Abstract
Adenosine regulates the interaction between lymphocytes and the vasculature and is important for controlling lymphocyte trafficking in response to tissue injury or infection. Adenosine can blunt the effects of T cell receptor (TCR) activation primarily by activating adenosine A2A receptors (A2AR) and signaling via cyclic AMP and protein kinase A (PKA). PKA reduces proximal TCR signaling by phosphorylation of C-terminal Src kinase (Csk), nuclear factor of activated T cells (NF-AT) and cyclic AMP response element binding protein (CREB). PKA activation can either enhance or inhibit the survival of T cells depending on the strength and duration of signaling. Inducible enzymes such as CD73 and CD39 regulate adenosine formation and degradation in vivo. The extravasation of lymphocytes through blood vessels is influenced by A2AR-mediated suppression of Intercellular Adhesion Molecule 1 (ICAM) expression on lymphocytes and diminished production of IFNγ and IFNγ-inducible chemokines that are chemotactic to activated lymphocytes. Adenosine also decreases the barrier function of vascular endothelium by activating A2BRs. In sum, adenosine signaling is influenced by tissue inflammation and injury through induction of receptors and enzymes and has generally inhibitory effects on lymphocyte migration into inflamed tissues due to PKA-mediated effects on adhesion molecules, IFNγ production and endothelial barrier function.
Keywords: adenosine, lymphocytes, T cells
Introduction
In addition to playing a central role in biochemical processes, adenosine is a generally anti-inflammatory1, 2 signaling molecule that is produced by all cells in proportion to metabolic activity, injury and hypoxia. Adenosine signaling is mediated by four G-protein coupled adenosine receptors (AR): A1, A2A, A2B and A33. These receptors are antagonized by naturally occurring and widely consumed methylxanthines, caffeine and theophylline, as well as by more potent synthetic antagonists3, 4. Adenosine produced as a byproduct of metabolic activity readily crosses most cell membranes on nucleoside transporters5. Extracellular adenosine is produced from the degradation of adenine nucleotides by exonucleases. ATP and ADP are converted to AMP and adenosine following nucleotide release to the extracellular space through membrane channels6, necrotic cell death, or as granular components of platelets, mast cells and neuronal synaptic granules.
The A2AR is the predominant adenosine receptor subtype found on lymphocytes. Stimulation of A2ARs on activated T cells acutely inhibits pro-inflammatory cytokine production and effector functions7, 8. In addition, both A2A and A2B receptors are found on antigen presenting cells (APCs) and strongly influence T cell activation. This review focuses on recent advances in our understanding of lymphocyte activation and the interaction of lymphocytes with the vasculature by enzymes that regulate adenosine metabolism, adenosine receptors, and cyclic AMP signaling.
Lymphocyte Activation
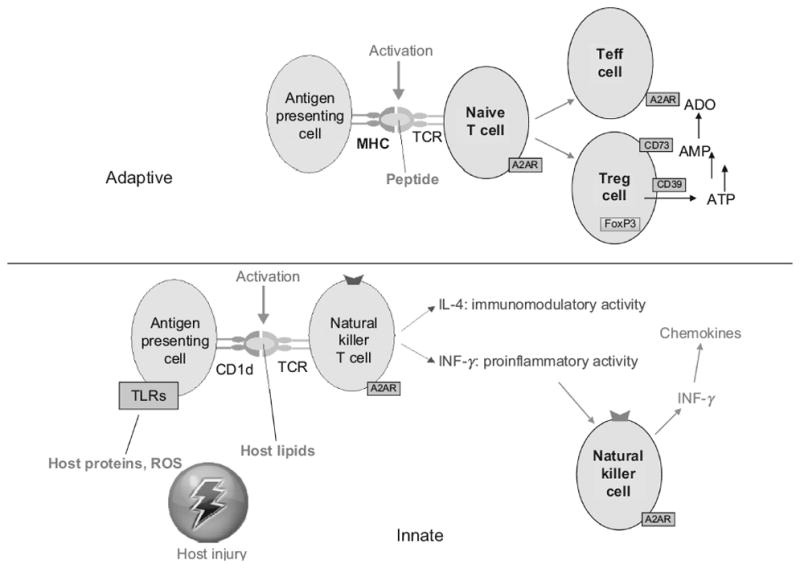
T cells can be activated as a result of antigen presentation by antigen presenting cells (APCs) such as dendritic cells (DCs) or macrophages. Antigenic molecules are displayed on the surface of APCs by major histocompatibility proteins (MHC) and activate T cell receptors on lymphocytes (Figure 1). Following TCR stimulation, lymphocyte activation can result in T cell differentiation, cytokine production, or cytotoxic activity. Antigens are presented by APCs to the ligand-binding portion (αβ subunits) of T cell receptors (Figure 1). TCR activation, known as “signal 1”, is transduced through γ,δ,ε and ζ chains of the CD3 portion of the TCR. Following stimulation, TCR signal transduction is initiated by lymphocyte-specific protein tyrosine kinase (Lck) phosphorylation of tyrosines on immunoreceptor tyrosine-based activation motifs (ITAMs) present in the tails of CD3 components. These phosphorylated residues provide docking sites for the SH2 domains of zeta-chain-associated protein kinase 70 (ZAP-70), which dock and phosphorylate tyrosines on Linker of Activated T cells (LAT). In the case of a small subset of lymphocytes known as invariant natural killer T (iNKT) cells, lipid antigens replace peptide antigens on APCs, and MHC is replaced by CD1 antigen presenting molecules9. Below we discuss how T cell activation influences lymphocyte adhesion to the endothelium and the production of chemotactic chemokines.
Figure 1. Cyclic AMP signaling inhibits TCR and CD28 signaling in lymphocytes.
Cyclic AMP accumulates in T cells in the region of lipid rafts in response to TCR activation, and more globally in response to strong Gs-coupled A2AR activation. Cyclic AMP inhibits proximal TCR signaling through a pathway involving activation of protein kinase A-1 (PKA-1) and C-terminal Src kinase (Csk) to inhibit lymphocyte-specific protein kinase (Lck) and to reduce recruitment to CD3 of zeta-chain-associated protein kinase 70 (zap-70). PKA-1 also phosphorylates (indicated by red dots) and inhibits NF-AT. NF-AT activation is reversed by the Ca2+-calmodulin-dependent phosphatase, calcineurin. TCR-induced accumulation of cAMP near lipid rafts is reduced upon CD28 stimulation due to the activation of phosphatidylinositol-3-kinase (PI3-K) to produce PIP3. This results in translocation from the cytosol to the lipid raft of a complex consisting of AKT, PDE4 and β-arrestin (β-arr) by binding of the plextrin homology (PH) domain of AKT to PIP3. PDE4 degrades cAMP to relieve inhibition of TCR signaling. Abbreviations: A2AR, adenosine A2A receptor; Ado, adenosine; Ino, inosine; ADA, adenosine deaminase; αsβγ, subunits of the heterotrimeric G protein, Gs; AC, adenylyl cyclase; AKT, a serine/threonine-specific protein kinase, also known as protein kinase B; PIP3, phosphatidylinositol (3,4,5)-triphosphate; PDE4, type 4 phosphodiesterase; MHC, major histocompatibility complex; Ag, antigen; RhoH, Ras Homolog, a small GTP hydrolyzing protein; AKAP, A kinase anchor protein; TCR, T cell receptor; LAT, linker for activation of T cells; PLC, phospholipase C; PKC, protein kinase C; CaM, calmodulin; NF-AT, nuclear factor of activated T cells; CREB, cAMP response element-binding protein.
Adenosine A2AR activation selectively inhibits cytokine production by T cell subsets
Depending on the environment during antigen encounter, CD4+ T cells can differentiate into T helper 1 cells (Th1) that secrete primarily type 1 cytokines including interleukin 2 (IL-2), interferon γ (IFN-γ) and TNFα; T helper 2 cells (Th2) that secrete primarily IL-4, IL-5, and IL-10; or Th17 cells that secrete IL-17A, IL-17F and IL-21. Type 1/type 2 cytokine polarization exists for both MHC class 2-restricted CD4+ T cells (Th1/Th2 subsets) and for MHC class 1-restricted cytotoxic CD8+ T cells (Tc1/Tc2 subsets). Agonist binding to A2ARs activates the heterotrimeric G protein, Gs, to catalyze cyclic AMP production. The protein kinase A (PKA) pathway negatively regulates the production of type 1 cytokines, with lessor effects on type 2 cytokines10. A2AR activation reduces production of IL-2, TNFα and IFNγ secretion from Tc1 and Tc2 cells, but does not affect IL-4 or IL-5 secretion11. A2AR activation also strongly inhibits the production of IFNγ by iNKT cells.12 A2AR activation has not been reported to directly influence cytokine release from purified Th-17 cells. When given in vivo or in mixed cell T cell development assays with antigen presenting cells, A2A agonists inhibit production of IL-6 and enhance production of IL-10. This results in indirect inhibition of Th1, Th2 and Th17 effector cell development.13, 14. In sum, the strongest direct effects of A2AR stimulation on lymphocytes is on type 1 cytokine production by Th1, Tc1 and iNKT cells8, 11, 12.
How Cyclic AMP and PKA mediate A2AR signaling in T cells
The activation of Gs following agonist binding to A2ARs stimulates adenylyl cyclase to produce cyclic AMP. Two downstream effectors, protein kinase A (PKA) and Exchange protein directly activated by cyclic AMP (Epac), are the principal mediators of cyclic AMP action in T cells. The cyclic AMP mimetic, 8-(4-chlorophenylthio)adenosine-3′,5′-cyclic monophosphate (8-CPT-cAMP) is a useful tool for distinguishing between these two pathways because it activates PKA but fails to activate Epac. Experiments using 8-CPT-cAMP indicate that most transcriptional effects of cyclic AMP in T cells are mediated by PKA. In addition to adenosine, several other Gs coupled receptors are found on T cells. These include β2-adrenergic15, prostaglandin E2 (PGE2), dopamine D116 and vasoactive intestinal peptide (VIP)17. Adenosine is particularly important for limiting lymphocyte activation because A2ARs are induced upon activation of T cells8, 18 and iNKT cells19. Cyclic AMP is degraded in T cells primarily by phosphodiesterase 4 (PDE4) and PDE4 inhibitors facilitate the actions of adenosine and other Gs-coupled receptor agonists.
Cyclic AMP regulates T cell cytokine secretion and proliferation by directly phosphorylating the transcription factors cAMP response element binding protein (CREB) and nuclear factor of activated T cells (NF-AT).20 Suppression of proximal T cell signaling pathways indirectly inhibits activation of another transcription factor, nuclear factor kappa B (NF-κB). The most abundant isoform of PKA found in T cells, PKA-1, activates C-terminal Src kinase (Csk), which inhibits the Src family tyrosine kinases Lck and Fyn and thus functions to check T cell activation (Figure 1). PKA-1 is targeted to the TCR-CD3 complex during T-cell activation via an A-kinase-anchoring protein (AKAP) that serves as a scaffold for the cAMP-PKA/Csk pathway in lipid rafts of the T cell plasma membrane. The small GTP binding protein RhoH also serves as an adaptor molecule for Lck and Zap-70 to regulate TCR signaling21. Protein kinase C theta (PKCθ) and PKA inversely affect cytokine expression, whereas other PKC isotypes do not influence TCR signaling. The opposing cAMP/PKA and PKCθ pathways converge at the level of NF-AT22. NF-AT proteins are retained in the cytoplasm following serine phospyorylation by PKA. After T cell activation, NF-AT proteins are dephosphorylated by the Ca2+-calmodulin activated protein phosphatase calcineurin. This dephosphorylation unmasks a nuclear localization signal, facilitating the rapid translocation of NF-AT proteins to the nucleus where they pair with AP-1 and bind to consensus NF-AT sites on DNA. The immunosuppressive drugs cyclosporin A and FK506 prevent the calcineurin-mediated dephosphorylation of NF-AT, accounting for some of their immunosuppressive effects on T cells. PKA also regulates T cell function at the level of other transcription factors and kinases including members of the mitogen-activated protein kinase pathway, RhoA and proteins involved in the control of cell cycle progression.23
Signal 2 and cAMP
Signal 1 activation of TCRs alone produces limited T cell activation because TCR engagement locally enhances cyclic AMP production and activates Csk in the region of the immunologic synapse. Activation of TCRs is amplified by signal 2, i.e. co-stimulation of CD28 by ligands expressed on the surface of APCs, B7.1 and B7.2 (CD80 and CD86). Upon TCR/CD28 co-stimulation PI3K activation leads to phosphatidylinositol-(3,4,5)-triphosphate (PIP3) production. This stimulates recruitment of an AKT/β-arrestin/PDE4 complex to the plasma membrane via the AKT plextrin homology (PH) domain, resulting in the degradation of cyclic AMP located near lipid rafts24, 25 It is not entirely clear how stimulation of the TCR results in elevated cAMP levels26, but recruitment of Gs to lipid rafts may be involved27. It is also possible that cell activation due to TCR signaling stimulates production of adenosine that exits the cell to act on autocrine or paracrine A2A receptors.
Adenosine signaling increases T cell tolerance and Treg development
Unlike the localized production of cyclic AMP that occurs as a result of signal 1, strong activation of A2ARs or other Gs-coupled receptors can produce whole cell increases in cyclic AMP that are not limited just to the region of lipid rafts. Thus, extracellular adenosine reduces the activation of T cells by APCs and modifies T cell differentiation, cytokine production and proliferation by preventing rapid tyrosine phosphorylation of ZAP-70 and downstream signaling such as activation of Akt and ERK1/2.28 Cyclic AMP elevation in naïve T cells also favors development of a regulatory phenotype (Treg) characterized by high expression of CD25, cytotoxic-T-lymphocyte-associated protein 4 (CTLA4), and Forkhead box protein 3 (FoxP3). CTLA4 is involved in suppressive activities by Tregs.29 Unlike T effector cells, Tregs also express ecto-enzymes CD39 and CD73 (Table 1) that metabolize adenine nucleotides in the extracellular space to adenosine that locally inhibits the activation of effector T cells and APCs.7
Table 1.
Enzymes involved in adenosine transport or metabolism
| Human gene | Protein | alias | substrate | Human gene location |
|---|---|---|---|---|
| ADA | ADA | Ado/2-deoxyado | 20q13.12 | |
| ADK | ADK | Ado/nucleosides | 10q22.2 | |
| DPP4 | DPP4 | CD26 | ADA binding | 2q24.2 |
| ENTPD1 | ENTP1 | CD39 | ATP/ADP | 10q24.1 |
| ENTPD2 | ENTP2 | CD39L1 | ATP/GTP | 9q34.3 |
| ENTPD3 | ENTP3 | CD39L3 | ATP/ADP | 3p22.1 |
| NT5E | 5NTD | CD73 | AMP nucleotides | 6q14.3 |
| SLC29A(1-4) | S29A(1-4) | ENT(1-4) | ado | 6p21.1/11q13/10q22.1/7p22.1 |
| SLC28A(1-3) | S28A(1-3) | CNT(1-3) | ado | 15q25.3/15q15/9q21.33 |
ADA, adenosine deaminase; ADK, adenosine kinase; Ado, adenosine; SLC28, solute carrier family 28 (sodium-coupled nucleoside transporter); ENTPD, ectonucleoside triphosphate diphosphohydrolase; DPP, dipeptidyl-peptidase; SLC29, solute carrier family 29 (nucleoside transporter); ENT, equilibrative nucleoside transporter; 5NTD, 5′ nucleotidase.
Paradoxical effects of PKA on T cells survival
Inhibition of apoptosis
Activation-induced cell death (AICD) describes an apoptotic program initiated by restimulation of previously activated peripheral T cells. A2AR activation reduces AICD in mouse CD4+ hybridomas and human Jurkat cells.30 A2AR activation reduces AICD by interfering with the production of factors that stimulate T cell activation, IL-2-and the downstream expression of the co-stimulatory molecules CD2 and CD28.31
Enhancement of apoptosis
In contrast to the anti-apoptotic effect of transient cyclic AMP elevation, prolonged elevation of cyclic AMP triggers T cell apoptosis. This property of persistent cyclic AMP elevation to kill T cells has been used to select for T cell lines that have mutations in the cyclic AMP signaling pathway32. Recent studies have identified the mechanism by which cyclic AMP triggers an apoptotic program in T cells. Treatment of wild type S49 T cells with the PKA activating cyclic AMP analog, 8-CPT-cAMP, increases the expression of cytotoxic T lymphocyte antigen-2α (CTLA-2α), a cathepsin L-like cysteine protease inhibitor that triggers apoptosis33. Treatment of kinase- S49 cells T cells that lack functional PKA, with 8-CPT-cAMP fails to stimulate CTLA-2α expression and apoptosis, indicating that the increase in CTLA-2α in wild type S49 cells is PKA-dependent34,35.
Effects of adenosine deaminase deficiency on T cell survival
Several investigators have sought to determine if PKA-mediated killing of T cells contributes to severe combined immunedeficiency (SCID) that occurs in individuals lacking adenosine deaminase (ADA). Since ADA deficiency causes adenosine concentrations to increase in the thymus and other tissues, it is reasonable to suspect that the resulting increase in A2AR signaling via adenosine, Gs and PKA in thymocytes might evoke T cell killing by PKA-induced apoptosis. Apasov et al36 concluded that a portion of thymocyte apoptosis that occurs in response to ADA deficiency can be attributed to A2AR activation. However, the primary cause of toxicity in developing human thymocytes is the accumulation of deoxyATP which triggers mitochondrial-dependent apoptosis.37
CD26 dampens adenosine signaling in T cells
In human cells a soluble form of adenosine deaminase (ADA) can bind to cell surface CD26 that is expressed by lymphocytes, epithelial cell and capillary endothelial cells. CD26 expression is strongly up regulated following T cell activation42. ADA binding to CD26 on human T cells results in ADA accumulation on the T-cell surface and is associated with T cell activation.43
Effects of adenosine metabolism and transport on lymphocyte activation
The concentration of adenosine in the extracellular space is regulated by adenosine transport as well as adenosine formation and degradation (Table 1). Nucleoside transporters are divided into two families; the Na+-dependent solute carrier family 28 (SLC28) and the equilibrative solute carrier family 29 (SLC29). SLC28 family transporters (CNT1-3) display subtype-selective expression patterns; CNT1 is localized primarily to epithelial tissues whereas CNT2 and CNT3 have more widespread distributions. SLC29 family transporters (ENTP1-4) are glycosylated proteins localized to the plasma and mitochondrial membranes. They are expressed in the heart, brain, mammary gland, erythrocytes and placenta, and also in fetal liver and spleen, and mediate nucleoside influx and efflux. Insulin and glucose induce changes in expression levels of nucleoside transporters in T lymphocytes.38
Lymphocyte CD39 and CD73 and immune regulation
T regs comprise a subset of T cells that inhibit the activation of effector T cells. CD39 (ENTP1) and CD73 (5NTD) are coexpressed on the surface of murine T regulatory but not effector cells, and together generate extracellular adenosine from ATP, ADP and AMP. Murine T regulatory cells are usually defined based on expression of CD4, CD25, and the transcription factor, FoxP3. However, these markers are not sufficient to uniquely define T cell subsets in humans. Liu et al39 found that the IL-7 receptor (CD127) is low on a subset of CD4+ T cells in peripheral human blood. CD39, independently of CD73, is expressed on human CD4+ CD25+ CD127lo Tregs, also characterized by high expression of Foxp3. A distinct population of human CD4+ CD39+ T lymphocytes does not express CD25 and FoxP3. The latter cells secrete proinflammatory cytokines such as IFNγ and IL-17. These cells are increased, with a concomitant decrease in Tregs, in the peripheral blood of patients exhibiting transplant rejection. Hence, CD39 may be a useful marker for the success of organ transplantation.40 Immunodeficiency from HIV is associated with a significant increase in CD39 expression on human T regs41. Treg inhibitory effects are enhanced by CD39 up regulation, and are replicated by activation of A2ARs on HIV patient T cells. A2ARs are expressed at higher than normal levels on the T cells of HIV patients. The expansion of CD39+ Treg cells correlates with the level of immune activation and low CD4+ T cell counts in HIV. A genetic association study identified a CD39 gene polymorphism that is associated with down-regulation of CD39 expression and slower progression to AIDS41.
Adenosine regulation of APCs
Independent of the effects of adenosine on T cells, dendritic cells and macrophages are highly susceptible to adenosine-mediated regulation. DCs and macrophages activated by LPS in the presence of adenosine have a reduced capacity to induce Th1 polarization of naive CD4+ T lymphocytes, diminished release TNFα and IL-12, and enhanced release of anti-inflammatory IL-1044–48. Inhibition of adenosine receptor signaling is needed to observe optimal activation of DC’s and T cell activation by pathogen-associated molecular patterns49, 50. DCs express all four types of adenosine receptors but their expression level varies depending on subtype, maturation status or progenitors from which they differentiate51–55. Immature DCs express A1 and A3 adenosine receptors52–54 that are thought to mediate chemotaxis53. In LPS-matured DCs A1 and A3 receptors are down regulated while A2A and A2B receptors are up regulated51–55. A2BR expression can be further induced by hypoxia51 or TNFα56. A2BR activation also inhibits DC-mediated T cell activation because A2BR stimulation reduces LPS-induced surface expression of MHCII and CD86 which results in decreased IL-2 expression by T cells 57. However, activation of the A2BR can be pro-inflammatory. In the absence of TLR signaling A2BR stimulation increases pro-inflammatory IL-6, which together with TGFβ can deviate naïve CD4+ T cells to a Th17 phenotype that favors chronic inflammation58.
Interface between lymphocytes and the Vasculature
Adenosine and ischemia
Vascular diseases, infections or tissue injury can lead to vaso-occlusion and tissue hypoxia that strongly influences adenosine signaling to immune cells. Part of this effect is due to inhibition of adenosine kinase in hypoxic cells, resulting in an increase in the cellular accumulation of adenosine59. In addition, hypoxia inducible factor (HIF) drives induction of immunosuppressive A2B receptors on APCs60 and CD73 on epithelial and endothelial cells61, 62. Elevated levels of CD73 produce elevated tissue and blood levels of adenosine due to enhanced conversion of AMP to adenosine.
Adenosine and ICAM-1
Tissue damage due to trauma or infection produces an inflammatory cascade resulting in chemotaxis into the inflamed tissue of lymphocytes and other leukocytes. Adenosine can inhibit this process. Part of the effect of adenosine has been attributed to inhibition of expression of intercellular adhesion molecule 1 (ICAM-1).63 ICAM-1 is expressed by T cells and can bind to macrophage adhesion ligand-1 (Mac-1), leukocyte function associated antigen-1 (LFA-1), and fibrinogen, all of which are expressed on endothelial cells and other leukocytes.
Adenosine and IFNγ-inducible chemokines
Chemokines contribute to lymphocyte extravasation into inflamed tissues. Tissue damage results in the activation of invariant natural killer T (iNKT) cells that rapidly produce large amounts of IFN-γ upon activation12. Widespread tissue damage and iNKT cell activation is produced in sickle cell anemia by rigid red cells that cause widespread microvascular occlusion and ischemia. NY1DD mice with sickle cell anemia have increased activation of iNKT cells, and high tissue levels of IFNγ and IFNγ-inducible chemokines (CXCL9, CXCL10 and CXCL11) that are chemotactic to activated lymphocytes that express CXCR364. Treating NY1DD mice with anti-CD1d antibody to inhibit CD1d-restricted iNKT cell activation reverses pulmonary dysfunction and blocks the accumulation of activated leukocytes. Neutralization of CXCR3 receptors also ameliorates pulmonary dysfunction64. Infusion of an A2A receptor agonist into NY1DD mice blocks iNKT IFNγ production, lung inflammation and lung injury8. CXCR3 also regulates NK- and T-cell trafficking during sepsis, and blockade of CXCR3 attenuates the pathogenesis of septic shock65. A2AR activation also can reduce inflammation and improve survival of mice with sepsis66.
Effects of CD73 on lymphocyte migration into lymph nodes
CD73 is expressed on the cell surface of endothelial cells. After an inflammatory stimulus, lymphocyte migration into draining lymph nodes increases dramatically to facilitate the encounter of naive T cells with antigen-loaded dendritic cells. Cd73-/- mice have 2.5-fold increased rates of L-selectin-dependent lymphocyte migration from the blood through high endothelial venules compared with wild-type mice after LPS administration67. The endothelial A2BR is a likely target of CD73-generated adenosine and inhibits the adhesion of lymphocytes67 and other leukocytes68, 69.
Effects of CD73 on allograft survival
In a heterotopic cardiac allotransplantation model, CD73 deficiency in either donor or recipient mice results in decreased graft survival and development of cardiac allograft vasculopathy, suggesting a contribution of CD73 on both graft-resident and circulating cells in preventing vasculopathy70. Lack of CD73 results in loss of cardiac graft barrier function and diminished graft expression of A2BR mRNA, with a concordant exacerbation of acute inflammatory and immune responses. Antagonism of the A2BR causes a significant increase in vascular leakage, and activation of A2BRs results in prolongation of graft survival and suppression of cardiac allograft vasculopathy. In another model, implantation of tracheal allografts from wild type mice into CD73-/- recipients caused a large increase in airway luminal obliteration that was associated with an increase in CD3+ lymphocytic infiltration71. The protective effect of CD73 was attributed to generation of adenosine and stimulation of the A2AR. Treatment of WT recipients with an A2AR agonist significantly reduced CD3+ lymphocyte infiltration and airway luminal obliteration; similar treatment of CD73-/- recipients rescued them from rejection. These data implicate CD73 acting through adenosine generation and its stimulation of A2A and A2B receptors as inhibitors of lymphocyte recruitment into allografts. In allo-mismatched in vitro co-culture experiments either genetic deletion or pharmacological blockade of CD73 increased transendothelial lymphocyte migration. These data suggest that CD73 on graft-resident or circulating cells diminishes transendothelial leukocyte trafficking and mitigates inflammation and rejection.
Summary
Adenosine generally inhibits the activation and extravasation of lymphocytes into damaged or infected tissues. This is due to a combination of effects on T cells, APCs, and endothelial cells. A2A and A2B receptors and enzymes that control adenosine metabolism can be rapidly induced in response to inflammation or hypoxia. Adenosine signaling functions to limit inflammation and tissue injury without producing excessive immunosuppression.
Footnotes
Disclosures
The authors have no disclosures to report.
References
- 1.Hoskin DW, Mader JS, Furlong SJ, Conrad DM, Blay J. Inhibition of t cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (review) Int J Oncol. 2008;32:527–535. [PubMed] [Google Scholar]
- 2.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Jackson EK, Johnson JT, Gorelik E, Lang S, Whiteside TL. Adenosine and prostaglandin e2 cooperate in the suppression of immune responses mediated by adaptive regulatory t cells. J Biol Chem. 2010;285:27571–27580. doi: 10.1074/jbc.M110.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fredholm BB, APIJ, Jacobson KA, Linden J, Muller CE. International union of basic and clinical pharmacology. Lxxxi. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev. 2011;63:1–34. doi: 10.1124/pr.110.003285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: Therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao SY, Ng AM, Cass CE, Baldwin SA, Young JD. Nucleobase transport by human equilibrative nucleoside transporter 1 (hent1) J Biol Chem. 2011;286:32552–32562. doi: 10.1074/jbc.M111.236117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in atp release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci U S A. 2007;104:6436–6441. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohta A, Madasu M, Kini R, Subramanian M, Goel N, Sitkovsky M. A2a adenosine receptor may allow expansion of t cells lacking effector functions in extracellular adenosine-rich microenvironments. Journal of immunology. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 8.Lappas CM, Rieger JM, Linden J. A2a adenosine receptor induction inhibits ifn-gamma production in murine cd4+ t cells. J Immunol. 2005;174:1073–1080. doi: 10.4049/jimmunol.174.2.1073. [DOI] [PubMed] [Google Scholar]
- 9.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, Tsuji M, Kawahara K, Wong CH, Kronenberg M. Recognition of bacterial glycosphingolipids by natural killer t cells. Nature. 2005;434:520–525. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 10.Munoz E, Zubiaga AM, Merrow M, Sauter NP, Huber BT. Cholera toxin discriminates between t helper 1 and 2 cells in t cell receptor-mediated activation: Role of camp in t cell proliferation. J Exp Med. 1990;172:95–103. doi: 10.1084/jem.172.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdmann AA, Gao ZG, Jung U, Foley J, Borenstein T, Jacobson KA, Fowler DH. Activation of th1 and tc1 cell adenosine a2a receptors directly inhibits il-2 secretion in vitro and il-2-driven expansion in vivo. Blood. 2005;105:4707–4714. doi: 10.1182/blood-2004-04-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine a2a receptor activation reduces hepatic ischemia reperfusion injury by inhibiting cd1d-dependent nkt cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zarek PE, Huang CT, Lutz ER, Kowalski J, Horton MR, Linden J, Drake CG, Powell JD. A2a receptor signaling promotes peripheral tolerance by inducing t-cell anergy and the generation of adaptive regulatory t cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, Deitch EA, Spolarics Z, Nemeth ZH, Hasko G. Adenosine a2a receptor activation inhibits t helper 1 and t helper 2 cell development and effector function. Faseb J. 2008;22:3491–3499. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham G, Schusser GF, Ungemach FR. Dexamethasone-induced increase in lymphocyte beta-adrenergic receptor density and camp formation in vivo. Pharmacology. 2003;67:1–5. doi: 10.1159/000066787. [DOI] [PubMed] [Google Scholar]
- 16.Huang Y, Qiu AW, Peng YP, Liu Y, Huang HW, Qiu YH. Roles of dopamine receptor subtypes in mediating modulation of t lymphocyte function. Neuro Endocrinol Lett. 2010;31:782–791. [PubMed] [Google Scholar]
- 17.Gonzalez-Rey E, Delgado M. Vasoactive intestinal peptide and regulatory t-cell induction: A new mechanism and therapeutic potential for immune homeostasis. Trends Mol Med. 2007;13:241–251. doi: 10.1016/j.molmed.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 18.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by cd39 and cd73 expressed on regulatory t cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wallace KL, Linden J. Adenosine a2a receptors induced on inkt and nk cells reduce pulmonary inflammation and injury in mice with sickle cell disease. Blood. 2010;116:5010–5020. doi: 10.1182/blood-2010-06-290643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez JL, Punzon C, Navarro J, Munoz-Fernandez MA, Fresno M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by t lymphocytes by inhibiting nuclear factor-kappab and nuclear factor of activated t cells activation. J Pharmacol Exp Ther. 2001;299:753–759. [PubMed] [Google Scholar]
- 21.Chae HD, Siefring JE, Hildeman DA, Gu Y, Williams DA. Rhoh regulates subcellular localization of zap-70 and lck in t cell receptor signaling. PLoS One. 2010;5:e13970. doi: 10.1371/journal.pone.0013970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hermann-Kleiter N, Thuille N, Pfeifhofer C, Gruber T, Schafer M, Zitt C, Hatzelmann A, Schudt C, Leitges M, Baier G. Pkctheta and pka are antagonistic partners in the nf-at transactivation pathway of primary mouse cd3+ t lymphocytes. Blood. 2006;107:4841–4848. doi: 10.1182/blood-2005-10-4044. [DOI] [PubMed] [Google Scholar]
- 23.Mosenden R, Tasken K. Cyclic amp-mediated immune regulation--overview of mechanisms of action in t cells. Cell Signal. 2011;23:1009–1016. doi: 10.1016/j.cellsig.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 24.Bjorgo E, Solheim SA, Abrahamsen H, Baillie GS, Brown KM, Berge T, Okkenhaug K, Houslay MD, Tasken K. Cross talk between phosphatidylinositol 3-kinase and cyclic amp (camp)-protein kinase a signaling pathways at the level of a protein kinase b/beta-arrestin/camp phosphodiesterase 4 complex. Mol Cell Biol. 2010;30:1660–1672. doi: 10.1128/MCB.00696-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tasken K, Stokka AJ. The molecular machinery for camp-dependent immunomodulation in t-cells. Biochem Soc Trans. 2006;34:476–479. doi: 10.1042/BST0340476. [DOI] [PubMed] [Google Scholar]
- 26.Ledbetter JA, Parsons M, Martin PJ, Hansen JA, Rabinovitch PS, June CH. Antibody binding to cd5 (tp67) and tp44 t cell surface molecules: Effects on cyclic nucleotides, cytoplasmic free calcium, and camp-mediated suppression. J Immunol. 1986;137:3299–3305. [PubMed] [Google Scholar]
- 27.Abrahamsen H, Baillie G, Ngai J, Vang T, Nika K, Ruppelt A, Mustelin T, Zaccolo M, Houslay M, Tasken K. Tcr- and cd28-mediated recruitment of phosphodiesterase 4 to lipid rafts potentiates tcr signaling. J Immunol. 2004;173:4847–4858. doi: 10.4049/jimmunol.173.8.4847. [DOI] [PubMed] [Google Scholar]
- 28.Linnemann C, Schildberg FA, Schurich A, Diehl L, Hegenbarth SI, Endl E, Lacher S, Muller CE, Frey J, Simeoni L, Schraven B, Stabenow D, Knolle PA. Adenosine regulates cd8 t-cell priming by inhibition of membrane-proximal t-cell receptor signalling. Immunology. 2009;128:e728–737. doi: 10.1111/j.1365-2567.2009.03075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson P, Gonzalez-Rey E. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human t cells at multiple levels. Mol Cell Biol. 2010;30:2537–2551. doi: 10.1128/MCB.01282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Himer L, Csoka B, Selmeczy Z, Koscso B, Pocza T, Pacher P, Nemeth ZH, Deitch EA, Vizi ES, Cronstein BN, Hasko G. Adenosine a2a receptor activation protects cd4+ t lymphocytes against activation-induced cell death. Faseb J. 2010;24:2631–2640. doi: 10.1096/fj.10-155192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler JJ, Mader JS, Watson CL, Zhang H, Blay J, Hoskin DW. Adenosine inhibits activation-induced t cell expression of cd2 and cd28 co-stimulatory molecules: Role of interleukin-2 and cyclic amp signaling pathways. J Cell Biochem. 2003;89:975–991. doi: 10.1002/jcb.10562. [DOI] [PubMed] [Google Scholar]
- 32.Coffino P, Gray JW. Regulation of s49 lymphoma cell growth by cyclic adenosine 3′:5′-monophosphate. Cancer Res. 1978;38:4285–4288. [PubMed] [Google Scholar]
- 33.Kurata M, Hirata M, Watabe S, Miyake M, Takahashi SY, Yamamoto Y. Expression, purification, and inhibitory activities of mouse cytotoxic t-lymphocyte antigen-2alpha. Protein Expr Purif. 2003;32:119–125. doi: 10.1016/S1046-5928(03)00222-5. [DOI] [PubMed] [Google Scholar]
- 34.Zambon AC, Zhang L, Minovitsky S, Kanter JR, Prabhakar S, Salomonis N, Vranizan K, Dubchak I, Conklin BR, Insel PA. Gene expression patterns define key transcriptional events in cell-cycle regulation by camp and protein kinase a. Proc Natl Acad Sci U S A. 2005;102:8561–8566. doi: 10.1073/pnas.0503363102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Yun H, Murray F, Lu R, Wang L, Hook V, Insel PA. Cytotoxic t lymphocyte antigen-2 alpha induces apoptosis of murine t-lymphoma cells and cardiac fibroblasts and is regulated by camp/pka. Cell Signal. 2011;23:1611–1616. doi: 10.1016/j.cellsig.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apasov SG, Blackburn MR, Kellems RE, Smith PT, Sitkovsky MV. Adenosine deaminase deficiency increases thymic apoptosis and causes defective t cell receptor signaling. The Journal of clinical investigation. 2001;108:131–141. doi: 10.1172/JCI10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joachims ML, Marble PA, Laurent AB, Pastuszko P, Paliotta M, Blackburn MR, Thompson LF. Restoration of adenosine deaminase-deficient human thymocyte development in vitro by inhibition of deoxynucleoside kinases. J Immunol. 2008;181:8153–8161. doi: 10.4049/jimmunol.181.11.8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakowicz M, Szutowicz A, Pawelczyk T. Insulin and glucose induced changes in expression level of nucleoside transporters and adenosine transport in rat t lymphocytes. Biochem Pharmacol. 2004;68:1309–1320. doi: 10.1016/j.bcp.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 39.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. Cd127 expression inversely correlates with foxp3 and suppressive function of human cd4+ t reg cells. The Journal of experimental medicine. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dwyer KM, Hanidziar D, Putheti P, Hill PA, Pommey S, McRae JL, Winterhalter A, Doherty G, Deaglio S, Koulmanda M, Gao W, Robson SC, Strom TB. Expression of cd39 by human peripheral blood cd4+ cd25+ t cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10:2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolova M, Carriere M, Jenabian MA, Limou S, Younas M, Kok A, Hue S, Seddiki N, Hulin A, Delaneau O, Schuitemaker H, Herbeck JT, Mullins JI, Muhtarova M, Bensussan A, Zagury JF, Lelievre JD, Levy Y. Cd39/adenosine pathway is involved in aids progression. PLoS Pathog. 2011;7:e1002110. doi: 10.1371/journal.ppat.1002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohnuma K, Dang NH, Morimoto C. Revisiting an old acquaintance: Cd26 and its molecular mechanisms in t cell function. Trends Immunol. 2008;29:295–301. doi: 10.1016/j.it.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Cordero OJ, Salgado FJ, Fernandez-Alonso CM, Herrera C, Lluis C, Franco R, Nogueira M. Cytokines regulate membrane adenosine deaminase on human activated lymphocytes. J Leukoc Biol. 2001;70:920–930. [PubMed] [Google Scholar]
- 44.Li K, Anderson KJ, Peng Q, Noble A, Lu B, Kelly AP, Wang N, Sacks SH, Zhou W. Cyclic amp plays a critical role in c3a-receptor-mediated regulation of dendritic cells in antigen uptake and t-cell stimulation. Blood. 2008;112:5084–5094. doi: 10.1182/blood-2008-05-156646. [DOI] [PubMed] [Google Scholar]
- 45.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via a2a and a2b but not the a3 adenosine receptor. J Pharmacol Exp Ther. 2006;317:172–180. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 46.Nemeth ZH, Lutz CS, Csoka B, Deitch EA, Leibovich SJ, Gause WC, Tone M, Pacher P, Vizi ES, Hasko G. Adenosine augments il-10 production by macrophages through an a2b receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2a adenosine receptors and c/ebpbeta are crucially required for il-10 production by macrophages exposed to escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits il-12 and tnf-[alpha] production via adenosine a2a receptor-dependent and independent mechanisms. Faseb J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 49.Desrosiers MD, Cembrola KM, Fakir MJ, Stephens LA, Jama FM, Shameli A, Mehal WZ, Santamaria P, Shi Y. Adenosine deamination sustains dendritic cell activation in inflammation. J Immunol. 2007;179:1884–1892. doi: 10.4049/jimmunol.179.3.1884. [DOI] [PubMed] [Google Scholar]
- 50.Flach TL, Pang W, Chau EM, Desrosiers MD, Shi Y. Adenosine primes resting stage dendritic cells before their activation. Biochem Biophys Res Commun. 2009;380:748–751. doi: 10.1016/j.bbrc.2009.01.114. [DOI] [PubMed] [Google Scholar]
- 51.Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X. Hif-dependent induction of adenosine receptor a2b skews human dendritic cells to a th2-stimulating phenotype under hypoxia. Immunol Cell Biol. 2010;88:165–171. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 52.Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood. 2004;103:1391–1397. doi: 10.1182/blood-2003-06-1959. [DOI] [PubMed] [Google Scholar]
- 53.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, Dichmann S, Norgauer J. Expression and function of adenosine receptors in human dendritic cells. Faseb J. 2001;15:1963–1970. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 54.Fossetta J, Jackson J, Deno G, Fan X, Du XK, Bober L, Soude-Bermejo A, de Bouteiller O, Caux C, Lunn C, Lundell D, Palmer RK. Pharmacological analysis of calcium responses mediated by the human a3 adenosine receptor in monocyte-derived dendritic cells and recombinant cells. Mol Pharmacol. 2003;63:342–350. doi: 10.1124/mol.63.2.342. [DOI] [PubMed] [Google Scholar]
- 55.Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J. Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the t-cell stimulatory capacity of human dendritic cells. Blood. 2003;101:3985–3990. doi: 10.1182/blood-2002-07-2113. [DOI] [PubMed] [Google Scholar]
- 56.Kolachala V, Asamoah V, Wang L, Obertone TS, Ziegler TR, Merlin D, Sitaraman SV. Tnf-alpha upregulates adenosine 2b (a2b) receptor expression and signaling in intestinal epithelial cells: A basis for a2br overexpression in colitis. Cell Mol Life Sci. 2005;62:2647–2657. doi: 10.1007/s00018-005-5328-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, Linden J, Ernst PB. The a2b adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009;182:4616–4623. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilson JM, Kurtz CC, Black SG, Ross WG, Alam MS, Linden J, Ernst PB. The a2b adenosine receptor promotes th17 differentiation via stimulation of dendritic cell il-6. J Immunol. 2011;186:6746–6752. doi: 10.4049/jimmunol.1100117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Decking UK, Schlieper G, Kroll K, Schrader J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ Res. 1997;81:154–164. doi: 10.1161/01.res.81.2.154. [DOI] [PubMed] [Google Scholar]
- 60.Yang M, Ma C, Liu S, Shao Q, Gao W, Song B, Sun J, Xie Q, Zhang Y, Feng A, Liu Y, Hu W, Qu X. Hif-dependent induction of adenosine receptor a2b skews human dendritic cells to a th2-stimulating phenotype under hypoxia. Immunology and cell biology. 2010;88:165–171. doi: 10.1038/icb.2009.77. [DOI] [PubMed] [Google Scholar]
- 61.Synnestvedt K, Furuta GT, Comerford KM, Louis N, Karhausen J, Eltzschig HK, Hansen KR, Thompson LF, Colgan SP. Ecto-5′-nucleotidase (cd73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li X, Zhou T, Zhi X, Zhao F, Yin L, Zhou P. Effect of hypoxia/reoxygenation on cd73 (ecto-5′-nucleotidase) in mouse microvessel endothelial cell lines. Microvasc Res. 2006;72:48–53. doi: 10.1016/j.mvr.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 63.Johnston A, Gudjonsson JE, Sigmundsdottir H, Ludviksson BR, Valdimarsson H. The anti-inflammatory action of methotrexate is not mediated by lymphocyte apoptosis, but by the suppression of activation and adhesion molecules. Clin Immunol. 2005;114:154–163. doi: 10.1016/j.clim.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 64.Wallace KL, Marshall MA, Ramos SI, Lannigan JA, Field JJ, Strieter RM, Linden J. Nkt cells mediate pulmonary inflammation and dysfunction in murine sickle cell disease through production of ifn-gamma and cxcr3 chemokines. Blood. 2009;114:667–676. doi: 10.1182/blood-2009-02-205492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Herzig DS, Driver BR, Fang G, Toliver-Kinsky TE, Shute EN, Sherwood ER. Regulation of lymphocyte trafficking by cxc chemokine receptor 3 during septic shock. Am J Respir Crit Care Med. 2012;185:291–300. doi: 10.1164/rccm.201108-1560OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moore CC, Martin EN, Lee GH, Obrig T, Linden J, Scheld WM. An a2a adenosine receptor agonist, atl313, reduces inflammation and improves survival in murine sepsis models. BMC Infect Dis. 2008;8:141. doi: 10.1186/1471-2334-8-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takedachi M, Qu D, Ebisuno Y, Oohara H, Joachims ML, McGee ST, Maeda E, McEver RP, Tanaka T, Miyasaka M, Murakami S, Krahn T, Blackburn MR, Thompson LF. Cd73-generated adenosine restricts lymphocyte migration into draining lymph nodes. Journal of immunology. 2008;180:6288–6296. doi: 10.4049/jimmunol.180.9.6288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Takahashi HK, Iwagaki H, Hamano R, Kanke T, Liu K, Sadamori H, Yagi T, Yoshino T, Sendo T, Tanaka N, Nishibori M. Effect of adenosine receptor subtypes stimulation on mixed lymphocyte reaction. Eur J Pharmacol. 2007;564:204–210. doi: 10.1016/j.ejphar.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 69.Sullivan GW, Lee DD, Ross WG, DiVietro JA, Lappas CM, Lawrence MB, Linden J. Activation of a2a adenosine receptors inhibits expression of alpha 4/beta 1 integrin (very late antigen-4) on stimulated human neutrophils. J Leukoc Biol. 2004;75:127–134. doi: 10.1189/jlb.0603300. [DOI] [PubMed] [Google Scholar]
- 70.Hasegawa T, Bouis D, Liao H, Visovatti SH, Pinsky DJ. Ecto-5′ nucleotidase (cd73)-mediated adenosine generation and signaling in murine cardiac allograft vasculopathy. Circ Res. 2008;103:1410–1421. doi: 10.1161/CIRCRESAHA.108.180059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ohtsuka T, Changelian PS, Bouis D, Noon K, Harada H, Lama VN, Pinsky DJ. Ecto-5′-nucleotidase (cd73) attenuates allograft airway rejection through adenosine 2a receptor stimulation. J Immunol. 2010;185:1321–1329. doi: 10.4049/jimmunol.0901847. [DOI] [PMC free article] [PubMed] [Google Scholar]


