Abstract
Background: exercise can reduce osteoporotic fracture risk by strengthening bone or reducing fall risk. Falls prevention exercise programmes can reduce fall incidence, and also include strengthening exercises suggested to load bone, but there is little information as to whether these programmes influence bone mineral density (BMD) and strength.
Objective: to evaluate the skeletal effects of home (Otago Exercise Programme, OEP) and group (Falls Exercise Management, FaME) falls prevention exercise programmes relative to usual care in older people.
Methods: men and women aged over 65 years were recruited through primary care. They were randomised by practice to OEP, FaME or usual care. BMD, bone mineral content (BMC) and structural properties were measured in Nottingham site participants before and after the 24-week intervention.
Results: participants were 319 men and women, aged mean(SD) 72(5) years. Ninety-two percentage of participants completed the trial. The OEP group completed 58(43) min/week of home exercise, while the FaME group completed 39(16) and 30(24) min/week of group and home exercise, respectively. Femoral neck BMD changes did not differ between treatment arms: mean (95% CI) effect sizes in OEP and FaME relative to usual care arm were −0.003(−0.011,0.005) and −0.002(−0.010,0.005) g cm−2, respectively; P = 0.44 and 0.53. There were no significant changes in BMD or BMC at other skeletal sites, or in structural parameters.
Conclusions: falls prevention exercise programmes did not influence BMD in older people. To increase bone strength, programmes may require exercise that exerts higher strains on bone or longer duration.
Keywords: osteoporosis; prevention; exercise; bone mineral density; X-ray absorptiometry; men; women; aged; primary care, older people
Introduction
Osteoporosis is increasingly prevalent: in the UK, it is estimated that over half of women, and one-fifth of men aged 50, will sustain a fragility fracture [1]. Physical activity may benefit bone mass, reducing the risk of osteoporotic fracture. The efficacy of exercise has received less attention relative to pharmaceutical treatments, although there is evidence that low load activities such as walking have at most a modest effect on bone mineral density (BMD) while high impact exercise or high intensity resistance training produce the greatest response [2, 3].
The risk of fracture depends also upon the risk of falling. Strength and balance training programmes such as the Otago Exercise Programme (OEP) and Falls Management Exercise (FaME) are designed to reduce fall risk in older people. OEP is a home-based programme, reported to be effective in reducing the number of falls and fall-related injuries, improving strength and balance and was cost-effective for those aged over 80 years [4–8]. FaME includes group exercise delivered by trained postural stability instructors and has been found to reduce the number of falls and injuries from falls [9, 10]. Both interventions include resistance exercises designed to load bone, and high load resistance exercise can benefit BMD in older people [2, 11]. Participation in FaME reduced bone loss at Ward's triangle in postmenopausal women [12]. Therefore, these falls prevention interventions could be effective for improving bone strength as well as reducing fall risk.
This study aimed to determine the effectiveness on BMD of community (FaME) and home-based (OEP) exercise interventions compared with usual care in older people.
Methods
Experimental design
Participants were recruited from the ProAct65+ trial, a major primary care-based trial comparing group exercise (FaME) and home-based exercise (OEP) with usual care in older people (registered as ISRCTN43453770). This trial recruited participants through general practices at two sites: London and Nottingham. Trial statisticians conducted randomisation by practice using minimisation for trial centre, practice size and the index of multiple deprivation [13]. At the baseline assessment (between 2009 and 2011), trial researchers invited all participants enrolled in the Nottingham site to participate in the bone sub-study. Volunteers attended additional visits at baseline and at the end of the intervention for measurements of BMD by bone study researchers. Bone study researchers conducting measurements and analysing data were not aware of participants' intervention allocation, although participants were aware which intervention they were receiving. The study was approved by the National Research Ethics Service and University Ethics Advisory Committee. All participants provided written informed consent.
Participants
Participants were recruited through general practices located in Nottinghamshire and Derbyshire, UK [13]. Inclusion criteria specified that participants were men and women aged 65 years or over, able to walk around at home and participate in an exercise programme. Participants were excluded if they had experienced three or more falls in the previous year, had unstable clinical conditions, would be unable to follow instructions about exercise safely, were receiving palliative care or were already exercising at or above the target level [13]. We estimated that 142 participants per group would be required to detect a difference in response in dual hip BMD between intervention and usual care group of 0.005(0.014) g cm−2 with power of 80%, 5% significance level, assuming a two-sided comparison.
Interventions
The interventions were home-based exercise (OEP) and community-based exercise (FaME), which were compared with usual care. Interventions were delivered for 6 months (24 weeks). The interventions have been described in more detail previously [13]; key features are summarised below.
The OEP programme consisted of three 30-min home exercise sessions and at least two 30-min sessions of walking at a moderate pace each week. The home exercise sessions were introduced by trial research staff in a one-off training session, and consisted of progressive leg strengthening and balance exercises. Each participant received an instruction booklet and ankle cuff weights at the initial session, and was followed up by a peer mentor, where available, during scheduled two home visits and eight telephone calls. The recruitment of peer mentors was difficult however, so the majority of participants did not have an assigned peer mentor [14].
The FaME programme consisted of one 60-min exercise class, two 30-min home exercise sessions based on the OEP, and at least two 30-min sessions of walking at a moderate pace each week. The exercise classes were delivered by a postural stability instructor and included progressive leg, arm and trunk muscle strengthening (using ankle cuff weights and Therabands); flexibility training; functional floor skills and adapted Tai Chi.
Participants in the usual care group were not offered the FaME or OEP programmes, but were permitted to participate in physical activity as they would if they had not been participating in the trial.
Outcome measures
Outcome measures were assessed prior to randomisation, and at the end of the intervention/usual care period (at least 6 months from the start and within 4 weeks of the end of the intervention). The primary outcome measure was femoral neck BMD. Secondary outcomes were bone measurements at other skeletal sites.
Dual X-ray absorptiometry (DXA) scans of the whole body, lumbar spine, proximal femur and distal forearm were conducted using GE Lunar Prodigy Advance bone densitometers using Encore version 13.2 software. All scans for each participant were performed by the same operator on the same scanner. The short-term root-mean-square coefficients of variation for femoral neck, upper neck, trochanter, total hip, lumbar spine, ultradistal radius and total body BMD were 1.3, 2.2, 1.2, 0.5, 1.2, 4.3 and 0.7%, respectively, while those for section modulus and strength index were 3.6 and 7.7%, respectively, and those for bone mass, fat mass and lean mass were 1.9, 1.9 and 1.5%, respectively.
During the baseline assessment at the participants' general practice, interviews were conducted to determine participants' age, ethnicity, socioeconomic status, health status and medication use [13]. Subjective habitual physical activity assessment included the CHAMPS questionnaire [15]. Dietary intake and supplement use [16], osteoporosis medication and age at menopause, were assessed by questionnaire during the additional bone sub-study assessment at baseline and follow-up visits.
All participants were requested to complete and return monthly diaries summarising their daily participation (in minutes) in FaME classes, home exercise sessions and walking.
Statistical analysis
Data for those that agreed to participate in the ProAct65+ bone study were compared with those in the main ProAct65+ trial who did not volunteer to evaluate possible bias, using unpaired t, Mann–Whitney or χ2 tests as appropriate. Descriptive statistics were calculated and compared descriptively between treatment arms. Data for participants who had commenced or ceased osteoporosis medication during the trial were excluded. Comparisons between treatment arms were made using random effects linear regression models to allow for clustering by practice, adjusted for baseline values, gender, anti-resorptive medication use and comorbidities (major surgery in previous year, asthma, diabetes, thyroid or oral steroid medication use). Analysis was repeated with participants with illness or medication that may substantively affect bone excluded (i.e. osteoporosis medication, oral steroids, major surgery in previous 12 months), and in compliers only, i.e. participants in exercise arms that completed at least 75% of the prescribed group/home exercise.
Economic analysis
The main ProAct65+ trial included an embedded economic analysis. Within the bone health study, the intention was to conduct a cost-effectiveness analysis to compare each intervention with the usual care condition with respect to the primary outcome (femoral neck BMD). The resources involved in the delivery of each intervention were gathered from study records, and included instructors, facilities (for FaME classes) and equipment. The full economic costs, from a health service perspective, were calculated in British pounds, 2011, using validated staff unit costs [17] and actual expenditures.
Results
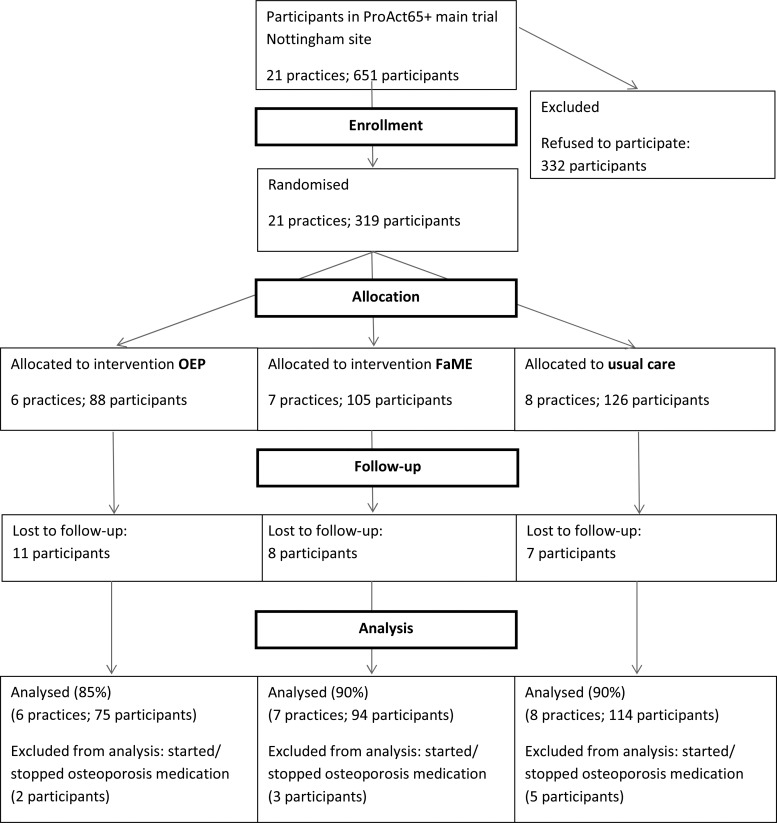
Of the 651 participants from 21 general practices in the Nottingham arm of the ProAct65+ study, 365 participants expressed an interest in the bone study. Of these, 319 agreed to participate and attended for baseline measurements and 293 for follow-up measurements (Figure 1).
Figure 1.
Flow of participants.
Participants in the ProAct65+ trial who volunteered for the bone study were younger, had fewer co-morbidities, higher levels of education and higher household income and physical activity than those who did not volunteer (Supplementary data, Table S1, are available in Age and Ageing online). Height, weight and the proportion of female participants were not significantly different between bone study participants and non-participants.
The characteristics of participants in each treatment arm are summarised in Table 1. The proportion of female participants was lowest in the usual care arm (54%) and highest in the OEP arm (68%). The proportion taking osteoporosis medication (all using anti-resorptive therapy) was higher in the FAME than in the usual care arm. Despite this, BMD and structural parameters were similar in the three treatment arms.
Table 1.
Baseline characteristics of bone study participants according to treatment arm: mean (SD) or number (%)
| OEP (n = 88) | FAME (n = 105) | Usual care (n = 126) | |
|---|---|---|---|
| Age (years) | 71.4 (4.9) | 71.8 (5.5) | 72.2 (5.5) |
| Height (cm) | 163.8 (9.6) | 164.8 (9.3) | 165.3 (8.7) |
| Weight (kg) | 71.8 (5.5) | 76.7 (14.4) | 78.2 (16.6) |
| % fat | 38.5 (8.8) | 36.6 (8.2) | 36.6 (8.9) |
| Female | 60 (68.2%) | 63 (60.0%) | 68 (54.0%) |
| Ethnicity | |||
| Asian | 1 (1.1%) | 2 (1.9%) | 3 (2.4%) |
| Other | 0 (0.0%) | 0 (0.0%) | 3 (2.4%) |
| White | 87 (98.9%) | 102 (97.1%) | 119 (94.4%) |
| Not specified | 0 (0.0%) | 1 (1.0%) | 1 (0.8%) |
| Osteoporosis medication | 5 (5.7%) | 11 (10.5%) | 3 (2.4%) |
| Diabetes medication | 7 (8.0%) | 10 (9.5%) | 13 (10.4%) |
| Thyroid medication | 8 (9.1%) | 6 (5.7%) | 9 (7.2%) |
| Oral steroids | 1 (1.1%) | 4 (3.8%) | 4 (3.2%) |
| Asthma medication | 3 (3.4%) | 9 (8.6%) | 15 (12.0%) |
| Oestrogen replacement | 2 (2.3%) | 0 (0.0%) | 2 (1.6%) |
| Major surgery/illness in past year | 4 (4.5%) | 7 (6.7%) | 2 (1.6%) |
| Total body BMD (g cm−2) | 1.113 (0.119) | 1.123 (0.123) | 1.133 (0.134) |
| Lumbar spine (L2–4) BMD (g cm−2) | 1.147 (0.206) | 1.161 (0.215) | 1.176 (0.234) |
| Ultradistal radius BMD (g cm−2) | 0.431 (0.100) | 0.448 (0.112) | 0.446 (0.101) |
| Femoral neck BMD (g cm−2) | 0.897 (0.128) | 0.889 (0.143) | 0.910 (0.150) |
| Trochanter BMD (g cm−2) | 0.789 (0.156) | 0.794 (0.166) | 0.814 (0.182) |
| Total hip BMD (g cm−2) | 0.946 (0.148) | 0.939 (0.160) | 0.959 (0.175) |
| Upper neck BMD (g cm−2) | 0.720 (0.133) | 0.723 (0.141) | 0.736 (0.146) |
| Femur strength index | 1.37 (0.35) | 1.35 (0.27) | 1.46 (0.37) |
| Section modulus (mm3) | 615 (191) | 644 (198) | 670 (212) |
| Started/stopped osteoporosis treatment during trial | 2 (2.5%) | 3 (3.1%) | 5 (4.1%) |
OEP participants averaged 58(43) min of home exercise and 166(172) minutes of walking each week. FaME participants reported less home exercise and walking [30(24) and 133(101) min] but also attended 39(16) min of FaME class each week. The usual care group also reported 15(32) min of home exercise and 121(121) min of walking per week. Adverse events were minimal and have been reported previously [18].
Ten participants started or stopped taking osteoporosis medication during the trial and their data were excluded from further analysis.
Femoral neck BMD (adjusted for baseline BMD, gender, major illness/surgery in last 12 months and osteoporosis, oral steroid, asthma, diabetes and thyroid medication use) did not differ between treatment arms, with effect sizes in OEP and FaME relative to usual care arms being −0.003 and −0.002 g cm−2 (P = 0.44 and 0.53), respectively, (−0.3 and −0.2% of the baseline value; Table 2; Supplementary data, Figure S1, are available in Age and Ageing online).
Table 2.
Random effects linear regression summarising effect size in FaME and OEP relative to usual care: differences between means, 95% confidence intervals and P-values
| OEP | FaME | |
|---|---|---|
| Femoral neck BMD (g cm−2) | −0.003 (−0.011, 0.005) P = 0.442 |
−0.002 (−0.010, 0.005) P = 0.526 |
| Trochanter BMD (g cm−2) | −0.005 (−0.032, 0.022) P = 0.701 |
0.000 (−0.025, 0.026) P = 0.980 |
| Total hip BMD (g cm−2) | −0.008 (−0.034, 0.019) P = 0.362 |
0.003 (−0.022, 0.028) P = 0.955 |
| Upper neck BMD (g cm−2) | 0.003 (−0.018, 0.023) P = 0.785 |
0.006 (−0.013, 0.026) P = 0.516 |
| Section modulus (mm3) | −8.5 (−21.7, 4.7) P = 0.205 |
−6.9 (−19.3, 5.6) P = 0.277 |
| Femur strength index | 0.008 (−0.035, 0.052) P = 0.704 | 0.036 (−0.005, 0.078) P = 0.084 |
| Lumbar spine (L2–4) BMD (g cm−2) | 0.003 (−0.012, 0.019) P = 0.649 |
0.005 (−0.010, 0.020) P = 0.497 |
| Distal radius BMD (g cm−2) | 0.001 (−0.008, 0.010) P = 0.857 |
−0.009 (−0.018, −0.000) P = 0.042 |
| Total body BMD (g cm−2) | 0.003 (−0.002, 0.008) P = 0.257 |
−0.003 (−0.007, 0.002) P = 0.206 |
| Total body BMC (g) | 0.8 (−22.0, 23.6) P = 0.945 |
−6.6 (−27.9, 14.7) P = 0.542 |
| Fat mass (kg) | 0.31 (−0.24, 0.87) P = 0.271 |
−0.57 (−1.09, 0.05) P = 0.033 |
| Lean mass (kg) | −0.17 (−0.62, 0.28) P = 0.446 |
−0.08 (−0.51, 0.36) P = 0.718 |
Adjusted for baseline value, gender, major illness/surgery in last 12 months, osteoporosis medication, oral steroids, asthma medication, diabetes medication, and thyroid medication. Bold values indicate significant differences relative to usual care (P < 0.05).
Similarly, there were no differences between treatment arms in BMD at other hip sites, lumbar spine or total body; or in structural parameters section modulus and femur strength index (Table 2). Distal radius BMD declined in the FaME arm relative to usual care (−2.0% relative to baseline value, Table 2). Body fat mass also reduced by 0.65 kg in FaME relative to the usual care arm, although there were no significant effects on lean mass.
A sensitivity analysis was conducted excluding participants without major surgery in the past 12 months, osteoporosis medication and/or oral steroids (n = 65, 78 and 94 in OEP, FaME and usual care, respectively). Effect sizes were very similar and there were no significant effects. The decline in ultradistal radius BMD in the FaME group relative to usual care was smaller (effect size [95% confidence intervals] −0.006 [−0.015, 0.003]) and no longer statistically significant (P = 0.182).
Analyses were repeated in 29 OEP and 27 FaME participants who had completed over 75% of the recommended duration of group and/or home exercise. There were again no significant effects on bone variables, with the effect size for femoral neck BMD in OEP relative to usual care being 0.000 [−0.009, 0.009] g cm−2, P = 0.98. Corresponding values in the FaME participants were 0.001 [−0.008, 0.010] g cm−2; P = 0.86.
The health service cost of the 6-month FaME exercise programme delivered in the trial was approximately £218 (77% of which was for the group instructors). The OEP programme cost approximately £36 per participant without a peer mentor, which was mostly for the induction session and ankle cuffs [18]. The corresponding cost for OEP participants with a peer mentor was £117. In the absence of significant improvement in bone density arising from either exercise programme, compared with usual care, a cost-effectiveness analysis was not warranted.
Discussion
This study demonstrates that the 6-month OEP and FaME exercise interventions did not benefit bone density in older people recruited through primary care.
The lack of skeletal benefit in this study may result from the intervention not providing adequate skeletal loading. According to mechanostat theory, bone adapts when stresses applied to bone generate strains (minute deformations) that exceed those to which bone is habituated [19]. Exercise that applies relatively high loading to bone can increase BMD even in older people [20]. The magnitudes of loading resulting from OEP and FaME exercises may not have exceeded that to which participants were habituated.
The duration of the intervention could also contribute to the lack of bone change in this study. The exercise interventions lasted only 6 months, which may have been inadequate to detect changes in mineralisation. While bone gains from resistance training have been observed within as little as 16 weeks [21] greater benefit has been reported after 12 months [22]. The FaME intervention that increased Ward's triangle BMD [12] involved a 9-month intervention in a frailer group of women. Differences in the progression of interventions, i.e. the rate of increasing magnitude of weights lifted, may also explain differences between trials. In practice, however, falls prevention programmes are not delivered for >6 months [23] so this study reflects prevailing clinical practice.
The unexpected reduction in BMD at the forearm in the FaME arm disappeared once participants with medical conditions or medications substantially affecting bone were excluded, so it seems likely that this apparent bone loss results from these factors, rather than the intervention. The FaME group also demonstrated a modest but statistically significant reduction in body fat content, which may confer further health benefits. Both findings however could be attributed to chance since their statistical significance was not strong, and a high number of variables were analysed.
Bone strength depends also upon structural parameters, which may change with physical activity and age [24]. Structural change may increase bone strength in the absence of any increase in BMD, so effects of exercise may be underestimated by using BMD alone [25]. We measured section modulus, which is related to the bone's strength in bending [26], and femur strength index, the estimated ratio of the strength of the bone relative to the stresses incurred in a fall [27]. Neither parameter showed statistically significant effects. Areal BMD measured by DXA does not separate cortical and trabecular compartments. Distinguishing these may be important as exercise increased the amount of cortical bone (measured by quantitative computed tomography, QCT) without any change in areal BMD by DXA in one study [28]. It is possible that QCT measures may have detected structural benefits not evident in this study.
This study is one of the largest exercise intervention trials to have been conducted on bone health. Although we did not quite achieve our target sample size, we should have been able to detect modest BMD changes of <1%. The study benefits from a primary care setting and more representative sample of participants than most previous trials, which may make findings more broadly generalizable, although participants may still have been more active than the older population in general. Limitations include the limited duration of the intervention, although this reflects the likely scenario in clinical practice, where it may not be feasible to introduce interventions of greater duration.
While group- or home-based exercise programmes have an important role in reducing fracture risk by reducing risk of falls, they do not increase BMD. To benefit bone density and strength as well as reducing fall risk, interventions may require a greater magnitude of progressive loading, and/or a longer duration.
Key points.
This study examined whether strength and balance training programmes influence BMD in older people.
319 participants were randomised to 6 months of group exercise, home exercise or usual care.
The exercise interventions had no significant effect on BMD or bone structural parameters.
To increase bone strength, exercise programmes may need to generate greater bone strains and/or have a longer duration.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Funding
The main ProAct65+ trial was supported by the National Institute of Health Research Health Technology Assessment Programme (grant number: 06/36/04). The ProAct65+ bone study was supported by an additional award from the National Osteoporosis Society (grant number NOS-2008-23). The financial sponsors had no role in study design, execution, analysis and interpretation of data or writing of the study.
Conflicts of interest
D.A.S. and S.D.Y. are directors of a not-for-profit training company, Later Life Training, who deliver training in the Otago and FaME exercises to health and fitness professionals.
Supplementary Material
References
- 1.Van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001; 29: 517–22. [DOI] [PubMed] [Google Scholar]
- 2.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. Physical activity and bone health. Med Sci Sports Exerc 2004; 36: 1985–96. [DOI] [PubMed] [Google Scholar]
- 3.Howe TE, Shea B, Dawson LJ, Downie F, Murray A, Ross C, Harbour RT, Caldwell LM, Creed G. Exercise for preventing and treating osteoporosis in postmenopausal women. The Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized controlled trial. J Am Geriatr Soc 1999; 47: 850–3. [DOI] [PubMed] [Google Scholar]
- 5.Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 2002; 50: 905–11. [DOI] [PubMed] [Google Scholar]
- 6.Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001; 30: 77–83. [DOI] [PubMed] [Google Scholar]
- 7.Robertson MC, Devlin N, Gardner MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. Br Med J 2001; 322: 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Tilyard MW, Buchner DM. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. Br Med J 1997; 315: 1065–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skelton DA, Dinan SM. Exercise for falls management: rationale for an exercise programme aimed a reducing postural instability. Physiother Theory Pract 1999; 15: 105–20. [Google Scholar]
- 10.Skelton DA, Dinan SM, Campbell MG, Rutherford OM. FaME (Falls Management Exercise): an RCT on the effects of a 9-month group exercise programme in frequently failing community dwelling women age 65 and over. J Aging Phys Act 2004; 12: 457–8. [Google Scholar]
- 11.Gianoudis J, Bailey CA, Ebeling PR, Nowson CA, Sanders KM, Hill K, Daly RM. Effects of a targeted multimodal exercise program incorporating high-speed power training on falls and fracture risk factors in older adults: a community-based randomized controlled trial. J Bone Miner Res 2014; 29: 182. [DOI] [PubMed] [Google Scholar]
- 12.Skelton DA, Stranzinger K, Dinan SM, Rutherford OM. BMD improvements following FaME (Falls Management Exercise) in frequently falling women age 65 and over: an RCT. J Aging Phys Act 2008; 16: S89–90. [Google Scholar]
- 13.Iliffe S, Kendrick D, Morris R, Skelton D, Gage H, Dinan S, Stevens Z, Pearl M, Masud T. Multi-centre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people aged 65 and over in primary care: protocol of the ProAct 65+trial. Trials 2010; 11: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens Z, Carpenter H, Gawler S, Belcher C, Haworth D, Kendrick D, Morris R, Masud T, Skelton D, Iliffe S. Lessons learnt during a complex, multicentre cluster randomised controlled trial: the ProAct65+ trial. Trials 2013; 14: 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc 2001; 33: 1126–41. [DOI] [PubMed] [Google Scholar]
- 16.McKeown NM, Day NE, Welch AA, Runswick SA, Luben RN, Mulligan AA, McTaggart A, Bingham SA. Use of biological markers to validate self-reported dietary intake in a random sample of the European Prospective Investigation into Cancer United Kingdom Norfolk cohort. Am J Clin Nutr 2001; 74: 188–96. [DOI] [PubMed] [Google Scholar]
- 17.Curtis L. Unit Costs of Health and Social Care 2012. Personal Social Services Research Unit, 2012. [Google Scholar]
- 18.Iliffe S, Kendrick D, Morris R, Masud T, Gage H, Skelton D, Dinan S, Bowling A, Griffin M, Haworth D. Multicentre cluster randomised trial comparing a community group exercise programme and home-based exercise with usual care for people aged 65 years and over in primary care. Health Technol Assess 2014; 18: 1–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat Rec 1987; 219: 1–9. [DOI] [PubMed] [Google Scholar]
- 20.Allison S, Folland J, Rennie W, Summers G, Brooke-Wavell K. High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone 2013; 53: 321–8. [DOI] [PubMed] [Google Scholar]
- 21.Menkes A, Mazel S, Redmond RA, Koffler K, Libanati CR, Gundberg CM, Zizic TM, Hagberg JM, Pratley RE, Hurley BF. Strength training increases regional bone mineral density and bone remodeling in middle-aged and older men. J Appl Physiol 1993; 74: 2478–84. [DOI] [PubMed] [Google Scholar]
- 22.Kerr D, Morton A, Dick I, Prince R. Exercise effects on bone mass in postmenopausal women are site-specific and load-dependent. J Bone Miner Res 1996; 11: 218–25. [DOI] [PubMed] [Google Scholar]
- 23.Royal College of Physicians. Older People's Experiences of Therapeutic Exercise as Part of a Falls Prevention Service—Patient and Public Involvement. London: RCP, 2012. [Google Scholar]
- 24.Kaptoge S, Jakes RW, Dalzell N, Wareham N, Khaw KT, Loveridge N, Beck TJ, Reeve J. Effects of physical activity on evolution of proximal femur structure in a younger elderly population. Bone 2007; 40: 506–15. [DOI] [PubMed] [Google Scholar]
- 25.Jarvinen TLN, Kannus P, Sievanen H. Have the DXA-based exercise studies seriously underestimated the effects of mechanical loading on bone? J Bone Miner Res 1999; 14: 1634–5. [DOI] [PubMed] [Google Scholar]
- 26.Beck T. Measuring the structural strength of bones with dual-energy X- ray absorptiometry: principles, technical limitations, and future possibilities. Osteoporos Int 2003; 14: S81–8. [DOI] [PubMed] [Google Scholar]
- 27.Faulkner KG, Wacker WK, Barden HS, Simonelli C, Burke PK, Ragi S, Del Rio L. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int 2006; 17: 593–9. [DOI] [PubMed] [Google Scholar]
- 28.Adami S, Gatti D, Braga V, Bianchini D, Rossini M. Site-specific effects of strength training on bone structure and geometry of ultradistal radius in postmenopausal women. J Bone Miner Res 1999; 14: 120–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.