Abstract
AIM: To study the effect of mobilized peripheral blood autologous CD34 positive (CD34+) cell infusion in patients with non-viral decompensated cirrhosis.
METHODS: Cirrhotic patients of non-viral etiology were divided into 2 groups based on their willingness to be listed for deceased donor liver transplant (DDLT) (control, n = 23) or to receive autologous CD34+ cell infusion through the hepatic artery (study group, n = 22). Patients in the study group were admitted to hospital and received granulocyte colony stimulating factor injections 520 μg/d for 3 consecutive days to mobilize CD34+ cells from the bone marrow. On day 4, leukapheresis was done and CD34+ cells were isolated using CliniMAC magnetic cell sorter. The isolated CD34+ cells were infused into the hepatic artery under radiological guidance. The patients were discharged within 48 h. The control group received standard of care treatment for liver cirrhosis and were worked up for DDLT as per protocol of the institute. Both groups were followed up every week for 4 wk and then every month for 3 mo.
RESULTS: In the control and the study group, the cause of cirrhosis was cryptogenic in 18 (78.2%) and 16 (72.72%) and alcohol related in 5 (21.7%) and 6 (27.27%), respectively. The mean day 3 cell count (cells/μL) was 27.00 ± 20.43 with a viability of 81.84 ± 11.99%. and purity of 80%-90%. Primary end point analysis revealed that at 4 wk, the mean serum albumin in the study group increased significantly (2.83 ± 0.36 vs 2.43 ± 0.42, P = 0.001) when compared with controls. This improvement in albumin was, however, not sustained at 3 mo. However, at the end of 3 mo there was a statistically significant improvement in serum creatinine in the study group (0.96 ± 0.33 vs 1.42 ± 0.70, P = 0.01) which translated into a significant improvement in the Model for End-Stage Liver Disease score (15.75 ± 5.13 vs 19.94 ± 6.68, P = 0.04). On statistical analysis of secondary end points, the transplant free survival at the end of 1 mo and 3 mo did not show any significant difference (P = 0.60) when compared to the control group. There was no improvement in aspartate transaminase, alanine transaminase, and bilirubin at any point in the study population. There was no mortality benefit in the study group. The procedure was safe with no procedural or treatment related complications.
CONCLUSION: Autologous CD 34+ cell infusion is safe and effectively improves liver function in the short term and may serve as a bridge to liver transplantation.
Keywords: CD34 cell infusion, Stem cell, Cirrhosis, Model for end-stage liver disease, Liver transplantation
Core tip: Cirrhosis of the liver is a chronic progressive disease with high morbidity and mortality without liver transplantation. Alternative stem cell based therapies have shown promising results. In our study, we used autologous CD34 positive (CD34+) cell infusion in decompensated cirrhotic patients of non-viral etiology. This is the first study which has compared these patients with controls who were selected from a waiting list of liver transplantation candidates. The results shows improvement in albumin at 1 mo and Model for End-Stage Liver Disease score at 3 mo. Though many questions still remain unanswered, stem cell therapy is a promising treatment modality and serves currently as a bridge to liver transplantation.
INTRODUCTION
The increasing prevalence of cirrhosis[1] coupled with paucity of donor tissues available for liver transplantation as well as high financial burden, have paved the way for finding alternative therapeutic options for this potentially life threatening condition. Cell based regenerative therapies, including adult hemopoietic stem cells (HSCs) and mesenchymal stem cell (MSC) based therapies, have evolved as the fore runner in the search for an alternative to whole-organ transplantation[2,3]. HSCs are cells that are capable of differentiating into multiple cell lineages including hepatocytes[4]. HSCs differentiating into hepatocytes was first demonstrated by Peterson et al[5], who showed that following a noxious insult to the murine liver, pluripotent stem cells in bone marrow contributed to liver regeneration. A phase I study by Gordon et al[6] on 5 patients with decompensated cirrhosis paved the way for use of peripherally mobilized CD34 positive (CD34+) cells in humans. Mobilization of bone marrow derived stem cells induced by granulocyte colony stimulating factor (G-CSF) has been shown to increase CD34+ cells along with increasing levels of hepatocyte growth factor, leading to hepatic progenitor cell proliferation in patients with alcoholic steatohepatitis[7]. However, the degree of hepatic engraftment in human liver is highly variable. A recent study by Garg et al[8] has shown that bone marrow stimulation with G-CSF in acute on chronic liver failure patients leads to a significant increase in CD34+ cells in liver tissues after 4 wk of G-CSF injection. Coinciding with this rise of CD34+ cells in liver tissues, there was a significant decrease in peripheral CD34+ cells, possibly due to migration and settlement of the CD34+ cells in the liver. This study therefore paved the way to explore whether direct infusion of CD34 cells into the hepatic artery in patients with cirrhosis would help in liver regeneration and improve prognosis. With the background of these studies, we designed a research protocol to prospectively study the effect of transfusion of mobilized autologous CD34+ cells into the hepatic artery of cirrhotic patients. This is the first research study which has used autologous transfusion of mobilized blood CD34+ cells in patients with decompensated cirrhotics of non-viral etiology and has compared them with patients receiving standard of care for cirrhosis.
This research protocol was designed to assess the effects of autologous CD34+ hematopoietic cell infusion in patients with decompensated cirrhosis of the liver. The primary endpoint of the study was improvement in the Model for End-Stage Liver Disease (MELD) score along with improvement in synthetic function of the liver as measured by serum albumin and INR at 3 mo with autologous CD34+ cell infusion compared with standard of care. The secondary endpoints were decrease in ascites as measured by ultrasonography and requirement of therapeutic paracentesis and improvement in transplantation free survival.
MATERIALS AND METHODS
Patient selection
The study protocol was approved by the Institutional Review Board, Institutional Ethics Committee and Institutional Committee for Stem Cell Research. This study was performed as per 2007 guidelines of Indian Council of Medical Research and Department of Biotechnology India utilizing autologous minimally manipulated hematopoietic stem cells under a permitted area of research.
Between July 2012 to June 2013, 100 cirrhotic patients who attended the Hepatology unit of the Institute were screened. All patients who required a liver transplantation were counseled for either living donor liver transplantation (LDLT) or deceased donor liver transplantation (DDLT). The patients who did not have the option of a living donor and who were unwilling for DDLT either due to the long waiting time or due to immediate financial constraints were counseled about autologous CD34+ cell infusion as a research tool which is being evaluated as an alternative or bridge to liver transplantation. These patients were considered as the study group. On the other hand, those patients who were willing only for DDLT were included in the institutional liver transplantation waiting list for DDLT. These patients were considered the control group.
Patients aged between 18-70 years, with clinically diagnosed hepatic cirrhosis, having a MELD score of > 14 and unwilling for immediate liver transplantation, with life expectancy of at least 3 mo (based on MELD score) and ability to give informed consent were included in the study group. All enrolled patients were counseled in detail about the nature of the research protocol to be followed and possible outcomes. An informed consent was obtained from all patients.
Patients with liver tumors or history of any other malignancy, active infections including human immunodeficiency virus (HIV), hepatitis B virus, hepatitis C virus, severe cardiac and pulmonary co-morbidities unrelated to cirrhosis, recent gastrointestinal bleed, acute kidney injury or hepatorenal syndrome, portal vein thrombosis, pregnancy, lactation, and inability to give informed consent were excluded from the study.
Patients with decompensated chronic liver disease between 18-70 years, who were enrolled in the waiting list during the study period for DDLT, were considered the control group. All other inclusion criteria were the same as the study group except that they were willing and eligible for DDLT and unwilling to be a part of the CD34+ cell infusion research protocol. Informed consent was taken from the control group for enrollment in the DDLT list as well as for regular follow up and comparison with autologous CD34+ cell infusion patients.
Patients in the study population were admitted to the hospital and a complete clinical examination was done. Investigations included complete blood counts, liver function test, serum creatinine, blood urea, alpha fetoprotein, coagulation profile, trans abdominal ultrasonography of the abdomen with doppler study of the spleno-portal axis, contrast enhanced computed topography scan for all patients except those with serum creatinine > 1.5 mg/dL, screening for hepatitis B and C, HIV, syphilis, cytomegalovirus. All other tests required to ascertain the cause of cirrhosis were done on a case to case basis.
The patients in the control group underwent complete clinical and laboratory work up as above, in addition to all other tests required for listing in the DDLT program as per the liver transplantation protocol of the Institute.
Refractory ascites was defined as ascites unresponsive to sodium restricted diet (less than 2 g/d) and high dose diuretic treatment (spironolactone 400 mg/d and frusemide 160 mg/d) or therapy limited by the complications of diuretics.
Treatment protocol
Patients in the study group were admitted to the hospital and received daily subcutaneous injections of human granulocyte-colony-stimulating factor 520 μg/day (G-CSF Neupogen, Filgrastim, Roche) for 3 consecutive days for mobilization of CD34+ cells from the bone marrow. This was based on an initial observation (n = 5) that peak CD34+ cell levels were achieved on day 3 followed by a steady decrease on day 4 and 5. Following G-CSF injections, daily monitoring of blood for complete blood counts, coagulation profile, creatinine and liver function tests were done. Any adverse effects were recorded. The peripheral concentration of CD34+ cells were measured daily prior to leukapheresis to ensure satisfactory levels (> 2 cells/μL). On day 4, leukapheresis was done using the MCS-3P magnetic cell separator (Hemaneics, USA) and 60-120 mL of peripheral blood was collected. Peripheral blood mononuclear cells were isolated from the leukapheresis products in the clean room. Mononuclear cells were isolated employing Hi-Sep method (HiSep LSM1077, LS001, Himedia). The mononuclear cells were washed with phosphate buffer saline (PBS) and diluted with CliniMACS buffer (Miltenyi Biotech, GmbH). The cells were centrifuged and incubated with CD34+ monoclonal antibodies directly labelled to micro beads (MACS, Miltney Biotech, GmbH171-01, Bergisch, Galdbach, Germany) for 30 min. After incubation the cells were washed with CliniMACS buffer and placed on a CliniMACs cell separator. The labelled cells were isolated using high gradient magnetic field and eluted from the column. At the end of the separation, the cells were counted under a microscope and viability was assessed by the trypan blue dye exclusion method. Purity of the cells was assessed by flowcytometry. The CD34+ cells were diluted with 10 mL of PBS with 2% human serum albumin in a sterile tube and were immediately infused through the hepatic artery under radiological guidance by the interventional radiologist. The patients were kept under observation for 24 h post procedure and discharged on the subsequent day. During the hospital stay, all clinical parameters and any adverse events were recorded.
Follow up
Following discharge from the hospital, the patients were followed up every week for one month and thereafter at end of 3 mo. During each visit, ascites was evaluated by ultrasonography and a need for therapeutic paracentesis due to ascites causing respiratory embarrassment was recorded. Laboratory tests at each visit included complete blood count, liver function test, coagulation profile, creatinine, blood urea, alpha feto-protein and doppler ultrasonography of whole abdomen.
The control group was followed up with a similar protocol for 3 mo.
Statistical analysis
The clinical and laboratory data at baseline between the study population and the control group and at 1 mo and the end of 3 mo were analyzed. The values are expressed as mean with standard deviation and as median with range wherever deemed appropriate. For categorical variables, Fischer’s exact test was used. Data was analyzed using online graphpad software 2014 and a P value (two tailed) of < 0.05 was considered to be statistically significant. The statistical analysis was per protocol analysis.
RESULTS
Of the 100 patient screened for eligibility, 55 patients were excluded. Thirty patients did not meet the inclusion criteria and 25 patients refused to be a part of the study. Twenty two patients were enrolled in the study population. At 1 mo, in the study group, all 22 patients were analyzed for response to CD34+ cell infusion. Between 1 mo to 3 mo, 2 patients despite being clinically stable decided to undergo LDLT, 1 patient died of hepatorenal syndrome and 1 patient was lost to follow up. In the control group, 23 patients were enrolled and all patients were analyzed at 1 mo. In between 1 mo to 3 mo, 3 patients died - 2 due to sepsis and 1 due to hepatorenal syndrome, 1 patient underwent DDLT and 2 patients were lost to follow up. In the final analysis, there were 17 and 18 patents in the control and study groups, respectively, at 3 mo (Figure 1).
Figure 1.
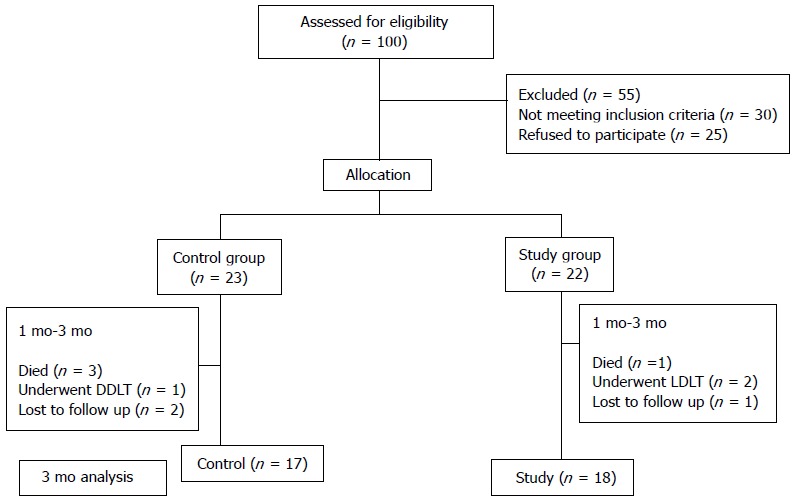
Consort flow diagram. LDLT: Living donor liver transplantation; DDLT: Deceased donor liver transplantation.
The baseline characteristics between the control group and the study population were identical (Table 1). In the control and study groups, the cause of cirrhosis was cryptogenic in 18 (78.2%) and 16 (72.72%) and alcohol related in 5 (21.7%) and 6 (27.27%), respectively. The MELD score in both groups was similar. The median period of alcohol abstinence in the control group was 7 mo (range, 5-11 mo) and in the study group was 6 mo (range, 4-12 mo). Among the patients with cryptogenic cirrhosis, 7 patients in the control group and 6 in the study group had type 2 diabetes mellitus with mean glycosylated hemoglobin (HBA1c) levels of 6.45 (range, 5.3-8.3) and 6.63 (range, 5.1-9), respectively. Twenty two (95.6%) patients in the control group and 22 (100%) in the study group had ascites. Refractory ascites requiring therapeutic paracentesis at least once every month for respiratory difficulty was 5 (22.7%) in the study group and 6 (21.7%) in the control group. In our population, the high maximum diuretic dose of 400 mg of spironolactone/d combined with furosemide 160 mg/d could not be given to any patients due to development of complications of diuretic therapy. All of our refractory ascites patients were therefore refractory due to development of diuretic therapy-related complications which limited their use at the maximal dose. The mean number of therapeutic paracentesis in the 3 mo period prior to enrollment was similar between the two groups. The presence of documented previous overt hepatic encephalopathy within the last 3 mo was 6 (26%) and 7 (31.81%) in the control and the study population, respectively.
Table 1.
Baseline characteristics between control and study group (mean ± SD)
| Parameter | Control group (n = 23) | Study group (n = 22) | P value |
| Age (yr) | 47.35 ± 12.54 | 48.91 ± 9.25 | 0.64 |
| Sex ratio (M:F) | 20:3 | 16:6 | 0.072 |
| Hemoglobin (g/dL) | 9.29 ± 1.86 | 9.15 ± 1.60 | 0.79 |
| Platelet count (Lakhs/mm3) | 0.92 ± 0.27 | 1.1 ± 0.72 | 0.24 |
| Total bilirubin (mg/dL) | 4.78 ± 4.06 | 3.55 ± 2.12 | 0.21 |
| AST (IU/mL) | 101.61 ± 174.41 | 67.14 ± 55.99 | 0.30 |
| ALT (IU/mL) | 77.87 ± 125.54 | 32.45 ± 17.07 | 0.10 |
| Albumin (mg/dL) | 2.7 ± 0.35 | 2.55 ± 0.35 | 0.16 |
| INR | 1.72 ± 0.53 | 1.80 ± 0.52 | 0.62 |
| Creatinine (mg/dL) | 1.08 ± 0.38 | 1.02 ± 0.29 | 0.56 |
| MELD score | 18.73 ± 5.29 | 18.28 ± 3.50 | 0.74 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; IU: International unit; INR: International normalized ratio; MELD: Model for end-stage liver disease score.
Mobilization of CD34+ cells after G-CSF
The mean (± SD) baseline cell count (cells/μL) was 2.3 ± 2.56 with baseline viability (%) of 48.17 ± 23.95. The day 3 cell count (cells/μL) was 27.00 ± 20.43 while the viability (%) was 81.84 ± 11.99. The purity of cells as assessed by enumerating CD34+ cells on the flow cytometer was 80%-90%.
Clinical results after CD34+ cell infusion
Following CD34+ cell infusion, patients were regularly monitored and clinical parameters were recorded. The liver and renal function tests during the course of stay in the hospital were normal. Following the procedure protocol Doppler ultrasound was performed and there was no evidence of any portal vein or hepatic artery thrombosis. All patients were discharged from hospital as planned in the protocol after 24 h of the procedure.
Primary end point analysis revealed that at 4 wk, the mean serum albumin in the study group increased significantly (2.83 ± 0.36 vs 2.43 ± 0.42, P = 0.001) when compared with controls. This improvement in albumin was however not sustained at 3 mo. (Tables 2 and 3). Serum bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) did not show any statistically significant improvement at 4 wk and at the end of 3 mo. However, at the end of 3 mo there was a statistically significant improvement in serum creatinine in the study group (0.96 ± 0.33 vs 1.42 ± 0.70, P = 0.01) which translated into a significant improvement in the MELD score (15.75 ± 5.13 vs 19.94 ± 6.68, P = 0.04). Platelet count and INR showed some improvement but did not reach statistical significance at any point of time.
Table 2.
Comparison between control and study group at 1 mo (mean ± SD)
| Parameter | Control group (n = 23) | Study group (n = 22) | P value |
| Platelet count (Lakhs/mm3) | 0.87 ± 0.23 | 0.94 ± 0.41 | 0.48 |
| Total bilirubin (mg/dL) | 4.63 ± 3.16 | 3.37 ± 1.91 | 0.11 |
| AST (IU/mL) | 80.78 ± 74.98 | 68 ± 24.98 | 0.45 |
| ALT (IU/mL) | 55.13 ± 61.34 | 41.73 ± 20.56 | 0.34 |
| Albumin (mg/dL) | 2.43 ± 0.42 | 2.83 ± 0.36 | 0.001 |
| INR | 1.78 ± 0.65 | 1.67 ± 0.49 | 0.50 |
| Creatinine (mg/dL) | 1.24 ± 0.50 | 1.01 ± 0.32 | 0.06 |
| MELD score | 19.42 ± 6.52 | 15.73 ± 3.35 | 0.02 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; IU: International unit; INR: International normalized ratio; MELD: Model for end-stage liver disease score.
Table 3.
Comparison between control and study group at 3 mo (mean ± SD)
| Parameter | Control group (n = 17) | Study group (n = 18) | P value |
| Platelet count (Lakhs/mm3) | 0.91 ± 0.36 | 1.16 ± 0.71 | 0.21 |
| Total bilirubin (mg/dL) | 5.89 ± 7.13 | 3.82 ± 3.30 | 0.27 |
| AST (IU/mL) | 70.29 ± 43.57 | 48.39 ± 15.79 | 0.05 |
| ALT (IU/mL) | 49.18 ± 66.59 | 39.44 ± 19.02 | 0.55 |
| Albumin (mg/dL) | 2.65 ± 0.53 | 2.83 ± 0.33 | 0.24 |
| INR | 1.88 ± 0.48 | 1.61 ± 0.30 | 0.05 |
| Creatinine (mg/dL) | 1.42 ± 0.70 | 0.96 ± 0.33 | 0.01 |
| MELD score | 19.94 ± 6.68 | 15.75 ± 5.13 | 0.04 |
| Frequency of tap in last 3 mo | 1.59 ± 1.06 | 1.28 ± 0.75 | 0.32 |
AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; IU: International unit; INR: International normalized ratio; MELD: Model for end-stage liver disease score; tap: Therapeutic paracentesis.
On statistical analysis of secondary end points, the transplant free survival with autologous CD34+ cell infusion at the end of 1 mo and 3 mo did not show any significant difference (P = 0.60) when compared to the control group. The mean requirement of therapeutic paracentesis was not significantly different at the end of 3 mo between the study and control groups (1.28 ± 0.75 vs 1.59 ± 1.06, P = 0.32) (Table 3). There was no patient for whom the requirement for therapeutic paracentesis increased after CD34 cell infusion.
Mortality data
In the study population, 1 patient developed sepsis with hepatorenal syndrome and died on 88th day post CD34+ cell infusion. In the control group, 3 patients died within 3 mo: 2 died due to sepsis and 1 due to a massive upper gastrointestinal bleed. The number of deaths between the study and the control groups was not statistically significant at 3 mo (P = 0.34)
Adverse events
The infusion of autologous CD34+ cells through the hepatic artery was safe with no specific treatment- or procedure-related side effects or mortality. The commonest complaint was slight discomfort at the peripheral catheter site and the pain score on the visual analog scale was always less than 4. One patient complained of chest discomfort 24 h after the procedure. However, the cardiac workup for ischemia was negative and it subsided within 1 h after administration of a proton pump inhibitor.
DISCUSSION
Cell based regenerative therapies for chronic liver disease are a promising new alternative to whole organ liver transplantation[9], where the lack of donor tissues and high cost[10] act as major obstacles. The attention towards bone marrow-derived stem cells for liver regeneration, started after a study by Theise et al[11] where Y chromosome positive liver cells were detected in autopsied women who received therapeutic bone marrow transplantation from male donors, suggested the existence of pluripotent stem cells among bone marrow cells. Previous studies[12-14] have demonstrated improvement in liver function with autologous CD34+ cell infusion in decompensated cirrhosis of varied etiology. In our study, we used peripheral CD34+ autologous stem cell infusion and this is the first study which has compared the results with controls in non-viral decompensated cirrhosis.
In our study population, mobilization of bone marrow-derived CD34+ cells was done using G-CSF for 3 consecutive days instead of the conventional 5 d therapy used in most other studies[12-14]. This was based on the initial pilot study done at our Institute on 5 patients, wherein it was observed that the mean cell counts reached a peak after the third dose of G-CSF injection and then showed a downward trend on day 4 and day 5 in non-viral cirrhosis patients. The mobilization of CD34+ cells by use of G-CSF was safe despite the presence of decompensation and portal hypertension in all the patients. In contrast to previous reports[15,16], there was no case of splenic rupture during the CD34+ mobilization procedure. The blood counts were monitored in all patients daily for 5 consecutive days and also every weekly post procedure up to one month. The cell counts returned to baseline in all patients after the end of 1 mo.
Liver stem cells are thought to be precursors to liver parenchymal cells or cholangiocytes[17-19]. The actual ability of these progenitor cells to differentiate into hepatocytes or cholangiocytes is not clear[20]. It has been suggested that conversion of stem cells to hepatocytes may occur via cell fusion[21,22]. Transplantation of stem cells by infusion into a peripheral or portal vein has shown successful engraftment and multiplication even in the setting of fibrosis in rodent models[23,24]. A recent human study on acute on chronic liver failure[8] has shown that G-CSF injections increases the homing of CD34+ cells in the liver. In our study, to improve hepatic homing of cells, which may involve chemo-attractants like stroma derived factor 1[25,26], the CD34+ cells were directly infused into the hepatic artery under radiological guidance. No procedural complications were observed in this process. Earlier studies had also safely used the hepatic artery for infusion[27]. Post procedure, there was no increase in portal vein or hepatic artery thrombosis nor was there any evidence of ischemic hepatitis.
The significant improvement in serum albumin which was observed at 1 mo coincides with the 4 wk period of maximal homing of CD34+ cells in the liver[8]. The 3 mo improvement in the MELD score in the study population may indicate that CD34+ cells which have homed in the liver may be exerting its peak regenerative effect at that time. This improvement in albumin and MELD score has been observed in previous studies[13,28]. Although studies in the past have shown improvement in bilirubin and transaminases, our study could not find any such statistical significance.
More patients in the study group (n = 2) underwent liver transplant. All of these patients had living donor liver transplantation and the cause of opting for LDLT after CD34+ cell infusion was purely based on the availability of a living donor, and wish for a curative treatment. The living donor as well as the family of the patients needed time for convincing themselves as well for arranging finances for a LDLT. In these patients, autologous CD34+ cell infusion served as a bridge to liver transplant. Only 1 patient in the control population underwent DDLT, reaffirming the paucity of deceased donor tissue for liver transplant. However, the mean time to liver transplant was not statistically different in both the groups.
Survival analysis to assess the transplant free survival was not statistically feasible as the number of patients were low. However, mortality was not statistically different in both the groups through the number of deaths in the control group was more (n = 3) than the study population (n = 1). The most common cause of death was hepatorenal syndrome and sepsis. This may suggest that the infusion of G-CSF causes restoration of neutrophil function[29,30] in decompensated cirrhosis, which has been attributed to development of sepsis and HRS in such patients. G-CSF infusion has also been shown to improve the development of antibodies to hepatitis B after hepatitis B vaccination in cirrhosis of non-viral etiology and thereby improves prognosis by preventing new hepatitis B infection in this patient population[31].
The limitations of this study was that 2 patients who enrolled for the study group later decided to go for LDLT thereby causing bias in the analysis of transplant free survival and overall mortality analysis. A larger study population would have given a clearer picture on the mortality data. Another limitation of the study was the lack of liver biopsy. Therefore, we could not objectively demonstrate the homing in and expansion of CD34+ cells in liver tissue.
The positives of this study, however, are that this is the first study in a non-viral decompensated cirrhosis of diverse etiology wherein the effects of CD34+ cell infusion has been analyzed for 3 mo and compared with controls. The improvement in MELD at 3 mo paves the way for identifying a potential window to delay transplantation. Long term data are required in this field.
In summary, autologous CD34+ cell infusion appears to be a safe and effective modality to delay the need for liver transplantation and thereby serve as a bridge to either DDLT or LDLT. The benefits of autologous CD34+ cell infusion indicates that there is a window during which a transplant may be still be required in a few subjects. Whether repeated infusion of stem cells can further delay the need for transplant merits evaluation in further trials.
COMMENTS
Background
Cirrhosis of the liver is a chronic liver disease with high morbidity and mortality. The most common causes of cirrhosis are alcohol, hepatitis B, hepatitis C and non-alcoholic fatty liver related. The only curative option for cirrhosis of the liver is a liver transplantation. However, the paucity of donor organs available for liver transplantation, coupled with the high costs involved in surgery have paved the way for a search for alternative cell-based therapies. Stem cell therapy has emerged as a fore-runner in this research effort and initial studies have shown promising results in cirrhosis of both viral and non-viral etiologies.
Research frontiers
Studies in the past have shown improvement in liver function by using stem cells from various sources. In this study, mobilization of a patient’s own stem cells from the bone marrow and subsequent isolation of these cells was done in the stem cell laboratory. The stem cell isolates were then infused back into the patient with the expectation that they will differentiate in the liver into liver cells and help in regeneration of the diseased liver.
Innovations and breakthroughs
Previous studies have compared the effect of autologous stem cell infusion in non-viral decompensated cirrhotic patients. This study is the first in this subset of patients wherein the authors have compared these patients with controls. The controls were patients waiting for liver transplantation. The authors directly infused the isolated cells into the hepatic artery of the patient with the hope that this will increase the availability of stem cells delivered to the liver. We found that there was an improvement in albumin at 1 mo. At 3 mo there was significant improvement in the MELD score and creatinine.
Applications
This study suggests that autologous infusion of CD34+ cells in patients with decompensated cirrhosis of non-viral etiology can be used safely to improve liver function in the short term.
Terminology
Decompensated cirrhosis means a cirrhotic patient in whom, due to the failing function of the liver, there is development of either jaundice or ascites (fluid accumulation in the abdomen). Liver transplantation is a surgical procedure in which the whole liver from a deceased person or a part of the liver from a related living donor is transplanted into a patient having cirrhosis of the liver after his own diseased liver is removed. Stem cells are the master cells of the human body which have the ability to differentiate into various tissue cell types including hepatocytes. CD34+ cells are stem cells of hemopoietic origin which are recognized in the laboratory by the presence of the marker CD34.
Peer-review
In this study, the authors have compared the use of autologous CD34+ cells in patients with decompensated liver cirrhosis of non-viral etiology with patients receiving the standard of care on the waiting list for liver transplantation. This idea is very good work, especially in the number of cases. The author needs to follow up the long term data of these patients to detect any long term side effects.
Footnotes
Supported by Grants from Asian Healthcare Foundation.
Ethics approval: The study was reviewed and approved by the by the Asian Institute of Gastroenterology Institutional review board, Asian Institute of Gastroenterology Institutional Ethics Committee and Institutional Committee for Stem Cell Research.
Informed consent: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest: All authors have received fees for serving as a consultant for Asian Institute of Gastroenterology and/or Asian Healthcare foundation. No conflict of interest exists in relation to the work submitted for consideration of publication.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at drmithunsharma@gmail.com Participants gave informed consent for data sharing
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: December 29, 2014
First decision: January 22, 2015
Article in press: March 19, 2015
P- Reviewer: Eshraghian A, El-Hawary AK, Zhao HT S- Editor: Yu J L- Editor: O’Neill M E- Editor: Zhang DN
References
- 1.Zatoński WA, Sulkowska U, Mańczuk M, Rehm J, Boffetta P, Lowenfels AB, La Vecchia C. Liver cirrhosis mortality in Europe, with special attention to Central and Eastern Europe. Eur Addict Res. 2010;16:193–201. doi: 10.1159/000317248. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, et al. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112–120. doi: 10.1111/j.1440-1746.2011.07024.x. [DOI] [PubMed] [Google Scholar]
- 3.Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820–828. doi: 10.1002/hep.24434. [DOI] [PubMed] [Google Scholar]
- 4.Zhan Y, Wang Y, Wei L, Chen H, Cong X, Fei R, Gao Y, Liu F. Differentiation of hematopoietic stem cells into hepatocytes in liver fibrosis in rats. Transplant Proc. 2006;38:3082–3085. doi: 10.1016/j.transproceed.2006.08.132. [DOI] [PubMed] [Google Scholar]
- 5.Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 6.Gordon MY, Levicar N, Pai M, Bachellier P, Dimarakis I, Al-Allaf F, M’Hamdi H, Thalji T, Welsh JP, Marley SB, et al. Characterization and clinical application of human CD34+ stem/progenitor cell populations mobilized into the blood by granulocyte colony-stimulating factor. Stem Cells. 2006;24:1822–1830. doi: 10.1634/stemcells.2005-0629. [DOI] [PubMed] [Google Scholar]
- 7.Spahr L, Lambert JF, Rubbia-Brandt L, Chalandon Y, Frossard JL, Giostra E, Hadengue A. Granulocyte-colony stimulating factor induces proliferation of hepatic progenitors in alcoholic steatohepatitis: a randomized trial. Hepatology. 2008;48:221–229. doi: 10.1002/hep.22317. [DOI] [PubMed] [Google Scholar]
- 8.Garg V, Garg H, Khan A, Trehanpati N, Kumar A, Sharma BC, Sakhuja P, Sarin SK. Granulocyte colony-stimulating factor mobilizes CD34(+) cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:505–512.e1. doi: 10.1053/j.gastro.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 9.Saito T, Tomita K, Haga H, Okumoto K, Ueno Y. Bone marrow cell-based regenerative therapy for liver cirrhosis. World J Methodol. 2013;3:65–69. doi: 10.5662/wjm.v3.i4.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palanisamy AP, Taber DJ, Sutter AG, Nadig SN, Dowden JE, McGillicuddy JW, Baliga PK, Chavin KD. Clinical outcomes and costs associated with in-hospital biliary complications after liver transplantation: a cross-sectional analysis. J Gastrointest Surg. 2015;19:282–289. doi: 10.1007/s11605-014-2675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Theise ND, Nimmakayalu M, Gardner R, Illei PB, Morgan G, Teperman L, Henegariu O, Krause DS. Liver from bone marrow in humans. Hepatology. 2000;32:11–16. doi: 10.1053/jhep.2000.9124. [DOI] [PubMed] [Google Scholar]
- 12.Mohamadnejad M, Alimoghaddam K, Mohyeddin-Bonab M, Bagheri M, Bashtar M, Ghanaati H, Baharvand H, Ghavamzadeh A, Malekzadeh R. Phase 1 trial of autologous bone marrow mesenchymal stem cell transplantation in patients with decompensated liver cirrhosis. Arch Iran Med. 2007;10:459–466. [PubMed] [Google Scholar]
- 13.Mohamadnejad M, Namiri M, Bagheri M, Hashemi SM, Ghanaati H, Zare Mehrjardi N, Kazemi Ashtiani S, Malekzadeh R, Baharvand H. Phase 1 human trial of autologous bone marrow-hematopoietic stem cell transplantation in patients with decompensated cirrhosis. World J Gastroenterol. 2007;13:3359–3363. doi: 10.3748/wjg.v13.i24.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pai M, Zacharoulis D, Milicevic MN, Helmy S, Jiao LR, Levicar N, Tait P, Scott M, Marley SB, Jestice K, et al. Autologous infusion of expanded mobilized adult bone marrow-derived CD34+ cells into patients with alcoholic liver cirrhosis. Am J Gastroenterol. 2008;103:1952–1958. doi: 10.1111/j.1572-0241.2008.01993.x. [DOI] [PubMed] [Google Scholar]
- 15.Falzetti F, Aversa F, Minelli O, Tabilio A. Spontaneous rupture of spleen during peripheral blood stem-cell mobilisation in a healthy donor. Lancet. 1999;353:555. doi: 10.1016/S0140-6736(99)00268-8. [DOI] [PubMed] [Google Scholar]
- 16.Ganetsky A, Kucharczuk C, Del Percio S, Frey N, Gill S. Spontaneous subcapsular splenic hematoma associated with filgrastim in a patient undergoing allogeneic hematopoietic stem cell transplantation. Ann Pharmacother. 2013;47:e22. doi: 10.1345/aph.1S005. [DOI] [PubMed] [Google Scholar]
- 17.Oertel M. Fetal liver cell transplantation as a potential alternative to whole liver transplantation? J Gastroenterol. 2011;46:953–965. doi: 10.1007/s00535-011-0427-5. [DOI] [PubMed] [Google Scholar]
- 18.Duncan AW, Dorrell C, Grompe M. Stem cells and liver regeneration. Gastroenterology. 2009;137:466–481. doi: 10.1053/j.gastro.2009.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forbes SJ, Vig P, Poulsom R, Alison MR, Wright NA. Bone marrow-derived liver stem cells: their therapeutic potential. Gastroenterology. 2002;123:654–655. doi: 10.1053/gast.2002.34225a. [DOI] [PubMed] [Google Scholar]
- 20.Lee KD, Kuo TK, Whang-Peng J, Chung YF, Lin CT, Chou SH, Chen JR, Chen YP, Lee OK. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- 22.Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- 23.Yovchev MI, Xue Y, Shafritz DA, Locker J, Oertel M. Repopulation of the fibrotic/cirrhotic rat liver by transplanted hepatic stem/progenitor cells and mature hepatocytes. Hepatology. 2014;59:284–295. doi: 10.1002/hep.26615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christ B, Brückner S, Stock P. Hepatic transplantation of mesenchymal stem cells in rodent animal models. Methods Mol Biol. 2011;698:315–330. doi: 10.1007/978-1-60761-999-4_24. [DOI] [PubMed] [Google Scholar]
- 25.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 26.Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol. 2009;15:2657–2664. doi: 10.3748/wjg.15.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan AA, Parveen N, Mahaboob VS, Rajendraprasad A, Ravindraprakash HR, Venkateswarlu J, Rao SG, Narusu ML, Khaja MN, Pramila R, et al. Safety and efficacy of autologous bone marrow stem cell transplantation through hepatic artery for the treatment of chronic liver failure: a preliminary study. Transplant Proc. 2008;40:1140–1144. doi: 10.1016/j.transproceed.2008.03.111. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Yan L, Han G, Zhou X, Hong L, Yin Z, Zhang X, Wang S, Wang J, Sun A, et al. Controlled trials in hepatitis B virus-related decompensate liver cirrhosis: peripheral blood monocyte transplant versus granulocyte-colony-stimulating factor mobilization therapy. Cytotherapy. 2008;10:390–396. doi: 10.1080/14653240802129901. [DOI] [PubMed] [Google Scholar]
- 29.Fiuza C, Salcedo M, Clemente G, Tellado JM. Granulocyte colony-stimulating factor improves deficient in vitro neutrophil transendothelial migration in patients with advanced liver disease. Clin Diagn Lab Immunol. 2002;9:433–439. doi: 10.1128/CDLI.9.2.433-439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang XM, Luo ZP, Zhou JH. Behavioral evidence for the role of noradrenaline in the putative anxiogenic actions of the inverse benzodiazepine receptor agonist methyl-4-ethyl-6,7-dimethoxy-beta-carboline-3-carboxylate. J Pharmacol Exp Ther. 1989;250:358–363. [PubMed] [Google Scholar]
- 31.Lankarani KB, Talebzadeh M, Eshraghian A, Malek-Hosseini SA. Granulocyte colony stimulating factor adjuvant role on the immunological response to hepatitis B vaccine in patients with cirrhosis: a double blind randomized placebo controlled trial. Hepat Mon. 2014;14:e15447. doi: 10.5812/hepatmon.15447. [DOI] [PMC free article] [PubMed] [Google Scholar]


