both pathologies have an additive effect on cognitive decline [34], which may support the possibility of a synergistic effect of anti-dementia and nootropic medications. However, further studies are needed to investigate the possible synergic effects of anti-dementia and nootropic medications.
In the NHI claim data, the prevalence of dementia among those aged 65 or above was 2.5% (2,646/103,797) in the year 2000, and increased to 6.6% (6,951/104,761) in the year 2010. These results are quite close to those of previous epidemiological studies conducted in Taiwan, where the prevalence rate in individuals aged 65 and over was reportedly 2.0–3.7% in the 1990s [35, 36]. and increased to 8% in 2011–2012 [37]. Therefore, the measurement of dementia cases should be valid.
The relative risk of mortality for patients with VaD is reportedly greater than the risk in AD, although this difference was not statistically significant [7, 38, 39]. In contrast, other reports have shown that patients with AD have a worse prognosis than those with VaD [40, 41]. In this study, patients with VaD tended to have a lower risk and slightly longer median survival time than patients with degenerative dementia. However, patients with VaD had a shorter survival time between the ages of 65 and 69. The risk reversed in those aged 85 and older, where degenerative dementia had a shorter survival time.
Our study revealed that the survival time among patients with dementia depends more on the age of the patient, which is consistent with a previous study [42]. Age and comorbidity remain the significant factors to mortality. Concomitant morbidities may increase mortality in most dementia cases [6]. Since concomitant illnesses commonly exist in elderly persons, it is difficult to investigate the direct associations between dementia and lifespan. In this study, we adjusted the models for CCI, where the elevation of one CCI score increased the mortality risk by 14%. The HRs of dementia decreased as the comorbidities were adjusted for in the full model. However, the relationship between dementia and mortality might be a marker of a global decline in health. Dementia is a complex series of symptoms with multiple causes, making it similar to most late-life chronic diseases.
Age-based stereotypes may lead to the underuse of medication based on the conclusion that an older patient’s malady is chronic and less susceptible to intervention [43]. Many people consider senility or dementia to be normal or an expected consequence of aging [44]. However, the impact of dementia on public health is enormous and will continue to grow, especially in an aging society. Dementia is a common and lengthy illness; therefore, even small treatment effects could have a substantial impact over time. In this study, patients with degenerative dementia who were using anti-dementia and/or nootropic medications had a more desirable outcome than those who were not using such medications, supporting the early use of these medications in dementia patients.
The results of this research should be viewed in the light of several limitations. First, the administrative data are subject to possible coding errors and under- or over-coding problems. Given that claims data have low sensitivity and high specificity for dementia diagnoses [45], the misclassification of some patients with milder dementia as being non-demented would underestimate our findings and imply that they are more conservative than they really are. Second, this study might suffer from certain inherent limitations because of the use of administrative data, which lack information on dementia severity. Third, drug therapy is usually not assigned randomly, so other unmeasured factors may influence the observed relationship between drug use and outcome. Behavioral and psychological symptoms of dementia (BPSD), such as psychosis, agitation, dysphoria, apathy, and disinhibition, occur in a majority of patients with dementia. The possible use of antipsychotics may be involved in an increase in mortality in these patients [46–48]. In this study, we focused on investigating the therapeutic effects of anti-dementia and nootropic medications on dementia-related mortality. Medication used for controlling BPSD was not investigated in our study. However, dementia patients who are treated with anti-dementia and nootropic agents may be more likely to be treated with their BPSD by their physicians than those who do not receive any treatment for dementia. Therefore, use of antipsychotic agents was not likely to be a cause of increased mortality in the non-treated group, which makes our results more conservative. Fourth, it was not possible to classify degenerative dementia into subtypes because of the data constraints, thus there is notable heterogeneity within this group. Fifth, although our analysis attempted to control for the severity of comorbidities, it is possible that there are residual confounding factors due to medical illness. Finally, the overall patterns of association between dementia and mortality for our population also reflect the organization of care for older people with dementia in Taiwan. The experience in other settings may be different if the care for this population is organized differently.
Conclusion
The strength of our study is that we described the mortality of a large, nationwide population-based cohort of older adults with incident dementia, which enabled us to evaluate the effects of anti-dementia and nootropic treatments on mortality. Families of participants newly diagnosed with dementia often ask the physicians how long people commonly live with their treatment conditions. Our data revealed the importance of early treatment of dementia. In particular, combination treatment with anti-dementia and nootropics may prolong the life of patients with dementia. It provide a balanced response based on data from representative population-based samples, and should be relevant to health care planning and policy making for the growing number of individuals with dementia.
Acknowledgments
We thank the Medical Science & Technology Building of Taipei Veterans General Hospital for providing us with the experimental space and facilities.
Data Availability
The data are available from the Taiwan National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare, and managed by National Health Research Institutes for researchers who meet the criteria for access to confidential data. Applicants must follow the Computer-Processed Personal Data Protection Law and related regulations of BNHI (Bureau of National Health Insurance) and NHRI (National Health Research Institutes), and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed by NHRI.
Funding Statement
This work was supported by grant number NSC 98-2314-B-075-029 from the National Science Council, Taiwan, and grant number VGH V103C–201 from Taipei Veterans General Hospital, Taipei, Taiwan.
References
- 1. Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366(9503):2112–7. 10.1016/S0140-6736(05)67889-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xie J, Brayne C, Matthews FE, Medical Research Council Cognitive F, Ageing Study c. Survival times in people with dementia: analysis from population based cohort study with 14 year follow-up. Bmj. 2008;336(7638):258–62. 10.1136/bmj.39433.616678.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garcia-Ptacek S, Farahmand B, Kareholt I, Religa D, Cuadrado ML, Eriksdotter M. Mortality Risk after Dementia Diagnosis by Dementia Type and Underlying Factors: A Cohort of 15,209 Patients based on the Swedish Dementia Registry. Journal of Alzheimer's disease: JAD. 2014;41(2):467–77. 10.3233/JAD-131856 . [DOI] [PubMed] [Google Scholar]
- 4. Helmer C, Joly P, Letenneur L, Commenges D, Dartigues JF. Mortality with dementia: results from a French prospective community-based cohort. American journal of epidemiology. 2001;154(7):642–8. . [DOI] [PubMed] [Google Scholar]
- 5. Ganguli M, Dodge HH, Shen C, Pandav RS, DeKosky ST. Alzheimer disease and mortality: a 15-year epidemiological study. Archives of neurology. 2005;62(5):779–84. 10.1001/archneur.62.5.779 . [DOI] [PubMed] [Google Scholar]
- 6. Lee M, Chodosh J. Dementia and life expectancy: what do we know? Journal of the American Medical Directors Association. 2009;10(7):466–71. 10.1016/j.jamda.2009.03.014 . [DOI] [PubMed] [Google Scholar]
- 7. Wolfson C, Wolfson DB, Asgharian M, M'Lan CE, Ostbye T, Rockwood K, et al. A reevaluation of the duration of survival after the onset of dementia. The New England journal of medicine. 2001;344(15):1111–6. 10.1056/NEJM200104123441501 . [DOI] [PubMed] [Google Scholar]
- 8. Matsui Y, Tanizaki Y, Arima H, Yonemoto K, Doi Y, Ninomiya T, et al. Incidence and survival of dementia in a general population of Japanese elderly: the Hisayama study. Journal of neurology, neurosurgery, and psychiatry. 2009;80(4):366–70. 10.1136/jnnp.2008.155481 . [DOI] [PubMed] [Google Scholar]
- 9. Di Santo SG, Prinelli F, Adorni F, Caltagirone C, Musicco M. A meta-analysis of the efficacy of donepezil, rivastigmine, galantamine, and memantine in relation to severity of Alzheimer's disease. Journal of Alzheimer's disease: JAD. 2013;35(2):349–61. 10.3233/JAD-122140 . [DOI] [PubMed] [Google Scholar]
- 10. Molino I, Colucci L, Fasanaro AM, Traini E, Amenta F. Efficacy of memantine, donepezil, or their association in moderate-severe Alzheimer's disease: a review of clinical trials. TheScientificWorldJournal. 2013;2013:925702 10.1155/2013/925702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rountree SD, Atri A, Lopez OL, Doody RS. Effectiveness of antidementia drugs in delaying Alzheimer's disease progression. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9(3):338–45. 10.1016/j.jalz.2012.01.002 . [DOI] [PubMed] [Google Scholar]
- 12. Lanni C, Lenzken SC, Pascale A, Del Vecchio I, Racchi M, Pistoia F, et al. Cognition enhancers between treating and doping the mind. Pharmacological research: the official journal of the Italian Pharmacological Society. 2008;57(3):196–213. 10.1016/j.phrs.2008.02.004 . [DOI] [PubMed] [Google Scholar]
- 13. Birks J, Grimley Evans J. Ginkgo biloba for cognitive impairment and dementia. The Cochrane database of systematic reviews. 2009;(1):CD003120 10.1002/14651858.CD003120.pub3 . [DOI] [PubMed] [Google Scholar]
- 14. Waegemans T, Wilsher CR, Danniau A, Ferris SH, Kurz A, Winblad B. Clinical efficacy of piracetam in cognitive impairment: a meta-analysis. Dementia and geriatric cognitive disorders. 2002;13(4):217–24. doi: 57700. . [DOI] [PubMed] [Google Scholar]
- 15. Chou YJ, Huang N, Lin IF, Deng CY, Tsai YW, Chen LS, et al. Do physicians and their relatives have a decreased rate of cesarean section? A 4-year population-based study in Taiwan. Birth. 2006;33(3):195–202. 10.1111/j.1523-536X.2006.00104.x . [DOI] [PubMed] [Google Scholar]
- 16. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. Journal of clinical epidemiology. 1993;46(10):1075–9; discussion 81–90. . [DOI] [PubMed] [Google Scholar]
- 17. Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, et al. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. Journal of internal medicine. 2014;275(3):251–83. 10.1111/joim.12191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Atri A, Rountree SD, Lopez OL, Doody RS. Validity, significance, strengths, limitations, and evidentiary value of real-world clinical data for combination therapy in Alzheimer's disease: comparison of efficacy and effectiveness studies. Neuro-degenerative diseases. 2012;10(1–4):170–4. 10.1159/000335156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Atri A, Shaughnessy LW, Locascio JJ, Growdon JH. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer disease and associated disorders. 2008;22(3):209–21. 10.1097/WAD.0b013e31816653bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lopez OL, Becker JT, Wahed AS, Saxton J, Sweet RA, Wolk DA, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. Journal of neurology, neurosurgery, and psychiatry. 2009;80(6):600–7. 10.1136/jnnp.2008.158964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Geldmacher DS, Provenzano G, McRae T, Mastey V, Ieni JR. Donepezil is associated with delayed nursing home placement in patients with Alzheimer's disease. Journal of the American Geriatrics Society. 2003;51(7):937–44. . [DOI] [PubMed] [Google Scholar]
- 22. Bond M, Rogers G, Peters J, Anderson R, Hoyle M, Miners A, et al. The effectiveness and cost-effectiveness of donepezil, galantamine, rivastigmine and memantine for the treatment of Alzheimer's disease (review of Technology Appraisal No. 111): a systematic review and economic model. Health technology assessment. 2012;16(21):1–470. 10.3310/hta16210 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopez OL, Becker JT, Wisniewski S, Saxton J, Kaufer DI, DeKosky ST. Cholinesterase inhibitor treatment alters the natural history of Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 2002;72(3):310–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rountree SD, Chan W, Pavlik VN, Darby EJ, Doody RS. Factors that influence survival in a probable Alzheimer disease cohort. Alzheimer's research & therapy. 2012;4(3):16 10.1186/alzrt119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vidal JS, Lacombe JM, Dartigues JF, Pasquier F, Robert P, Tzourio C, et al. Memantine therapy for Alzheimer disease in real-world practice: an observational study in a large representative sample of French patients. Alzheimer disease and associated disorders. 2008;22(2):125–30. 10.1097/WAD.0b013e31815a9e10 . [DOI] [PubMed] [Google Scholar]
- 26. Wattmo C, Londos E, Minthon L. Risk Factors That Affect Life Expectancy in Alzheimer's Disease: A 15-Year Follow-Up. Dementia and geriatric cognitive disorders. 2014;38(5–6):286–99. 10.1159/000362926 . [DOI] [PubMed] [Google Scholar]
- 27. Gasper MC, Ott BR, Lapane KL. Is donepezil therapy associated with reduced mortality in nursing home residents with dementia? The American journal of geriatric pharmacotherapy. 2005;3(1):1–7. . [DOI] [PubMed] [Google Scholar]
- 28. Froestl W, Muhs A, Pfeifer A. Cognitive enhancers (nootropics). Part 1: drugs interacting with receptors. Journal of Alzheimer's disease: JAD. 2012;32(4):793–887. 10.3233/JAD-2012-121186 . [DOI] [PubMed] [Google Scholar]
- 29. Meguro K, Kasai M, Akanuma K, Meguro M, Ishii H, Yamaguchi S. Donepezil and life expectancy in Alzheimer's disease: a retrospective analysis in the Tajiri Project. BMC neurology. 2014;14:83 10.1186/1471-2377-14-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nordstrom P, Religa D, Wimo A, Winblad B, Eriksdotter M. The use of cholinesterase inhibitors and the risk of myocardial infarction and death: a nationwide cohort study in subjects with Alzheimer's disease. European heart journal. 2013;34(33):2585–91. 10.1093/eurheartj/eht182 . [DOI] [PubMed] [Google Scholar]
- 31. Wattmo C, Londos E, Minthon L. Response to cholinesterase inhibitors affects lifespan in Alzheimer's disease. BMC neurology. 2014;14:173 10.1186/s12883-014-0173-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fang Y, Qiu Z, Hu W, Yang J, Yi X, Huang L, et al. Effect of piracetam on the cognitive performance of patients undergoing coronary bypass surgery: A meta-analysis. Experimental and therapeutic medicine. 2014;7(2):429–34. 10.3892/etm.2013.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazza M, Capuano A, Bria P, Mazza S. Ginkgo biloba and donepezil: a comparison in the treatment of Alzheimer's dementia in a randomized placebo-controlled double-blind study. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2006;13(9):981–5. 10.1111/j.1468-1331.2006.01409.x . [DOI] [PubMed] [Google Scholar]
- 34. Attems J, Jellinger KA. The overlap between vascular disease and Alzheimer's disease—lessons from pathology. BMC medicine. 2014;12:206 10.1186/s12916-014-0206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin RT, Lai CL, Tai CT, Liu CK, Yen YY, Howng SL. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. Journal of the neurological sciences. 1998;160(1):67–75. . [DOI] [PubMed] [Google Scholar]
- 36. Liu HC, Lin KN, Teng EL, Wang SJ, Fuh JL, Guo NW, et al. Prevalence and subtypes of dementia in Taiwan: a community survey of 5297 individuals. Journal of the American Geriatrics Society. 1995;43(2):144–9. . [DOI] [PubMed] [Google Scholar]
- 37. Sun Y, Lee HJ, Yang SC, Chen TF, Lin KN, Lin CC, et al. A nationwide survey of mild cognitive impairment and dementia, including very mild dementia, in Taiwan. PloS one. 2014;9(6):e100303 10.1371/journal.pone.0100303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koopmans RT, Ekkerink JL, van Weel C. Survival to late dementia in Dutch nursing home patients. Journal of the American Geriatrics Society. 2003;51(2):184–7. . [DOI] [PubMed] [Google Scholar]
- 39. Nilsson K, Gustafson L, Hultberg B. Survival in a large elderly population of patients with dementia and other forms of psychogeriatric diseases. Dementia and geriatric cognitive disorders. 2011;32(5):342–50. 10.1159/000335728 . [DOI] [PubMed] [Google Scholar]
- 40. Belloni-Sonzogni A, Tissot A, Tettamanti M, Frattura L, Spagnoli A. Mortality of demented patients in a geriatric institution. Archives of gerontology and geriatrics. 1989;9(2):193–7. . [DOI] [PubMed] [Google Scholar]
- 41. Hogan DB, Thierer DE, Ebly EM, Parhad IM. Progression and outcome of patients in a Canadian dementia clinic. The Canadian journal of neurological sciences Le journal canadien des sciences neurologiques. 1994;21(4):331–8. . [DOI] [PubMed] [Google Scholar]
- 42. Waring SC, Doody RS, Pavlik VN, Massman PJ, Chan W. Survival among patients with dementia from a large multi-ethnic population. Alzheimer disease and associated disorders. 2005;19(4):178–83. . [DOI] [PubMed] [Google Scholar]
- 43. Kane MN. Awareness of ageism, motivation, and countertransference in the care of elders with Alzheimer's disease. American journal of Alzheimer's disease and other dementias. 2002;17(2):101–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jolley DJ, Benbow SM. Stigma and Alzheimer's disease: causes, consequences and a constructive approach. International journal of clinical practice. 2000;54(2):117–9. . [PubMed] [Google Scholar]
- 45. Jin YP, Gatz M, Johansson B, Pedersen NL. Sensitivity and specificity of dementia coding in two Swedish disease registries. Neurology. 2004;63(4):739–41. . [DOI] [PubMed] [Google Scholar]
- 46. Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, et al. Antipsychotic drug use and mortality in older adults with dementia. Annals of internal medicine. 2007;146(11):775–86. . [DOI] [PubMed] [Google Scholar]
- 47. Langballe EM, Engdahl B, Nordeng H, Ballard C, Aarsland D, Selbaek G. Short- and long-term mortality risk associated with the use of antipsychotics among 26,940 dementia outpatients: a population-based study. The American journal of geriatric psychiatry: official journal of the American Association for Geriatric Psychiatry. 2014;22(4):321–31. 10.1016/j.jagp.2013.06.007 . [DOI] [PubMed] [Google Scholar]
- 48. Rossom RC, Rector TS, Lederle FA, Dysken MW. Are all commonly prescribed antipsychotics associated with greater mortality in elderly male veterans with dementia? Journal of the American Geriatrics Society. 2010;58(6):1027–34. 10.1111/j.1532-5415.2010.02873.x . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are available from the Taiwan National Health Insurance Research Database provided by the National Health Insurance Administration, Ministry of Health and Welfare, and managed by National Health Research Institutes for researchers who meet the criteria for access to confidential data. Applicants must follow the Computer-Processed Personal Data Protection Law and related regulations of BNHI (Bureau of National Health Insurance) and NHRI (National Health Research Institutes), and an agreement must be signed by the applicant and his/her supervisor upon application submission. All applications are reviewed by NHRI.


