Abstract
AIM: To investigate the effects of activated rat hepatic stellate cells (HSCs) on rat Th1/Th2 profile in vitro.
METHODS: Growth and survival of activated HSCs and CD4+ T lymphocytes cultured alone or together was assessed after 24 or 48 h. CD4+ T lymphocytes were then cultured with or without activated HSCs for 24 or 48 h and the proportion of Th1 [interferon (IFN)-γ+] and Th2 [interleukin (IL)-4+] cells was assessed by flow cytometry. Th1 and Th2 cell apoptosis was assessed after 24 h of co-culture using a caspase-3 staining procedure. Differentiation rates of Th1 and Th2 cells from CD4+ T lymphocytes that were positive for CD25 but did not express IFN-γ or IL-4 were also assessed after 48 h of co-culture with activated HSCs. Galectin-9 expression in HSCs was determined by immunofluorescence and Western blotting. ELISA was performed to assess galectin-9 secretion from activated HSCs.
RESULTS: Co-culture of CD4+ T lymphocytes with activated rat HSCs for 48 h significantly reduced the proportion of Th1 cells compared to culture-alone conditions (-1.73% ± 0.71%; P < 0.05), whereas the proportion of Th2 cells was not altered; the Th1/Th2 ratio was significantly decreased (-0.44 ± 0.13; P < 0.05). In addition, the level of IFN-γ in Th1 cells was decreased (-65.71 ± 9.67; P < 0.01), whereas the level of IL-4 in Th2 cells was increased (82.79 ± 25.12; P < 0.05) by co-culturing, as measured by mean fluorescence intensity by flow cytometry. Apoptosis rates in Th1 (12.27% ± 0.99%; P < 0.01) and Th2 (1.71% ± 0.185%; P < 0.01) cells were increased 24 h after co-culturing with activated HSCs; the Th1 cell apoptosis rate was significantly higher than in Th2 cells (P < 0.01). Galectin-9 protein expression was significantly decreased in HSCs only 24 h after co-culturing (P < 0.05) but not after 48 h. Co-culture for 48 h significantly increased the differentiation of Th1 and Th2 cells; however, the increase in the proportion of Th2 cells was significantly higher than that of Th1 cells (1.85% ± 0.48%; P < 0.05).
CONCLUSION: Activated rat HSCs lower the Th1/Th2 profile, inhibiting the Th1 response and enhancing the Th2 response, and this may be a novel pathway for liver fibrogenesis.
Keywords: Hepatic stellate cells, Th1 cells, Th2 cells, Galectin-9
Core tip: The Th1/Th2 profile plays a pivotal role in the development of fibrosis and may be impacted by hepatic stellate cell activation. However, little is known about the effect of fibroblasts on CD4+ T lymphocyte profiles. This study shows that co-culture with activated rat hepatic stellate cells shifts the Th1/Th2 profile of CD4+ T lymphocytes by inhibiting the Th1 response and enhancing the Th2 response.
INTRODUCTION
Liver fibrosis can result from sustained activation of myofibroblasts and other matrix-producing cells, such as hepatic stellate cells (HSCs)[1]. As fibrosis develops, there is an infiltration of inflammatory cells that secrete proinflammatory and profibrotic cytokines. Innate and adaptive immune cells, such as natural killer (NK) and Kupffer cells, play roles in the reversal of fibrosis[2]. In addition, T lymphocytes can interact with myofibroblasts and secrete various cytokines to modulate the fibrogenic process[3]. Type 1 helper T (Th1) lymphocytes secrete high levels of antifibrotic interferon (IFN)-γ, whereas type 2 (Th2) cells release profibrogenic interleukin (IL)-4 and IL-13[3-5].
The switch from an early Th1 response to a sustained Th2 response is thought to facilitate hepatic fibrogenesis in alcoholic liver disease[6] and schistosomiasis[7]. Therefore, the Th1/Th2 profile is pivotal in the development of liver fibrosis and presents a possible therapeutic target. However, the mechanisms involved in regulating Th1/Th2 balance during fibrogenesis are still unclear. Some evidence suggests that decreased production of IL-12 by antigen-presenting cells in the liver[6] and regulatory T cells[4,8] play roles in this balance. However, activated HSCs have also been observed adjacent to and engulfing infiltrating lymphocytes and NK cells in the liver following fibrosis induction[2,9,10], suggesting a direct interaction between liver myofibroblasts and infiltrating lymphocytes. HSCs are activated by immune cells[3,4,11] and can then influence lymphocyte infiltration, activation and proliferation via secretion of various proinflammatory cytokines and chemokines[2]. To gain a better understanding of this relationship, T lymphocytes were co-cultured with activated HSCs and the effect on the Th1/Th2 profile was examined in vitro.
MATERIALS AND METHODS
Activation of rat HSCs
Male Wistar rats (250 g each) were used in this study. Rat livers were digested with collagenase and HSCs were obtained by fractionation of the cell suspension using continuous Percoll density gradients, as described previously[12]. HSCs were cultured in RPMI 1640 (Gibco of Thermo Fisher Scientific, Waltham, MA, United States) containing penicillin (100 U/mL), streptomycin (100 μg/mL) and 20% fetal bovine serum (FBS; Gibco) at 37 °C with 5% CO2. After reaching about 100% confluency (10-14 d), the cells were subcultured for co-culture or further examination. Activation of subcultured cells was confirmed by immunostaining for anti-desmin (1:50; Santa Cruz Biotechnology Inc., Dallas, TX, United States) and anti-α-smooth muscle actin (1:800; Abcam, Cambridge, United Kingdom) using fluorescein isothiocyanate (FITC)-conjugated anti-rabbit (1:200; ZhongShan-Golden Bridge, Beijing, China) or anti-mouse (1:500; Abcam) secondary antibodies.
Isolation of CD4+ T lymphocytes
Rat spleen lymphocytes were isolated as previously described[13] and cultured in RPMI 1640 containing 10% FBS for 2 h to remove adherent monocytes. Rat CD4 microbeads (Miltenyi Biotech, Bergisch Gladbach, Germany) were used to isolate CD4+ T lymphocytes by magnetic cell sorting according to the manufacturer’s instructions. The purity was 98.23% ± 0.27% as detected by flow cytometry (FCM) using anti-CD4-FITC (Becton, Dickinson and Company, Franklin Lakes, NJ, United States).
Co-culture of activated HSCs and CD4+ T lymphocytes
After culturing for 24 h in 6-well culture plates, activated rat HSCs (2 × 105 cells/well) were co-cultured with freshly isolated CD4+ T lymphocytes (4 × 106 cells/well) using Millipore cell culture inserts (0.4 µm; Millipore, Billerica, MA, United States) in RPMI 1640 containing 10% FBS. CD4+ T lymphocytes from the same rat, cultured alone were used as a control and in each experiment, HSCs and T lymphocytes were from the same rat. The sample size was n = 10 rats per group.
A CFSE cell proliferation assay (Invitrogen of Thermo Fisher Scientific) was used to determine whether CD4+ T lymphocytes could proliferate in vitro in the absence of mitogens.
An Annexin V/Dead Cell Apoptosis Kit (Invitrogen) was used to determine the proportion of viable T lymphocytes by FCM; cells negative for both Annexin V and propidium iodide were considered viable.
Additionally, activated HSCs (5 × 103 cells/well) were cultured alone or with CD4+ T lymphocytes (5 × 104 cells/well) in 96-well culture plates. At the beginning and end of a 24 or 48 h co-culture, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to detect the proliferation of activated HSCs, as described previously[14].
Apoptosis and FCM determination of Th1 and Th2 cells
After 24 h of co-culture, apoptosis of CD4+ T lymphocytes was assessed using a Fluorescein Active Caspase-3 Staining Kit (BioVision, Mountain View, CA, United States) according to the manufacturer’s instructions.
After 48 h of co-culture, the proportion of Th1 cells (IFN-γ+) and Th2 cells (IL-4+) was determined by FCM using anti-IFN-γ-PE and anti-IL-4-PE antibodies, with an isotype antibody control (Becton, Dickinson and Company), as previously described[13].
Galectin-9 expression in activated HSCs
Galectin-9 in supernatants of activated HSCs was detected by ELISA (Uscn Life Science, Wuhan, China) according to the manufacturer’s instructions. Galectin-9 protein expression in activated HSCs was assessed by immunofluorescence using anti-galectin-9 (1:200; Abcam) and FITC-conjugated goat anti-rabbit (1:200; Zhongshan-Golden Bridge) antibodies. Protein levels were assessed by Western blotting using anti-galectin-9 (1:1000; Abcam) and anti-β-actin (1:4000; Sigma-Aldrich, St. Louis, MO, United States) antibodies with horseradish peroxidase-conjugated anti-rabbit (1:9000) or anti-mouse (1:8000; both from Zhongshan-Golden Bridge) secondary antibodies, as previously described[15].
Differentiation of Th1 and Th2 cells
After freshly isolated CD4+ T lymphocytes were cultured in medium with 5 μg/mL concanavalin A for 2 wk, the CD4+ T lymphocytes with differentiation potential (CD25+/IFN-γ-/IL-4-) were obtained. These T lymphocytes were co-cultured with activated HSCs in 6-well culture plates, as described above. After 48 h of co-culture, proportions of Th1 and Th2 cells were detected by FCM. CD4+ T lymphocytes from the same rat were cultured alone as a control and in each experiment, HSCs and T lymphocytes were from the same rat (n = 10).
Statistical analysis
Data were analyzed using SPSS 14.0 (SPSS Inc., Chicago, IL, United States). Paired Student’s t tests and randomized block design analysis of variance were applied; P < 0.05 was considered statistically significant. The data are expressed as mean ± SD.
RESULTS
Co-culturing affects HSC and T cell viability
The proliferation rate of activated HSCs detected by MTT assay was significantly increased when co-cultured for 24 or 48 h, compared with those cultured alone (P < 0.05) (Figure 1A); there were no changes in the morphology of HSCs. At the same time, the proportion of viable CD4+ T cells decreased after 48 h when co-cultured with activated HSCs compared to culturing alone without mitogens (55.27% ± 3.88% vs 61.28% ± 3.84%, P < 0.01) (Figure 1B).
Figure 1.
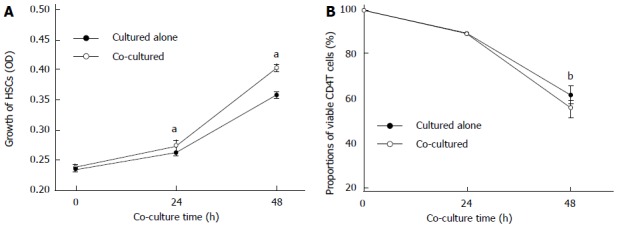
Effect of co-culturing on viability of activated hepatic stellate cells and CD4+ T lymphocytes. A: Proliferation of activated hepatic stellate cells (HSCs); B: Proportion of viable CD4+ T lymphocytes when cultured alone or co-cultured together (n = 3). OD: Optical density; data are expressed as mean ± SD. aP < 0.05 vs cultured alone; bP < 0.01 vs control.
Co-culturing with activated HSCs alters the Th1/Th2 profile
After 48 h of co-culture with activated HSCs, the proportion of Th1 cells significantly decreased compared to culturing alone (-1.73% ± 0.71%; P < 0.05), while the proportion of Th2 cells did not change (Figure 2A). Thus, co-culturing lowered the Th1/Th2 ratio (-0.44 ± 0.13; P < 0.05). Furthermore, the mean fluorescence intensity of IFN-γ in Th1 cells was decreased (-65.71 ± 9.67, P < 0.01) and the mean fluorescence intensity of IL-4 in Th2 cells was increased (82.79 ± 25.12; P < 0.05) after 48 h in co-culture conditions (Figure 2B).
Figure 2.
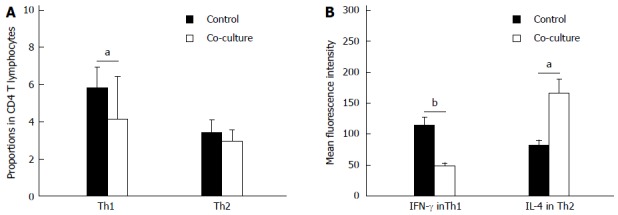
Effect of co-culturing on Th1/Th2 profile. A: Proportion of CD4+ T lymphocytes identified as Th1 and Th2 cells; B: Mean fluorescence intensity of interferon (IFN)-γ in Th1 cells and interleukin (IL)-4 in Th2 cells assessed by flow cytometry when cultured alone or co-cultured for 48 h with activated hepatic stellate cells (n = 10). Data are expressed as mean ± SD. aP < 0.05, bP < 0.01 vs control.
Co-culturing with activated HSCs induces apoptosis of Th1 cells
Co-culture with activated HSCs for 24 h significantly increased the apoptosis rates in Th1 and Th2 cells (P < 0.01; Figure 3). Moreover, the increased rate of apoptosis in Th1 cells was significantly higher than in Th2 cells (12.27% ± 0.99% vs 1.71% ± 0.185%, P < 0.01).
Figure 3.
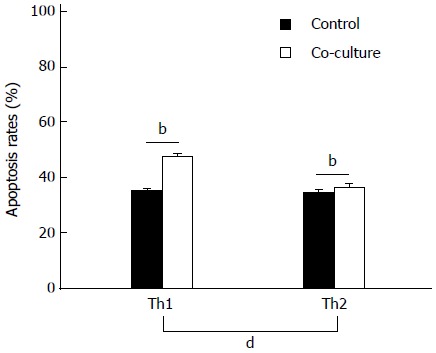
Effect of co-culturing on apoptosis of Th1 and Th2 cells. Apoptosis rates were assessed after 24 h by flow cytometric analysis of caspase-3 staining in CD4+ T lymphocytes cultured alone or co-cultured with activated hepatic stellate cells (n = 10). Data are expressed as mean ± SD; bP < 0.01 vs control.
Co-culturing transiently decreases galectin-9 expression in activated rat HSCs
As demonstrated by immunofluorescence staining, activated rat HSCs express galectin-9 (Figure 4A). Quantification of galectin-9 protein showed that levels were significantly reduced after co-culturing with CD4+ T lymphocytes for 24 h (P < 0.05) but not after 48 h (Figure 4B). However, galectin-9 was undetectable by ELISA in the supernatants of activated rat HSCs cultured alone or co-cultured with CD4+ T lymphocytes.
Figure 4.
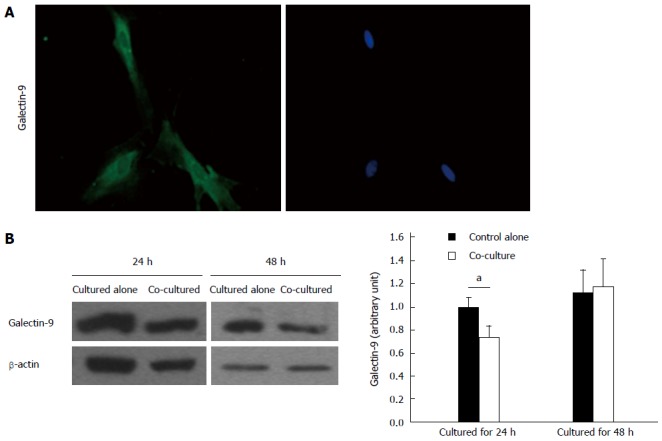
Galectin-9 expression in activated hepatic stellate cells. A: Immunofluorescence analysis of galectin-9 expression (left panel, green) in activated rat hepatic stellate cells (HSCs) (right panel, nuclear costain) (magnification × 400); B: Western blot analysis of galectin-9 expression in activated rat HSCs cultured alone or co-cultured with CD4+ T lymphocytes (n = 5). Data are expressed as mean ± SD. aP < 0.05, cultured alone vs co-cultured.
Co-culturing alters the differentiation of CD4+ T lymphocytes
CD4+ T lymphocytes with differentiation potential (CD25+/IFN-γ-/IL-4-) were also co-cultured with activated rat HSCs for 48 h. Flow cytometry showed that proportions of both IFN-γ+ (Th1) and IL-4+ (Th2) cells increased but the increase observed in the proportion of Th2 cells (1.85% ± 0.48%) was significantly higher than was observed for Th1 cells (P < 0.05) (Figure 5).
Figure 5.
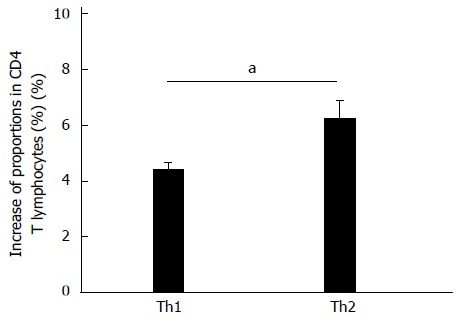
Effect of co-culturing on Th1 and Th2 cell differentiation. CD4+ T lymphocytes with differentiation potential were co-cultured for 48 h with activated hepatic stellate cells (n = 5). Data are expressed as mean ± SD; aP < 0.05, Th1 vs Th2.
DISCUSSION
Cytokines released from leukocytes can promote the activation of myofibroblasts in injured liver, which in turn can regulate immune cells and promote chronic inflammation[16,17]. This activation switches HSCs from a fibrogenic to a proinflammatory phenotype, which then continues to modulate local inflammatory responses long after they remodel the extracellular matrix[18]. Therefore, repeated activation of myofibroblasts can result in sustained chronic inflammation and fibrosis[2]. The results from the present study show that activated HSCs lower the Th1/Th2 profile of CD4+ T lymphocytes, thus suggesting a mechanism by which activated myofibroblasts promote fibrogenesis.
CD4+ T lymphocytes play an important role in the pathogenesis of fibrogenesis. IFN-γ released by Th1 cells decreases HSC activation and extracellular matrix deposition during rat liver fibrosis[19] and promotes the function of NK cells, which can induce HSC death[20]. Moreover, Th1 cells can inhibit the differentiation and maturation of Th17 and Th2 cells[21], which have proinflammatory and profibrogenic effects. This study shows that the relationship is reciprocal as the proportion of Th1 cells, as well as the amount of IFN-γ they express, is reduced by co-culture with activated HSCs. Therefore, activated HSCs might promote chronic inflammation and fibrosis by inhibiting the Th1 response. In line with this, the development of liver fibrosis was determined to be facilitated by a shift from a Th1 to a Th17 response in an animal model with an IL-12 gene knockout[22].
In the present study, we also found that activated HSCs disproportionately increase the apoptosis of CD4+ T cells, showing a more profound effect on Th1 cells. These results are consistent with a study that reported apoptosis of infiltrating T cells in hepatic fibrotic tissue, which was thought to be due to the interaction with myofibroblast-like cells[23].
Galectin-9 is known to induce apoptosis of Th1 cells[24] and is expressed by human lung fibroblasts and dermal fibroblasts[15,25]. Galectin-9 is detected on Kupffer cells, fibroblasts and histiocytes in biopsy specimens of drug-induced liver disease patients[26]. Although galectin-9 was expressed in the activated rat HSCs in this study, supernatant levels were below the ELISA detection limit, suggesting that that the activated HSCs were unable to secrete galectin-9 in vitro. This finding does not support the hypothesis that HSCs promote Th1 cell apoptosis via galectin-9 secretion. Further study is needed to ascertain the factors that induce in vivo galectin-9 secretion from activated HSCs.
In the present study, the differentiation of CD4+ T lymphocytes into Th2 cells was more strongly affected by co-culturing with activated HSCs as the increase in the proportion of Th2 cells was greater than for Th1 cells. This suggests that, in addition to proliferation and apoptosis of T lymphocytes, activated HSCs might also modulate Th1/Th2 balance by altering their differentiation rates. Moreover, the expression of IL-4 in Th2 cells was enhanced by co-culture with activated HSCs.
A limitation of this study is that only the in vitro effects were examined and thus may not fully reflect the in vivo condition. Future in vivo studies are needed to verify these results and to explore the underlying mechanisms.
In summary, the present study shows that activated HSCs decrease the Th1/Th2 ratio in vitro via promoting apoptosis and inhibiting IFN-γ production in Th1 cells, while promoting IL-4 production and differentiation of Th2 cells. These data suggest that a mechanism by which activated HSCs promote liver fibrosis is by altering the Th1/Th2 balance.
COMMENTS
Background
Hepatic stellate cells (HSCs) can be activated by various inflammatory cytokines and promote the development of liver fibrosis. HSCs can also influence the infiltration, activation and proliferation of lymphocytes, such as CD4+ T cells. Th1 cells play an anti-fibrotic role, whereas Th2 cells play a profibrogenic role. This study aimed to examine the effect of HSC activation on the Th1/Th2 profile and explored the potential mechanism of this effect.
Research frontiers
The authors found that activated rat HSCs may promote liver fibrosis via inhibition of the Th1 response and enhancement of the Th2 response.
Innovations and breakthroughs
This study found a direct interaction between HSCs and Th1/Th2 cells. Activated HSCs can regulate function, apoptosis and proliferation of Th1 and Th2 cells to change the Th1/Th2 immune status.
Applications
The findings suggest that activated rat HSCs alter the Th1/Th2 profile of CD4+ T lymphocytes as a novel mechanism for liver fibrogenesis.
Terminology
Galectin-9 is a ligand that inhibits T cell activation and proliferation and regulates their expression and secretion of cytokines.
Peer-review
This study addresses a very important aspect of immune regulation in liver diseases. Using an in vitro approach with co-cultures, these authors showed that the Th1/Th2 profile changed when the cells were co-cultured with activated HSCs. The idea is novel and interesting.
Footnotes
Supported by Major State Basic Research Development Program of China, No. 2007CB512802; and the National Natural Science Foundation of China, No. 30500425.
Ethics approval: The study was reviewed and approved by the Yan Tai Yu Huang Ding Hospital Review Board.
Institutional animal care and use committee: All animal experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of China Academy of Chinese Medical Sciences.
Conflict-of-interest: The authors declare that they have no conflicts of interest to this work.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 30, 2014
First decision: December 11, 2014
Article in press: April 9, 2015
P- Reviewer: Boscá L, La Mura V, Marcos R S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Ma S
References
- 1.Friedman SL. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–862, x. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marra F, Aleffi S, Galastri S, Provenzano A. Mononuclear cells in liver fibrosis. Semin Immunopathol. 2009;31:345–358. doi: 10.1007/s00281-009-0169-0. [DOI] [PubMed] [Google Scholar]
- 4.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shao DD, Suresh R, Vakil V, Gomer RH, Pilling D. Pivotal Advance: Th-1 cytokines inhibit, and Th-2 cytokines promote fibrocyte differentiation. J Leukoc Biol. 2008;83:1323–1333. doi: 10.1189/jlb.1107782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 7.Burke ML, Jones MK, Gobert GN, Li YS, Ellis MK, McManus DP. Immunopathogenesis of human schistosomiasis. Parasite Immunol. 2009;31:163–176. doi: 10.1111/j.1365-3024.2009.01098.x. [DOI] [PubMed] [Google Scholar]
- 8.Kitani A, Fuss I, Nakamura K, Kumaki F, Usui T, Strober W. Transforming growth factor (TGF)-beta1-producing regulatory T cells induce Smad-mediated interleukin 10 secretion that facilitates coordinated immunoregulatory activity and amelioration of TGF-beta1-mediated fibrosis. J Exp Med. 2003;198:1179–1188. doi: 10.1084/jem.20030917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muhanna N, Horani A, Doron S, Safadi R. Lymphocyte-hepatic stellate cell proximity suggests a direct interaction. Clin Exp Immunol. 2007;148:338–347. doi: 10.1111/j.1365-2249.2007.03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melhem A, Muhanna N, Bishara A, Alvarez CE, Ilan Y, Bishara T, Horani A, Nassar M, Friedman SL, Safadi R. Anti-fibrotic activity of NK cells in experimental liver injury through killing of activated HSC. J Hepatol. 2006;45:60–71. doi: 10.1016/j.jhep.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Jeong WI, Gao B. Innate immunity and alcoholic liver fibrosis. J Gastroenterol Hepatol. 2008;23 Suppl 1:S112–S118. doi: 10.1111/j.1440-1746.2007.05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weiskirchen R, Gressner AM. Isolation and culture of hepatic stellate cells. Methods Mol Med. 2005;117:99–113. doi: 10.1385/1-59259-940-0:099. [DOI] [PubMed] [Google Scholar]
- 13.Caraher EM, Parenteau M, Gruber H, Scott FW. Flow cytometric analysis of intracellular IFN-gamma, IL-4 and IL-10 in CD3(+)4(+) T-cells from rat spleen. J Immunol Methods. 2000;244:29–40. doi: 10.1016/s0022-1759(00)00249-0. [DOI] [PubMed] [Google Scholar]
- 14.Shi H, Guan SH. Increased apoptosis in HepG2.2.15 cells with hepatitis B virus expression by synergistic induction of interferon-gamma and tumour necrosis factor-alpha. Liver Int. 2009;29:349–355. doi: 10.1111/j.1478-3231.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- 15.Asakura H, Kashio Y, Nakamura K, Seki M, Dai S, Shirato Y, Abedin MJ, Yoshida N, Nishi N, Imaizumi T, et al. Selective eosinophil adhesion to fibroblast via IFN-gamma-induced galectin-9. J Immunol. 2002;169:5912–5918. doi: 10.4049/jimmunol.169.10.5912. [DOI] [PubMed] [Google Scholar]
- 16.Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- 17.Marra F. Hepatic stellate cells and the regulation of liver inflammation. J Hepatol. 1999;31:1120–1130. doi: 10.1016/s0168-8278(99)80327-4. [DOI] [PubMed] [Google Scholar]
- 18.Schnabl B, Purbeck CA, Choi YH, Hagedorn CH, Brenner D. Replicative senescence of activated human hepatic stellate cells is accompanied by a pronounced inflammatory but less fibrogenic phenotype. Hepatology. 2003;37:653–664. doi: 10.1053/jhep.2003.50097. [DOI] [PubMed] [Google Scholar]
- 19.Baroni GS, D’Ambrosio L, Curto P, Casini A, Mancini R, Jezequel AM, Benedetti A. Interferon gamma decreases hepatic stellate cell activation and extracellular matrix deposition in rat liver fibrosis. Hepatology. 1996;23:1189–1199. doi: 10.1002/hep.510230538. [DOI] [PubMed] [Google Scholar]
- 20.Notas G, Kisseleva T, Brenner D. NK and NKT cells in liver injury and fibrosis. Clin Immunol. 2009;130:16–26. doi: 10.1016/j.clim.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M, Zhang W, Yang GX, Tsuneyama K, Ando Y, Kawata K, Park O, Leung PS, Coppel RL, Ansari AA, et al. Deletion of interleukin (IL)-12p35 induces liver fibrosis in dominant-negative TGFβ receptor type II mice. Hepatology. 2013;57:806–816. doi: 10.1002/hep.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi S, Seki S, Kawada N, Morikawa H, Nakatani K, Uyama N, Ikeda K, Nakajima Y, Arakawa T, Kaneda K. Apoptosis of T cells in the hepatic fibrotic tissue of the rat: a possible inducing role of hepatic myofibroblast-like cells. Cell Tissue Res. 2003;311:353–364. doi: 10.1007/s00441-002-0670-4. [DOI] [PubMed] [Google Scholar]
- 24.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 25.Igawa K, Satoh T, Hirashima M, Yokozeki H. Regulatory mechanisms of galectin-9 and eotaxin-3 synthesis in epidermal keratinocytes: possible involvement of galectin-9 in dermal eosinophilia of Th1-polarized skin inflammation. Allergy. 2006;61:1385–1391. doi: 10.1111/j.1398-9995.2006.01130.x. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi Y, Fukusato T, Kobayashi Y, Akiyama S, Tamatani T, Shiga J, Mori S. High expression of eosinophil chemoattractant ecalectin/galectin-9 in drug-induced liver injury. Liver Int. 2006;26:106–115. doi: 10.1111/j.1478-3231.2005.01189.x. [DOI] [PubMed] [Google Scholar]


