Abstract
Background and Objective
Conflicting data have been reported on the association between tumor necrosis factor (TNF) –308G>A and nitric oxide synthase 3 (NOS3) +894G>T polymorphisms and migraine. We performed a meta-analysis of case-control studies to evaluate whether the TNF –308G>A and NOS3 +894G>T polymorphisms confer genetic susceptibility to migraine.
Method
We performed an updated meta-analysis for TNF –308G>A and a meta-analysis for NOS3 +894G>T based on studies published up to July 2014. We calculated study specific odds ratios (OR) and 95% confidence intervals (95% CI) assuming allele contrast, dominant model, recessive model, and co-dominant model as pooled effect estimates.
Results
Eleven studies in 6682 migraineurs and 22591 controls for TNF –308G>A and six studies in 1055 migraineurs and 877 controls for NOS3 +894G>T were included in the analysis. Neither indicated overall associations between gene polymorphisms and migraine risk. Subgroup analyses suggested that the “A” allele of the TNF –308G>A variant increases the risk of migraine among non-Caucasians (dominant model: pooled OR = 1.82; 95% CI 1.15 – 2.87). The risk of migraine with aura (MA) was increased among both Caucasians and non-Caucasians. Subgroup analyses suggested that the “T” allele of the NOS3 +894G>T variant increases the risk of migraine among non-Caucasians (co-dominant model: pooled OR = 2.10; 95% CI 1.14 – 3.88).
Conclusions
Our findings appear to support the hypothesis that the TNF –308G>A polymorphism may act as a genetic susceptibility factor for migraine among non-Caucasians and that the NOS3 +894G>T polymorphism may modulate the risk of migraine among non-Caucasians.
Introduction
Migraine is characterized by recurrent, moderate to severe, throbbing headache attacks, associated with photophobia, phonophobia, nausea, and vomiting. Migraine is remarkably common, affecting approximately 11% of the adult population around the world [1] and 9.3% in China [2]. This condition is associated with high financial costs, reported to cost $18.5 billion Euros per year in Europe [3], and is listed as one of the top 20 most debilitating disorders according to the World Health Organization [4]. Migraine is therefore a public health problem that has a major impact on both the individual and society.
The etiology of migraine is complex, involving both multiple genetic and environmental factors [5]. It is now acknowledged that changes in immune homeostasis leading to changes in cytokine profiles can contribute to migraine [6].
Sterile meningeal inflammation is a key mechanism that may underlie the sustained activation and sensitization of perivascular meningeal nociceptors. Cytokines and nitric oxide (NO) have been implicated in the pathogenesis of migraine in both animal and human studies [7].
Tumor necrosis factor-alpha (TNF-α) is an important cytokine that can promote powerful hyperalgesia by causing prostanoid release, increasing nerve growth factor (NGF) and bradykinin receptor expression, or by modulation of activity within sympathetic fibers. Several studies have indicated changes in serum [8], plasma [9], and urine [10] concentrations of TNF-α as well as altered serum concentrations of the soluble TNF-α receptor [11] among migraineurs either during or outside of attacks. Furthermore, TNF-α can stimulate transcription of calcitonin gene-related peptide (CGRP), which plays a pivotal role in the pathophysiology of migraine [12]. Exaggerated serum concentrations of TNF reverted to normality after drug therapy [13].
NO is a potent endogenous vasodilator, which is a key molecule affecting the pain associated with migraine [14], as it was shown to cause immediate headache in migraineurs and less often in control subjects [15–17]. Impaired release of NO can lead to vascular/coagulative dysfunction [18] with subsequent variation in cerebral blood flow, and activate cortical spreading depression and the trigeminovascular system, which mediate the pain [19].
NO is synthesized from l-arginine and molecular oxygen by the NO synthase (NOS) family. In endothelial cells, NO is synthesized by the endothelial NOS (eNOS). Deregulation of eNOS influences the plasma level of NO, which has an anti-inflammatory effect. The haplotype of the NOS3 gene is associated with variability in endogenous NO formation [20].
Migraine is a complex genetic disorder in which several genes play a role [21–23]. Identification of susceptibility genes will enable a better understanding of the mechanisms underlying the disease processes. The gene encoding TNF-α is located in the class III gene cluster of the major histocompatibility complex (MHC) on chromosome 6p21.3. eNOS is encoded by a gene of 26 exons (NOS3) located on chromosome 7 [17]. Variants in these genes have been shown to modulate production of TNF-α [24] and NO [20, 25]. Therefore, various polymorphisms in TNF and NOS3 among migraineurs have been investigated. The most widely studied variants are TNF –308G>A and NOS3 +894G>T. A number of recent observations concerning the association between TNF –308G>A and NOS3 +894G>T and migraine support a significant genetic component to predisposition toward this frequently undiagnosed disabling disorder [26–33], but the results are controversial [34–38]. A single study may not have sufficient power to completely demonstrate this complicated genetic relationship because of relatively small sample sizes, and larger studies could overcome these disadvantages. There have been two previous meta-analyses of TNF –308G>A, and the scenario remained virtually unchanged [39, 40]. To clarify this association, we performed a meta-analysis of case-control studies to evaluate whether the TNF –308G>A and NOS3 +894G>T polymorphisms confer genetic susceptibility to migraine.
Materials and Methods
Literature sources and search strategy
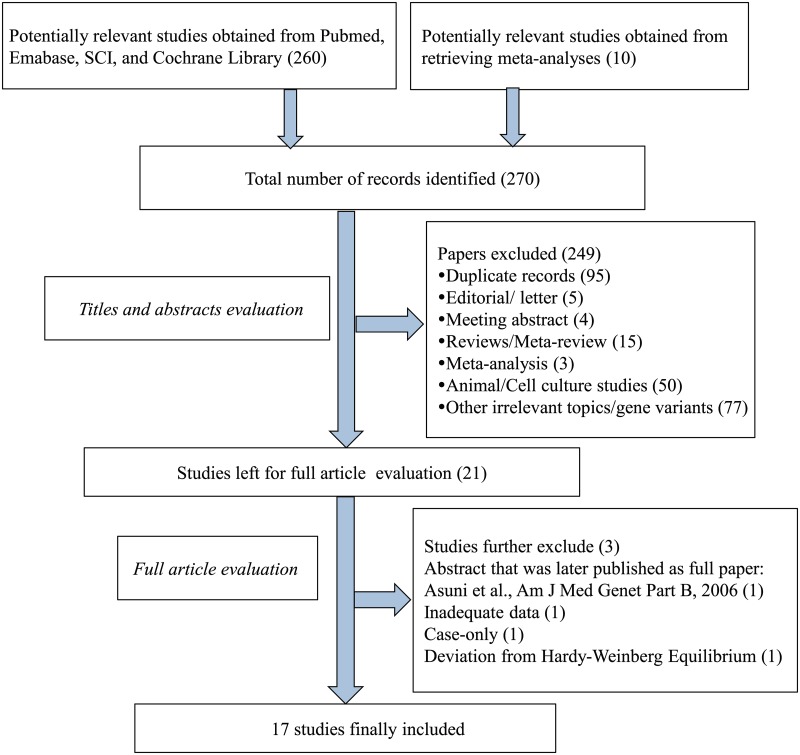
Two of the authors (MC and WT) searched the PubMed, EMBASE, Science Citation Index (SCI), and Cochrane Library electronic databases for tumor necrosis factor and endothelial nitric oxide synthase (“tumor necrosis factor-α” OR ‘‘tumor necrosis factor-alpha” OR “TNF-α” OR “TNF-alpha” OR “endothelial nitric oxide synthase” OR “eNOS”) AND “headache” OR “headache disorder” OR “migraine” OR “migraine disorder” OR “migraine with aura” OR “migraine without aura” AND “gene” OR “polymorphism” OR “genetic variation” OR “polymorphisms” OR “rs1800629” OR TNF –308G>A” OR “rs1799983” OR “Glu298Asp” OR “NOS3 +894G/T.” The search was performed without any restrictions, except that the studies were conducted in humans. The full electronic search strategy of TNF –308G>A for PubMed was (“migraine disorders” [MeSH Terms] OR “migraine disorders” [All Fields] OR “migraine” [All Fields] OR “migraine disorder” [All Fields] OR “headache disorders” [MeSH Terms] OR “headache disorders” [All Fields] OR “headache” [All Fields] OR “headache disorder” [All Fields])) OR “migraine with aura” [MeSH Terms] OR “migraine with aura” [All Fields] OR “migraine without aura” [MeSH Terms] OR “migraine without aura” [All Fields]) AND (“tumor necrosis factor-alpha” [MeSH Terms] OR “tumor necrosis factor-alpha” [All Fields] OR “tumor necrosis factor alpha” [All Fields] OR “tumor necrosis factors” [MeSH Terms] OR “tumor necrosis factors” [All Fields] OR “tnf” [All Fields] OR “tnf alpha” [All Fields]) AND (“genetic” [All Fields] OR “genetics” [All Fields] OR “genetics” [MeSH Terms] OR “polymorphism,” “genetic” [MeSH Terms] OR “genetic polymorphism” [All Fields] OR (“polymorphism” [All Fields] AND “genetic” [All Fields]) OR “variant” [All Fields] OR “variants” [All Fields] OR “rs1800629” OR “TNF –308G>A”) AND “humans” [MeSH Terms]. In addition, the reference lists of selected papers and potentially relevant studies in the previous meta-analyses were also screened to further identify potentially relevant papers through reference association. The last search was updated in July 2014. The selection process is shown as a flow chart in Fig 1.
Fig 1. Flow chart of the study selection process.
Inclusion criteria
The meta-analysis included only articles (i) that evaluated the association of the TNF –308G>A or NOS3 +894G>T polymorphism and migraine risk based on a case-control or cohort design, and (ii) that contained information on the sample sizes, distribution of genotypes, and allele frequencies for the polymorphisms investigated among migraineurs and non-migraineurs allowing for calculation of crude risks for migraine. In publications with overlapping cases and/or controls, the most recent or largest population was chosen.
In the first step of the analysis, two of the investigators (MC and WT) independently identified all studies not meeting any of the pre-specified criteria by screening the titles and abstracts, and these studies were excluded. In the second step, the same investigators independently evaluated the full-text publications of the remaining studies. Studies were excluded if they did not meet all of the criteria. Any discrepancies were adjudicated by a third reviewer (ZD).
Exclusion criteria
Studies were excluded if one of the following existed: (1) studies with insufficient genotyping data of the participants; (2) case-only studies; (3) review articles; (4) animal studies; (5) editorials; (6) abstracts; (7) studies that reported associations between gene polymorphisms and recovery from a migraine attack; (8) studies that reported associations between gene polymorphisms and response to any therapy; (9) studies that deviated from Hardy—Weinberg equilibrium.
Data extraction and contact with authors
Two of the investigators (MC and WT) independently reviewed, extracted, and entered data from the studies in a standardized data extraction form. Discrepancies were resolved by consensus. The items recorded were authors’ names, year of publication, ethnicity of subjects, study design, genotyping method, migraine status (migraine, migraine with aura [MA], and migraine without aura [MO]), diagnostic criterion of cases, gender of individuals in the study population, study size, allele and genotype frequencies, and additional potentially relevant information. We attempted to collect genetic information for all migraineurs and migraineurs with and without aura separately as well as for the whole study population and for females and males separately. If not presented in the article, allele and genotype frequencies were calculated when possible. For studies that did not allow extraction of all relevant information, including genotype and allele frequencies, we e-mailed the corresponding authors to obtain the missing information. Authors not responding within 1 week were sent up to two reminders by e-mail.
Quality score assessment
The quality of studies was assessed by the same two reviewers (MC and WT) independently using a modified 10-point Newcastle—Ottawa Scale (NOS) (http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp). The quality of each study was assessed and awarded stars based on indicators of quality, including selection of study population, comparability, and exposure assessment independently. The quality score ranged from 0 (worst) to 10 points (best). Any discrepancies were adjudicated by a third reviewer (LH).
Statistical methods
All analyses were performed using STATA version 12.0 (Stata Corporation, College Station, TX). Unless otherwise stated, P < 0.05 was taken to indicate statistical significance. Hardy—Weinberg equilibrium (HWE) for each study was determined using the goodness-of-fit χ 2 test. The strengths of the associations between the TNF –308G>A and NOS3 +894G>T polymorphisms and migraine risk were examined by ORs and corresponding 95% CIs. The pooled ORs were calculated for co-dominant model (AA vs. GG and GA vs. GG for TNF –308G>A, TT vs. GG and GT vs. GG for NOS3 +894G>T), dominant model (AA+GA vs. GG for TNF –308G>A, TT+GT vs. GG for NOS3 +894G>T), recessive model (AA vs. GA + GG for TNF –308G>A, TT vs. GT+GG for NOS3 +894G>T), and allele contrast (A vs. G for TNF –308G>A, T vs. G for NOS3 +894G>T). Heterogeneity across studies was detected by the χ 2 test-based Q-statistic (P < 0.10 was deemed as evidence of heterogeneity) as well as I 2 statistic, which takes values between 0% and 100% (I 2 < 25% represents absence of heterogeneity; I 2 = 25%– 50% moderate heterogeneity; I 2 > 50% large heterogeneity) [41]. When the heterogeneity was indicated to be non-significant (P > 0.10), the fixed-effects model (Mantel—Haenszel method) [42] was applied; otherwise the random-effects model (DerSimonian and Laird method) [43] was performed. Stratified analyses were performed by ethnicity, type of migraine, and gender to handle heterogeneity. We used Galbraith plots to visually examine the impact of individual studies on the overall homogeneity test statistic. Leave-one-out sensitivity analysis was carried out to determine the extent of influence of single studies on the combined results. Begg’s funnel test and Egger’s test were applied to evaluate publication bias across studies (P < 0.10 was taken to indicate statistical significance).
Results
Eligible studies
Fig 1. summarizes the process of identifying eligible studies. A total of 270 potential studies were identified by electronic and manual searches. After reviewing titles and abstracts, 249 were removed. Among the remaining 21 papers, 12 papers focused only on TNF –308G>A [26, 28–31, 34–37, 44–46], eight focused only on NOS3 +894G>T [33, 38, 47–52], and one dealt with both TNF –308G>A and NOS3 +894G>T [27]. After further examination, among the 12 papers investigating only TNF –308G>A, we excluded one abstract [46] because detailed results were later published in full [37]. Another study was excluded because of deviation from HWE and the method used for SNP genotyping (SSP-PCR), which is particularly susceptible to errors [29]. Among the eight papers regarding only NOS3 +894G>T, one was excluded because no information about the distribution of genotypes was provided [47], and one was excluded because of its case-only design and therapy response evaluation [52]. In the paper regarding both TNF –308G>A and NOS3 +894G>T [27], information about TNF –308G>A was available but not that for NOS3 +894G>T. MC and WT independently read the articles, and 11 studies were finally included for TNF –308G>A and 6 studies were included for NOS3 +894G>T. Kappa statistic for agreement of TNF –308G>A between the two reviewers was 0.9, and that for NOS3 +894G>T was 0.86. Any discrepancies were adjudicated by a third reviewer (ZD).
Contact with authors
Among the 11 papers focusing on TNF –308G>A, 7 [27, 28, 31, 34, 36, 37, 44] reported genotype and allele frequencies for all participants and for overall migraine, as well as stratified by gender and aura; however, the remaining 4 papers did not [26, 30, 35, 45]. After contacting the authors of these papers, we obtained complete data for one study [35]. Among the eight papers discussing NOS3 +894G>T, six reported genotype and allele frequencies for all participants and for overall migraine as well as those stratified by aura [33, 38, 49–51, 53], while the remaining two did not [27, 47]. We attempted to contact the authors, but they did not respond and so we excluded these two studies from the analysis.
Study characteristics
The detailed characteristics of each study included in the meta-analysis are presented in Table 1. These studies were published between 2002 and 2014. In one study, migraine was self-reported [27], while the rest were based on recognized diagnostic criteria [26, 28–31, 33–36, 38, 44, 45, 48–51, 54]. Among the studies included in the analysis, 11 evaluated TNF –308G>A polymorphisms [26–31, 34–37, 44, 45] and 6 evaluated NOS3 +894G>T polymorphisms [33, 50, 51, 53, 55, 56].
Table 1. Characteristics of the eligible studies in this meta-analysis.
| First Author (year) | Ethnicity | Gender | Sample size of controls | Sample size of cases | Diagnostic criteria of cases | Design of study | Detecting method of polymorphism | Source of controls | HWE | mNOS (*) |
|---|---|---|---|---|---|---|---|---|---|---|
| (A) Studies of TNF –308G>A polymorphism | ||||||||||
| Trabace, 2002 [32] | Caucasians | F/M | 101 | 79 | IHS | Case-control | PCR-RFLP | Population | Y | 7 |
| Rainero, 2004 [26] | Caucasians | F/M | 306 | 299 | IHS | Case-control | PCR-RFLP | Blood donors | Y | 7 |
| Herken, 2005 [45] | Non-Caucasians | F/M | 60 | 60 | IHS | Case-control | PCR-RFLP | Population | Y | 6 |
| Lee, 2007 [34] | Non-Caucasians | F | 382 | 439 | IHS | Case-control | PCR | Not mentioned | Y | 6 |
| Asuni, 2009 [37] | Caucasians | F/M | 278 | 299 | IHCD-II | Case-control | PCR-RFLP | Blood donors | Y | 7 |
| Schurks, 2009 [65] | Caucasians | F | 20,425 | 4577 | Self-reported | Cohort | PCR | Population | Y | 6 |
| Ghosh, 2010 [31] | Non-Caucasians | F/M | 216 | 216 | IHS | Case-control | PCR-RFLP | Population | Y | 7 |
| Yilmaz, 2010 [28] | Non-Caucasians | F/M | 96 | 67 | IHCD-II | Case-control | PCR-RFLP | Population, hospital,family members of patients | Y | 6 |
| Pappa, 2010 [36] | Caucasians | F/M | 178 | 103 | IHCD-II | Case-control | PCR-RFLP | Population | Y | 8 |
| Ates, 2011 [30] | Non-Caucasians | F/M | 202 | 203 | IHS | Case-control | ARMS-PCR | Hospital | Y | 6 |
| Stuart, 2013[35] | Caucasians | F/M | 345 | 340 | IHS | case-control | SNPs | Hospital | Y | 6 |
| (B) Studies for NOS3 +894G>T polymorphism | ||||||||||
| Borroni, 2006 [33] | Caucasians | F/M | 125 | 156 | IHS | Case-control | PCR | Healthy volunteers | Y | 6 |
| Toriello, 2008 [38] | Caucasians | F/M | 337 | 341 | IHS | Case-control | R-T PCR | Patients’ friends and healthy volunteers. | Y | 6 |
| Gruber, 2010 [53] | Caucasians | F/M | 76 | 54 | IHS | Case-control | R-T PCR | Patients’ friends and healthy volunteers | Y | 6 |
| Goncalves, 2011 [49] | Non-Caucasians | F | 117 | 178 | IHS | Case-control | RT-PCR | Population | Y | 7 |
| Goncalves, 2012 [50] | Non-Caucasians | F | 99 | 150 | IHS | Case-control | RT-PCR | Population | Y | 7 |
| Eroz, 2014 [51] | Non-Caucasians | F/M | 123 | 176 | IHCD-II | Case-control | PCR-RFLP | Blood donors | Y | 4 |
ICHD-II: International Classification of Headache Disorders-II; HIS: International Headache Society; HWE: Hardy-Weinberg equilibrium; PCR: polymerase chain reaction; RFLP: restriction fragment length polymorphism; ARMS: amplification refractory mutation system; SNPs: single nucleotide polymorphisms; RT-PCR: real-time polymerase chain reaction.
Y: consistent with HWE; F: female; M: male. NOS: Newcastle—Ottawa Quality Assessment Scale for Case Control Studies.
For TNF –308G>A, all studies were of a case-control design [26, 28–31, 34–36, 44–46] except one that had a cohort design [27]. One study was performed in children [36], and the others in adults [26–32, 34, 35, 45, 46]. Six studies were performed in Caucasians [26, 27, 32, 35, 36, 46] and five in non-Caucasians [28, 30, 31, 34, 45]. For NOS3 +894G>T, all studies were of a case-control design, and all studies were performed in adults. Three studies were performed in Caucasians [33, 53, 56] and three in non-Caucasians [50, 51, 55].
The distributions of genotypes and alleles for TNF –308G>A and NOS3 +894G>T among migraineurs and controls are listed in S1 and S2 Tables.
Tables 2 and 3 summarize the pooled effect estimates, measures of heterogeneity, and tests for TNF –308G>A and NOS3 +894G>T polymorphisms, respectively.
Table 2. Main results of the pooled data for the TNF –308G>A polymorphism.
| Subgroups | AA vs. GG | AA+GA vs. GG | AA vs. GA+GG | A vs. G | GA vs. GG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | ||
| Total | 0.84 (0.45,1.56) | 0.080 | 41.7 | 1.14 (0.84,1.55) | 0.000 | 83.2 | 0.92 (0.77,1.11) | 0.143 | 33.1 | 1.11 (0.83,1.47) | 0.000 | 84.8 | 1.15 (0.86,1.55) | 0.000 | 80.1 | |
| Ethnicity | ||||||||||||||||
| Caucasians | 0.91 (0.76,1.09) | 0.204 | 30.9 | 0.81 (0.56,1.17) | 0.015 | 67.8 | 0.90 (0.75,1.08) | 0.280 | 20.3 | 0.79 (0.56,1.12) | 0.000 | 84.3 | 0.85 (0.59, 1.20) | 0.037 | 60.8 | |
| Non-Caucasians | 1.60 (0.20,12.5) | 0.063 | 58.8 | 1.82 (1.15,2.87) | 0.000 | 84.3 | 1.46 (0.21,10.2) | 0.088 | 54.1 | 1.74 (1.13,2.67) | 0.012 | 69.1 | 1.78 (1.17,2.72) | 0.000 | 81.8 | |
| Type of migraine | ||||||||||||||||
| MA | Total | 1.16 (0.87,1.54) | 0.960 | 0 | 1.17 (1.05,1.30) | 0.195 | 30.6 | 1.11 (0.84,1.48) | 0.956 | 0 | 1.13 (1.03,1.24) | 0.299 | 17.1 | 1.17 (1.05,1.31) | 0.123 | 40.2 |
| Caucasians | 1.16 (0.87,1.55) | 0.806 | 0 | 1.15 (1.03,1.28) | 0.335 | 11.7 | 1.12 (0.84,1.49) | 0.799 | 0 | 1.12 (1.02,1.23) | 0.403 | 0 | 1.15 (1.02,1.29) | 0.256 | 25.9 | |
| Non-Caucasians | 0.93 (0.11,8.20) | 0.967 | 0 | 1.62 (1.03,2.53) | 0.230 | 31.9 | 0.84 (0.10,7.42) | 0.990 | 0 | 1.49 (0.97,2.28) | 0.284 | 20.5 | 1.69 (1.08,2.65) | 0.212 | 35.4 | |
| MO | Total | 0.89 (0.43,1.82) | 0.088 | 40.4 | 0.98 (0.69,1.38) | 0.000 | 80.4 | 0.96 (0.75,1.22) | 0.156 | 31.6 | 0.98 (0.70,1.36) | 0.000 | 82.4 | 0.99 (0.71,1.37) | 0.000 | 76.1 |
| Caucasians | 0.88 (0.68,1.14) | 0.275 | 21.1 | 0.74 (0.49,1.12) | 0.000 | 81.5 | 0.90 (0.69,1.16) | 0.367 | 7.7 | 0.73 (0.49,1.09) | 0.000 | 83.1 | 0.77 (0.52,1.15) | 0.000 | 77.6 | |
| Non-Caucasians | 2.41 (0.34,17.1) | 0.086 | 54.5 | 1.65 (0.922.97) | 0.043 | 63.1 | 2.48 (0.91,6.76) | 0.110 | 50.2 | 1.65 (0.92,2.99) | 0.019 | 69.8 | 1.53 (1.11,2.11) | 0.116 | 49.3 | |
| Gender | ||||||||||||||||
| female | Migraine | 0.95 (0.79,1.15) | 0.133 | 35.7 | 1.13 (0.80,1.58) | 0.000 | 79.8 | 0.94 (0.78,1.1) | 0.210 | 26.3 | 1.09 (0.80, 1.50) | 0.000 | 81.5 | 1.14 (0.82,1.57) | 0.000 | 75.8 |
| MA | 1.16 (0.85,1.60) | 0.981 | 0 | 1.65 (1.02,2.68) | 0.067 | 58.1 | 1.11 (0.81,1.5) | 0.965 | 0 | 1.16 (1.05,1.28) | 0.146 | 44.2 | 1.80 (1.03,3.14) | 0.032 | 65.9 | |
| MO | 1.02 (0.78,1.33) | 0.319 | 14.5 | 1.23 (0.87,1.74) | 0.018 | 60.8 | 1.02 (0.78,1.3) | 0.435 | 0 | 1.21 (0.85,1.72) | 0.006 | 66.5 | 1.21 (0.88,1.65) | 0.061 | 50.1 | |
| male | Migraine | 0.76 (0.32,1.81) | 0.685 | 0 | 0.83 (0.58,1.18) | 0.179 | 32.7 | 0.76 (0.32,1.8) | 0.786 | 0 | 0.81 (0.59,1.12) | 0.122 | 40.3 | 0.87 (0.60,1.26) | 0.302 | 16.8 |
| MA | * | * | * | 1.06 (0.28,4.09) | 0.623 | 0 | * | * | * | 1.02 (0.28,3.74) | 0.710 | 0 | 1.10 (0.28,4.24) | 0.552 | 0 | |
| MO | 1.61 (0.35,7.35) | 0.872 | 0 | 0.97 (0.55,1.70) | 0.456 | 0 | 1.61 (0.35,7.3) | 0.912 | 0 | 0.96 (0.57,1.63) | 0.352 | 9.5 | 0.99 (0.56,1.75) | 0.588 | 0 | |
MA: migraine with aura; MO: migraine without aura; OR: odds ratio; 95% CI: 95% confidence interval;
*effect estimates for some studies could not be calculated due to the small number of studies.
Table 3. Main results of the pooled data for the NOS3 +894G>T polymorphism.
| Subgroups | TT vs. GG | TT+GT vs. GG | TT vs. GT+GG | T vs. G | GT vs. GG | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | OR (95% CI) | Ph | I 2 (%) | ||
| Total | 1.29 (0.95,1.75) | 0.372 | 6.9 | 1.03 (0.66,1.60) | 0.000 | 80.8 | 1.27 (0.96,1.68) | 0.639 | 0 | 1.05 (0.82,1.34) | 0.015 | 64.4 | 0.97 (0.59,1.59) | 0.000 | 82.8 | |
| Ethnicity | ||||||||||||||||
| Caucasians | 1.08 (0.76,1.54) | 0.699 | 0 | 0.96 (0.75,1.24) | 0.000 | 0 | 1.13 (0.82,1.56) | 0.514 | 0 | 1.02 (0.86,1.21) | 0.827 | 0 | 0.93 (0.71,1.21) | 0.625 | 0 | |
| Non-Caucasians | 2.10 (1.14,3.88) | 0.534 | 0 | 1.09 (0.40,2.99) | 0.002 | 92.1 | 1.84 (1.02,3.33) | 0.953 | 0 | 1.11 (0.63,1.94) | 0.002 | 84.5 | 0.99 (0.33,2.99) | 0.000 | 92.9 | |
| Type of migraine | ||||||||||||||||
| MA | Total | 1.61 (1.12,2.31) | 0.700 | 0 | 1.10 (0.65,1.87) | 0.001 | 76.4 | 1.50 (1.08,2.09) | 0.782 | 0 | 1.18 (0.90,1.55) | 0.057 | 53.4 | 0.97 (0.52,1.79) | 0.000 | 79.8 |
| Caucasians | 1.43 (0.94,2.17) | 0.665 | 0 | 1.11 (0.81,1.51) | 0.996 | 0 | 1.41, (0.98,2.04) | 0.450 | 0 | 1.17 (0.95,1.44) | 0.787 | 0 | 0.99 (0.71,1.39) | 0.775 | 0 | |
| Non-Caucasians | 2.37 (1.12,4.99) | 0.656 | 0 | 1.07 (0.30,3.88) | 0.000 | 90.5 | 1.93 (0.94 3.97) | 0.866 | 0 | 1.11 (0.56,2.19) | 0.007 | 80.0 | 0.93 (0.21,4.02) | 0.000 | 91.6 | |
| MO | Total | 1.06 (0.73,1.54) | 0.233 | 26.9 | 0.94 (0.64,1.39) | 0.012 | 65.7 | 1.10 (0.78, 1.55) | 0.390 | 4.1 | 0.97 (0.75,1.24) | 0.058 | 53.1 | 0.93 (0.61,1.42) | 0.008 | 68.3 |
| Caucasians | 0.80 (0.51,1.27) | 0.408 | 0 | 0.84 (0.62,1.14) | 0.832 | 0 | 0.89 (0.59, 1.35) | 0.299 | 17.1 | 0.89 (0.71,1.10) | 0.624 | 0 | 0.86 (0.62,1.19) | 0.627 | 0 | |
| Non-Caucasians | 1.90 (0.97,3.73) | 0.625 | 0 | 1.05 (0.46,2.394) | 0.001 | 85.3 | 1.81 (0.95, 3.47) | 0.973 | 0 | 1.09 (0.67,1.78) | 0.018 | 75.2 | 0.96 (0.39,2.35) | 0.001 | 86.4 | |
MA: migraine with aura; MO: migraine without aura; OR: odds ratio; 95% CI: 95% confidence interval
Association between TNF –308G>A polymorphism and migraine
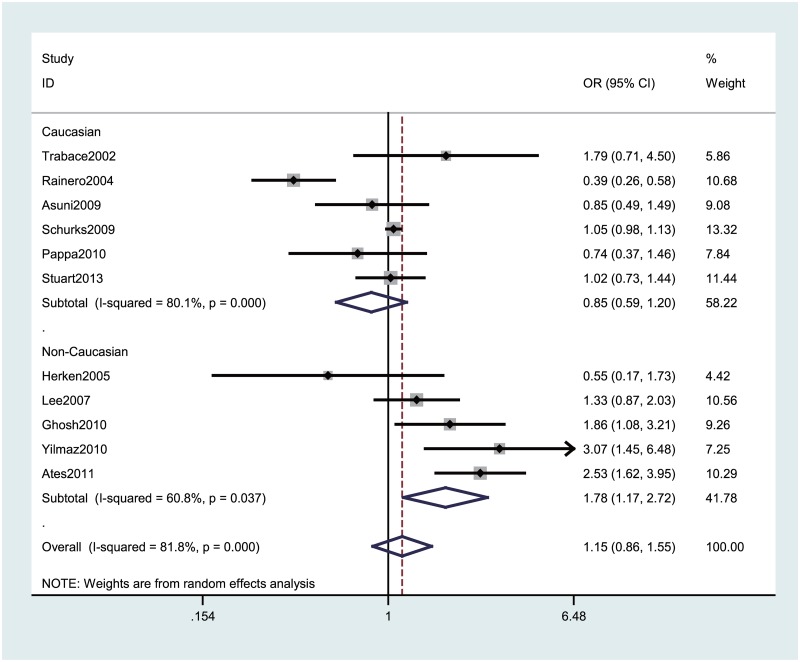
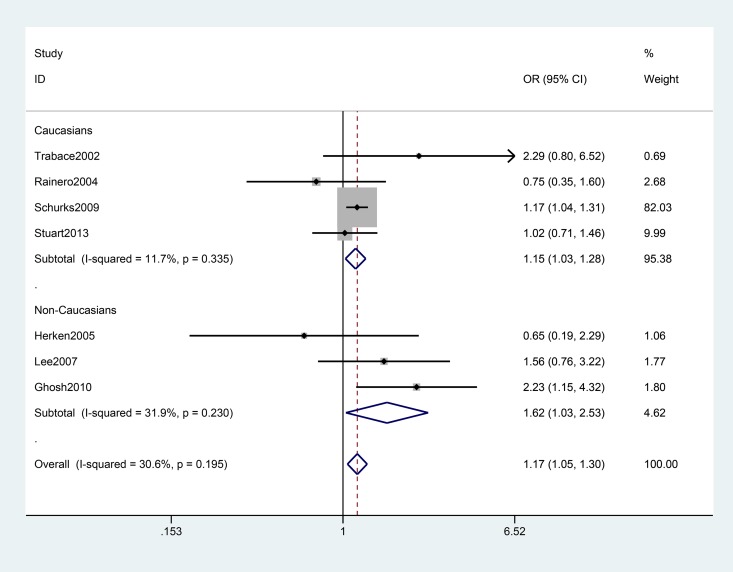
Table 2 lists the main meta-analysis results, which were calculated with the fixed-effects model when Ph > 0.10 and the random-effects model when the Ph < 0.10. Based on data from 11 studies for TNF –308G>A with 6682 genotyped migraine cases and 22591 controls, no significant association between TNF –308G>A polymorphism and migraine risk was observed under any of the genetic models. Strikingly, the meta-analysis provided an OR of 1.74 (95% CI: 1.13–2.67, Ph = 0.012) under A vs. G, an OR of 1.82 (95%CI: 1.15–2.87, Ph = 0.000) under AA+GA vs. GG, and an OR of 1.78 (95% CI: 1.17–2.72, Ph = 0.000) under GA vs. GG among non-Caucasians, while none of the contrast models showed a significant association in Caucasian populations (Fig 2). When stratifying the data by type of migraine, we observed an increased risk of migraine with aura (MA) among all participants (AA+GA vs. GG: pooled OR = 1.17, 95% CI: 1.05–1.30 Ph = 0.195; GA vs. GG: pooled OR = 1.17, 95% CI: 1.05–1.31, Ph = 0.123), especially in non-Caucasians (Table 2 and Fig 3). The association was stronger among females than males.
Fig 2. Forest plot of migraine risk associated with the TNF –308G>A polymorphism stratified by ethnicity under the GA vs. GG model.
The boxes and horizontal lines represent the OR and the corresponding 95% CI. The areas of the boxes indicates the weight (inverse of the variance). The diamonds correspond to the summary OR and 95% CI.
Fig 3. Forest plot of migraine with aura risk associated with the TNF –308G>A polymorphism stratified by ethnicity under the AA+GA vs. GG model.
The boxes and horizontal lines represent the OR and the corresponding 95% CI. The areas of the boxes indicate the weight (inverse of the variance). The diamonds correspond to the summary OR and 95% CI.
Association between NOS3 +894G>T polymorphism and migraine
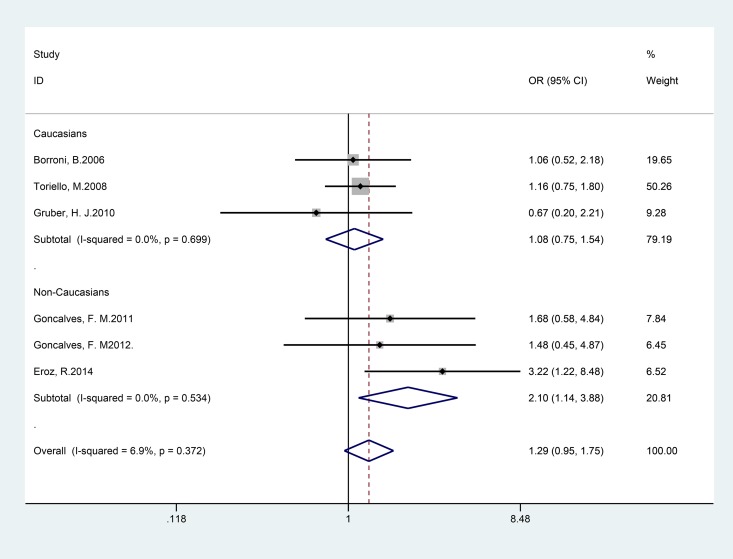
By pooling the six selected studies with 1055 genotyped migraine cases and 877 controls for NOS3 +894G>T, no significant association between NOS3 +894G>T polymorphism and migraine risk was observed under any of the genetic models examined. Subgroup analyses suggested that the T allele of the NOS3 +894G>T variant increased the risk for migraine among non-Caucasians, which was driven by associations for MA (co-dominant model TT vs. GG: pooled OR = 2.10; 95% CI 1.14–3.88), as shown in Table 3 and Fig 4).
Fig 4. Forest plot of migraine risk associated with the NOS3 +894G>T polymorphism stratified by ethnicity under the TT vs. GG model.
The boxes and horizontal lines represent the OR and the corresponding 95% CI. The areas of the boxes indicate the weight (inverse of the variance). The diamonds correspond to the summary OR and 95% CI.
Heterogeneity and sensitivity analyses
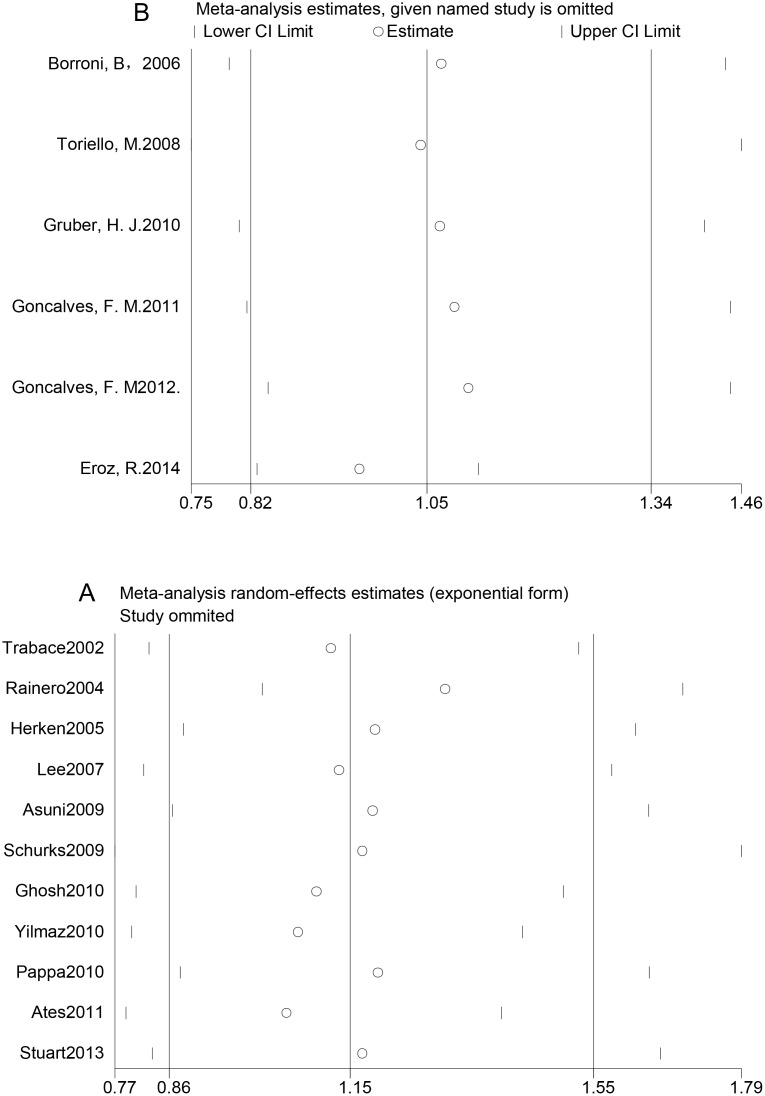
There was no significant heterogeneity among studies of TNF –308G>A polymorphism and MA (P > 0.10 for all genetic models). Nevertheless, the reverse effects were observed in studies of the TNF –308G>A polymorphism and migraine without aura (MO). Using Galbraith plots, we identified individual studies as important sources of heterogeneity [26, 28, 30, 31, 51], as shown in S1A and S1B Fig Among the five studies that were sources of heterogeneity, one had a low NOS scale, indicating low quality [51], and the other three had study populations with heterogeneous ethnic backgrounds [28, 30, 31]. Therefore, we performed sensitivity analyses by excluding studies that fell outside the margin set by the z score ± 2 standard deviation. We conducted sensitivity analysis to explore the potential sources of heterogeneity in the overall results. The initial ORs were not significantly influenced by sequential removal of each study from the total analysis (Fig 5A and 5B).
Fig 5. One-way sensitivity analyses of the associations between genetic polymorphisms and migraine risk.
A: One-way sensitivity analysis of the association between the TNF –308G>A polymorphism and migraine risk under the GA vs. GG model. B: One-way sensitivity analysis of the association between the NOS3 +894G>T polymorphism and migraine risk under the T vs. G model.
Publication bias
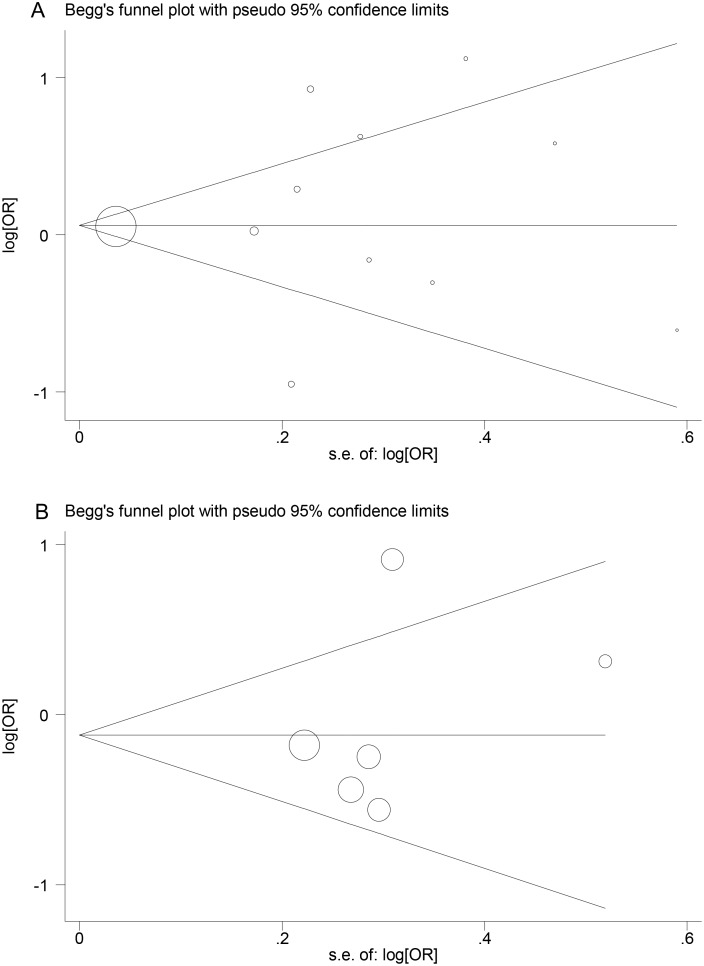
Publication bias was determined using Begg’s funnel plots and Egger’s test. Neither Egger’s test nor Begg’s funnel plots indicated any significant publication bias (P = 0.699 and 1.000, respectively, for TNF –308G>A, P = 0.707 and 0.508, respectively, for NOS3 +894G>T) (Fig 6A and 6B).
Fig 6. Begg’s funnel plots for genetic polymorphisms.
A: Begg’s funnel plot for the TNF –308G>A polymorphism. B: Begg’s funnel plot for the NOS3 +894G>T polymorphism.
Discussion
The overall results of this meta-analysis did not indicate associations between any of the polymorphisms investigated and migraine. However, the associations differed by ethnicity and clinical phenotype.
Among studies investigating the TNF –308G>A polymorphism, stratified analysis by ethnicity suggested an increased risk of migraine among non-Caucasians. When stratifying the data by type of migraine, we observed an increased risk of MA among both non-Caucasians and Caucasians, but especially among non-Caucasians.
Subgroup analyses suggested that the T allele of NOS3 +894G>T variant increased the risk of migraine among non-Caucasians, which was driven by associations for MA. Assuming an allele T frequency of 10.64% among controls and using the TT vs. GT+GG genetic model, this study had about 84.3% power at a significance level of α = 0.05 to detect an effect size of 1.84 among non-Caucasians [57].
“Neurogenic inflammation” around the dural trigeminal afferents is known to play an important role in the generation of migraine attacks [7] and to contribute to the activation and sensitization of perivascular meningeal afferents during migraine attacks [58]. TNF-α and NO, potential noxious pain mediators in neurovascular inflammation, are involved in the initiation and maintenance of a migraine attack [59]. Furthermore, TNF-α contributes to the development of central sensitization by enhancing excitatory and reducing inhibitory currents and by activating induction of COX-2, which plays an important role in the development of inflammatory hyperalgesia [60], a phenomenon related to allodynia experienced by some migraineurs. In addition, Eroz et al. reported that patients with the NOS3 +894G>T genotype GT have an increased risk of allodynia [51].
The levels of TNF-α and NO are under genetic control. An “A” at position –308 in the TNF promoter has been shown to be associated with a high level of TNF-α expression [61, 62]. A functional polymorphism hypothesis links TNF responses to certain HLA alleles, which have high linkage disequilibrium with TNF. Individuals carrying the “A” allele as part of the HLA Al, B8, DR3 haplotypes, which are population-specific within the human MHC region [63], may have higher levels of TNF-α expression. The different results obtained after stratifying by ethnicity may have been due to the influence of linkage disequilibrium pattern. Studies included in the present meta-analysis had similar results, with the exception of the study by Rainero et al. [26], in which subjects homozygous for the G allele had a higher risk of developing the disease. This discrepancy may have been due to differences in the ethnicity of the study populations.
The associations of the TNF –308G>A and NOS3 +894G>T polymorphisms with predisposition to migraine have been examined in a number of studies, which reported inconsistent results. Due to the failure of independent association studies in identifying a role for the TNF –308G>A polymorphism in migraine, two meta-analyses were performed to reach definitive and reliable conclusions. Gu et al. [40] performed a meta-analysis associating the TNF –308G>A polymorphism with migraine based on six studies in Asian populations (985 cases and 958 controls); their results indicated that the TNF –308G>A polymorphism was associated with migraine risk in Asians. Subgroup analysis suggested that there was a statistically significant result for MA but not for MO. Another meta-analysis conducted by Schuerks et al. [64] based on 10 studies available for the TNF –308G>A polymorphism indicated no overall association with migraine. Subgroup analyses suggested that the “A” allele of the TNF –308G>A variant increases the risk of migraine among non-Caucasian populations, which was driven by associations for MO. In our study, subgroup analyses suggested that the risk of migraine with aura (MA) was increased among both Caucasians and non-Caucasians. Performance of a genome-wide association study (GWAS) enables identification of genetic variants in many multi-pathogenic diseases. To date, neither the TNF –308G>A nor the NOS3 +894G>T polymorphism has been included in GWAS.
Despite their plausible associations with migraine, this meta-analysis revealed no general associations between the TNF –308G>A or NOS3 +894G>T polymorphism and migraine risk. There are two possible explanations for the observed overall lack of associations. Either, there may be really be no association and the positive results in some studies may have occurred by chance or due to certain study characteristics, or the pattern of association may be more complex and involve additional factors. In this context, considerations such as the heterogeneity among studies, different sources of controls, and variable sample sizes among the studies must be addressed.
Specifically, there were moderate to high degrees of heterogeneity among the studies investigating the association of the TNF –308G>A polymorphism with migraine and MO, which may have been due to differences between subgroups. Furthermore, assuming that the frequency of the “A” allele is 2.74% among controls and using the A vs. G genetic model, this study had only about 61.5% power at a significance level of = 0.05 to detect an effect size of 1.74 among non-Caucasians [57]. To achieve 80% power, the study would require 1522 cases. Moreover, with regard to NOS3 +894G>T, not all information was available, which would have been valuable to draw clear associations with migraine among populations of different ethnicities.
Inherent bias, such as sampling bias, selection bias, publication bias, and within-study bias, cannot be avoided in a meta-analysis of observational studies. The following biases must be taken into consideration when interpreting the results of the present study. First, all of the studies included in the meta-analysis were published in English, so selection bias may have occurred. Second, we did not include fugitive literature, including academic dissertations and conference papers, so there may have been bias in provision of data. Third, there was a lack of unified sources of controls. The controls were selected from three sources: hospital-based, healthy population, and family members of the migraineurs, so selection bias may have occurred especially among the studies with hospital-based controls. Fourth, there was heterogeneity across the studies, which may have masked significance in the present study. Another limitation of our study was that we did not analyze the gene-to-gene or gene-environment interactions that may contribute to modification of migraine risk due to a lack of data among the existing publications eligible for this meta-analysis.
Conclusions
In summary, the general results of our meta-analysis do not support a strong overall effect, but provide statistical evidence that the TNF –308G>A polymorphism in the TNF region may represent a risk for migraine among non-Caucasians and MA among both Caucasians and non-Caucasians. The NOS3 +894G>T polymorphism of eNOS may represent a risk for migraine among non-Caucasians. However, given the heterogeneity among study designs, the results of this meta-analysis should be interpreted with caution. Further cohort studies based on pedigree are warranted to evaluate population-specific effects, including population stratification.
Supporting Information
(DOCX)
(DOC)
(XLSX)
A: S1A Fig Galbraith plot for TNF –308G>A. B: S1B Fig Galbraith plot for NOS3 +894G>T.
(EPS)
MA: migraine with aura; MO: migraine without aura
(DOCX)
MA: migraine with aura; MO: migraine without aura
(DOCX)
Acknowledgments
We are grateful to Lyn R. Griffiths, Shani Stuart, and their colleagues, who supported this meta-analysis by kindly providing additional data from their published studies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was financially supported by the National Science Foundation Committee (NSFC) in China (no. 81171058). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia: an international journal of headache. 2007;27(3):193–210. Epub 2007/03/27. 10.1111/j.1468-2982.2007.01288.x [DOI] [PubMed] [Google Scholar]
- 2. Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache. 2012;52(4):582–91. Epub 2012/05/17. . [DOI] [PubMed] [Google Scholar]
- 3. Olesen J, Gustavsson A, Svensson M, Wittchen HU, Jonsson B. The economic cost of brain disorders in Europe. European journal of neurology: the official journal of the European Federation of Neurological Societies. 2012;19(1):155–62. Epub 2011/12/20. 10.1111/j.1468-1331.2011.03590.x . [DOI] [PubMed] [Google Scholar]
- 4. Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). The journal of headache and pain. 2005;6(6):429–40. Epub 2006/01/03. 10.1007/s10194-005-0252-4 ; PubMed Central PMCID: PMCPmc3452308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulder EJ, Van Baal C, Gaist D, Kallela M, Kaprio J, Svensson DA, et al. Genetic and environmental influences on migraine: a twin study across six countries. Twin research: the official journal of the International Society for Twin Studies. 2003;6(5):422–31. Epub 2003/11/20. 10.1375/136905203770326420 . [DOI] [PubMed] [Google Scholar]
- 6. Fidan I, Yuksel S, Ymir T, Irkec C, Aksakal FN. The importance of cytokines, chemokines and nitric oxide in pathophysiology of migraine. J Neuroimmunol. 2006;171(1–2):184–8. Epub 2005/12/06. 10.1016/j.jneuroim.2005.10.005 . [DOI] [PubMed] [Google Scholar]
- 7. Longoni M, Ferrarese C. Inflammation and excitotoxicity: role in migraine pathogenesis. Neurological sciences: official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2006;27 Suppl 2:S107–10. Epub 2006/05/12. 10.1007/s10072-006-0582-2 . [DOI] [PubMed] [Google Scholar]
- 8. Covelli V, Massari F, Fallacara C, Munno I, Pellegrino NM, Jirillo E, et al. Increased spontaneous release of tumor necrosis factor-alpha/cachectin in headache patients. A possible correlation with plasma endotoxin and hypothalamic-pituitary-adrenal axis. The International journal of neuroscience. 1991;61(1–2):53–60. Epub 1991/11/01. . [DOI] [PubMed] [Google Scholar]
- 9. Perini F, D'Andrea G, Galloni E, Pignatelli F, Billo G, Alba S, et al. Plasma cytokine levels in migraineurs and controls. Headache. 2005;45(7):926–31. Epub 2005/06/30. 10.1111/j.1526-4610.2005.05135.x . [DOI] [PubMed] [Google Scholar]
- 10. Mueller L, Gupta AK, Stein TP. Deficiency of tumor necrosis factor alpha in a subclass of menstrual migraineurs. Headache. 2001;41(2):129–37. Epub 2001/03/17. . [DOI] [PubMed] [Google Scholar]
- 11. Guldiken S, Guldiken B, Demir M, Kabayel L, Ozkan H, Turgut N, et al. Soluble CD40 ligand and prolactin levels in migraine patients during interictal period. The journal of headache and pain. 2011;12(3):355–60. Epub 2011/02/19. 10.1007/s10194-011-0306-8 ; PubMed Central PMCID: PMCPmc3094677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durham PL. Calcitonin gene-related peptide (CGRP) and migraine. Headache. 2006;46 Suppl 1:S3–8. Epub 2006/08/25. ; PubMed Central PMCID: PMCPmc3134175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Covelli V, Munno I, Pellegrino NM, Marinaro MR, Gesario A, Massari F, et al. In vivo administration of propranolol decreases exaggerated amounts of serum TNF-alpha in patients with migraine without aura. Possible mechanism of action. Acta neurologica. 1992;14(4–6):313–9. Epub 1992/08/01. . [PubMed] [Google Scholar]
- 14. Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia: an international journal of headache. 2000;20(10):907–18. Epub 2001/04/17. . [DOI] [PubMed] [Google Scholar]
- 15. Moncada S, Higgs EA. Endogenous nitric oxide: physiology, pathology and clinical relevance. European journal of clinical investigation. 1991;21(4):361–74. Epub 1991/08/01. . [DOI] [PubMed] [Google Scholar]
- 16. Akerman S, Williamson DJ, Kaube H, Goadsby PJ. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. British journal of pharmacology. 2002;137(1):62–8. Epub 2002/08/17. 10.1038/sj.bjp.0704842 ; PubMed Central PMCID: PMCPmc1573468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lassen LH, Christiansen I, Iversen HK, Jansen-Olesen I, Olesen J. The effect of nitric oxide synthase inhibition on histamine induced headache and arterial dilatation in migraineurs. Cephalalgia: an international journal of headache. 2003;23(9):877–86. Epub 2003/11/18. . [DOI] [PubMed] [Google Scholar]
- 18. Gkaliagkousi E, Douma S, Zamboulis C, Ferro A. Nitric oxide dysfunction in vascular endothelium and platelets: role in essential hypertension. Journal of hypertension. 2009;27(12):2310–20. Epub 2009/10/20. 10.1097/HJH.0b013e328330e89a . [DOI] [PubMed] [Google Scholar]
- 19. Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2010;7(2):183–90. Epub 2010/05/01. 10.1016/j.nurt.2010.03.006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metzger IF, Sertorio JT, Tanus-Santos JE. Modulation of nitric oxide formation by endothelial nitric oxide synthase gene haplotypes. Free radical biology & medicine. 2007;43(6):987–92. Epub 2007/08/19. 10.1016/j.freeradbiomed.2007.06.012 . [DOI] [PubMed] [Google Scholar]
- 21. Maher BH, Griffiths LR. Identification of molecular genetic factors that influence migraine. Molecular genetics and genomics: MGG. 2011;285(6):433–46. Epub 2011/04/27. 10.1007/s00438-011-0622-3 . [DOI] [PubMed] [Google Scholar]
- 22. de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM. Molecular genetics of migraine. Human genetics. 2009;126(1):115–32. Epub 2009/05/21. 10.1007/s00439-009-0684-z [DOI] [PubMed] [Google Scholar]
- 23. Russell MB, Ducros A. Sporadic and familial hemiplegic migraine: pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet neurology. 2011;10(5):457–70. Epub 2011/04/05. 10.1016/s1474-4422(11)70048-5 [DOI] [PubMed] [Google Scholar]
- 24. Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, et al. Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clinical and experimental immunology. 1998;113(3):401–6. Epub 1998/09/16. ; PubMed Central PMCID: PMCPmc1905064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, et al. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. Journal of hypertension. 2002;20(10):2023–7. Epub 2002/10/03. . [DOI] [PubMed] [Google Scholar]
- 26. Rainero I, Grimaldi LME, Salani G, Valfre W, Rivoiro C, Savi L, et al. Association between the tumor necrosis factor-alpha-308 G/A gene polymorphism and migraine. Neurology. 2004;62(1):141–3. WOS:000188010100038. [DOI] [PubMed] [Google Scholar]
- 27. Schurks M, Kurth T, Buring JE, Zee RY. A candidate gene association study of 77 polymorphisms in migraine. The journal of pain: official journal of the American Pain Society. 2009;10(7):759–66. Epub 2009/06/30. 10.1016/j.jpain.2009.01.326 ; PubMed Central PMCID: PMCPmc2704575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yilmaz IA, Ozge A, Erdal ME, Edgunlu TG, Cakmak SE, Yalin OO. Cytokine polymorphism in patients with migraine: some suggestive clues of migraine and inflammation. Pain medicine (Malden, Mass). 2010;11(4):492–7. Epub 2010/02/02. 10.1111/j.1526-4637.2009.00791.x . [DOI] [PubMed] [Google Scholar]
- 29. Mazaheri S, Hajilooi M, Rafiei A. The G-308A promoter variant of the tumor necrosis factor-alpha gene is associated with migraine without aura. Journal of neurology. 2006;253(12):1589–93. 10.1007/s00415-006-0270-4. WOS:000243465700010. [DOI] [PubMed] [Google Scholar]
- 30. Ates O, Kurt S, Altinisik J, Karaer H, Sezer S. Genetic Variations in Tumor Necrosis Factor Alpha, Interleukin-10 Genes, and Migraine Susceptibility. Pain Medicine. 2011;12(10):1464–9. 10.1111/j.1526-4637.2011.01200.x. WOS:000296349600004. [DOI] [PubMed] [Google Scholar]
- 31. Ghosh J, Joshi G, Pradhan S, Mittal B. Investigation of TNFA 308G > A and TNFB 252G > A polymorphisms in genetic susceptibility to migraine. Journal of neurology. 2010;257(6):898–904. Epub 2009/12/26. 10.1007/s00415-009-5430-x . [DOI] [PubMed] [Google Scholar]
- 32. Trabace S, Brioli G, Lulli P, Morellini M, Giacovazzo M, Cicciarelli G, et al. Tumor necrosis factor gene polymorphism in migraine. Headache. 2002;42(5):341–5. Epub 2002/06/06. . [DOI] [PubMed] [Google Scholar]
- 33. Borroni B, Rao R, Liberini P, Venturelli E, Cossandi M, Archetti S, et al. Endothelial nitric oxide synthase (Glu298Asp) polymorphism is an independent risk factor for migraine with aura. Headache. 2006;46(10):1575–9. Epub 2006/11/23. 10.1111/j.1526-4610.2006.00614.x . [DOI] [PubMed] [Google Scholar]
- 34. Lee K-A, Jang SY, Sohn K-M, Won HH, Kim MJ, Kim J-W, et al. Association between a polymorphism in the lymphotoxin_a promoter region and migraine. Headache. 2007;47(7):1056–62. 10.1111/j.1526-4610.2007.00847.x. WOS:000248083000010. [DOI] [PubMed] [Google Scholar]
- 35. Stuart S, Maher BH, Sutherland H, Benton M, Rodriguez A, Lea RA, et al. Genetic variation in cytokine-related genes and migraine susceptibility. Twin research and human genetics: the official journal of the International Society for Twin Studies. 2013;16(6):1079–86. Epub 2013/09/24. 10.1017/thg.2013.63 . [DOI] [PubMed] [Google Scholar]
- 36. Pappa S, Hatzistilianou M, Kouvatsi A, Pantzartzi C, Sakellaropoulou A, Pavlou E, et al. Tumour necrosis factor gene polymorphisms and migraine in Greek children. Archives of medical science: AMS. 2010;6(3):430–7. Epub 2010/06/30. 10.5114/aoms.2010.14267 ; PubMed Central PMCID: PMCPmc3282523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Asuni C, Stochino ME, Cherchi A, Manchia M, Congiu D, Manconi F, et al. Migraine and tumour necrosis factor gene polymorphism. An association study in a Sardinian sample. Journal of neurology. 2009;256(2):194–7. Epub 2009/03/03. 10.1007/s00415-009-0961-8 . [DOI] [PubMed] [Google Scholar]
- 38. Toriello M, Oterino A, Pascual J, Castillo J, Colas R, Alonso-Arranz A, et al. Lack of association of endothelial nitric oxide synthase polymorphisms and migraine. Headache. 2008;48(7):1115–9. Epub 2008/08/09. 10.1111/j.1526-4610.2008.01181.x . [DOI] [PubMed] [Google Scholar]
- 39. Schurks M, Rist PM, Zee RY, Chasman DI, Kurth T. Tumour necrosis factor gene polymorphisms and migraine: a systematic review and meta-analysis. Cephalalgia: an international journal of headache. 2011;31(13):1381–404. Epub 2011/10/18. 10.1177/0333102411419022 ; PubMed Central PMCID: PMCPmc3303222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gu L, Yan Y, Long J, Su L, Hu Y, Chen Q, et al. The TNF-alpha-308G/A polymorphism is associated with migraine risk: A meta-analysis. Experimental and therapeutic medicine. 2012;3(6):1082–6. Epub 2012/09/13. 10.3892/etm.2012.533 ; PubMed Central PMCID: PMCPmc3438673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed). 2003;327(7414):557–60. Epub 2003/09/06. 10.1136/bmj.327.7414.557 ; PubMed Central PMCID: PMCPmc192859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute. 1959;22(4):719–48. Epub 1959/04/01. . [PubMed] [Google Scholar]
- 43. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–88. Epub 1986/09/01. . [DOI] [PubMed] [Google Scholar]
- 44. Trabace S, Brioli G, Lulli P, Morellini M, Giacovazzo M, Cicciarelli G, et al. Tumor necrosis factor gene polymorphism in migraine. Headache. 2002;42(5):341–5. 10.1046/j.1526-4610.2002.02104.x. WOS:000176036600003. [DOI] [PubMed] [Google Scholar]
- 45. Herken H, Erdal ME, Yilmaz M, Savasoglu K, Bayazit YA. The-308 G/A polymorphism of tumor necrosis factor alpha gene is not associated with migraine. Pain Clinic. 2005;17(4):389–93. 10.1163/156856905774482814. BIOSIS:PREV200600176105. [DOI] [Google Scholar]
- 46. Asuni C, Stochino ME, Cherchi A, Manconi M, Congiu D, Del Zompo M. Migraine and tumor necrosis factor gene polymorphism: An association study in a Sardinian sample. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2006;141B(7):785-. WOS:000240877700448. [DOI] [PubMed] [Google Scholar]
- 47. MacClellan LR, Howard TD, Cole JW, Stine OC, Giles WH, O'Connell JR, et al. Relation of candidate genes that encode for endothelial function to migraine and stroke: the Stroke Prevention in Young Women study. Stroke; a journal of cerebral circulation. 2009;40(10):e550–7. Epub 2009/08/08. 10.1161/strokeaha.109.557462 ; PubMed Central PMCID: PMCPmc2753702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gruber HJ, Bernecker C, Lechner A, Weiss S, Wallner-Blazek M, Meinitzer A, et al. Increased nitric oxide stress is associated with migraine. Cephalalgia: an international journal of headache. 2010;30(4):486–92. Epub 2009/08/14. 10.1111/j.1468-2982.2009.01964.x . [DOI] [PubMed] [Google Scholar]
- 49. Goncalves FM, Martins-Oliveira A, Speciali JG, Luizon MR, Izidoro-Toledo TC, Silva PS, et al. Endothelial nitric oxide synthase haplotypes associated with aura in patients with migraine. DNA and cell biology. 2011;30(6):363–9. Epub 2011/02/22. 10.1089/dna.2010.1152 . [DOI] [PubMed] [Google Scholar]
- 50. Goncalves FM, Luizon MR, Speciali JG, Martins-Oliveira A, Dach F, Tanus-Santos JE. Interaction among nitric oxide (NO)-related genes in migraine susceptibility. Molecular and cellular biochemistry. 2012;370(1–2):183–9. Epub 2012/08/07. 10.1007/s11010-012-1409-5 . [DOI] [PubMed] [Google Scholar]
- 51. Eroz R, Bahadir A, Dikici S, Tasdemir S. Association of Endothelial Nitric Oxide Synthase Gene Polymorphisms (894G/T, -786T/C, G10T) and Clinical Findings in Patients with Migraine. Neuromolecular medicine. 2014;16(3):587–93. Epub 2014/05/23. 10.1007/s12017-014-8311-0 . [DOI] [PubMed] [Google Scholar]
- 52. Molana A, Mehrpour M, Vousooghi N, Hajighasem MR, Joghataei MT. Effect of NOS3 gene polymorphism on response to Tricyclic antidepressants in migraine attacks. Iranian journal of neurology. 2014;13(3):154–9. Epub 2014/11/26. ; PubMed Central PMCID: PMCPmc4240933. [PMC free article] [PubMed] [Google Scholar]
- 53. Gruber HJ, Bernecker C, Lechner A, Weiss S, Wallner-Blazek M, Meinitzer A, et al. Increased nitric oxide stress is associated with migraine. Cephalalgia: an international journal of headache. 2010;30(4):486–92. 10.1111/j.1468-2982.2009.01964.x [DOI] [PubMed] [Google Scholar]
- 54. Asuni C, Stochino ME, Cherchi A, Manchia M, Congiu D, Manconi F, et al. Migraine and tumour necrosis factor gene polymorphism. Journal of neurology. 2009;256(2):194–7. 10.1007/s00415-009-0961-8. WOS:000264176700005. [DOI] [PubMed] [Google Scholar]
- 55. Goncalves FM, Martins-Oliveira A, Speciali JG, Luizon MR, Izidoro-Toledo TC, Silva PS, et al. Endothelial Nitric Oxide Synthase Haplotypes Associated with Aura in Patients with Migraine. DNA and cell biology. 2011;30(6):363–9. 10.1089/dna.2010.1152. WOS:000291164000004. [DOI] [PubMed] [Google Scholar]
- 56. Toriello M, Oterino A, Pascual J, Castillo J, Colas R, Alonso-Arranz A, et al. Lack of association of endothelial nitric oxide synthase polymorphisms and migraine. Headache. 2008;48(7):1115–9. 10.1111/j.1526-4610.2008.01181.x [DOI] [PubMed] [Google Scholar]
- 57. Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Controlled clinical trials. 1990;11(2):116–28. Epub 1990/04/01. . [DOI] [PubMed] [Google Scholar]
- 58. Levy D. Migraine pain and nociceptor activation—where do we stand? Headache. 2010;50(5):909–16. Epub 2010/06/16. 10.1111/j.1526-4610.2010.01670.x . [DOI] [PubMed] [Google Scholar]
- 59. Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annual review of physiology. 2013;75:365–91. Epub 2012/11/30. 10.1146/annurev-physiol-030212-183717 . [DOI] [PubMed] [Google Scholar]
- 60. Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. British journal of pharmacology. 1992;107(3):660–4. Epub 1992/11/01. ; PubMed Central PMCID: PMCPmc1907751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34(5):391–9. Epub 1997/04/01. . [DOI] [PubMed] [Google Scholar]
- 62. Wilson AG, Symons JA, McDowell TL, McDevitt HO, Duff GW. Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activation. Proc Natl Acad Sci U S A. 1997;94(7):3195–9. Epub 1997/04/01. ; PubMed Central PMCID: PMCPmc20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lam TH, Shen M, Chia JM, Chan SH, Ren EC. Population-specific recombination sites within the human MHC region. Heredity. 2013;111(2):131–8. Epub 2013/05/30. 10.1038/hdy.2013.27 ; PubMed Central PMCID: PMCPmc3716270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schuerks M, Rist PM, Zee RYL, Chasman DI, Kurth T. Tumour necrosis factor gene polymorphisms and migraine: A systematic review and meta-analysis. Cephalalgia: an international journal of headache. 2011;31(13):1381–404. 10.1177/0333102411419022. WOS:000295887500007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schuerks M, Kurth T, Buring JE, Zee RYL. A Candidate Gene Association Study of 77 Polymorphisms in Migraine. Journal of Pain. 2009;10(7):759–66. 10.1016/j.jpain.2009.01.326. WOS:000267712500010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
(XLSX)
A: S1A Fig Galbraith plot for TNF –308G>A. B: S1B Fig Galbraith plot for NOS3 +894G>T.
(EPS)
MA: migraine with aura; MO: migraine without aura
(DOCX)
MA: migraine with aura; MO: migraine without aura
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.