Abstract
Mast cells (MCs) have been implicated in orchestrating the host's early innate immune and adaptive immune responses in several models of acute bacterial infections. Most of this activity results in early clearance of the bacteria and timely resolution of infection. However, during chronic infections because of the prolonged nature of MC-bacterial interactions, the role of the MC in determining the fate of infection is markedly more complex. Depending on the nature of the pathogen, severity of infection and its association with a preexisting inflammatory disease, MCs may promote rather than contain chronic infections and exacerbate their pathological sequellae.
Introduction
When infectious bacteria colonize a host, they first encounter and are adapted to penetrate varied environments at the interface of the host and the external environment. However, just as bacteria are adapted to invade through specific pathways and tissues, so the host's immune system has adapted to meet each unique challenge. Whether bacteria prefer to colonize the skin, such as Staphylococcus aureus, the gut mucosae, as does Salmonella typhimurium, the lungs, in the case of Klebsiella pneumonaie, or the urinary tract, like Escherichia coli, each of these potential pathogens are likely to quickly encounter a cornerstone of the host's innate defense system, the mast cell (MC). MCs are able to recognize various pathogens or their products, and rapidly degranulate, or release pre-stored inflammatory mediators (Figure 1). While MCs can directly kill bacteria, most of their antimicrobial activity is linked to their ability to recruit neutrophils and antigen presenting cells, such as dendritic cells, to the site of infection. Neutrophils are essential for direct killing of bacteria and the antigen presenting cells are subsequently drained from the infection site to the lymph nodes, where adaptive immune responses are galvanized. As a result, MCs promote the development of a vigorous and largely appropriate innate and adaptive immune responses to the pathogens during acute infection. However, in the case of chronic and persistent infection, which often involves the persistence of intracellular bacteria, MCs are largely implicated in promoting pathological sequellae. This is especially true when these infections occur at sites with preexisting inflammatory disease. MCs possess several traits that uniquely enable them to exacerbate persistent inflammatory diseases and these include: (i) their ability to achieve sustained mediator release (ii) their ability to congregate and proliferate at sites of inflammation, and (iii) their longevity. In this article, we will review the MC recognition mechanisms of bacterial pathogens and describe their seemingly divergent roles in acute and chronic bacterial infections.
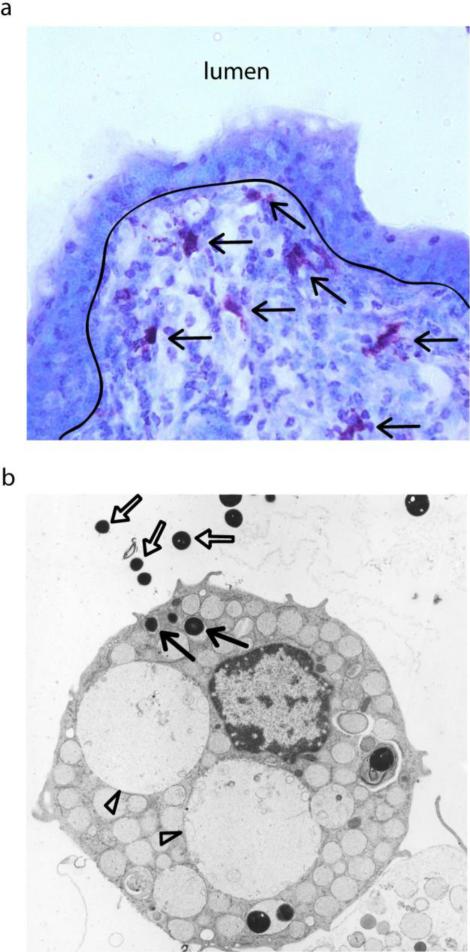
Figure 1. Mast Cells as Sentinels of Immune Surveillance.
(a) Toluidine blue stain of porcine small intestine (40x magnification). Mast cells, stained deep purple (black arrows), are strategically located underneath the gut epithelium. (b) TEM of a mast cell interacting with Staphlococcus. Some of the external bacteria (white arrows) have been endocytosed within the mast cell (black arrow). Additionally, the mast cell has undergone the degranulation process due to the presence of large empty granule vacuoles (white arrowheads).
Pleiotropic MC Responses to Microbial Pathogens and their Products
Like other immune surveillance cells, MCs are capable of directly recognizing a range of bacterial pathogens or secreted products. Additionally, they can indirectly recognize and interact with bacteria bound by various host defense proteins, including immunoglobulins, complement components and surfactant lipoproteins, which serve as opsonins. The MC membrane is also replete with pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), which recognize structurally conserved pathogen-associated molecular patterns (PAMPs) [1] even without opsonisation, variously affecting the nature and magnitude of the MC response to bacteria. TLR2 on rodent MCs recognizes and bind peptidogylcans found on Gram-positive bacteria, triggering MC degranulation and cytokine production, while Gram-negative lipopolysaccharide (LPS) stimulates TLR4, promoting a vigorous cytokine response without degranulation [2]. Interestingly, upon bacterial activation of MC TLRs, only proinflammatory responses were observed to be elicited, but not type I interferon [3]. In addition to conventional PRRs, other pathogen receptors are also present on the MC cell surface. One such receptor, CD48, detects widely divergent bacteria from type-1 fimbriated E. coli and K. pneumonia to Mycobacterium tuberculosis and S. aureus [4-6], prompting MC degranulation. Notably, physical contact between bacteria and the MC is unnecessary for a MC response. It is much more likely that bacterial toxins and cell wall components released by bacteria attempting to penetrate the epithelial barrier activate MCs located in the submucosae. Responses to toxins also differ according to the specific ligand-receptor interaction. The toxin of Vibrio cholerae which binds GM1 selectively amplifies production of certain cytokines, such as interleukin-6 (IL-6), while inhibiting the production of others [7]. Furthermore, Clostriudium difficile toxin binding to neurokinin-1 induces MC degranulation and proinflammation [8] whereas Bordetella pertussis toxin binding of G proteins suppresses MC degranulation and histamine release [9]. Since each of these bacterial products are known virulence determinants, it is conceivable that the MC responses evoked by each may have unique consequences to the host's immune responses.
With the expression of multiple Fc receptors (FcRs), including FcγRII and the high-affinity receptor FcεRI, MCs are able to bind both immunoglobulin G (IgG) and IgE. Although IgE is not commonly generated against bacteria, IgE specific to Helicobacter pylori and S. aureus have been reported in patients with chronic peptic ulcers and atopic dermatitis, respectively [10,11]. Stimulation of both FcεRI and TLRs on MCs synergistically augments proinflammatory cytokine production through the activation of mitogen-activated protein kinases (MAPK) [12]. MC FcRs can also be activated by bacterial superantigens, such as protein A from S. aureus [13]. Complement receptors on MCs can also greatly enhance the mast cell response by synergism with the complement system. The cleaved and activated C3a and C5a complement proteins are potent MC activators and chemoattractants during inflammation [14]. Peritoneal neutrophilia during S. aureus infection has been recently shown to be partially MC dependent, with concomitant TLR2 and C5aR activation contributing to immune responses [15]. The CR3 receptor for the iC3b fragment is also suggested to mediate MC binding to Salmonella [16]. This synergy of MC and complement is highlighted in C3-deficient mice, which exhibit greatly reduced MC degranulation and TNF-α production upon acute septic peritonitis, impairing neutrophil recruitment and bacterial clearance [17]. Thus, MCs have a broad repertoire of receptors that are capable of directly or indirectly binding pathogens or their products, with specific interactions evoking strikingly different MC responses and enhanced MC responses through involvement of multiple receptors.
MC Mediated Immune Responses to Acute Bacterial Infections
Knowledge of the contribution of MCs to outcomes of bacterial infection largely comes from comparing acute infections in wild type and MC-deficient mice. These studies reveal that MCs primarily function as proinflammatory sentinels mediating the rapid recruitment of immune cells to sites of infection and simultaneously mobilizing the adaptive immune response in distal lymph nodes (Figure 2). Studies with different bacterial pathogens implicate MC products such as TNF [18,19], MC protease-6 [20], IL-4 [21] and IL-6 [22] in promoting leukocyte-mediated bacterial clearance either through neutrophil recruitment or enhancing antibacterial activities of neutrophils and macrophages. These local and long distance effects are achieved in part through the release of their pre-stored mediators packaged in small submicron sized, heparin-based particles (granules) [23]. These particles release their contents into the surroundings in a regulated fashion, maximizing the potency of their cargo. The released mediators promote the recruitment of neutrophils and antigen presenting cells to the site of infection. Interestingly, a portion of the MCs granules released into the surrounding tissue also gain access into lymphatic vessels and rapidly drain into the neighboring lymph nodes [23]. The various MC mediators eluted from exocytosed MC particles trigger extensive mobilization of select dendritic cell subtypes, and lymphocytes which are necessary for the development of pathogen-specific immune responses [24,25]. A potent immunomodulator in MC granules is TNF, which initiates neutrophil and dendritic cell migration into tissues and lymphocyte recruitment in nodes by upregulating E-selectin [24] and VCAM-1 [26], respectively, on neighboring blood vessels. In addition to promoting the maturation and migration of antigen presenting cells at sites of infection, MCs, themselves, can serve as antigen presenting cells. This was demonstrated with antigen-pulsed MCs, which were capable of inducing the proliferation of antigen-specific CD8+ T cells and increasing their cytotoxic activity [27].
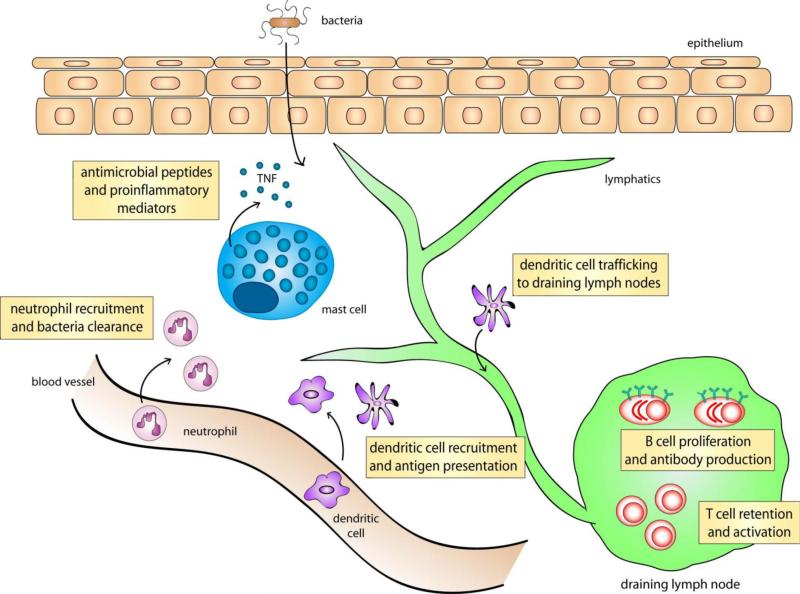
Figure 2. Mast Cell Mediated Immune Response Upon Bacterial Infection.
Mast cells have been shown to be crucial in the recruitment of neutrophils for bacterial clearance during infection. They are also important for dendritic cell extravagation, as well as their migration to the draining lymph nodes where initiation of adaptive immunity occurs. Mast cells are also crucial for lymph node hypertrophy, where there is retention of circulating T cells and subsequently, greater B cell proliferation and antibody production. However, at sites predisposed with chronic bacterial infection, these MC mediated recruitment of immune cells can become exaggerated and lead to disease pathology.
Direct killing of bacteria is achieved by the release of cathelicidins, broad-spectrum antimicrobial peptides highly effective against group A streptococci skin infections [28]. Cathelicidins are produced constitutively by MCs but can be induced in response to bacterial products, including LPS [29] and pneumolysin of Streptococcus pneumoniae [30]. While primarily exocytic in activity, MCs can kill bacteria by endocytosis, an activity supported by their ability to generate reactive oxygen species [31].
It is not always clear how MCs function to protect against bacteria. MC-sufficient mice have improved morbidity and survival following Mycoplasma pulmonis infection compared to MC-deficient mice. The bacterial load and lymph node hypertrophy of MC-sufficient mice were also markedly reduced, as was the extent of progressive pneumonia and airway occlusion [32]. A recent study examining M. pulmonis lung infections under allergic conditions suggested the importance of IL-6 for bacterial clearance, but paradoxically MC-derived IL-6 was not required [33].
In view of the importance of MCs in orchestrating effective immune responses against a broad spectrum of bacteria, it is unsurprising that bacteria have evolved strategies in subverting MC function. For reasons that are not immediately clear, certain commensal E.coli strains appear able to inactivate MCs, inhibiting degranulation for days after exposure [34]. B. pertussis is able to block histamine release by MCs and selectively inhibit MC IL-6 release through pertussis toxin [35]. Certain pathogens even appear to have evolved mechanisms to destroy MCs. Exotoxin A of Pseudomonas aeruginosa, for instance, induces human MC apoptosis by down-regulating anti-apoptotic proteins and activating of caspase-8 and -3 pathways [36].
Diverse MC Mediated Immune Responses to Chronic infection
Incomplete clearance of bacterial infections may result in chronic disease. Often, these infections are limited to a particular physiological site. There is considerably less known regarding the role of MCs in chronic infections compared to acute infections. To date, data suggest that MCs display widely divergent effects depending on the nature of the bacterial pathogen and whether or not the infection is associated with a preexisting inflammatory disorder. During mycobacterial infections of the lungs, MCs appear to play a critical role by encouraging the development of granulomas, which contain mycobacterial spread [37]. MCs aggregate around granulomas, promoting their growth and enhance homing of activated CD8+ T cells to the lungs by regulating the local cytokine milieu [37]. Many chronic infections are closely linked to preexisting inflammatory disorders and, in these contexts, MCs appear to be more harmful than beneficial. These include Staphylococcal infections in atopic dermatitis [38], P. aeroginosa infections of inflamed cystic fibrotic lungs [39], and H. pylori infections in chronic gastritis [40]. These and other examples are listed in Table 1. In these cases, MCs exacerbate the inflammatory condition through hypersecretion of proinflammatory mediators such as IL-1α and β [39] and anomalous recruitment of cytotoxic immune cells including neutrophils and eosinophils. Often, the MC-activating bacterial factors are exotoxins, such as strepolysin O for Group A Streptococci and α- toxin for S. aureus in chronic urticaria [41], and the cytotoxin VacA of H. pylori in chronic gastritis [42], which lead to enhanced apoptosis of epithelial cells and increase in tissue fibrosis in the antral gastric mucosa [40]. Hyperactivation of MCs by concurrent infection and inflammatory disorders can lead to pathological tissue damage.
Table 1.
Inflammatory Diseases Exacerbated by MC-bacterial interactions.
| Disease | Bacteria | Additional Immune cells associated with pathology |
|---|---|---|
| Asthma | Mycoplasma Chlamydia | eosinophil T cell |
| Atopie Dermatitis | Staphylococcus Streptococcus | eosinophil dendritic cell T cell |
| Cystitic Fibrosis | Pseudomonas | neutrophil |
| Chronic Gastritis | Helicobacter | eosinophil neutrophil |
| Irritable Bowel Syndrome | commensal gut microflora | macrophage neutrophil T cell B cell macrophage |
| Interstitial Cystitis | chronic urinary tract infections | T cell B cell macrophage |
Mast cell activation and hyperplasia have been observed and correlated to the worsening of pathology in numerous diseases, many of which are also predisposed to bacterial infections.
The capacity of MCs to aggregate and proliferate at sites of inflammation contributes to their ability to exacerbate chronic infections in an inflamed tissue environment. Unlike other immune cells that migrate to inflamed locations, MCs that are already located in tissues can actively proliferate in spite of their terminal differentiation, thereby increasing their local numbers. MCs are also able to mediate multiple cycles of mediator release of pre-stored and de novo synthesized mediators. Since in chronic infections the interactions between bacteria and the MC are likely to be prolonged and frequent, this property of MCs has significant relevance. Interestingly, it has been suggested that subsequent MC degranulation responses may be enhanced over the original response, as the regranulation process in an inflamed site can change in response to pathogen encounter or exposure to inflammatory mediators, resulting in a different composition of mediators within new MC granules [43]. With large bacterial loads, dysregulated MC secretory responses may contribute to pathogenesis. For example, MCs exhibit a protective role in a cecal ligation and puncture (CLP) model of moderate septic peritonitis but in severe CLP or in a S. typhimurium peritonitis infection with high bacterial load, MC responses conversely led to increased mouse mortality [44]. These harmful effects of MCs may in part be related to their physical location adjacent to blood vessels. MCs lining blood vessels may be susceptible to activation by bacteria transported in the circulation and the resulting outpouring of mediators can potentially have lethal effects on the host. Mediators released by hyperactive MCs rapidly gain access into blood vessels resulting in severe systemic effects in which mast cell stabilization can potentially improve survival [45]. The functional importance of systemic MC degranulation during infection was recently evaluated by compartment-specific MC reconstitution of MC-deficient mice. These studies compared the morbidity associated with CLP in MC-deficient mice following MC repletion of the peritoneal compartment alone and repletion of both the peritoneal systemic compartments. While morbidity after CLP in mice locally repleted with MCs markedly improved, reconstitution in peritoneal and systemic compartments decreased survival. Additionally, mice systemically reconstituted with IL-6−/− MCs had improved survival compared to controls, suggesting the contribution of IL-6 to the detrimental effects of systemic MC activation [46]. These results indicate that while immediate and local MC activation during infection is beneficial, systemic and sustained MC activation may not be. Thus, both temporal and spatial aspects of MC activation appear to be additional determinants of the beneficial or harmful contributions of MC during infection.
Therapeutic Strategies Employing Modulators of MCs or their Products
The extensive control of MCs on host innate and adaptive immune responses has led to various strategies to modulate host immunity for therapeutic benefit. Recently, the feasibility of employing small molecule activators of MCs to boost immune responses to various vaccine antigens was examined at a nasal mucosal site in mice [47]. These small molecule activators of MCs served as potent vaccine adjuvants evoking systemic as well as mucosal immunity against a variety of vaccine antigens. This was also shown to be protective against both infectious agents and their toxins in rodent and rabbit models [48,49]. Additionally, it is conceivable that pharmacologic ‘stabilizers” of MCs might be an effective adjunct to current treatment regimens to reduce MC contribution to the pathology of chronic infections in inflammatory disorders. Several FDA approved pharmacological agents exist, acting by either blocking activity of MCs or MC products such as histamine [50], leukotrienes [51] or TNF [52], that can potentially be used to manage chronic infections or their MC-promoted symptoms. For example, in the mouse model, MC stabilization was shown to be effective in ameliorating S. aureus-induced diarrhea, which is promoted by PGN activation of MCs in a manner dependent on TLR2 and NOD2 [53]. In each case of either purposeful MC activation or stabilization, the powerful control of the MC on tissue homeostasis and success of pathogen clearance emphasizes the importance of proceeding with caution when exploiting or suppressing their functions.
Conclusion
MCs have several unique attributes that make them powerful immune surveillance cells. Upon recognition of various pathogens or their products, they are capable of orchestrating protective immunity by activating and recruiting immune cells involved in the early innate and adaptive immune responses. The critical nature of these MC-promoted events is clearly illustrated by the numerous models of infection where the presence of MCs can prevent lethal infection outcomes. However, MC functions can also be detrimental chronic infections, especially at sites with a preexisting inflammatory disorder. Thus, MCs play widely disparate functional roles depending on the nature and location of bacterial infections. A greater understanding of these pathogen-specific roles that MCs play within tissues is necessary to develop unique strategies to promote bacterial clearance, prevent persistent infection, and reduce collateral tissue damage.
Highlights.
➢ Mast cells directly and indirectly bind bacterial pathogens or secreted products
➢ Mast cells can mediate acute and adaptive immune responses upon bacterial infection
➢ Immediate and local mast cell activation during acute infection is beneficial
➢ Systemic and sustained mast cell activation can exacerbate pathological sequellae
➢ Activate/suppressing mast cells can modulate host immunity for therapeutic benefit
Acknowledgements
We thank Adam Moeser (N.C State University) for acquiring the image in Figure 1a and Wei Xin Gladys Ang (Duke University Medical Center) for critical reading of this manuscript. Our studies are supported by the US National Institutes of Health grants R01 A135678, R01 DK077159, R01 A150021, R37 DK50814 and R21 A1056101.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 2.Supajatura V, Ushio H, Nakao A, Akira S, Okumura K, Ra C, Ogawa H. Differential responses of mast cell Toll-like receptors 2 and 4 in allergy and innate immunity. J Clin Invest. 2002;109:1351–1359. doi: 10.1172/JCI14704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietrich N, Rohde M, Geffers R, Kröger A, Hauser H, Weiss S, Gekara NO. Mast cells elicit proinflammatory but not type I interferon responses upon activation of TLRs by bacteria. Proc. Natl. Acad. Sci. U.S.A. 2010;107:8748–8753. doi: 10.1073/pnas.0912551107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malaviya R, Gao Z, Thankavel K, van der Merwe PA, Abraham SN. The mast cell tumor necrosis factor alpha response to FimH-expressing Escherichia coli is mediated by the glycosylphosphatidylinositol-anchored molecule CD48. Proc Natl Acad Sci U S A. 1999;96:8110–8115. doi: 10.1073/pnas.96.14.8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munoz S, Hernandez-Pando R, Abraham SN, Enciso JA. Mast cell activation by Mycobacterium tuberculosis: mediator release and role of CD48. J Immunol. 2003;170:5590–5596. doi: 10.4049/jimmunol.170.11.5590. [DOI] [PubMed] [Google Scholar]
- 6.Rocha-de-Souza CM, Berent-Maoz B, Mankuta D, Moses AE, Levi-Schaffer F. Human mast cell activation by Staphylococcus aureus: interleukin-8 and tumor necrosis factor alpha release and the role of Toll-like receptor 2 and CD48 molecules. Infect Immun. 2008;76:4489–4497. doi: 10.1128/IAI.00270-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leal-Berumen I, Snider DP, Barajas-Lopez C, Marshall JS. Cholera toxin increases IL-6 synthesis and decreases TNF-alpha production by rat peritoneal mast cells. J. Immunol. 1996;156:316–321. [PubMed] [Google Scholar]
- 8.Meyer GK, Neetz A, Brandes G, Tsikas D, Butterfield JH, Just I, Gerhard R. Clostrdium difficile Toxins A and B directly stimulate human mast cells. IAI. 2007;75:3868–3876. doi: 10.1128/IAI.00195-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito S, Okajima F, Molski TF, Sha'afi RI, Ui M, Ishizaka T. Effects of ADP-ribosylation of GTP-binding protein by pertussis toxin on immunoglobulin E-dependent and independent histamine release from mast cells and basophils. J. Immunol. 1987;138:3927–3934. [PubMed] [Google Scholar]
- 10.Aceti A, Celestino D, Caferro M, Casale V, Citarda F, Conti EM, Grassi A, Grilli A, Pennica A, Sciarretta F, et al. Basophil-bound and serum immunoglobulin E directed against Helicobacter pylori in patients with chronic gastritis. Gastroenterology. 1991;101:131–137. doi: 10.1016/0016-5085(91)90469-2. [DOI] [PubMed] [Google Scholar]
- 11.Leung DY, Harbeck R, Bina P, Reiser RF, Yang E, Norris DA, Hanifin JM, Sampson HA. Presence of IgE antibodies to staphylococcal exotoxins on the skin of patients with atopic dermatitis. Evidence for a new group of allergens. J. Clin. Invest. 1993;92:1374–1380. doi: 10.1172/JCI116711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao H, Andrade MV, Lisboa FA, Morgan K, Beaven MA. FcepsilonR1 and toll-like receptors mediate synergistic signals to markedly augment production of inflammatory cytokines in murine mast cells. Blood. 2006;107:610–618. doi: 10.1182/blood-2005-06-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genovese A, Bouvet JP, Florio G, Lamparter-Schummert B, Bjorck L, Marone G. Bacterial immunoglobulin superantigen proteins A and L activate human heart mast cells by interacting with immunoglobulin E. Infect Immun. 2000;68:5517–5524. doi: 10.1128/iai.68.10.5517-5524.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J. Immunol. 1996;157:1693–1698. [PubMed] [Google Scholar]
- 15.Mullaly SC, Kubes P. The role of TLR2 in vivo following challenge with Staphylococcus aureus and prototypic ligands. J. Immunol. 2006;177:8154–8163. doi: 10.4049/jimmunol.177.11.8154. [DOI] [PubMed] [Google Scholar]
- 16.Sher A, Hein A, Moser G, Caulfield JP. Complement receptors promote the phagocytosis of bacteria by rat peritoneal mast cells. Lab Invest. 1979;41:490–499. [PubMed] [Google Scholar]
- 17.Prodeus AP, Zhou X, Maurer M, Galli SJ, Carroll MC. Impaired mast cell-dependent natural immunity in complement C3-deficient mice. Nature. 1997;390:172–175. doi: 10.1038/36586. [DOI] [PubMed] [Google Scholar]
- 18.Echtenacher B, Männel DN, Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. doi: 10.1038/381075a0. [DOI] [PubMed] [Google Scholar]
- 19.Malaviya R, Ikeda T, Ross E, Abraham SN. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-alpha. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 20.Thakurdas SM, Melicoff E, Sansores-Garcia L, Moreira DC, Petrova Y, Stevens RL, Adachi R. The mast cell-restricted tryptase mMCP-6 has a critical immunoprotective role in bacterial infections. J Biol Chem. 2007;282:20809–20815. doi: 10.1074/jbc.M611842200. [DOI] [PubMed] [Google Scholar]
- 21.Ketavarapu JM, Rodriguez AR, Yu JJ, Cong Y, Murthy AK, Forsthuber TG, Guentzel MN, Klose KE, Berton MT, Arulanandam BP. Mast cells inhibit intramacrophage Francisella tularensis replication via contact and secreted products including IL-4. Proc. Natl. Acad. Sci. U.S.A. 2008;105:9313–9318. doi: 10.1073/pnas.0707636105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sutherland RE, Olsen JS, McKinstry A, Villalta SA, Wolters PJ. Mast cell IL-6 improves survival from Klebsiella pneumonia and sepsis by enhancing neutrophil killing. J Immunol. 2008;181:5598–5605. doi: 10.4049/jimmunol.181.8.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kunder CA, St. John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J. Exp. Med. 2009;206:2455–2467. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shelburne CP, Nakano H, St. John A, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dawicki W, Jawdat DW, Xu N, Marshall JS. Mast cells, histamine, and IL-6 regulate the selective influx of dendritic cell subsets into an inflamed lymph node. J Immunol. 2010;184:2116–2123. doi: 10.4049/jimmunol.0803894. [DOI] [PubMed] [Google Scholar]
- 26.Meng H, Tonnesen MG, Marchese MJ, Clark RA, Bahou WF, Gruber BL. Mast cells are potent regulators of endothelial cell adhesion molecule ICAM-1 and VCAM-1 expression. J. Cell. Phys. 2005;160:40–53. doi: 10.1002/jcp.1041650106. [DOI] [PubMed] [Google Scholar]
- 27.Stelekati E, Bahri R, D'Orlando O, Orinska Z, Mittrücker HW, Langenhaun R, Glatzel M, Bollinger A, Paus R, Bulfone-Paus S. Mast cell-mediated antigen presentation regulates CD8+ T cell effector functions. Immunity. 2009;31:665–676. doi: 10.1016/j.immuni.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Di Nardo A, Yamasaki K, Dorschner RA, Lai Y, Gallo RL. Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin. J Immunol. 2008;180:7565–7573. doi: 10.4049/jimmunol.180.11.7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li G, Domenico J, Jia Y, Lucas JJ, Gelfand EW. NF-kappaB-dependent induction of cathelicidin-related antimicrobial peptide in murine mast cells by lipopolysaccharide. Int. Arc Alelrgy Immunol. 2009;150:122–132. doi: 10.1159/000218115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruse G, Fernandes VE, de Salort J, Pankhania D, Marinas MS, Brewin H, Andrew PW, Bradding P, Kadioglu A. Human lung mast cells mediate pneumococcal cell death in response to activation by pneumolysin. J. Immunol. 2010;184:7108–7115. doi: 10.4049/jimmunol.0900802. [DOI] [PubMed] [Google Scholar]
- 31.Swindle EJ, Metcalfe DD, Coleman JW. Rodent and human mast cells produce functionally significant intracellular reactive oxygen species but not nitric oxide. J. Biol. Chem. 2004;279:48751–48759. doi: 10.1074/jbc.M409738200. [DOI] [PubMed] [Google Scholar]
- 32.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM, Hawgood S, Caughey GH. Am J Respir Crit Care Med. 2006;Mast cells protect mice from Mycoplasma pneumonia.173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michels NM, Chu HW, LaFasto SC, Case SR, Minor MN, Martin RJ. Mast cells protect against airway Mycoplasma pneumoniae under allergic conditions. Clin Exp Allergy. 2010;40:1406–1413. doi: 10.1111/j.1365-2222.2010.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mager M, Lammer V, Siebenhaar F, Zuberbier T, Metz M, Maurer M. Non-pathogenic commensal Escherichia coli can inhibit degranulation of mast cells. Exp. Derm. 2008;17:427–435. doi: 10.1111/j.1600-0625.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 35.Mielcarek N, Hörnquist EH, Johansson BR, Locht C, Abraham SN, Holmgren J. Interaction of Bordetella pertussis with mast cells, modulation of cytokine secretion by pertussis toxin. Cell Microbiol. 2001;3:181–188. doi: 10.1046/j.1462-5822.2001.00106.x. [DOI] [PubMed] [Google Scholar]
- 36.Jenkins CE, Swiatoniowski A, Issekutz AC, Lin TJ. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and -3-dependent mechanism. J Biol Chem. 2004;279:37201–37207. doi: 10.1074/jbc.M405594200. [DOI] [PubMed] [Google Scholar]
- 37.Carlos D, Frantz FG, Souza-Júnior DA, Jamur MC, Oliver C, Ramos SG, Quesniaux VF, Ryffel B, Silva CL, Bozza MT, Faccioli LH. TLR2-dependent mast cell activation contributes to the control of Mycobacterium tuberculosis infection. Microbes Infect. 2009;11:770–778. doi: 10.1016/j.micinf.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 38.Werfel T. The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. Invest. Derm. 2009;129:1878–1891. doi: 10.1038/jid.2009.71. [DOI] [PubMed] [Google Scholar]
- 39.Lin TJ, Garduno R, Boudreau RT, Issekutz AC. Pseudomonas aeruginosa activates human mast cells to induce neutrophil transendothelial migration via mast cell-derived IL-1 alpha and beta. J Immunol. 2002;169:4522–4530. doi: 10.4049/jimmunol.169.8.4522. [DOI] [PubMed] [Google Scholar]
- 40.Hofman V, Lassalle S, Selva E, Kalem K, Steff A, Hebuterne X, Sicard D, Auberger P, Hofman P. Involvement of mast cells in gastritis caused by Helicobacter pylori: a potential role in epithelial cell apoptosis. J Clin Pathol. 2007;60:600–607. doi: 10.1136/jcp.2006.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metz M, Magerl M, Kuhl NF, Valeva A, Bhakdi S, Maurer M. Mast cells determine the magnitude of bacterial toxin-induced skin inflammation. Exp Dermatol. 2009;18:160–166. doi: 10.1111/j.1600-0625.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- 42.Supajatura V, Ushio H, Wada A, Yahiro K, Okumura K, Ogawa H, Hirayama T, Ra C. Cutting edge: VacA, a vacuolating cytotoxin of Helicobacter pylori, directly activates mast cells for migration and production of proinflammatory cytokines. J Immunol. 2002;168:2603–2607. doi: 10.4049/jimmunol.168.6.2603. [DOI] [PubMed] [Google Scholar]
- 43.Ghildyal N, McNeil HP, Gurish MF, Austen KF, Stevens RL. Transcriptional regulation of the mucosal mast cell-specific protease gene, MMCP-2, by interleukin 10 and interleukin 3. J Biol Chem. 1992;267:8473–8477. [PubMed] [Google Scholar]
- 44.Piliponsky AM, Chen CC, Grimbaldeston MA, Burns-Guydish SM, Hardy J, Kalesnikoff J, Contag CH, Tsai M, Galli SJ. Mast cell-derived TNF can exacerbate mortality during severe bacterial infections in C57BL/6-KitW-sh/W-sh mice. Am. J. Pathol. 2010;176:926–938. doi: 10.2353/ajpath.2010.090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramos L, Peña G, Cai B, Deitch EA, Ulloa L. Mast cell stabilization improves survival by preventing apoptosis in sepsis. J. Immunol. 2010;185-:709–716. doi: 10.4049/jimmunol.1000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seeley EJ, Sutherland RE, Kim SS, Wolters PJ. Systemic mast cell degranulation increases mortality during polymicrobial septic peritonitis in mice. J. Leukoc. Biol. 2011:90. doi: 10.1189/jlb.0910531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 48.Staats HF, Fielhauer JR, Thompson AL, Tripp AA, Sobel AE, Maddaloni M, Abraham SN, Pascual DW. Mucosal targeting of a BoNT/A subunit vaccine adjuvanted with a mast cell activator enhances induction of BoNT/A neutralizing antibodies in rabbits. PLoS One. 2009;6:e16532. doi: 10.1371/journal.pone.0016532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowen AL, Hale LP, Shelburne CP, Abraham SN, Staats HF. The mast cell activator compound 48/80 is safe and effective when used as an adjuvant for intradermal immunization with Bacillus anthracis protective antigen. Vaccine. 2009;27:3544–3552. doi: 10.1016/j.vaccine.2009.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estelle F, Simons R. Advances in H1-antihistamines. NEJM. 2004;351:2203–2217. doi: 10.1056/NEJMra033121. [DOI] [PubMed] [Google Scholar]
- 51.Lipworth BJ. Leukotriene-receptor antagonists. LANCET. 1999;353:57–62. doi: 10.1016/S0140-6736(98)09019-9. [DOI] [PubMed] [Google Scholar]
- 52.Colombel JF, Sandborn WJ, Reinisch W, Mantzaris GJ, Kornbluth A, Rachmilewitz D, Lichtiger S, D'Haens G, Diamond RH, Broussard DL, et al. Infliximab, Azathioprine, or combination therapy for crohn's disease. NEJM. 2010;362:1383–1395. doi: 10.1056/NEJMoa0904492. [DOI] [PubMed] [Google Scholar]
- 53.Feng BS, He SH, Zheng PY, Wu L, Yang PC. Mast cells play a crucial role in Staphylococcus aureus peptidoglycan-induced diarrhea. Am. J. Pathol. 2007;171:537–547. doi: 10.2353/ajpath.2007.061274. [DOI] [PMC free article] [PubMed] [Google Scholar]