Abstract
In all three domains of life, N-glycosylation begins with the assembly of glycans on phosphorylated polyisoprenoid carriers. Like eukaryotes, archaea also utilize phosphorylated dolichol for this role, yet whereas the assembled oligosaccharide is transferred to target proteins from dolichol pyrophosphate in eukaryotes, archaeal N-linked glycans characterized to date are derived from a dolichol monophosphate carrier, apart from a single example. In this study, glycan-charged dolichol phosphate from the hyperthermophile Pyrococcus furiosus was identified and structurally characterized. Normal and reverse phase liquid chromatography-electrospray ionization mass spectrometry revealed the existence of dolichol phosphate charged with the heptasaccharide recently described in in vitro studies of N-glycosylation on this species. As with other described archaeal dolichol phosphates, the α- and ω-terminal isoprene subunits of the P. furiosus lipid are saturated, in contrast to eukaryal phosphodolichols that present only a saturated α-position isoprene subunit. Interestingly, an additional 1-4 of the 12-14 isoprene subunits comprising P. furiosus dolichol phosphate are saturated, making this lipid not only the longest archaeal dolichol phosphate described to date but also the most highly saturated.
Introduction
The polyisoprenols are a family of hydrophobic polymers comprising up to more than 100 isoprene subunits bearing a terminal hydroxyl group at the α-terminus of the molecule [1–3]. The two main groups of polyisoprenoids, polyprenols and dolichols, can be distinguished by the presence of an unsaturated (polyprenols) or saturated (dolichols) α-isoprene subunit. While various biological roles have been suggested for the polyisoprenoid alcohols, these remain to be clearly delineated [1–5]. On the other hand, phosphorylated polyprenols and dolichols are well established as central players in N-glycosylation, namely the covalent linkage of glycans to select asparagine residues of target proteins [6–8].
In N-glycosylation, the glycan moiety is initially assembled on a phosphorylated polyisoprenol, from where it is transferred to the protein target by the actions of an oligosaccharyltransferase (OST). Whereas bacterial N-linked glycosylation generally relies on polyprenol phosphate species, such as C55 undecaprenol phosphate (UndP), as the glycan carrier, eukaryotes and archaea instead rely on phosphorylated dolichols for this purpose [6–8]. Various traits, however, serve to distinguish between the phosphorylated dolichols used in eukaryal and archaeal N-glycosylation. In eukaryal N-glycosylation, dolichols of various lengths are found, with C80, C90 and C95 phosphorylated dolichol predominating in yeast, rat and humans, respectively [1]. Archaeal N-glycosylation also involves dolichol species of multiple lengths, although these tend to be shorter than their eukaryal counterparts, containing only 8–12 five-carbon isoprene units [9–13]. In addition, whereas a phosphosugar is added to dolichol phosphate (DolP) in the first step of the eukaryotic pathway to generate a dolichol pyrophosphate (DolPP)-bound oligosaccharide, archaeal N-glycosylation assembles oligosaccharides on DolP (although in the haloarchaeon Halobacterium salinarum, DolP and DolPP have both been proposed to serve this role) [8,14]. Most strikingly, phosphorylated dolichols in eukaryotes and archaea differ in their degrees of saturation. While phosphorylated dolichols contain a saturated α-isoprene unit in organisms belonging to each domain, archaeal DolP also contains a saturated ω-isoprene unit [10,11,13].
In research designed to further our understanding of archaeal N-glycosylation, the OST from Pyrococcus furiosus, a hyperthermophilic archaeon isolated from a marine solfatara off the coast of southern Italy that grows optimally at 100°C [15], was addressed. In these studies, in vitro enzymatic experiments showed that upon incubation with an extracted pool of lipid-linked oligosaccharides and the full-length OST, an added hexapeptide containing an asparagine-based N-glycosylation signal (a ‘sequon’) was modified by a novel heptasaccharide of recently defined composition [16–18]. Although the glycan-bearing lipid moiety was not characterized in these studies, it was assumed to be DolPP [18].
In the present report, liquid chromatography-electrospray ionization mass spectrometry (LC-ESI MS) was employed to structurally characterize the lipid species bearing the complete glycan recruited in P. furiosus N-glycosylation, as well as to verify the composition of this heptasaccharide.
Materials and Methods
P. furiosus lipid extraction
P. furiosus DSM 3638 cells were a gift from Prof. Michael Adams (University of Georgia) [15]. The cell pellet (11 g) was added to a 250 ml round bottom flask and extracted with 2:1:0.8 methanol/chloroform/pellet (50 ml, total volume) for 24 h, stirring at room temperature. The mixture was vacuum-filtered through a Büchner funnel and three filter papers (Whatman Grade 1) and the filtrate was dried down using a rotary evaporator at 30°C. The dried lipid extract (52.3 mg) was stored at -20°C until further analyzed.
Normal phase (NP)LC-ESI MS
NPLC-ESI MS of the P. furiosus lipid extract was performed using an Agilent 1200 Quaternary LC system coupled to a high resolution TripleTOF5600 mass spectrometer (Applied Biosystem, Foster City, CA). An Ascentis Si HPLC column (5 μm, 25 cm × 2.1 mm) was used. Mobile phase A consisted of chloroform/methanol/aqueous ammonium hydroxide (800:195:5, v/v/v). Mobile phase B consisted of chloroform/methanol/water/aqueous ammonium hydroxide (600:340:50:5, v/v/v/v). Mobile phase C consisted of chloroform/methanol/water/aqueous ammonium hydroxide (450:450:95:5, v/v/v/v).
The elution program consisted of the following: 100% mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 11 min. The LC gradient was then changed to 100% mobile phase C over 3 min and held at 100% C for 3 min, then returned to 100% A over 0.5 min and held at 100% A for 5 min. The LC eluent (with a total flow rate of 300 μl/min) was introduced into the ESI source of the mass spectrometer. Instrument settings for negative ion ESI/MS and MS/MS analysis of lipid species were as follows: Ion spray voltage (IS) = -4500 V; Curtain gas (CUR) = 20 psi; Ion source gas 1 (GS1) = 20 psi; De-clustering potential (DP) = -55 V; Focusing potential (FP) = -150 V. The MS/MS analysis used nitrogen as the collision gas. Data acquisition and analysis were performed using the Analyst TF1.5 software (Applied Biosystem, Foster City, CA).
Reverse phase (RP)LC-ESI/MS
RPLC-ESI/MS of the P. furiosus lipid extract was performed using a Shimadzu LC system (comprising a solvent degasser, two LC-10A pumps and a SCL-10A system controller) coupled to a TripleTOF5600 mass spectrometer (Applied Biosystems, Foster City, CA). LC was operated at a flow rate of 200 μl/min with a linear gradient as follows: 100% of mobile phase A was held isocratically for 2 min and then linearly increased to 100% mobile phase B over 14 min and held at 100% B for 4 min. Mobile phase A consisted of methanol/acetonitrile/aqueous 1 mM ammonium acetate (60/20/20, v/v/v). Mobile phase B consisted of 100% ethanol containing 1 mM ammonium acetate. A Zorbax SB-C8 reversed-phase column (5 μm, 2.1 x 50 mm) was obtained from Agilent (Palo Alto, CA). The MS operating conditions were as described above.
Results
P. furiosus DolP is charged with a heptasaccharide and its precursors
In several archaea, including Hbt. salinarum [14] and Sulfolobus acidocaldarius [12], DolP is charged with the same glycans (or their precursors) as those N-linked to glycoproteins in these organisms. In other instances, such as Haloferax volcanii [11] and Methanococcus voltae [19], evidence for the delivery of oligosaccharides or single sugars from DolP carriers to target Asn residues has been provided. Although in vitro efforts revealed that lipid-linked oligosaccharides are required for P. furiosus N-glycosylation, the identity of the lipid carrier was not defined [16–18]. To thus determine the nature of this heptasaccharide-charged lipid carrier in P. furiosus, RPLC-ESI MS was performed.
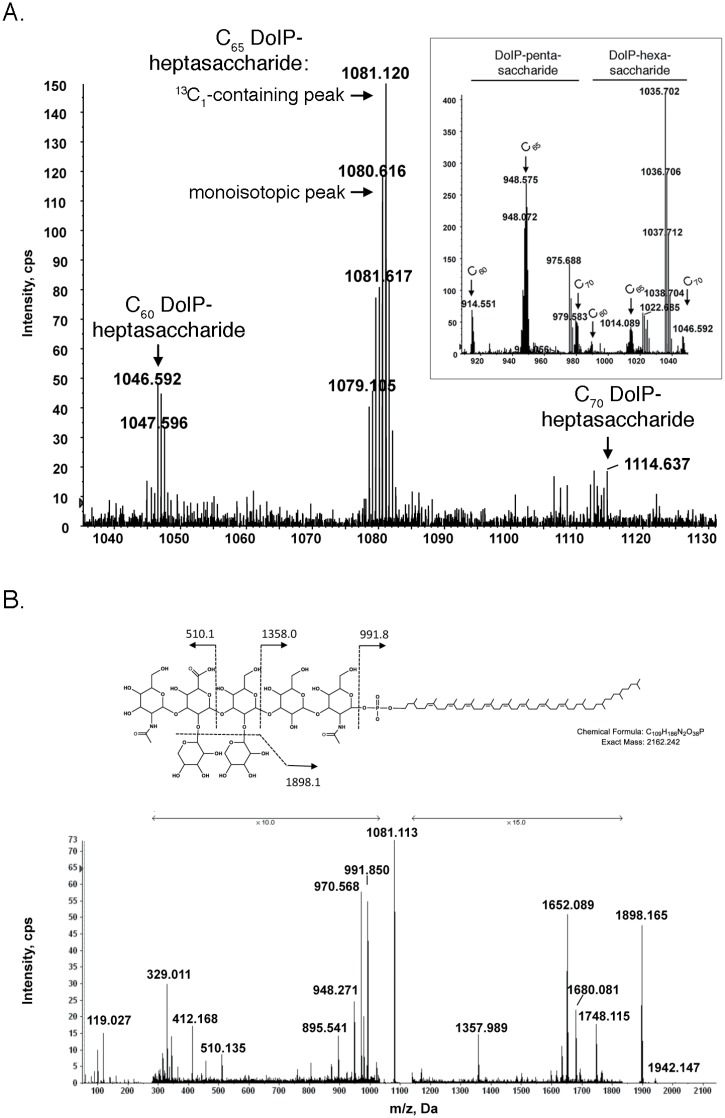
When the MS profile of the RPLC fraction with a retention time of 8.5–9.5 min was examined, a doubly de-protonated [M-2H]2- ion peak of m/z 1081.120 was observed (Fig 1A). The mass of this ion (observed mass 2164.256 Da) matches with C65 DolP linked to the heptasaccharide that P. furiosus AglB was previously shown to add to sequon-containing peptides (13C1-containing isotopic exact mass 2164.250 Da). In the same window of retention time, monoisotopic [M-2H]2- peaks of m/z 1046.592 and 1114.637, corresponding to heptasaccharide-charged C60 and C70 DolP, were also observed, albeit at lesser intensities. The same profile also included 13C1-containing isotopic [M-2H]2- peaks of m/z 914.551, 948.575 and 979.583, corresponding to C60, C65 and C70 DolP attached to the pentasaccharide precursor of the complete heptasaccharide, as well as [M-2H]2- peaks of m/z 980.585, 1014.089 and 1048.16, corresponding to C60, C65 and C70 DolP attached to the hexasaccharide precursor of the same heptasaccharide (Fig 1A, inset). Note that the m/z values reported correspond to either the monoisotopic or 13C1-containing isotopic peak, depending on which appeared as the highest peak in the mass spectrum of each species.
Fig 1. P. furiosus contains heptasaccharide-charged DolP.
A. The mass spectrum of the RPLC fraction with a retention time of 8.5–9.5 min contains [M-2H]2- peaks of m/z 1046.592, 1081.120 and 1114.637, corresponding to heptasaccharide-charged C60, C65 and C70 DolP, respectively. The 13C1-containing isotopic and monoisotopic [M-2H]2- peaks of heptasaccharide-charged C65 DolP are indicated. The inset shows [M-2H]2- peaks of m/z 914.551, 948.575 and 979.583, corresponding to C60, C65 and C70 DolP attached to the pentasaccharide precursor of the complete heptasaccharide, as well as [M-2H]2- peaks of m/z 980.585, 1014.089 and 1048.116, corresponding to C60, C65 and C70 DolP attached to the hexasaccharide precursor of the same heptasaccharide. B. The chemical structure (based on the N-linked glycan) and MS/MS fragmentation scheme are shown in the panel top. The MS/MS spectrum of the [M-2H]2- peak of m/z 1081.12 corresponding to heptasaccharide-charged C65 DolP is presented in the panel bottom. The arrows indicating x10 and x15 reflect magnification of the ion peaks in the corresponding region of m/z values on the spectrum.
The heptasaccharide added to sequon-containing peptides in the in vitro assay introduced above was shown to comprise a linear pentasaccharide containing N-acetylgalactosamine, two mannoses, glucuronic acid and N-acetylmannosamine, with xyloses attached to the second mannose and the glucuronic acid [18]. To determine whether the same glycan was attached to P. furiosus DolP in the present study, the monoisotopic [M-2H]2- peak of m/z 1081.12 thought to correspond to heptasaccharide-charged C65 DolP was further analyzed by tandem mass spectrometry (MS/MS). The fragmentation pattern obtained is consistent with the previously described N-linked glycan (Fig 1B). No sugar stereochemistry is drawn in the chemical structure due to the lack of such information from the MS analysis. Such structural information has, however, been reported by Kohda and co-workers [18].
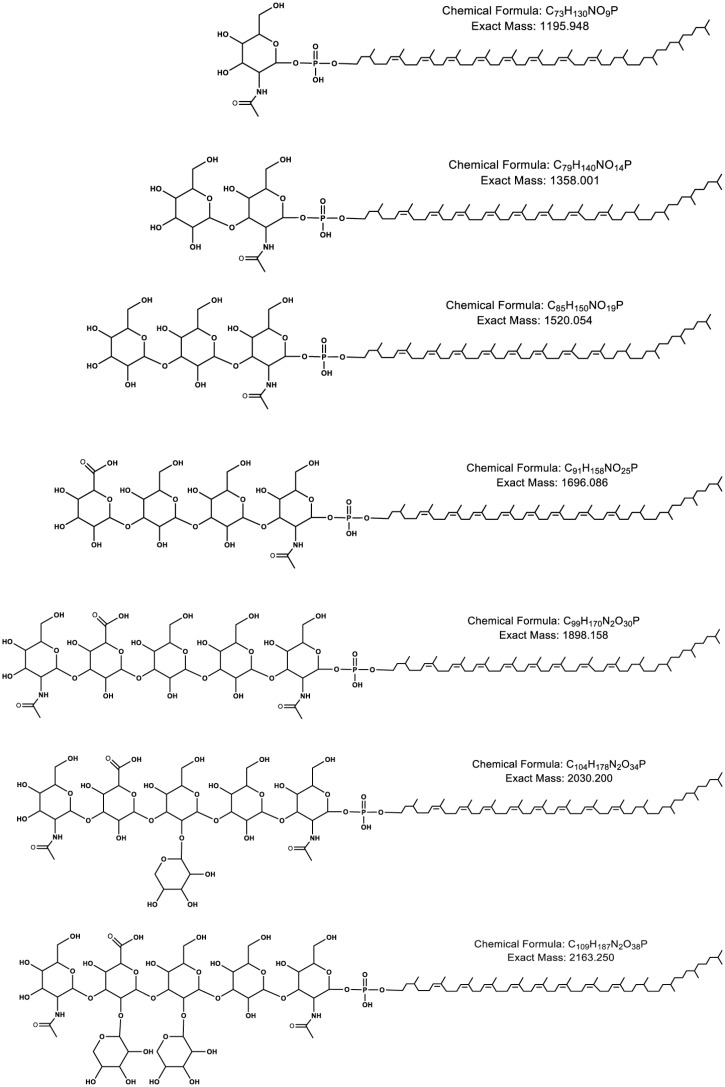
In addition to heptasaccharide-charged DolP, as well as the hexa- and pentasaccharide-charged precursors, RPLC-ESI MS also revealed the presence of tetra-, tri-, di- and monosaccharide-charged DolP in the P. furiosus lipid extract. The chemical structures and exact masses of different glycan-charged C65 DolP species are presented in Fig 2.
Fig 2. The glycan-charged DolP species detected in the P. furiosus lipid extract.
The predicted chemical structures and calculated masses of C65 DolP charged with the mono-, di-, tri-, tetra-, penta-, hexasaccharide-, and the complete heptasaccharide are shown (top to bottom, respectively).
Notably, no DolPP-linked glycan species were detected in the total lipid extracts.
P. furiosus DolP is highly saturated
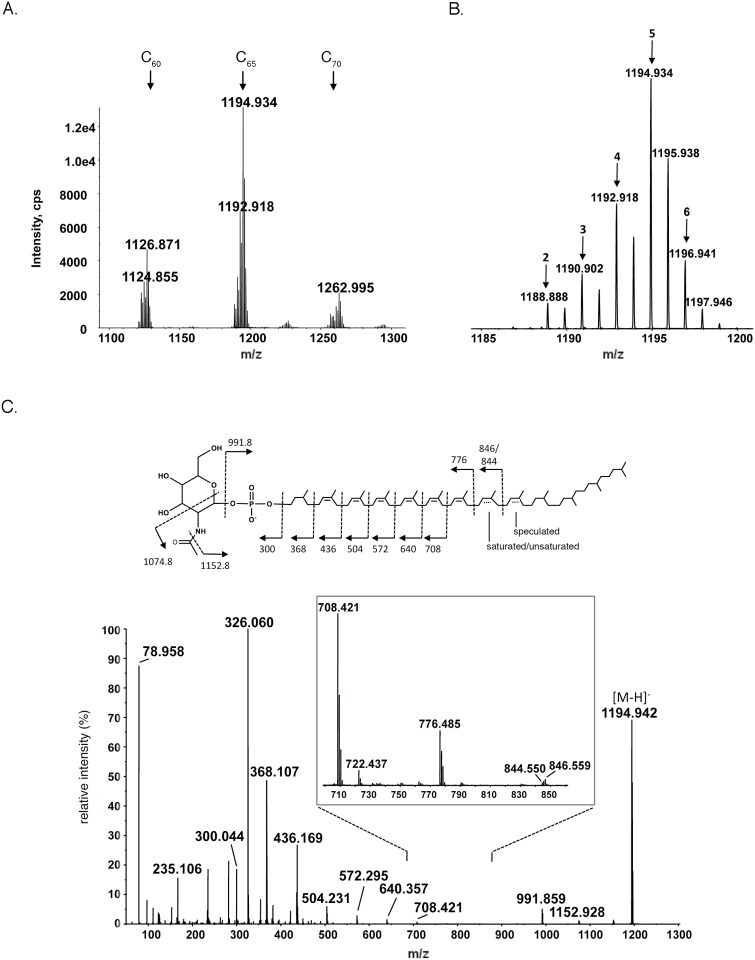
To better characterize the lipid carrier to which the heptasaccharide and its precursors are bound, NPLC-ESI MS was performed. With a retention time of 14–14.5 min, [M-H]- peaks corresponding to monosaccharide (N-acetylhexosamine)-charged C6o, C65 and C70 DolP (m/z 1126.871, 1194.934 and 1262.995, respectively) were observed (Fig 3A). Each species comprises a series of DolP variants showing differing degrees of isoprene subunit saturation (Fig 3B). When MS/MS analysis was performed on the [M-H]- ion of C65 DolP at m/z 1194.934 (Fig 3C), a fragmentation pattern consistent with a DolP molecule presenting a saturated isoprene subunit at the α-position, as well as multiple saturated isoprene subunits close to the ω-end of the polyisoprenoid chain, was obtained The results show that some isoprene units are only partially saturated. For example, the m/z 846 and 844 fragments suggest that isoprene-6 (from the ω-end) is only partially saturated. Such heterogeneity makes complete characterization difficult, even with the most advanced MS/MS techniques. Indeed, to the best of our knowledge, no better characterization of dolichols has been previously reported. In addition, a peak at m/z 78.950, corresponding to a phosphate group, was noted.
Fig 3. P. furiosus DolP is highly saturated.
A. The MS spectrum of the NPLC fraction with a retention time of 14–14.5 min contains [M-H]- ion peaks corresponding to N-acetylhexosamine-linked C6o, C65 and C70 DolP (m/z 1126.871, 1194.934 and 1262.995, respectively). Each DolP species includes 7–11 unsaturated isoprene subunits. B. Zoomed-in spectrum of N-acetylhexosamine-charged C65 DolP [M-H]- ion peaks showing the varying degree of dolichol saturation. Peaks corresponding to species containing dolichol that includes 2–6 saturated bonds are indicated accordingly. C. MS/MS spectrum of the [M-H]- ion of C65 DolP at m/z 1194.934. The fragmentation scheme (inset) shows the distribution of the saturated bonds (mostly near the ω-terminus).
Finally, [M-H]- peaks of m/z 983.811, 987.827, 989.844, 991.853, corresponding to C65 DolP containing 11, 10, 9 and 8 unsaturated isoprene subunits, respectively, were observed. However, the levels detected were insufficient for MS/MS (not shown).
Discussion
Across evolution, N-linked glycans are initially assembled on cytoplasmically-oriented phosphorylated polyisoprenoids before delivery to target proteins in the lumen of the endoplasmic reticulum in eukaryotes, in the periplasm in bacteria and on the external surface of the cell in archaea [8,20,21]. Comparison of these lipid carriers, however, reveals domain-specific traits. In bacteria, a nucleotide-charged version of the linking sugar is reacted with UndP to afford an undecaprenol pyrophosphate (UndPP)-linked sugar. Further glycosylation of the resulting UndPP-linked sugar ensues [22]. In higher eukaryotes, a similar process takes place on the cytoplasmic face of the endoplasmic reticulum (ER) membrane and results in the generation of a heptasaccharide-charged DolPP core. Additional individual sugars are subsequently transferred to the DolPP-linked glycan from DolP carriers on the luminal face of the ER membrane [6]. In archaea where lipids involved in N-glycosylation have been studied, it was reported that glycans are attached to DolP carriers [9,11,13,15,19,23,24]. The sole confirmed exception reported to date is Hbt. salinarum, where one of the two glycans N-linked to the S-layer glycoprotein is derived from a DolPP carrier. Of the 11 Asn residues decorated by glycans in this protein, only Asn-2 bears the unique DolPP-derived glycan; 10 present a common oligosaccharide derived from a DolP carrier [14].
Still, just as archaeal N-linked glycosylation shows diversity in terms of glycan composition and architecture not seen in eukaryotes or bacteria [25], the DolP carrier used in archaeal N-glycosylation also shows considerable variety. Like its eukaryal counterpart, archaeal DolP is saturated at the α-position isoprene but is also saturated at the ω-position isoprene [10,11]. In at least one case, namely Sulfolobus acidocaldarius, a thermoacidophilic archaeon that grows optimally at 80°C and pH 2 [26], not only are the α- and ω-isoprenes saturated, so are several internal isoprene units [12]. Indeed, prior to the present study, no other DolPs presenting a similar extent of saturation had been reported. Moreover, archaeal DolPs reported prior to the present report were shown to be shorter than their eukaryal counterparts [27]. In the case of the S. acidocaldarius, DolP contains only nine isoprene units, many of which are saturated [12]. In this report, it was shown that P. furiosus DolP isoprenes at both the α- and ω-positions are saturated, as are 1–4 internal isoprenes. At the same time, P. furiosus DolP is longer than any known archaeal DolP, comprising 12–14 isoprenes, with C65 DolP predominating. As such, it would appear that the degree of DolP saturation, but not Dol length, corresponds to an adaptation to life at high temperatures. Indeed, the relative shortness of S. acidocaldarius DolP (C45) could reflect the monolayer nature of its plasma membrane [28]. Moreover, while S. acidocaldarius belongs to the Crenarchaeota, a major archaeal phyla, P. furiosus is assigned to the Euryarchaeota, a second major archaeal phylum [29]. Hence, comparison of phosphorylated dolichols in organisms belonging to these two fundamental branches of the archaeal tree could provide evolutionary insight into the biosynthesis of this molecule.
One possible reason why archaeal N-glycosylation may favor DolP rather than DolPP as the glycan carrier (with Hbt. salinaurum providing the sole exception to date [14]), may be as an adaptation to the harsh environments archaea can inhabit, since DolPP-glycan derivatives are chemically more labile than the corresponding DolP-glycan derivatives. Indeed, studies addressing the behavior of components of the Methanococcus voltae N-glycosylation pathway revealed that sugar-charged DolP but not DolPP served as substrate for AglB and other enzymes [19]. At the same time, computer-based fitting (not shown) revealed that both P. furiosus DolP-heptasaccharide as characterized in this study and modeled DolPP-heptasaccharide could be accommodated within the solved crystal structure of the P. furiosus AglB soluble domain [17], implying that steric considerations cannot explain the preferential use of DolP as lipid glycan carrier. Accordingly, Hbt. salinarum only encodes a single version of AglB that is apparently able to process distinct DolP- and DolPP-bound glycans [30].
Describing the chemical structure of the phosphorylated dolichol purportedly used for N-glycosylation in this organism could potentially be integrated with earlier crystallographic analysis of the C-terminal domain of P. furious AglB (PF0156) which includes part of the catalytic machinery of this enzyme [17] to provide new structural and mechanistic insight into this protein-processing event. AglB is the archaeal oligosaccharyltransferase, responsible for transferring the phosphorylated dolichol-bound glycan to target protein asparagine residues [31,32]. Although structural information on AglBs from other archaea (i.e. Archaeoglobus fulgidus [33–35]) and Pyrococcus horikoshii [36]) is available, nothing is yet known of the lipid-linked glycan carrier in these species. To date, the interaction between an oligosaccharyltransferase and the corresponding lipid-linked oligosaccharide substrate has only been proposed based on molecular modeling of the bacterial oligosaccharyltransferase PglB [37].
Finally, DolP modified by the complete heptasaccharide recruited for N-glycosylation in P. furious [17,18], as well as the hexa- through monosaccharide precursors of this glycan, was detected. As such, it is possible that assembly of the N-linked heptasaccharide on the DolP carrier begins with the sequential addition of the first five heptasaccharide sugars (N-acetylgalactosamine, mannose, mannose, glucuronic acid, N-acetylmannosamine) followed by the addition of xyloses to the second mannose and then to the glucuronic acid. Indeed, glycopeptides modified by the N-linked heptasaccharide as well as by a hexa- and a pentasaccharide respectively lacking one or two xyloses were reported [18]. Still, the techniques used in that study and the present report cannot distinguish between precursors or breakdown products of the hexasaccharide. As such, additional genetic or biochemical studies will be required to delineate the pathway of N-glycosylation in P. furious.
Further insight into the evolution and biology of archaeal phosphodolichols will be possible as more archaeal genomic sequences are revealed and tools for their manipulation become available, along with the growing number of solved structures of AglB proteins [33–36] and in vitro assays for studying the assembly and processing of lipid-linked oligosaccharides [19]. Such efforts could prove important for questions beyond archaeal N-glycosylation.
Acknowledgments
The authors thank Prof. Mike Adams and Farris Poole from the University of Georgia for the gift of a P. furiosus cell pellet.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by the National Institutes of Health (NIH) through grant GM-039334 to B.I. and by the Israel Science Foundation through grant 8/11 to J.E. The mass spectrometry facility in the Department of Biochemistry of the Duke University Medical Center and Z.G. were supported by the NIH through the LIPID MAPS Large Scale Collaborative Grant number GM-069338 to Z.G.
References
- 1. Swiezewska E, Danikiewicz W (2005) Polyisoprenoids: structure, biosynthesis and function. Prog Lipid Res 44: 235–258. [DOI] [PubMed] [Google Scholar]
- 2. Jones MB, Rosenberg JN, Betenbaugh MJ, Krag SS (2009) Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim Biophys Acta 1790: 485–494. 10.1016/j.bbagen.2009.03.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hartley MD, Imperiali B (2012) At the membrane frontier: a prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch Biochem Biophys 517: 83–97. 10.1016/j.abb.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGarvey DJ, Croteau R (1995) Terpenoid metabolism. Plant Cell 7: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajda A, Konopka-Postupolska D, Krzymowska M, Hennig J, Skorupinska-Tudek K, Surmacz L, et al. (2009) Role of polyisoprenoids in tobacco resistance against biotic stresses. Physiol Plant 135: 351–364. 10.1111/j.1399-3054.2009.01204.x [DOI] [PubMed] [Google Scholar]
- 6. Burda P, Aebi M (1999) The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta 1426: 239–257. [DOI] [PubMed] [Google Scholar]
- 7. Reid CW, Stupak J, Szymanski CM (2010) Characterization of lipid-linked oligosaccharides by mass spectrometry. Methods Mol Biol 600: 187–197. 10.1007/978-1-60761-454-8_13 [DOI] [PubMed] [Google Scholar]
- 8. Jarrell KF, Ding Y, Meyer BH, Albers SV, Kaminski L, Eichler J (2014) N-linked glycosylation in Archaea: a structural, functional, and genetic analysis. Microbiol Mol Biol Rev 78: 304–341. 10.1128/MMBR.00052-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lechner J, Wieland F, Sumper M (1985) Transient methylation of dolichyl oligosaccharides is an obligatory step in halobacterial sulfated glycoprotein biosynthesis. J Biol Chem 260: 8984–8989. [PubMed] [Google Scholar]
- 10. Kuntz C, Sonnenbichler J, Sonnenbichler I, Sumper M, Zeitler R (1997) Isolation and characterization of dolichol-linked oligosaccharides from Haloferax volcanii. Glycobiology 7: 897–904. [DOI] [PubMed] [Google Scholar]
- 11. Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J (2010) Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol Microbiol 78: 1294–1303. 10.1111/j.1365-2958.2010.07405.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Guan Z, Meyer BH, Albers SV, Eichler J (2011) The thermoacidophilic archaeon Sulfolobus acidocaldarius contains an unusually short, highly reduced dolichyl phosphate. Biochim Biophys Acta 1811: 607–616. 10.1016/j.bbalip.2011.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen-Rosenzweig C, Guan Z, Shaanan B, Eichler J (2014) Substrate promiscuity: AglB, the archaeal oligosaccharyltransferase, can process a variety of lipid-linked glycans. Appl Environ Microbiol 80: 486–496. 10.1128/AEM.03191-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lechner J, Wieland F (1989) Structure and biosynthesis of prokaryotic glycoproteins. Annu Rev Biochem 58: 173–194. [DOI] [PubMed] [Google Scholar]
- 15. Fiala G, Stetter KO (1986) Pyrococcus-Furiosus Sp-Nov Represents a Novel Genus of Marine Heterotrophic Archaebacteria Growing Optimally at 100-Degrees C. Archives of Microbiology 145: 56–61. [Google Scholar]
- 16. Kohda D, Yamada M, Igura M, Kamishikiryo J, Maenaka K (2007) New oligosaccharyltransferase assay method. Glycobiology 17: 1175–1182. [DOI] [PubMed] [Google Scholar]
- 17. Igura M, Maita N, Kamishikiryo J, Yamada M, Obita T, Maenaka K, et al. (2008) Structure-guided identification of a new catalytic motif of oligosaccharyltransferase. EMBO J 27: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujinami D, Matsumoto M, Noguchi T, Sonomoto K, Kohda D (2014) Structural elucidation of an asparagine-linked oligosaccharide from the hyperthermophilic archaeon, Pyrococcus furiosus. Carbohydr Res 387: 30–36. 10.1016/j.carres.2014.01.021 [DOI] [PubMed] [Google Scholar]
- 19. Larkin A, Chang MM, Whitworth GE, Imperiali B (2013) Biochemical evidence for an alternate pathway in N-linked glycoprotein biosynthesis. Nat Chem Biol 9: 367–373. 10.1038/nchembio.1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nothaft H, Szymanski CM (2010) Protein glycosylation in bacteria: sweeter than ever. Nat Rev Microbiol 8: 765–778. 10.1038/nrmicro2383 [DOI] [PubMed] [Google Scholar]
- 21. Breitling J, Aebi M (2013) N-Linked Protein Glycosylation in the Endoplasmic Reticulum. Cold Spring Harbor Perspectives in Biology 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wood AC, Oldfield NJ, O'Dwyer CA, Ketley JM (1999) Cloning, mutation and distribution of a putative lipopolysaccharide biosynthesis locus in Campylobacter jejuni. Microbiology-Uk 145: 379–388. [DOI] [PubMed] [Google Scholar]
- 23. Calo D, Guan Z, Eichler J (2011) Glyco-engineering in Archaea: differential N-glycosylation of the S-layer glycoprotein in a transformed Haloferax volcanii strain. Microb Biotechnol 4: 461–470. 10.1111/j.1751-7915.2011.00250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kaminski L, Guan Z, Yurist-Doutsch S, Eichler J (2013) Two distinct N-glycosylation pathways process the Haloferax volcanii S-layer glycoprotein upon changes in environmental salinity. MBio 4: e00716–00713. 10.1128/mBio.00716-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eichler J (2013) Extreme sweetness: protein glycosylation in archaea. Nature Reviews Microbiology 11: 151–156. 10.1038/nrmicro2957 [DOI] [PubMed] [Google Scholar]
- 26. Brock TD, Brock KM, Belly RT, Weiss RL (1972) Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol 84: 54–68. [DOI] [PubMed] [Google Scholar]
- 27. Guan Z, Eichler J (2011) Liquid chromatography/tandem mass spectrometry of dolichols and polyprenols, lipid sugar carriers across evolution. Biochim Biophys Acta 1811: 800–806. 10.1016/j.bbalip.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. De Rosa M, Gambacorta A, Gliozzi A (1986) Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol Rev 50: 70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brochier-Armanet C, Forterre P, Gribaldo S (2011) Phylogeny and evolution of the Archaea: one hundred genomes later. Curr Opin Microbiol 14: 274–281. 10.1016/j.mib.2011.04.015 [DOI] [PubMed] [Google Scholar]
- 30. Magidovich H, Eichler J (2009) Glycosyltransferases and oligosaccharyltransferases in Archaea: putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol Lett 300: 122–130. 10.1111/j.1574-6968.2009.01775.x [DOI] [PubMed] [Google Scholar]
- 31. Abu-Qarn M, Eichler J (2006) Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol Microbiol 61: 511–525. [DOI] [PubMed] [Google Scholar]
- 32. Chaban B, Voisin S, Kelly J, Logan SM, Jarrell KF (2006) Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea. Mol Microbiol 61: 259–268. [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto S, Igura M, Nyirenda J, Matsumoto M, Yuzawa S, Noda N, et al. (2012) Crystal structure of the C-terminal globular domain of oligosaccharyltransferase from Archaeoglobus fulgidus at 1.75 A resolution. Biochemistry 51: 4157–4166. 10.1021/bi300076u [DOI] [PubMed] [Google Scholar]
- 34. Matsumoto S, Shimada A, Nyirenda J, Igura M, Kawano Y, Kohda D (2013) Crystal structures of an archaeal oligosaccharyltransferase provide insights into the catalytic cycle of N-linked protein glycosylation. Proceedings of the National Academy of Sciences of the United States of America 110: 17868–17873. 10.1073/pnas.1309777110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Matsumoto S, Shimada A, Kohda D (2013) Crystal structure of the C-terminal globular domain of the third paralog of the Archaeoglobus fulgidus oligosaccharyltransferases. Bmc Structural Biology 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nyirenda J, Matsumoto S, Saitoh T, Maita N, Noda NN, Inagaki F, et al. (2013) Crystallographic and NMR Evidence for Flexibility in Oligosaccharyltransferases and Its Catalytic Significance. Structure 21: 32–41. 10.1016/j.str.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 37. Lizak C, Gerber S, Numao S, Aebi M, Locher KP (2011) X-ray structure of a bacterial oligosaccharyltransferase. Nature 474: 350–U377. 10.1038/nature10151 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.