Abstract
Introduction
Right ventricular failure remains a major cause of mortality during acute pulmonary embolism. Right ventricular function can be assessed with transesophageal echocardiography. However, due to the complex right ventricular anatomy, only a few echocardiographic parameters are reliable and easily obtainable intraoperatively. Tricuspid annular plane systolic excursion is a validated parameter of global right ventricular function.
Methods
Data from 81 patients with acute pulmonary embolus undergoing pulmonary embolectomy were evaluated. Transesophageal echocardiography derived parameters of right ventricular function were obtained and compared to tricuspid annular plane systolic excursion measurements. Patients were then divided into two groups (TAPSE < 18 mm and ≥18 mm).
Results
The patient population consisted of 46 males and 35 females, mean age 61.0 ± 12.9 years. Patients in the TAPSE <18 mm group had significantly larger diastolic (p=0.0015) and systolic (p=0.0031) right ventricular diameters, lower right ventricular fractional area change (p=0.0065) and greater degrees of tricuspid regurgitation (p=0.0001) compared to patients with TAPSE ≥18 mm. In addition, all patients who needed intraoperative cardiopulmonary resuscitation (11/81) or died intraoperatively (8/81) belonged to the TAPSE <18 mm group. Logistic regression analysis confirmed TAPSE <18 mm as an independent risk factor for intraoperative cardiopulmonary resuscitation and death.
Conclusions
Transesophageal echocardiography derived TAPSE is easily obtainable and correlates well with other standardized parameters of right ventricular function. TAPSE <18 mm is an independent predictor of intraoperative cardiopulmonary resuscitation and death in patients undergoing emergent pulmonary embolectomy.
Keywords: pulmonary embolism, TAPSE, transesophageal echocardiography, right ventricle, cardiopulmonary resuscitation
Introduction
Massive acute pulmonary embolism (PE) remains a life threatening event, associated with significant morbidity and mortality [1,2,3]. Conservative treatment of PE includes administration of thrombolytics, however, in hemodynamically unstable patients, emergent surgical embolectomy may be indicated. Hemodynamic instability is mostly due to an acute increase in right ventricular (RV) afterload resulting in ventricular strain, dilatation and ultimately ventricular failure with cardiac arrest and death if the cycle is not interrupted in a timely manner.
Intraoperative transesophageal echocardiography (TEE) is commonly used during cardiac surgery as a monitor of cardiac performance and a diagnostic tool for visualizing pulmonary emboli within the main or right pulmonary arteries [4, 5]. Indirect echocardiographic signs of acute PE include a dilated right atrium (RA) or RV with bowing of the interatrial septum, tricuspid regurgitation (TR) as well as underfilling of the left ventricle (LV) [6]. Thus, TEE can provide useful information for the peri- and intra-operative management of patients with acute PE [7, 8].
Echocardiographic assessment of RV function remains difficult because of its complex geometry. Standard echocardiographic parameters of RV function, such as fractional area change (RVFAC) and ejection fraction (RVEF), are excellent theoretical entities but, due to suboptimal RV endocardial definition, may have limited value in current clinical practice [9].
Tricuspid annular plane systolic excursion (TAPSE) is a parameter of global RV function which describes apex-to-base shortening [10, 11]. TAPSE correlates closely with the RVEF [12], and has been found to be both highly specific and easy to measure [13]. In non-surgical patients, TAPSE has been shown to be an excellent parameter for assessing RV global function and predicting poor prognosis in patients with acute inferior wall myocardial infarction [14,15,16].
In the following study, we sought to determine whether TAPSE performed with TEE during acute pulmonary embolectomy correlates with established parameters of RV function and if it has a prognostic value.
Methods
Study design and patient population. Approval for this retrospective study was obtained from the Institutional Review Board (IRB). A consecutive series of 81 patients who underwent emergent pulmonary embolectomies at Brigham and Women’s Hospital were enrolled 1997-2006 and evaluated.
Definition of preoperative medical co-morbidities. Demographic data and co-morbidities were obtained from the computerized institutional database and included age, gender, body mass index (BMI), history of malignancy, myocardial infarction (MI), pulmonary disease, cerebrovascular disease, arterial hypertension and pulmonary hypertension, and were defined according to Society of Thoracic Surgery (STS) standard definitions.
TEE exam. A comprehensive intraoperative TEE exam was performed following the induction of general anesthesia and prior to the institution of cardiopulmonary bypass (CPB), using multiplane TEE probes (Acuson, Mountain View, CA). All TEE exams were independently assessed off-line by two cardiac anaesthesiologists certified in perioperative TEE.
The following TEE derived parameters were evaluated:
- RV diameters were measured in a mid-esophageal (ME) four chamber view at end-systole (defined as the end of the T-wave on electrocardiogram) and end-diastole (defined as the R-wave on electrocardiogram) corresponding to the minor axis [17].
- RVFAC was assessed in a ME four chamber view [9] and calculated as: RVFAC = [RV end-diastolic area - RV end systolic area]/RV end-diastolic area x 100%.
- TAPSE was measured in a ME four-chamber view by placing the 2D cursor at the tricuspid lateral annulus and measuring the distance of systolic annular RV excursion along a longitudinal line defining the end of systole as the end of the T wave in the electrocardiogram [18]. Secondary echocardiographic signs of acute pulmonary artery obstruction such as RV hypokinesis, RV enlargement, flattening of the interventricular septum, leftward bowing of the interatrial septum were assessed using standardized criteria [4]. While a TAPSE as low as 16 mm has been shown to correlate with RV systolic dysfunction in a patient population with coronary artery disease [12, 18], patients in this present study were divided into two groups: TAPSE 18 mm [19]. A ROC analysis that was performed and showed the TAPSE cut-off of 18 mm to be highly sensitive of impending death and, on the other hand, useful in ruling out the risk of perioperative death (negative predictive value: 31/31=100%).
- Tricuspid regurgitation was evaluated in a ME four-chamber view and ME RV inflow-outflow view by color flow Doppler and graded as mild, moderate or severe according to the vena contracta width and the direction and size of the TR jet [20, 21].
- Bowing of the interatrial septum was defined as a leftward curvature of the interatrial septum which persisted throughout the cardiac cycle, thereby suggesting the presence of right atrial hypertension [22].
- LV diameterswere measured at end-systole and end-diastole in a ME four chamber view.
- LV Fractional area change (LVFAC) was assessed in a ME 4-chamber view by tracing around the LV cavity in end-diastole and end-systole excluding the papillary muscles using the formula LVFAC = [LV end-diastolic area - LV end systolic area]/LV end-diastolic area x 100%.
Statistical analysis. Categorical data are reported as numbers and percentages, and continuous data are summarized with the median and interquartile range if not otherwise indicated. Differences between the TAPSE < 18 mm and ≥ 18 mm groups were tested using the Mann-Whitney U test, and statistical significance was assumed for p < 0.05. In addition, the strength and direction of relationships between TAPSE and measures of ventricular function were analyzed using Spearman’s rank correlation coefficient. To investigate the relationship between TAPSE and binary outcome variables (death, need for perioperative cardio-pulmonary resuscitation), logistic regression analysis was performed, and unadjusted odds ratios were calculated as well as estimates adjusted for other known predictors (age, sex, BMI, and CPB duration). Data analysis was performed with the JMP® 10.0 statistical software (SAS Institute Inc., Cary, NC).
Results
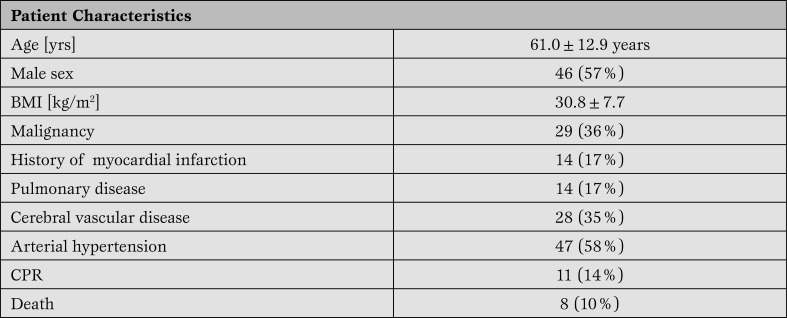
Study population. A comprehensive TEE exam including all RV measurements was obtained in all 81 patients (61.0±12.9 years, 46 males). Co-morbidities included malignancies (n=29; 36%), MI (n=14; 17%), systemic hypertension (n=47; 58%), pulmonary disease (n=14; 17%) and cerebral disorders (n=28; 35%). 11 patients (11/81, 14%) required intraoperative cardio-pulmonary resuscitation (CPR), and 8 patients (8/81, 10%) died intraoperatively. Patient characteristics and co-morbidities are shown in Table 1.
Table 1.
Patient characteristics and co-morbidities.
*BMI= body mass index; CPR=cardiopulmonary resuscitation.
TAPSE and CPB times. Cardiopulmonary bypass times did not differ between groups: TAPSE <18 mm (median 57 min, interquartile range (IQR) 35 to 99 min) vs. TAPSE ≥ 18 mm (median 58 min, IQR 37 to 97 min), (p=0.63).
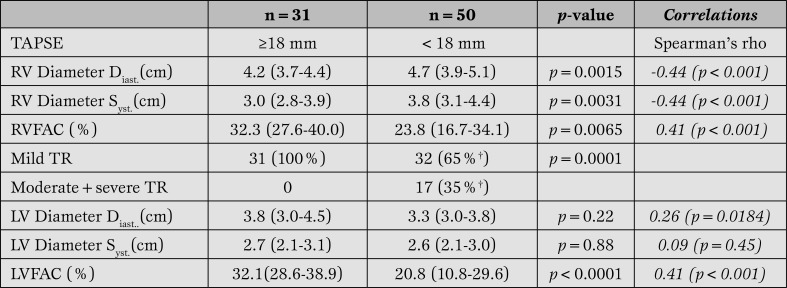
TAPSE and right ventricular echocardiographic data (Table 2).
Table 2.
TEE derived parameters of right ventricular and left ventricular function.
†1 patient with missing indication of TR. RV function data are reported with the median and interquartile range (IQR).
TAPSE = Tricuspid Annular Plane Systolic Excursion; RV = right ventricular; RVFAC = right ventricular fractional area change; TR = tricuspid regurgitation; LV = left ventricular; LV = left ventricular; LVFAC = leftventricular fractional area change.
Measured parameters of RV function including diastolic and systolic diameters and RVFAC differed significantly between patients with TAPSE < 18 mm or TAPSE ≥ 18 mm. Both systolic and diastolic RV diameters were significantly larger in the TAPSE < 18 mm population compared to the TAPSE ≥18 mm population (RVdiast. p=0.0015, RVsyst. p=0.0031). In addition, RVFAC was significantly reduced in patients with TAPSE < 18 mm compared to those with TAPSE ≥ 18 mm (p=0.0065).
A moderate to good correlation of TAPSE with established parameters of RV function was observed (Table 2).
All patients with moderate or severe TR had TAPSE measurements of < 18 mm (p=0.0001). Mild TR was associated only with equivocal TAPSE values.
TAPSE and left ventricular data (Table 2). Neither systolic nor diastolic LV diameter measurements were statistically different between the TAPSE groups < 18 mm and ≥ 18 mm (LV diameter Diast, p=0.22, LV diameters Syst, p=0.88). LVFAC was significantly reduced in the TAPSE < 18 mm group (p < 0.0001). The correlation of TAPSE with LV diameters was weak.
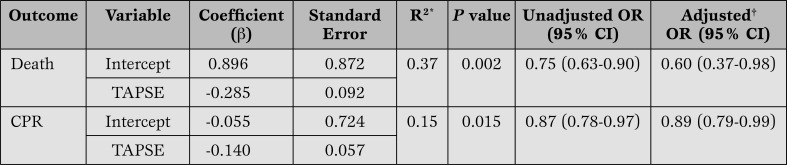
TAPSE and death and intraoperative CPR (Table 3).
Table 3.
Logistic regression analysis predicting death and CPR from TAPSE measurements (n=81 patients).
R2 (Nagelkerke), †adjusted for age, sex, BMI, and CPB time.
CPR = cardio-pulmonary resuscitation; TAPSE = Tricuspid Annular Plane Systolic Excursion, OR = odds ratio; CI = confidence interval; CPB = cardiopulmonary bypass.
Patients who died intraoperatively had a TAPSE of 6.8 mm ±3.5 which was significantly lower (p < 0.0001) compared to 15.7±6.1 in the group with surviving patients. Descriptively, all patients that died had a TAPSETable 3), a TAPSE
Patients who required intraoperative CPR had markedly decreased TAPSE values (10.2 mm ±5.9, p=0.0102) compared to the population that did not need CPR (15.5 mm ±6.3). Descriptively, only 2 patients out of 8 that needed CPR intraoperatively had a TAPSE >18 mm. Seven patients that ended up needing intraoperative CPR did not die. Again, logistic regression analysis showed TAPSE < 18 mm to be an independent predictor of impending CPR (p=0.015) (Table 3). When adjusted for age, sex, BMI, and CPB time logistic regression showed that each mm increase in TAPSE decreased the risk for intraoperative CPR by the factor 0.89.
Out of the 8 patients that died 4 patients received CPR intraoperatively.
Discussion
Massive, acute PE is a life threatening disease which may result in acute RV failure and death, when not diagnosed and treated promptly [23]. Because a failing RV is the number one cause for mortality in acute PE, an accurate and quick assessment of RV function can be critically important and potentially life-saving. Echocardiography has become increasingly important in RV functional assessment. However, echocardiographic evaluation of the RV remains challenging due to its complex geometry. Consequently, the few established echocardiographically derived parameters that can assess global RV function are not always easy to obtain, especially in an emergency situation.
TAPSE is a widely recognized, easily obtainable and clinically useful echocardiographic measure of global RV function, which has been shown to have prognostic value in patients with myocardial infarction and pulmonary hypertension [8, 10]. The value of TAPSE as a highly sensitive and specific parameter reflecting RV global function was previously shown for non-surgical patients with chronic pulmonary hypertension by Forfia et al. [19].
In this study we confirm that TAPSE correlates well with RV dimensions and RVFAC in a population of cardiac surgery patients undergoing emergent pulmonary embolectomy. We also show that decreased TAPSE values (i.e. < 18 mm) serve as independent predictors of impending intraoperative resuscitation and death.
Our data in patients with acute massive PE is consistent with prior reports from Lopez-Candales et al, who demonstrated a strong correlation between TAPSE and reduced RVFAC in patients undergoing routine transthoracic echocardiographic (TTE) examinations for various clinical indications [24].
Similar to our patient population with a TAPSE < 18 mm, these investigators also demonstrated that significant reductions in TAPSE values correlate directly with LV function, correlation that did not apply to our patient population.
Rydman et al. [25] recently found TAPSE to be a useful tool in evaluating RV function in patients with acute PE. However, echocardiographic evaluation of the patients in this study was limited to TTE examination and the patients were treated conservatively, consequently not undergoing emergent pulmonary embolectomy. Kucher et al. retrospectively evaluated 1035 patients with acute PE and preserved systemic arterial pressure in the ICOPER - study, and found RV hypokinesis to be an independent predictor of early death.
Unfortunately, it is unclear how echocardiography was used to objectively diagnose RV hypokinesis in this study [26]. Recently Lobo et al. conducted a study on more than 700 patients with acute pulmonary embolism examining parameters of right ventricular function.
In this study Lobo et al. were able to confirm TAPSE < 1,6 cm as a prognostic marker for death in this patient population. Contrary to our patient population echocardiographic data were obtained through TTE and not TEE as in our study; also, none of the patients was hemodynalmically unstable or underwent surgical pulmonary embolectomy.
We believe that our data is in keeping with and add to the aforementioned findings by studying a patient population that not only undergoes the various complex hemodynamic changes associated with acute pulmonary embolism that ultimately lead to increased right ventricular afterload, but suffers from hemodynamic instability necessitating immediate surgery [27].
The second important finding in our study is the prognostic value for increased mortality and impending CPR associated with TAPSE in patients with massive acute PE who require emergent pulmonary embolectomy. Forfia et al. [19] demonstrated that a TAPSE < 18 mm was associated with a nearly fourfold increased risk of death in medical patients with pulmonary hypertension after a 19 month follow up period (TAPSE mean =11.7 mm).
In our study we can confirm the prognostic value of TAPSE using the same criteria as Forfia et al., also showing that patients with TAPSE < 18 mm are more likely to die intraoperatively than patients with TAPSE ≥18 mm.
While our study provides novel insight into the diagnosis of RV dysfunction using echocardiography, certain limitations are worth noting including the relatively small patient population and the limited longer term follow up.
Conclusion
In summary, we confirm the described correlation between echocardiographic TAPSE measurements and RV function in the setting of acute pulmonary embolectomy using TEE. We also introduce TAPSE as a predictive parameter for poor perioperative clinical outcome in this surgical setting.
Understanding the rapid downward spiral associated with acute PE and RV failure and the availability of a simple, TEE-derived prognostic parameter such as TAPSE could influence clinical practice by enabling improved risk stratification and more efficient definitive intervention.
Acknowledgments
PS, SS, PR, MNM designed the study; ES, GB, CS, PR, SS, MNM reaviewed and analyzed data; JNH, ES, SK, MNM wrote the manuscript.
Footnotes
Source of Support Nil.
Disclosures Except for Dr. Stanton K. Shernan who discloses a relationship with "Philips Healthcare, Inc." none of the authors have any conflicts of interest.
Cite as: Schmid E, Hilberath JN, Blumenstock G, Shekar PS, Kling S, Shernan SK, Rosenberge P, Nowak-Machen M. Tricuspid annular plane systolic excursion (TAPSE) predicts poor outcome in patients undergoing acute pulmonary embolectomy. Heart, Lung and Vessels. 2015; 7(2): 151-158
References
- Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P. et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276–2315. doi: 10.1093/eurheartj/ehn310. [DOI] [PubMed] [Google Scholar]
- Tortosa J A, Hernández-Palazón J. Fatal massive intra-operative pulmonary embolism while placing a patient in the surgical position. Eur J Anaesthesiol. 1999;16:350–350. doi: 10.1046/j.1365-2346.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Chen H L, Wong C S, Ho S T, Chang F L, Hsu C H, Wu C T. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg. 2002;95:1060–1062. doi: 10.1097/00000539-200210000-00049. [Table of contents] [DOI] [PubMed] [Google Scholar]
- Rosenberger P, Shernan S K, Body S C, Eltzschig H K. Utility of intraoperative transesophageal echocardiography for diagnosis of pulmonary embolism. Anesth Analg. 2004;99:12–16. doi: 10.1213/01.ANE.0000117284.25696.64. [DOI] [PubMed] [Google Scholar]
- Rosenberger P, Shernan S K, Mihaljevic T, Eltzschig H K. Transesophageal echocardiography for detecting extrapulmonary thrombi during pulmonary embolectomy. Ann Thorac Surg. 2004;78:862–866. doi: 10.1016/j.athoracsur.2004.02.069. [Discussion 866] [DOI] [PubMed] [Google Scholar]
- Niimi Y, Hiki M, Ishiguro Y, Goto T, Morita S. Determination of right ventricular function by transesophageal echocardiography: impact of proximal right coronary artery stenosis. J Clin Anesth. 2004;16:104–110. doi: 10.1016/j.jclinane.2003.05.009. [DOI] [PubMed] [Google Scholar]
- Rosenberger P, Shernan S K, Mihaljevic T, Eltzschig H K. Transesophageal echocardiography for pulmonary embolectomy. Ann Thorac Surg. 2005;79:1092–1093. doi: 10.1016/j.athoracsur.2004.04.103. [Author reply 1093] [DOI] [PubMed] [Google Scholar]
- Rosenberger P, Shernan S K, Weissmüller T, Eltzschig H K. Role of intraoperative transesophageal echocardiography for diagnosing and managing pulmonary embolism in the perioperative period. Anesth Analg. 2005;100:292–293. doi: 10.1213/01.ANE.0000141274.42848.40. [Author reply 293] [DOI] [PubMed] [Google Scholar]
- Haddad F, Couture P, Tousignant C, Denault A Y. The right ventricle in cardiac surgery, a perioperative perspective: I. Anatomy, physiology, and assessment. Anesth Analg. 2009;108:407–421. doi: 10.1213/ane.0b013e31818f8623. [DOI] [PubMed] [Google Scholar]
- Alam M, Wardell j, Andersson E, Samad B A, Nordlander R. Characteristics of mitral and tricuspid annular velocities determined by pulsed wave Doppler tissue imaging in healthy subjects. J Am Soc Echocardiogr. 1999;12:618–628. doi: 10.1053/je.1999.v12.a99246. [DOI] [PubMed] [Google Scholar]
- Ueti O M, Camargo E E, Ueti Ade A, de Lima-Filho E C, Nogueira E A. Assessment of right ventricular function with Doppler echocardiographic indices derived from tricuspid annular motion: comparison with radionuclide angiography. Heart. 2002;88:244–248. doi: 10.1136/heart.88.3.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul S, Tei C, Hopkins J M, Shah P M. Assessment of right ventricular function using two-dimensional echocardiography. Am Heart J. 1984;107:526–531. doi: 10.1016/0002-8703(84)90095-4. [DOI] [PubMed] [Google Scholar]
- Lee S, Kamdar F, Madlon-Kay R, Boyle A, Colvin-Adams M, Pritzker M. et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant. 2010;29:209–215. doi: 10.1016/j.healun.2009.11.599. [DOI] [PubMed] [Google Scholar]
- Samad B A, Alam M, Jensen-Urstad k. Prognostic impact of right ventricular involvement as assessed by tricuspid annular motion in patients with acute myocardial infarction. Am J Cardiol. 2002;90:778–781. doi: 10.1016/s0002-9149(02)02612-7. [DOI] [PubMed] [Google Scholar]
- Niemann P S, Pinho L, Balbach T, Galuschky C, Blankenhagen M, Silberbach M. et al. Anatomically oriented right ventricular volume measurements with dynamic three-dimensional echocardiography validated by 3-Tesla magnetic resonance imaging. J Am Coll Cardiol. 2007;50:1668–1676. doi: 10.1016/j.jacc.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Tamborini G, Muratori M, Brusoni D, Celeste F, Maffessanti F, Caiani E G. et al. Is right ventricular systolic function reduced after cardiac surgery? A two- and three-dimensional echocardiographic study. Eur J Echocardiogr. 2009;10:630–634. doi: 10.1093/ejechocard/jep015. [DOI] [PubMed] [Google Scholar]
- Lang R M, Bierig M, Devereux R B, Flachskampf F A, Foster E, Pellikka P A. et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Rudski L G, Lai W W, Afilalo J, Hua L, Handschumacher M D, Chandrasekaran K. et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [Quiz 786-8] [DOI] [PubMed] [Google Scholar]
- Forfia P R, Fisher M R, Mathai S C, Housten-Harris T, Hemnes A R, Borlaug B A. et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- Lancellotti P, Moura L, Pierard L A, Agricola E, Popescu B A, Tribouilloy C. et al. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr. 2010;11:307–332. doi: 10.1093/ejechocard/jeq031. [DOI] [PubMed] [Google Scholar]
- Rogers J H, Bolling S F. The tricuspid valve: current perspective and evolving management of tricuspid regurgitation. Circulation. 2009;119:2718–2725. doi: 10.1161/CIRCULATIONAHA.108.842773. [DOI] [PubMed] [Google Scholar]
- Royse C F, Royse A G, Soeding P F, Blake D W. Shape and movement of the interatrial septum predicts change in pulmonary capillary wedge pressure. Ann Thorac Cardiovasc Surg. 2001;7:79–83. [PubMed] [Google Scholar]
- Aklog L, Williams C S, Byrne J G, Goldhaber S Z. Acute pulmonary embolectomy: a contemporary approach. Circulation. 2002;105:1416–1419. doi: 10.1161/01.cir.0000012526.21603.25. [DOI] [PubMed] [Google Scholar]
- López-Candales A, Rajagopalan N, Saxena N, Gulyasy B, Edelman K, Bazaz R. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol. 2006;98:973–977. doi: 10.1016/j.amjcard.2006.04.041. [DOI] [PubMed] [Google Scholar]
- Rydman R, Larsen F, Caidahl K, Alam M. Right ventricular function in patients with pulmonary embolism: early and late findings using Doppler tissue imaging. J Am Soc Echocardiogr. 2010;23:531–537. doi: 10.1016/j.echo.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Kucher N, Rossi E, De Rosa M, Goldhaber S Z. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165:1777–1781. doi: 10.1001/archinte.165.15.1777. [DOI] [PubMed] [Google Scholar]
- Lobo J L, Holley A, Tapson V, Moores L, Oribe M, Barrón M. et al. Prognostic significance of tricuspid annular displacement in normotensive patients with acute symptomatic pulmonary embolism. J Thromb Haemost. 2014;12:1020–1027. doi: 10.1111/jth.12589. [DOI] [PubMed] [Google Scholar]