Abstract
AIM: To evaluate mucosal healing in patients with small bowel plus colonic Crohn’s disease (CD) with a single non-invasive examination, by using PillCam COLON 2© (PCC2).
METHODS: Patients with non-stricturing nonpenetrating small bowel plus colonic CD in sustained corticosteroid-free remission were included. At diagnosis, patients had undergone ileocolonoscopy to identify active CD lesions, such as ulcers and erosions, and small bowel capsule endoscopy to assess the Lewis Score (LS). After ≥ 1 year of follow-up, patients underwent entire gastrointestinal tract evaluation with PCC2. The primary endpoint was assessment of CD mucosal healing, defined as no active colonic CD lesions and LS < 135.
RESULTS: Twelve patients were included (7 male; mean age: 32 years), and mean follow-up was 38 mo. The majority of patients (83.3%) received immunosuppressive therapy. Three patients (25%) achieved mucosal healing in both the small bowel and the colon, while disease activity was limited to either the small bowel or the colon in 5 patients (42%). It was possible to observe the entire gastrointestinal tract in 10 of the 12 patients (83%) who underwent PCC2.
CONCLUSION: Only three patients in sustained corticosteroid-free clinical remission achieved mucosal healing in both the small bowel and the colon, highlighting the limitations of clinical assessment when stratifying disease activity, and the need for pan-enteric endoscopy to guide therapeutic modification.
Keywords: Crohn’s disease, Mucosal healing, Capsule endoscopy, Small bowel diseases, Inflammatory bowel disease
Core tip: Our study reports for the first time the use of PillCam COLON2 Capsule (PCC2) to evaluate mucosal healing of the entire intestinal tract in small bowel plus colonic Crohn’s disease. Only 25% of our patients in corticosteroid-free clinical remission achieved mucosal healing in both the small bowel and the colon, while in 42% there was disease activity limited to either the small bowel or the colon. Endoscopic evaluation of the entire gastrointestinal tract with PCC2 was both feasible and safe. Our results highlight the limitations of clinical assessment when stratifying disease activity and emphasize the need for pan-enteric endoscopy in order to guide therapeutic adjustment.
INTRODUCTION
Crohn’s disease (CD) is a chronic inflammatory bowel disease whose prevalence has been rising over the past decades[1]. In CD, there is a transmural inflammatory process that may affect the entire gastrointestinal tract, from the mouth to the anus, and in half the patients there is involvement of both the small bowel and colon[2]. Although the terminal ileum, easily accessible through ileocolonoscopy, is the most commonly affected small bowel segment in CD (around 80%)[2], up to half of the patients suffering from ileal CD have concomitant jejunal mucosal damage[3]. Furthermore, one third of patients present with isolated proximal lesions, associated with an increased risk of relapse and poorer disease outcomes[3], but whose observation has often been challenging or incomplete.
Capsule endoscopy first became available in 2001[4], revolutionizing the investigation of small bowel diseases. In the most recent European Crohn’s and Colitis Organization guidelines[2], small bowel capsule endoscopy (SBCE) was established as a valid and important diagnostic tool in the diagnosis and evaluation of CD. SBCE was shown to be superior to small bowel follow-through and computed tomography enterography (CT-E) in the evaluation of the small bowel[5]. When compared with magnetic resonance enterography (MRI-E), small studies showed that SBCE had better sensitivity for proximal small bowel mucosal lesions[6]. Capsule endoscopy is highly sensitive for superficial mucosal lesions, with a strong negative predictive value[7,8]. In established CD, capsule endoscopy may be used to evaluate the extent and activity of disease[3,9], often having an impact on therapeutic decisions[10-12].
Mucosal healing, defined as the resolution of active inflammatory lesions in the gut[13], is now recognized as a major determinant on the outcome of CD[13]. Ileocolonoscopy is the gold standard for mucosal healing evaluation, but it is an invasive procedure, associated with discomfort and pain often requiring sedation and analgesia, and reaching only to the terminal ileum. Procedural risks such as perforation are significantly increased in patients with severe disease[14], and such patients would benefit from a less invasive diagnostic procedure. Capsule endoscopy, particularly when coupled with scoring systems such as the Lewis Score (LS)[2] or the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv Score)[2], has the potential for assessing and quantifying mucosal healing in small bowel CD[2].
Recently, a capsule aimed at colonic observation has been developed[15], PillCam COLON 2© (PCC2, Given Imaging©), which has been primarily used for colorectal cancer screening in average risk populations or when colonoscopy is contraindicated or incomplete[16-18]. Some recent studies have focused on the potential role of colon capsule endoscopy in patients with ulcerative colitis[19,20] and colonic CD[21]. PCC2 allows for the continuous and non-invasive observation of the entire intestinal tract (pan-endoscopy), and new studies are emerging to analyze its effectiveness in such evaluation[22]. Although it still requires bowel preparation, colon capsule endoscopy does not require insufflation or sedation; the risks associated with the procedure are minimal, although capsule retention and potential bowel obstruction are significantly more frequent in patients with established CD[4,17].
We aimed to evaluate mucosal healing in patients with small bowel plus colonic CD on corticosteroid-free clinical remission, with at least 1 year of follow-up after diagnosis, using PCC2 for pan-endoscopy.
MATERIALS AND METHODS
This retrospective single center study, based on prospectively collected data, included patients with small bowel plus colonic CD at diagnosis, with a non-stricturing, non-penetrating phenotype (Montreal Classification L3, B1[1]), who were in corticosteroid-free remission (defined for an Harvey-Bradshaw Index < 5[1]), had at least 1 year of follow-up, and were aged > 18 years. Exclusion criteria were pregnancy, known intestinal obstruction or current obstructive symptoms, non-steroidal anti-inflammatory drug use in the 4 wk prior to enrolment as well as previous intestinal surgery. Inclusion and exclusion criteria are summarized in Table 1. Small bowel radiological imaging was not mandatory prior to inclusion in this study; patients with no clinical features of stricturing or penetrating disease and no stricture at the index ileocolonoscopy were allowed to undergo capsule endoscopy without previous small bowel imaging.
Table 1.
Inclusion and exclusion criteria
| Inclusion and exclusion criteria |
| Inclusion criteria (all of the following) |
| Small bowel plus colonic Crohn's disease |
| Non-stricturing, non-penetrating phenotype |
| Corticosteroid-free remission (Harvey-Bradshaw Index < 5) |
| Follow-up ≥ 1 yr |
| Age ≥ 18 yr |
| Exclusion criteria (any of the following) |
| Pregnancy |
| Intestinal obstruction/obstructive symptoms |
| Intestinal surgery |
| Non-steroidal anti-inflammatory drug use within 4 wk of enrolment |
All patients underwent both SBCE (PillCam SB2, Given Imaging©) and ileocolonoscopy at diagnosis as per the department protocol. Small bowel disease activity was assessed using the LS. The LS is calculated through a specific formula using the presence of villous edema, ulcers and stenosis, and classifies small bowel inflammatory activity in 3 grades: LS < 135 (no activity), 135 ≤ LS < 790 (mild activity) and LS ≥ 790 (moderate to severe activity); in this study, patients with LS ≥ 135 were included[23,24]. Capsule observation was performed by 3 physicians with experience in capsule endoscopy, and the images were read at a maximum of 10 frames per second. Colonic lesions included were ulcers and aphthous ulcerations, while other lesions such as pseudopolyps, granularity without mucosal breaks or nodularity were considered non-active CD. Disease activity, measured with the Harvey-Bradshaw Index (HBI), therapy (salicylates, corticosteroids, immunomodulators and anti-tumor necrosis factor-α) and disease complications were evaluated during follow-up.
Assessment of mucosal healing was performed with the PCC2, using our own modified protocol from Herrerías-Gutiérrez et al[25] and Cotter et al[26]. Patients were instructed to have a low-fiber diet and ingest at least 10 glasses of water 2 d before the procedure; on the day before the procedure, a clear liquid diet (water, tea, transparent beverages) was prescribed, as well as 1 L of polyethylene glycol solution plus 500 mL of water between 7 and 9 pm; on the day of the procedure, another liter of this solution plus 500 mL of water was ingested (between 6:30 and 8:30 am), and fasting was warranted afterwards. At 9 am patients were instructed to ingest the capsule. Prior to the ingestion, real-time viewing (Rapid Access Real Time; Given Imaging©) was initiated and the adaptive frame rate mode was activated to ensure visualization of the entire small bowel (Figure 1).
Figure 1.
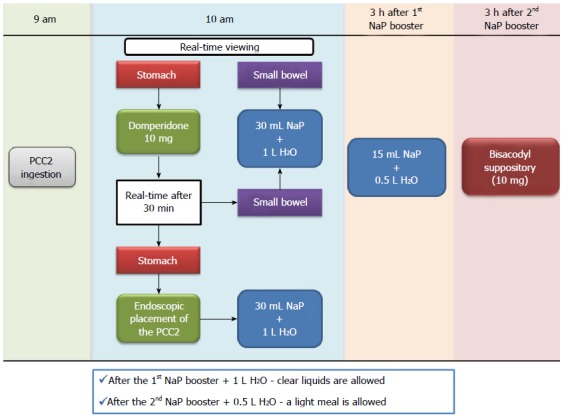
Department protocol for pan-enteric evaluation with PCC2©[40]. PCC2: Pillcam colon capsule 2©; NaP: Sodium phosphate.
One hour later, using the real-time viewing system, capsule progression to the small-bowel was confirmed, and 10 mg of domperidone was administered if the capsule was still in the stomach. Thirty minutes later, capsule progression was assessed, and, in the case of delayed stomach emptying, endoscopic capsule placement in the small bowel was performed. When the small bowel was reached, a booster of 30 mL of sodium phosphate solution (Fleet Phospho Soda; Casen-Fleet Laboratories©) was administered, followed by ingestion of 1 L of water; 3 h later a second booster of sodium phosphate (15 mL) was administered (plus 500 mL of water) if the capsule was not excreted by then, and after an additional 3 h, a bisacodyl suppository was given. In the event of an incomplete examination, unless the patient reported capsule excretion, an abdominal X-ray was performed after a period of 15 d or if obstructive symptoms developed.
Capsule observation was performed by a physician with experience in capsule endoscopy, blinded to both the initial endoscopic procedures and current therapy. The images were read at a maximum of 10 frames per second, using both cameras sequentially for colonic evaluation and a single camera for the remainder of the gastrointestinal tract. Small bowel, colon (segmented as follows: cecum, ascending, transverse, descending/sigmoid colon, and rectum) and upper gastrointestinal CD lesions were described, and LS was calculated. Gastric transit time (from the first gastric frame to first duodenum frame), small bowel transit time (from the first duodenum frame to first cecum frame) and colonic transit time (from the first cecum frame to last rectal frame) were noted in minutes. In the case of incomplete examination (capsule not excreted during battery time) a colon transit time was defined from the first cecal frame to the last registered colon frame. Small bowel and colon preparation quality was classified with a graded scale ranging from 1 to 4, where 1 was excellent (no more than small bits of residue), 2 was good (some residue, not enough to interfere with the examination), 3 was fair (enough residue to preclude a completely reliable examination) and 4 was poor (large amount of residue)[25].
A blood panel was performed both at diagnosis and on the day of the pan-endoscopy (complete blood count, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin and albumin). Anemia was defined as hemoglobin < 12 g/dL in women and < 13 g/dL in men; iron deficiency was defined as ferritin levels < 100 μg/L, leukocytosis was defined as a white blood count > 11.000/μL, elevated CRP was defined as levels ≥ 3 mg/L, elevated ESR was defined as > 20 mm/h, and hypoalbuminemia was defined as levels < 3.5 mg/dL, according to our laboratory reference range.
The Ethics Committee of the Centro Hospitalar do Alto Ave, E.P.E approved this study. All patients gave their written informed consent before enrolment. Data were analyzed anonymously to preserve patient confidentiality. Statistical analysis of frequencies was performed using the SPSS v.21.0 (IBM Corp., Armonk, NY, United States).
RESULTS
Twelve patients were included at baseline, and all completed the study protocol. Five patients were female (41.7%); the mean age was 32 years (range: 18-50 years), and mean follow-up was 40 mo (14-65 mo). Mean HBI at diagnosis was 9.64 (range: 6-17). Two patients reported regular tobacco consumption (10-20 cigarettes/d), 3 were ex-smokers and 7 were non-smokers.
On baseline SBCE, mean LS was 1022 ± 810 (range: 168-2980), and was highest in the third tercile (mean: 994 ± 838; range: 112-2980); mild inflammatory activity (135 ≤ LS < 790) was present in 5 patients and moderated to severe inflammatory activity (LS ≥ 790) was found in the other 7 patients. No small bowel stenoses were observed. On baseline ileocolonoscopy, 8 patients (66.7%) presented with a segmental pattern of colonic lesions, the majority in the right colon (n = 5); in 4 patients, there was extensive mucosal damage throughout the entire colon. The laboratory results at diagnosis and at the time of the PCC2 are summarized in Table 2.
Table 2.
Hematological results at diagnosis and follow-up (mean ± SD)
| Result | Diagnosis | Follow-up | Reference |
| Hemoglobin (g/dL) | 13.2 ± 2.8 | 14.2 ± 1.4 | 12.0-18.0 |
| Leucocytes (103/μL) | 10.100 ± 3.200 | 7.800 ± 2.800 | 4.8-10.8 |
| Platelets (103/μL) | 363 ± 194 | 265 ± 124 | 150-350 |
| Erythrocyte sedimentation rate (mm/h) | 28.6 ± 23.8 | 12.3 ± 8.3 | 0-14 |
| C-reactive protein (mg/L) | 42.6 ± 36.4 | 17.2 ± 17.0 | < 2.9 |
| Ferritin (ng/mL) | 120 ± 112 | 74 ± 49 | 26-388 |
| Albumin (ng/mL) | 4.1 ± 0.6 | 3.9 ± 0.3 | 3.1-17.5 |
The majority of patients (83.3%) were medicated with immunosuppressive therapy. Two patients were treated with combination immunomodulation therapy (adalimumab plus azathioprine), 8 with azathioprine in monotherapy and 2 with oral mesalazine
Mean gastric transit time was 45 ± 38 min (range: 3-90 min); domperidone administration was warranted in 4 patients, and endoscopic placement was needed in 3 of them, with no subsequent complications. Mean small bowel transit time was 90 ± 37 min (range: 21-162 min). Mean colon transit time was 321 ± 308 min (range: 20-936). Two pan-endoscopy procedures were incomplete (17%), the splenic flexure being reached in both of them. In these patients, the colon transit time was 895 and 936 min (total battery time for the PillCam COLON 2© is 1020 min).
Small bowel cleanliness was excellent in 5 (42%) patients, good in 6 (50%), and fair in 1 (8%), while the colonic preparation was excellent in 2 patients (17%), good in 6 (50%) patients, fair in 2 (17%), and poor in 2 (17%).
PCC2 findings in the small bowel were as follows: complete mucosal healing of the small bowel (LS < 135) was achieved in 4 patients (33%), 3 of them under azathioprine monotherapy, while the other patient was treated with mesalazine; one patient (8%) with previous moderate to severe inflammatory activity treated with anti-tumor necrosis-α plus azathioprine presented with mild inflammatory activity, and moderate to severe inflammatory activity was found in the remaining patients (n = 7, 58%). In 4 patients (33%), a single stenosis was found on the small bowel, all on the third tercile, and was ulcerated in 2 of them; the stenoses were traversed in all patients, causing no obstructive symptoms.
Mean LS was 1551 ± 1999 (range: 0-5392), and was highest in the third tercile (1126 ± 1213; range: 0-3040). Colonic lesions were found in half the patients (n = 6); 2 patients presented with ulcers throughout the entire colon, while segmental inflammatory activity was found in the remaining 4. Six patients (50%) achieved complete mucosal healing of the colon; in 3, there was concomitant small bowel mucosal healing. Patient characteristics, CD therapy, and endoscopic findings are summarized in Table 3.
Table 3.
Patient characteristics, Crohn's disease therapy and endoscopic findings
| Patient | Age (yr) | Sex | Follow-up (mo) | Therapy | SBCE LS | Index colonoscopy colon findings | PCC2 LS | PCC2 colon findings |
| 1 | 21 | Male | 48 | Azathioprine 2.5 mg/kg per day | 2980 | Extensive ulcers and erythema in entire colon | 1068 | Extensive ulcers in entire colon |
| 2 | 21 | Female | 63 | Azathioprine 2.5 mg/kg per day Adalimumab 40 mg eow | 1440 | Extensive ulcers and erythema in entire colon | 2336 | No lesions |
| 3 | 26 | Male | 57 | Azathioprine 2.5 mg/kg per day Adalimumab 40 mg eow | 1350 | Aphtoid ulcers and erythema in the sigmoid | 562 | No lesions |
| 4 | 48 | Male | 33 | Azathioprine 2.5 mg/kg per day | 1240 | Aphtoid ulcers and erythema in the cecum | 5392 | Extensive ulcers in the ascending and transverse colon |
| 5 | 24 | Male | 39 | Mesalazine 3 g/d | 1104 | Aphtoid ulcers in the cecum | 1518 | Aphtoid ulcers in the cecum |
| 6 | 27 | Female | 14 | Azathioprine 2.5 mg/kg per day | 900 | Extensive ulcers and erythema proximal to the splenic flexure | 0 | Ulcers in the transverse colon |
| 7 | 18 | Male | 14 | Azathioprine 2.5 mg/kg per day | 1690 | Extensive ulcers and erythema in entire colon | 2336 | Ulcers in the cecum, ascending, transverse and descending colon |
| 8 | 35 | Female | 22 | Azathioprine 2.5 mg/kg per day | 314 | Aphtoid ulcers and erythema in the cecum | 5392 | No lesions |
| 9 | 32 | Male | 22 | Mesalazine 3 g/d | 458 | Extensive ulcers and erythema in entire colon | 0 | No lesions |
| 10 | 46 | Female | 49 | Azathioprine 2.5 mg/kg per day | 393 | Extensive ulcers and erythema proximal to the splenic flexure | 0 | No lesions |
| 11 | 50 | Female | 65 | Azathioprine 2.5 mg/kg per day | 225 | Ulcers in the sigmoid | 8 | Ulcerative lesions in the splenic flexure; |
| 12 | 39 | Male | 62 | Mesalazine 3 g/d | 168 | Aphtoid ulcers in the cecum and ascending colon | 0 | No lesions |
SBCE: Small bowel capsule endoscopy; LS: Lewis Score; eow: Every other week; PCC2: PillCam COLON 2©.
DISCUSSION
In our study of patients in corticosteroid-free clinical remission, we found significant inflammatory activity in 9/12 (75%), and crucially, in 5 patients (42%), disease with previous both small bowel and colonic involvement was limited to one of those segments on follow-up, highlighting the limitations of clinical disease assessment when stratifying disease activity, and the need for therapeutic modification.
Moreover, in 3 out of 6 of the patients with normal colonic mucosa, there was involvement of the proximal small bowel, an independent risk factor for disease relapse[3]. Conversely, 2 patients, in whom no significant inflammatory activity was found in the small bowel (LS < 135), were shown to have multiple ulcers in the colonic mucosa. Finally, moderate to severe activity in the small bowel, as well as colon disease, was confirmed in a third of our patients.
Adequate small bowel preparation was achieved in all 12 patients, and in only 2 was colonic preparation poor. These results are comparable to those reported in the literature for both the small bowel and the colon[4], but warrant consideration of whether it is possible to further optimize colon preparation.
Although this was a single center retrospective study with a small number of patients, it was based on prospectively collected data, with strict inclusion criteria, and it focused on a very relevant hot topic in CD that has not previously been investigated - the simultaneous evaluation of post-treatment mucosal healing in both the small bowel and colon with a single non-invasive endoscopic examination.
In CD, symptom remission has not been shown to alter the natural course of the disease[13], particularly regarding complication and surgical rates[27], arguably because the correlation between clinical and inflammatory activities is poor[28]. In the era of biologic therapy, a new concept, mucosal healing, has arisen. In contrast to clinical symptoms, mucosal healing has been associated with significantly reduced rates of surgery[29] and hospitalization[30] as well as with the achievement of long term steroid-free remission[31].
Optimal assessment of mucosal healing is still debated. Ileocolonoscopy is currently the gold standard for evaluating mucosal healing, but it is an invasive procedure and restricted to the colon and distal ileum[32]. Several surrogate markers for mucosal healing exist, but none are without limitations. Fecal markers, such as calprotectin, have shown promising discriminating power to predict disease relapse[33], but the results regarding ileal disease are unremarkable[34]. Cross-sectional imaging, particularly CT-E and MR-E, allows the evaluation of the small bowel and colonic mucosa, as well as deep-tissue assessment, and has shown good correlation with both clinical and endoscopic activity[13]. However, CT poses a cumulative radiation risk, and MR-E is expensive and not widely available in clinical practice. SBCE has shown superior diagnostic accuracy to cross-sectional imaging in small bowel CD[6,35,36], particularly in detecting proximal and superficial lesions[5,6], and was recently reported to be safe in established small bowel CD, even in patients with previously known stenotic lesions[32], but does not allow for colon observation.
The colon capsule was recently developed for colon observation, particularly in patients who refuse colonoscopy or in whom such a procedure is not possible[17]. Although it still requires colon preparation, there is no need for insufflation or sedation, and the risks associated with the procedure are minimal[4,17]. Colon capsule endoscopy to detect mucosal inflammation in the colon was previously described in ulcerative colitis patients[19,20], and was recently shown to have a good correlation with colonoscopy when evaluating colon mucosal damage in patients with colonic CD[21]. The use of colon capsule endoscopy for observation of the whole intestinal tract was previously reported by Remes-Troche et al[22] and by Negreanu et al[37]. In both studies, PCC2 allowed for a thorough examination of both the small bowel and the colon, with very good tolerability and no complications
No stenosis was encountered during SBCE at diagnosis, but single small bowel stenoses were found in 4 patients on PCC2. These patients reported no obstructive symptoms either before or during the procedure, and such results are consistent with those reported by Niv et al[32], where 6 patients with ulcerated small bowel stenoses underwent repeated SBCE with no case of retention or complications.
The cecum was reached in all patients, allowing for the crucially important observation of the terminal ileum, ileocecal valve, and the cecum. Despite the optimized protocol and prolonged battery life (maximum 17 h), the procedure was incomplete in 2 patients.
Gastric transit time was per protocol always under 90 min. In contrast to other colon capsule preparation regimens[22,25], we used real-time viewing to adjust drug administration, allowing for criterious use of prokinetic drugs only in patients with delayed gastric emptying, as well as for determination of the ideal timing for sodium phosphate booster delivery.
Small bowel transit time was under 3 h in all patients. Despite some evidence that the small bowel transit time correlates with diagnostic yield in SBCE[38], the possibility that the shortening of small bowel transit time might reduce the diagnostic yield with PCC2 would probably not be an issue as its dynamic frame rate allows for the capture of up to 35 frames per second in accelerated movement.
Colon transit times averaged 320 min, and we encountered 2 incomplete studies, whose colon transit times were 895 and 936 min - a completion rate of 83.3%. No colon lesions were found in these patients despite the splenic flexure being reached in both of them. Both patients reported the excretion of the capsule on the following day with no complications. The completion rate for colon capsule endoscopy is reported to range between 76%-100%[18,19,22,39], comparable to our own findings (83%). Finally, we had no technical failures.
Our study has some limitations: it was an exploratory single center study, which included a limited number of patients due to strict inclusion criteria, particularly the requirement of both ileocolonoscopy and SBCE at diagnosis, a minimum follow-up of 1 year after the initiation of CD therapy, and corticosteroid-free clinical remission, prior to mucosal healing assessment with PCC2. Secondly, as the objective of the study was to demonstrate the feasibility of PCC2 for the evaluation of small bowel and colonic mucosal healing, no control group or gold standard was employed. Prospective multicenter studies would enable the inclusion of a significantly larger number of patients to validate our preliminary results, with the primary outcome of assessment of complete mucosal healing in both small bowel and colonic mucosa; ideally, new studies should use new or adapted scoring systems that could measure inflammatory activity both in the small bowel and the colon, as this novel concept becomes widespread in the investigation of this pan-enteric disease, based on a compromise of high diagnostic accuracy, less invasiveness, and convenience for patients. Further investigations should be able to evaluate whether complete (absence of endoscopically visible lesions) vs partial (improvement with lower inflammatory activity scores) mucosal healing in each and/or both “segments” (small bowel and/or colon) have a significant prognostic value, by following patients to assess for endpoints such as the rate of clinical relapses, hospitalization, or surgery. Although the possibility of full assessment of mucosal healing during the course of CD by means of a single non-invasive procedure seems a very attractive concept, there is currently not enough evidence that such a strategy could positively impact on disease outcomes; moreover, key practical drawbacks such as the high cost of each examination, the time required to read the videos, and the relatively scarce availability of adequately trained medical staff to read PCC2 videos, are some of the issues that currently limit its widespread generalizability for use in clinical practice, beyond those patients unwilling or unable to undergo conventional ileocolonoscopy.
In conclusion, the PillCam COLON 2© allows for a new concept of non-invasive, safe, and well tolerated examination of the entire gastrointestinal tract. Additionally, in a population currently in corticosteroid-free clinical remission, we found significant inflammatory activity in all but 3 patients (25%); of relevance, 5 patients (42%) with previous activity in both the small bowel and colon presented with disease limited to one of these, reinforcing the importance of entire gut visualization before any management decisions regarding CD patients. In the future, this procedure may be used to evaluate mucosal healing in the small bowel, particularly with proximal distribution, and colonic CD.
COMMENTS
Background
Crohn’s disease (CD) is a chronic inflammatory bowel disease characterized by periods of remission and periods of relapse. Both the small bowel and colonic mucosa are affected in half of CD patients, and mucosal healing has been recently shown to be associated with improved clinical outcomes. Capsule endoscopy has been developed for the study of the small bowel and colon, and with the new Pillcam Colon Capsule 2© (PCC2), it is now possible to observe the entire intestinal tract (pan-endoscopy).
Research frontiers
In this study, the authors aimed to evaluate the presence of mucosal healing in patients with small bowel plus colonic CD in clinical remission, with at least 1 year of follow-up after diagnosis, using PCC2 for pan-endoscopy.
Innovations and breakthroughs
The authors report for the first time the use of capsule endoscopy to assess mucosal healing of the entire intestinal tract in patients with CD. Mucosal healing for both the small bowel and the colon was achieved in 25% of the patients, and in 42%, there was disease activity limited to either the small bowel or the colon. Pan-endoscopy with PCC2 was safe and feasible in our study.
Applications
This study highlights the limitations of clinical assessment when stratifying disease activity. Moreover, in patients with previous small bowel and colonic CD, we found mucosal healing to be limited to one segment in almost half the patients, emphasizing the need for pan-enteric endoscopy to guide therapeutic modification in patients with established CD.
Terminology
Pan-endoscopy refers to the endoscopic assessment of both the small bowel and colon. Mucosal healing corresponds to the resolution of active inflammatory lesions in the gut (erosions, ulcers, friability, hemorrhage). Capsule endoscopy is a recent endoscopic technique where a small device with a camera is swallowed by the patient in order to visualize the mucosa of the gastrointestinal tract.
Peer-review
This is an interesting study performed by Carvalho et al reporting on a new technique - the use of colon capsule in the assessment of mucosal healing in CD patients. The manuscript is well written, and carefully designed. The procedures are described in great details in the material and methods section.
Footnotes
Ethics approval: The study was reviewed and approved by the Centro Hospitalar do Alto Ave Institutional Review Board.
Informed consent: All patients provided written consent to undergo total colonoscopy, small bowel capsule endoscopy and pan-enteric endoscopy with PCC2.
Conflict-of-interest: The authors have no conflict of interests regarding this manuscript.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 11, 2015
First decision: February 10, 2015
Article in press: April 17, 2015
P- Reviewer: Formica V, Ma L, Rolle U S- Editor: Qi Y L- Editor: Cant MR E- Editor: Liu XM
References
- 1.Van Assche G, Dignass A, Panes J, Beaugerie L, Karagiannis J, Allez M, Ochsenkühn T, Orchard T, Rogler G, Louis E, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Definitions and diagnosis. J Crohns Colitis. 2010;4:7–27. doi: 10.1016/j.crohns.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Flamant M, Trang C, Maillard O, Sacher-Huvelin S, Le Rhun M, Galmiche JP, Bourreille A. The prevalence and outcome of jejunal lesions visualized by small bowel capsule endoscopy in Crohn’s disease. Inflamm Bowel Dis. 2013;19:1390–1396. doi: 10.1097/MIB.0b013e31828133c1. [DOI] [PubMed] [Google Scholar]
- 4.Ladas SD, Triantafyllou K, Spada C, Riccioni ME, Rey JF, Niv Y, Delvaux M, de Franchis R, Costamagna G. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small-bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220–227. doi: 10.1055/s-0029-1243968. [DOI] [PubMed] [Google Scholar]
- 5.Dionisio PM, Gurudu SR, Leighton JA, Leontiadis GI, Fleischer DE, Hara AK, Heigh RI, Shiff AD, Sharma VK. Capsule endoscopy has a significantly higher diagnostic yield in patients with suspected and established small-bowel Crohn’s disease: a meta-analysis. Am J Gastroenterol. 2010;105:1240–1248; quiz 1249. doi: 10.1038/ajg.2009.713. [DOI] [PubMed] [Google Scholar]
- 6.Jensen MD, Nathan T, Rafaelsen SR, Kjeldsen J. Diagnostic accuracy of capsule endoscopy for small bowel Crohn’s disease is superior to that of MR enterography or CT enterography. Clin Gastroenterol Hepatol. 2011;9:124–129. doi: 10.1016/j.cgh.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Tukey M, Pleskow D, Legnani P, Cheifetz AS, Moss AC. The utility of capsule endoscopy in patients with suspected Crohn’s disease. Am J Gastroenterol. 2009;104:2734–2739. doi: 10.1038/ajg.2009.404. [DOI] [PubMed] [Google Scholar]
- 8.Figueiredo P, Almeida N, Lopes S, Duque G, Freire P, Lérias C, Gouveia H, Sofia C. Small-bowel capsule endoscopy in patients with suspected Crohn’s disease-diagnostic value and complications. Diagn Ther Endosc. 2010;2010 doi: 10.1155/2010/101284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park SK, Yang SK, Park SH, Park SH, Kim JW, Yang DH, Jung KW, Kim KJ, Ye BD, Byeon JS, et al. Long-term prognosis of the jejunal involvement of Crohn’s disease. J Clin Gastroenterol. 2013;47:400–408. doi: 10.1097/MCG.0b013e3182705f9e. [DOI] [PubMed] [Google Scholar]
- 10.Kalla R, McAlindon ME, Drew K, Sidhu R. Clinical utility of capsule endoscopy in patients with Crohn’s disease and inflammatory bowel disease unclassified. Eur J Gastroenterol Hepatol. 2013;25:706–713. doi: 10.1097/MEG.0b013e32835ddb85. [DOI] [PubMed] [Google Scholar]
- 11.Cotter J, Dias de Castro F, Moreira MJ, Rosa B. Tailoring Crohn’s disease treatment: the impact of small bowel capsule endoscopy. J Crohns Colitis. 2014;8:1610–1615. doi: 10.1016/j.crohns.2014.02.018. [DOI] [PubMed] [Google Scholar]
- 12.Long MD, Barnes E, Isaacs K, Morgan D, Herfarth HH. Impact of capsule endoscopy on management of inflammatory bowel disease: a single tertiary care center experience. Inflamm Bowel Dis. 2011;17:1855–1862. doi: 10.1002/ibd.21571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Cruz P, Kamm MA, Prideaux L, Allen PB, Moore G. Mucosal healing in Crohn’s disease: a systematic review. Inflamm Bowel Dis. 2013;19:429–444. doi: 10.1002/ibd.22977. [DOI] [PubMed] [Google Scholar]
- 14.Riccioni ME, Urgesi R, Cianci R, Bizzotto A, Spada C, Costamagna G. Colon capsule endoscopy: Advantages, limitations and expectations. Which novelties? World J Gastrointest Endosc. 2012;4:99–107. doi: 10.4253/wjge.v4.i4.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eliakim R, Fireman Z, Gralnek IM, Yassin K, Waterman M, Kopelman Y, Lachter J, Koslowsky B, Adler SN. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963–970. doi: 10.1055/s-2006-944832. [DOI] [PubMed] [Google Scholar]
- 16.Romero-Vázquez J, Argüelles-Arias F, García-Montes JM, Caunedo-Álvarez Á, Pellicer-Bautista FJ, Herrerías-Gutiérrez JM. Capsule endoscopy in patients refusing conventional endoscopy. World J Gastroenterol. 2014;20:7424–7433. doi: 10.3748/wjg.v20.i23.7424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK, et al. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527–536. doi: 10.1055/s-0031-1291717. [DOI] [PubMed] [Google Scholar]
- 18.Triantafyllou K, Viazis N, Tsibouris P, Zacharakis G, Kalantzis C, Karamanolis DG, Ladas SD. Colon capsule endoscopy is feasible to perform after incomplete colonoscopy and guides further workup in clinical practice. Gastrointest Endosc. 2014;79:307–316. doi: 10.1016/j.gie.2013.07.061. [DOI] [PubMed] [Google Scholar]
- 19.Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117–124. doi: 10.1111/1751-2980.12005. [DOI] [PubMed] [Google Scholar]
- 20.Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T, et al. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28:1174–1179. doi: 10.1111/jgh.12203. [DOI] [PubMed] [Google Scholar]
- 21.D'Haens GR, Franchimont D, Lowenberg M, Ponsioen C, Bonssuyt P, Amininejad L, Van Gossum AM. Assessment of the Performance of the Colonic PillCam Pcce-2 in Patients With Active Crohn's Disease: a Pilot Study. AGA. 2014;5 Suppl:AB574. [Google Scholar]
- 22.Remes-Troche JM, Jiménez-García VA, García-Montes JM, Hergueta-Delgado P, Roesch-Dietlen F, Herrerías-Gutiérrez JM. Application of colon capsule endoscopy (CCE) to evaluate the whole gastrointestinal tract: a comparative study of single-camera and dual-camera analysis. Clin Exp Gastroenterol. 2013;6:185–192. doi: 10.2147/CEG.S45215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosa B, Moreira MJ, Rebelo A, Cotter J. Lewis Score: a useful clinical tool for patients with suspected Crohn’s Disease submitted to capsule endoscopy. J Crohns Colitis. 2012;6:692–697. doi: 10.1016/j.crohns.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Gralnek IM, Defranchis R, Seidman E, Leighton JA, Legnani P, Lewis BS. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment Pharmacol Ther. 2008;27:146–154. doi: 10.1111/j.1365-2036.2007.03556.x. [DOI] [PubMed] [Google Scholar]
- 25.Herrerías-Gutiérrez JM, Argüelles-Arias F, Caunedo-Álvarez A, San-Juan-Acosta M, Romero-Vázquez J, García-Montes JM, Pellicer-Bautista F. PillCamColon Capsule for the study of colonic pathology in clinical practice. Study of agreement with colonoscopy. Rev Esp Enferm Dig. 2011;103:69–75. doi: 10.4321/s1130-01082011000200004. [DOI] [PubMed] [Google Scholar]
- 26.Cotter J, de Castro FD, Magalhães J, Moreira MJ, Rosa B. Finding the solution for incomplete small bowel capsule endoscopy. World J Gastrointest Endosc. 2013;5:595–599. doi: 10.4253/wjge.v5.i12.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cosnes J, Nion-Larmurier I, Beaugerie L, Afchain P, Tiret E, Gendre JP. Impact of the increasing use of immunosuppressants in Crohn’s disease on the need for intestinal surgery. Gut. 2005;54:237–241. doi: 10.1136/gut.2004.045294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Modigliani R, Mary JY, Simon JF, Cortot A, Soule JC, Gendre JP, Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn’s disease. Evolution on prednisolone. Groupe d’Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990;98:811–818. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- 29.Allez M, Lemann M, Bonnet J, Cattan P, Jian R, Modigliani R. Long term outcome of patients with active Crohn’s disease exhibiting extensive and deep ulcerations at colonoscopy. Am J Gastroenterol. 2002;97:947–953. doi: 10.1111/j.1572-0241.2002.05614.x. [DOI] [PubMed] [Google Scholar]
- 30.Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009;15:1295–1301. doi: 10.1002/ibd.20927. [DOI] [PubMed] [Google Scholar]
- 31.Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463–468; quiz e10-e11. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 32.Niv E, Fishman S, Kachman H, Arnon R, Dotan I. Sequential capsule endoscopy of the small bowel for follow-up of patients with known Crohn’s disease. J Crohns Colitis. 2014;8:1616–1623. doi: 10.1016/j.crohns.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Louis E, Mary JY, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology. 2012;142:63–70.e5; quiz e31. doi: 10.1053/j.gastro.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Sipponen T, Kärkkäinen P, Savilahti E, Kolho KL, Nuutinen H, Turunen U, Färkkilä M. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther. 2008;28:1221–1229. doi: 10.1111/j.1365-2036.2008.03835.x. [DOI] [PubMed] [Google Scholar]
- 35.Voderholzer WA, Beinhoelzl J, Rogalla P, Murrer S, Schachschal G, Lochs H, Ortner MA. Small bowel involvement in Crohn’s disease: a prospective comparison of wireless capsule endoscopy and computed tomography enteroclysis. Gut. 2005;54:369–373. doi: 10.1136/gut.2004.040055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gee MS, Harisinghani MG. MRI in patients with inflammatory bowel disease. J Magn Reson Imaging. 2011;33:527–534. doi: 10.1002/jmri.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negreanu L, Smarandache G, Mateescu RB. Role of capsule endoscopy Pillcam COLON 2 in patients with known or suspected Crohn’s disease who refused colonoscopy or underwent incomplete colonoscopic exam: a case series. Tech Coloproctol. 2014;18:277–283. doi: 10.1007/s10151-013-1054-3. [DOI] [PubMed] [Google Scholar]
- 38.Westerhof J, Koornstra JJ, Hoedemaker RA, Sluiter WJ, Kleibeuker JH, Weersma RK. Diagnostic yield of small bowel capsule endoscopy depends on the small bowel transit time. World J Gastroenterol. 2012;18:1502–1507. doi: 10.3748/wjg.v18.i13.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alarcón-Fernández O, Ramos L, Adrián-de-Ganzo Z, Gimeno-García AZ, Nicolás-Pérez D, Jiménez A, Quintero E. Effects of colon capsule endoscopy on medical decision making in patients with incomplete colonoscopies. Clin Gastroenterol Hepatol. 2013;11:534–540.e1. doi: 10.1016/j.cgh.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Carvalho PB, Rosa B, Cotter J. Mucosal healing in Crohn's disease - are we reaching as far as possible with capsule endoscopy? J Crohns Colitis. 2014;8:1566–1567. doi: 10.1016/j.crohns.2014.06.008. [DOI] [PubMed] [Google Scholar]


