Abstract
AIM: To analyze the available evidence about the risk of extrapancreatic malignancies and pancreatic ductal adenocarcinoma associated to pancreatic intraductal papillary mucinous tumors (IPMNs).
METHODS: A systematic search of literature was undertaken using MEDLINE, EMBASE, Cochrane and Web-of-Science libraries. No limitations for year of publication were considered; preference was given to English papers. All references in selected articles were further screened for additional publications. Both clinical series and Literature reviews were selected. For all eligible studies, a standard data extraction form was filled in and the following data were extracted: study design, number of patients, prevalence of pancreatic cancer and extrapancreatic malignancies in IPMN patients and control groups, if available.
RESULTS: A total of 805 abstracts were selected and read; 25 articles were considered pertinent and 17 were chosen for the present systematic review. Eleven monocentric series, 1 multicentric series, 1 case-control study, 1 population-based study and 3 case report were included. A total of 2881 patients were globally analyzed as study group, and the incidence of pancreatic cancer and/or extrapancreatic malignancies ranged from 5% to 52%, with a mean of 28.71%. When a control group was analyzed (6 papers), the same incidence was as low as 9.4%.
CONCLUSION: The available Literature is unanimous in claiming IPMNs to be strongly associated with pancreatic and extrapancreatic malignancies. The consequences in IPMNs management are herein discussed.
Keywords: Intraductal papillary mucinous neoplasm, Pancreas, Diagnosis, Follow-up, Tumors, Computed tomography scan, 18FDG-PET
Core tip: This paper enter a clinical debate which has real relevance in the daily practice in the field of pancreatic cystic neoplasm, as a number of asymptomatic intraductal papillary mucinous tumors (IPMNs) are diagnosed every day by abdominal imaging, and the actual most common clinical attitude provides for a weak diagnostic pathway. However, in the light of an unanimous literature, this attitude would be a while dangerous, as up to 30%-40% of IPMN cases would develop a second malignancy in their life. The same should be underlined when discussing the follow-up protocols.
INTRODUCTION
Intraductal papillary mucinous tumors (IPMNs) belong to the group of cystic neoplasms of the pancreas, and are characterized by a communication with the Wirsung duct and by the absence of ovarian-type stroma[1]; the cystic expansion of the main or a branch pancreatic duct is due to a papillary growth of the epithelium, with rich mucin production. IPMNs were firstly described in 1982[2], and subsequently classified as adenoma, borderline or carcinoma (in situ or invasive) tumors[3,4]. In the following years, from a number of clinical series[5-9] based both upon surgical treatment[10-13] and radiological follow-up[14-17], clinical and pathological features characteristic of IPMNs clearly emerged: the neoplasm is symptomatic in many cases, recurrent acute pancreatitis without recognised biliary or alcoholic etiology being the most frequent expression. The diagnosis is based on CT and MRI imaging, which further distinguish 2 different variants, the main duct tumor (MDT) and the branch duct tumor (BDT), having different incidence of malignancy and prognosis. The natural history of IPMNs remains partially unclear. At diagnosis, 10%-20% of cases harbour invasive carcinoma, 10%-20% in situ carcinoma and the remaining 60%-80% intraductal or intracystic papillomatosis with simple dysplasia. In the published series, resectability was 90%-100%, while mortality and morbidity were similar to those related to pancreatic surgery for ductal adenocarcinoma; 5-year survival is 100% for adenomas and border-line tumours, 80%-90% for in situ carcinomas and 50%-70% for invasive carcinomas.
One of the most surprising and most interesting characteristic of IPMNs is to be frequently associated with other neoplasms, both pancreatic and extrapancreatic. Some initial reports of this feature date back at least 20 years ago[18], and were later confirmed by numerous studies. Though it is not yet known the underlying mechanism, however this figure substantially has never been questioned ever since; it has been reported that patients affected by IPMN would be more likely to die of other neoplasm than from IPMN itself[17]; of course this entails major practical implications, particularly with respect to the diagnostic phase and to the follow-up.
The present article discusses the literature focused on this subject, and commits the clinical questions, to date unsolved, resulting therefrom.
MATERIALS AND METHODS
Search methods
A systematic search of literature was performed using MEDLINE, EMBASE, Cochrane and Web-of-Science libraries. No limitations for year of publication were considered. Search terms were: IPMN, IPMN-associated cancers, extrapancreatic neoplasm. MeSH terms were: Adenocarcinoma, Mucinous/diagnosis, Adenocarcinoma, Mucinous/surgery, Adenocarcinoma, Mucinous/prognosis, Pancreas/pathology, Pancreatic Neoplasms/diagnosis, Pancreatic Neoplasms/prognosis, Pancreatic Neoplasms/surgery.
Preference has been given to English publications, but abstracts from other languages were also included. All references in selected articles were further screened for additional publications. Articles were retrieved according to the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses guidelines (Figure 1).
Figure 1.
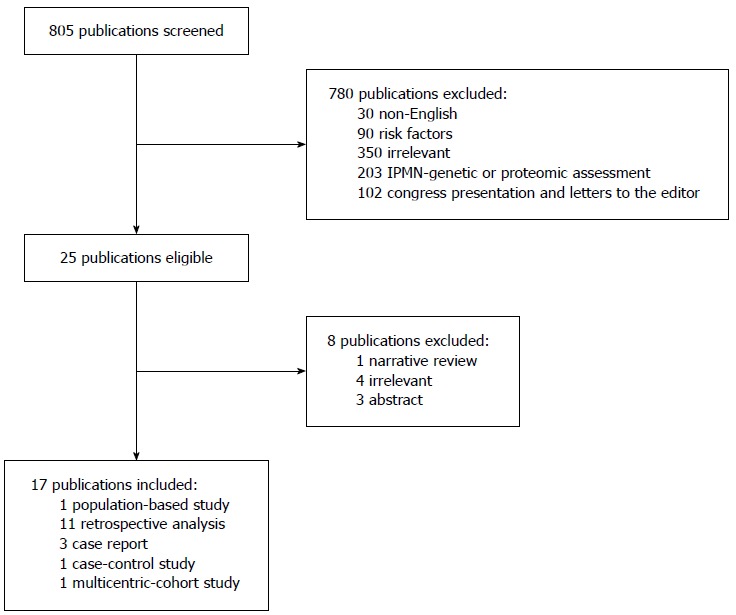
Articles retrieval strategy, according to the Preferred Items for Reporting of Systematic Reviews and Meta-Analyses guidelines. IPMN: Intraductal papillary mucinous tumor.
Study selection
Articles were selected if their abstract showed an association between IPMN and pancreatic adenocarcinoma and/or extrapancreatic neoplasms before, after or at the same time of IPMN diagnosis. Both clinical series and Literature reviews were selected. Papers reporting genetic characteristic or proteomic assessment in IPMN, studies of risk factors for malignancy, congress presentations and letters to the editor were excluded.
Data extraction
For all eligible studies, a standard data extraction form was filled in and the following data were extracted: study design, number of patients, prevalence of pancreatic cancer (PC) and extrapancreatic malignancies (EPM) in IPMN patients and control groups, if available. For statistical analysis, it was considered significant P < 0.05, when published.
RESULTS
The titles and abstracts of a total of 805 articles were screened separately by 2 authors (Gian Luca Baiocchi, Sarah Molfino) for eligibility. Out of these, 780 articles were excluded, either for having abstract unavailable (n = 30), or for reporting risk factors for malignancy (n = 90), or being irrelevant to our topic (n = 355), or because they described other features such as genetic proteomic assessment of IPMN (n = 203); excluded congress presentations and letters to the editor were 102. This resulted in 25 articles, 8 of which were further excluded after full text examination, either for being narrative review (n = 1), or comments to other papers (n = 4), or only available in abstract form (n = 3).
The remaining 17 articles were fully analyzed and data were extracted as summarized in Table 1[19-35].
Table 1.
Studies reporting pancreatic cancer and extrapancreatic malignancies incidence in patients with intraductal papillary mucinous tumors
| Ref. | Year | Study design | n (IPMN) | Prevalence EPM | Colon Ca | Gastric Ca | NET | Group 23 | n (group 2) | Prevalence EPM - group 2 (%) | P vaule |
| Lubezky et al[19] | 2012 | Retrospective | 82 | 20% | 31% | NR | NR | Pancreatic ductal adenocarcinoma | 150 | 6 | 0.002 |
| Riall et al[20] | 2007 | Population-based | 992 | 10% | 3% | 0.1% | NR | Other neoplasm1 | 18655 | 10 | NS |
| Yoon et al[21] | 2008 | Retrospective | 210 | 33.8% | 7% | 14% | NR | Non-IPMN cystic pancreatic neoplasm | 175 | 12 | < 0.001 |
| Kamisawa et al[22] | 2005 | Retrospective | 79 | 35% | 9% | 15% | NR | ||||
| Ishida et al[23] | 2008 | Retrospective | 61 | 24.6% | 8% | 10% | NR | ||||
| Ishida et al[24] | 2013 | Case report | 1 | 100% | 0 | 0 | 100 | ||||
| Goh et al[25] | 2006 | Case report | 3 | 100% | 0 | 0 | 100 | ||||
| Eguchi et al[26] | 2006 | Retrospective | 69 | 42% | 12% | 6% | NR | Other neoplasm2 | 301 | 12 | < 0.001 |
| Choi et al[27] | 2006 | Retrospective | 61 | 39% | 7% | 13% | NR | Other pancreatic neoplasm | 38 | 8 | < 0.001 |
| Calculli et al[28] | 2010 | Retrospective | 142 | 14.1% | NR | ||||||
| Reid-Lombardo et al[29] | 2010 | Retrospective | 471 | 52% | 4% | 0 | NR | ||||
| Oh et al[30] | 2009 | Retrospective | 37 | 38% | 8% | 8% | NR | ||||
| Baumgaertner et al[31] | 2008 | Case-control | 178 | 17% | 10% | NR | NR | Aged and gender-matched control | 356 | 8.4 | 0.003 |
| Tanno et al[32] | 1999 | Retrospective | 42 | 48% | 12% | 10% | NR | ||||
| Larghi et al[33] | 2013 | Multicentric cohort | 390 | 23.6% | 12.4% | NR | NR | ||||
| Tewari et al[34] | 2013 | Case report | 3 | 100% | 0 | 0 | 100% | ||||
| Uehara et al[35] | 2008 | Retrospective | 60 | 54 | NR | NR | NR |
Data from SEER Tumor Registry;
Data from Osaka Cancer Registry;
Control-group, if present;
Pancreatic adenocarcinoma. NR: Not reported; IPMN: Intraductal papillary mucinous tumor.
A total of 2881 patients were globally analyzed as study group, and the mean incidence of PC/EPM was 28.71%. When a control group was analyzed (6 papers), the same incidence was as low as 9.4%.
The majority of studies are retrospective (n = 11), while a case-control study and a multicentric-cohort were also included. Prevalence of PC/EPM in patients with IPMN was 14.1%-52%, with the exception of Uehara et al[35] (2008), reporting a prevalence of PC of only 5% (but this paper does not consider the extrapancreatic malignancies). Another paper reporting a somewhat lower incidence of EPM was published in 2007 by Riall and Coll.; in this study data of more than 18500 patients with pancreatic cancer were collected from the SEER Tumor Registry, and in this group the incidence of EPM was compared between patients with pancreatic adenocarcinoma and patients with IPMN. Thus, only malignant or invasive IPMNs were included.
Six studies reported a control group analysis; in all of them, a significantly higher prevalence of PC/EPM in IPMN patients than in control-group was reported; furthermore, in all the studies the incidence of EPM exceeded the expected rate of the same malignancies in the general population. When reported, in the majority of cases, the extrapancreatic neoplasm associated to IPMN patients were colorectal cancers (3%-12%).
DISCUSSION
Pancreatic IPMNs are observed with increasing frequency in asymptomatic patients[36], and still represent in the majority of cases a clinical dilemma. Identifyng the small subgroup pf malignant cases is of upmost importance: from one side, resecting all the IPMNs cases would represent an over-treatment in more than 75% of patients, but on the other side unrecognising malignancy would significantly worsen the prognosis of those cases. Thus, while in presence of clear malignant features the surgical indication is supported by good survival results, nevertheless a number of controversies remain for IPMNs at low risk of malignancy, relative to the diagnostic protocol to be adopted and to the surgical indication. Finally, it remains still not clear what kind of follow-up is indicated in both patients with resected IPMN and patients not submitted to surgery, as the likelihood of developing malignant recurrence and of progress into malignancy, respectively, is very low from the available series.
Comprehensive imaging, including ultrasonography (US) and contrast-enhanced US, CT scan, magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound, is highly sensitive and very low specific (down to about 20%[37]) in recognising malignancy, when the Sendai criteria, evidence-based treatment guidelines, published by a consortium of the International Association of Pancreatology in 2006 and 2012[38,39], are strictly applied. Endo-US fine-needle cytology significantly increases the specificity, but it is fairly invasive. 18FDG-PET was also investigated in a few published series, whit a reported accuracy rate of 85%-95%[40-44], and a specificity rate up to 100%[37]. However, Sendai guidelines do not take into account 18FDG-PET, which is besides very expensive and finally poorly employed in the majority of Centres.
When evaluating a rationale strategy for IPMNs staging and follow-up, an important and unique feature should be taken into consideration: it appears from the Literature that patients harbouring IPMNs are at higher risk of developing a second malignancy, both before, concurrent or after IPMN diagnosis[17,18].
An extensive literature search does not reveal a clear aetiopathogenetic explanation. The merely assumption that patients, once diagnosed to harbor IPMN, are subjected to instrumental examinations which therefore detect with greater frequency other malignancies, is not confirmed either by the fact that most of the associated tumors were diagnosed before the IPMN, either because the examination currently regarded as the gold standard for IPMNs, namely the MRCP, is an organ-specific and much less panoramic test compared to other investigations (including CT, which is less often used for the great number of necessary examinations and then the elevated radiation dose). The only speculative indications come from the analysis of risk factors for PC/EPC in IPMNs: female gender, white race, and above all advanced age [as early as 55-64 years, but with a peak to 75-84 years; for this age group the odds ratio for EPM, compared to the general population, is as high as 4.0 (95%CI: 2.4-6.7)]. Very few studies sought to analyze the molecular characteristics of IPMNs cases with PC/EPC; among them, Lee and Coll. found only correlation with the expression of the MUC2 gene and not of other genes, including p53[45]. As in other fields of oncology, the thorough study of patients with IPMN and associated malignancies would provide an interesting model for the study of carcinogenesis. Unfortunately, currently available data does not allow the precise identification of the subset of IPMN patients at greater risk of developing PC/EPM. Even in patients with small BDT, high incidences have been reported.
The most significant claim coming from the analysis of this data, to some extent surprising and still partially unexplained, concerns the clinical pathway. Indeed, the concept that at least one third of patients with IPMN develop in the course of their live PC/EPM cannot be overlooked. The first implication is related to the initial management of patients with newly diagnosed IPMN. There is currently a strong tendency to consider the BDT- IPMNs, without clinical and radiological signs which lead them in groups “high risk stigmata” and “worrisome features” according to Sendai guidelines, a disease of limited clinical relevance, for which no further investigation are warranted[46]. Although such a trend appears to be fully justified regarding the IPMN itself, what is described in the present paper leads to a reflection about the opportunity to submit these patients to instrumental examinations whit total-body value.
The second consideration concerns the IPMNs at high risk of malignancy (“high risk stigmata” according to Sendai): in these cases, since surgery is frequently performed, it might be smart to subject the patients to an even more comprehensive diagnostic preoperative work-up, including for example a colonoscopy and gastroscopy, in sight of a possible combined intervention.
The third consideration refers to the follow-up protocols: despite the low risk of malignant degeneration of resected IPMNs, and of not resected IPMNs without Sendai criteria, it appears at least careless to abandon these patients and end the follow-up, even though it is clearly expensive.
Of course, the points made above have consequences on the most widely used imaging modality in IPMNs: in addition to the MRCP, other panoramic examinations such as thorax-abdomen contrast medium enhanced CT and 18FDG-PET become relevant.
In conclusion, the literature appear unanimous in reporting that patients with IPMNs have a high risk, in the order of at least 30%, to develop PC and EPM; hence the opportunity to deepen, at the time of the first finding, and in any case preoperatively, the search for such tumors; and to maintain an high index of suspicion, and a constant follow-up, in all IPMNs patients, with total body examinations.
COMMENTS
Background
Intraductal papillary mucinous tumors (IPMNs) belong to the group of cystic neoplasms of the pancreas, and are characterized by a communication with the Wirsung duct and by the absence of ovarian-type stroma.
Research frontiers
One of the most surprising and most interesting characteristic of IPMNs is to be frequently associated with other neoplasms, both pancreatic and extrapancreatic. Some initial reports of this feature date back at least 20 years ago[18], and were later confirmed by numerous studies.
Innovations and breakthroughs
Comprehensive imaging, including ultrasonography (US) and contrast-enhanced US, computed tomography (CT) scan, magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound, is highly sensitive and very low specific (down to about 20%) in recognising malignancy, when the Sendai criteria, evidence-based treatment guidelines, published by a consortium of the International Association of Pancreatology in 2006 and 2012, are strictly applied.
Applications
The points made above have consequences on the most widely used imaging modality in IPMNs: in addition to the MRCP, other panoramic examinations such as thorax-abdomen contrast medium enhanced CT and 18FDG-PET become relevant.
Peer-review
This in an interesting review article presented by Baiocchi et al which focuses on intraductal papillary mucinous neoplasmas of the pancreas and their association with pancreatic and extrapancreatic malignancies.
Footnotes
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Data sharing: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 29, 2015
First decision: March 10, 2015
Article in press: May 7, 2015
P- Reviewer: Koukourakis GV, Li CY S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.Klöppel G. Clinicopathologic view of intraductal papillary-mucinous tumor of the pancreas. Hepatogastroenterology. 1998;45:1981–1985. [PubMed] [Google Scholar]
- 2.Itai Y, Ohhashi K, Nagai H, Murakami Y, Kokubo T, Makita K, Ohtomo K. “Ductectatic” mucinous cystadenoma and cystadenocarcinoma of the pancreas. Radiology. 1986;161:697–700. doi: 10.1148/radiology.161.3.3786719. [DOI] [PubMed] [Google Scholar]
- 3.Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin LH. WHO: histological typing of tumors of the exocrine pancreas. 2nd ed. Springer: Berlin; 1996. [Google Scholar]
- 4.Longnecker DS, Hruban RH, Kloppel G. Intraductal-papillary mucinous neoplasms of the pancreas. In: Hamilton SR, Aaltonen LA, eds , editors. World Health Organization classification of tumors. Pathology and genetics of tumors of the digestive system. Lyon: IARC press; 2000. pp. 237–240. [Google Scholar]
- 5.Traverso LW, Peralta EA, Ryan JA, Kozarek RA. Intraductal neoplasms of the pancreas. Am J Surg. 1998;175:426–432. doi: 10.1016/s0002-9610(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 6.Rivera JA, Fernández-del Castillo C, Pins M, Compton CC, Lewandrowski KB, Rattner DW, Warshaw AL. Pancreatic mucinous ductal ectasia and intraductal papillary neoplasms. A single malignant clinicopathologic entity. Ann Surg. 1997;225:637–644; discussion 644-646. doi: 10.1097/00000658-199706000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Falconi M, Salvia R, Bassi C, Zamboni G, Talamini G, Pederzoli P. Clinicopathological features and treatment of intraductal papillary mucinous tumour of the pancreas. Br J Surg. 2001;88:376–381. doi: 10.1046/j.1365-2168.2001.01720.x. [DOI] [PubMed] [Google Scholar]
- 8.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT, et al. Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 2002;123:1500–1507. doi: 10.1053/gast.2002.36552. [DOI] [PubMed] [Google Scholar]
- 9.Doi R, Fujimoto K, Wada M, Imamura M. Surgical management of intraductal papillary mucinous tumor of the pancreas. Surgery. 2002;132:80–85. doi: 10.1067/msy.2002.125386. [DOI] [PubMed] [Google Scholar]
- 10.Maire F, Hammel P, Terris B, Paye F, Scoazec JY, Cellier C, Barthet M, O’Toole D, Rufat P, Partensky C, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–722. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugiyama M, Izumisato Y, Abe N, Masaki T, Mori T, Atomi Y. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg. 2003;90:1244–1249. doi: 10.1002/bjs.4265. [DOI] [PubMed] [Google Scholar]
- 12.Jang JY, Kim SW, Ahn YJ, Yoon YS, Choi MG, Lee KU, Han JK, Kim WH, Lee YJ, Kim SC, et al. Multicenter analysis of clinicopathologic features of intraductal papillary mucinous tumor of the pancreas: is it possible to predict the malignancy before surgery? Ann Surg Oncol. 2005;12:124–132. doi: 10.1245/ASO.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 13.Wang SE, Shyr YM, Chen TH, Su CH, Hwang TL, Jeng KS, Chen JH, Wu CW, Lui WY. Comparison of resected and non-resected intraductal papillary mucinous neoplasms of the pancreas. World J Surg. 2005;29:1650–1657. doi: 10.1007/s00268-005-0035-8. [DOI] [PubMed] [Google Scholar]
- 14.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636; discussion 637. doi: 10.1016/j.amjsurg.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt CM, White PB, Waters JA, Yiannoutsos CT, Cummings OW, Baker M, Howard TJ, Zyromski NJ, Nakeeb A, DeWitt JM, et al. Intraductal papillary mucinous neoplasms: predictors of malignant and invasive pathology. Ann Surg. 2007;246:644–651; discussion 651-654. doi: 10.1097/SLA.0b013e318155a9e5. [DOI] [PubMed] [Google Scholar]
- 16.Baiocchi GL, Portolani N, Missale G, Baronchelli C, Gheza F, Cantù M, Grazioli L, Giulini SM. Intraductal papillary mucinous neoplasm of the pancreas (IPMN): clinico-pathological correlations and surgical indications. World J Surg Oncol. 2010;8:25. doi: 10.1186/1477-7819-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeuchi N, Itoi T, Sofuni A, Itokawa F, Tsuchiya T, Kurihara T, Ishii K, Tsuji S, Umeda J, Moriyasu F, et al. Prognosis of cancer with branch duct type IPMN of the pancreas. World J Gastroenterol. 2010;16:1890–1895. doi: 10.3748/wjg.v16.i15.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugiyama M, Atomi Y. Extrapancreatic neoplasms occur with unusual frequency in patients with intraductal papillary mucinous tumors of the pancreas. Am J Gastroenterol. 1999;94:470–473. doi: 10.1111/j.1572-0241.1999.879_h.x. [DOI] [PubMed] [Google Scholar]
- 19.Lubezky N, Ben-Haim M, Lahat G, Marmor S, Solar I, Brazowski E, Nackache R, Klausner JM. Intraductal papillary mucinous neoplasm of the pancreas: associated cancers, family history, genetic predisposition? Surgery. 2012;151:70–75. doi: 10.1016/j.surg.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 20.Riall TS, Stager VM, Nealon WH, Townsend CM, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803–813; discussion 813-814. doi: 10.1016/j.jamcollsurg.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Yoon WJ, Ryu JK, Lee JK, Woo SM, Lee SH, Park JK, Kim YT, Yoon YB. Extrapancreatic malignancies in patients with intraductal papillary mucinous neoplasm of the pancreas: prevalence, associated factors, and comparison with patients with other pancreatic cystic neoplasms. Ann Surg Oncol. 2008;15:3193–3198. doi: 10.1245/s10434-008-0143-4. [DOI] [PubMed] [Google Scholar]
- 22.Kamisawa T, Tu Y, Egawa N, Nakajima H, Tsuruta K, Okamoto A. Malignancies associated with intraductal papillary mucinous neoplasm of the pancreas. World J Gastroenterol. 2005;11:5688–5690. doi: 10.3748/wjg.v11.i36.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishida M, Egawa S, Kawaguchi K, Aoki T, Sakata N, Mikami Y, Motoi F, Abe T, Fukuyama S, Katayose Y, et al. Synchronous and metachronous extrapancreatic malignant neoplasms in patients with intraductal papillary-mucinous neoplasm of the pancreas. Pancreatology. 2008;8:577–582. doi: 10.1159/000159844. [DOI] [PubMed] [Google Scholar]
- 24.Ishida M, Shiomi H, Naka S, Tani T, Okabe H. Concomitant intraductal papillary mucinous neoplasm and neuroendocrine tumor of the pancreas. Oncol Lett. 2013;5:63–67. doi: 10.3892/ol.2012.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goh BK, Ooi LL, Kumarasinghe MP, Tan YM, Cheow PC, Chow PK, Chung YF, Wong WK. Clinicopathological features of patients with concomitant intraductal papillary mucinous neoplasm of the pancreas and pancreatic endocrine neoplasm. Pancreatology. 2006;6:520–526. doi: 10.1159/000097361. [DOI] [PubMed] [Google Scholar]
- 26.Eguchi H, Ishikawa O, Ohigashi H, Tomimaru Y, Sasaki Y, Yamada T, Tsukuma H, Nakaizumi A, Imaoka S. Patients with pancreatic intraductal papillary mucinous neoplasms are at high risk of colorectal cancer development. Surgery. 2006;139:749–754. doi: 10.1016/j.surg.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Choi MG, Kim SW, Han SS, Jang JY, Park YH. High incidence of extrapancreatic neoplasms in patients with intraductal papillary mucinous neoplasms. Arch Surg. 2006;141:51–56; discussion 56. doi: 10.1001/archsurg.141.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Calculli L, Pezzilli R, Brindisi C, Morabito R, Casadei R, Zompatori M. Pancreatic and extrapancreatic lesions in patients with intraductal papillary mucinous neoplasms of the pancreas: a single-centre experience. Radiol Med. 2010;115:442–452. doi: 10.1007/s11547-010-0502-7. [DOI] [PubMed] [Google Scholar]
- 29.Reid-Lombardo KM, Mathis KL, Wood CM, Harmsen WS, Sarr MG. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251:64–69. doi: 10.1097/SLA.0b013e3181b5ad1e. [DOI] [PubMed] [Google Scholar]
- 30.Oh SJ, Lee SJ, Lee HY, Paik YH, Lee DK, Lee KS, Chung JB, Yu JS, Yoon DS. [Extrapancreatic tumors in intraductal papillary mucinous neoplasm of the pancreas] Korean J Gastroenterol. 2009;54:162–166. doi: 10.4166/kjg.2009.54.3.162. [DOI] [PubMed] [Google Scholar]
- 31.Baumgaertner I, Corcos O, Couvelard A, Sauvanet A, Rebours V, Vullierme MP, Hentic O, Hammel P, Lévy P, Ruszniewski P. Prevalence of extrapancreatic cancers in patients with histologically proven intraductal papillary mucinous neoplasms of the pancreas: a case-control study. Am J Gastroenterol. 2008;103:2878–2882. doi: 10.1111/j.1572-0241.2008.02142.x. [DOI] [PubMed] [Google Scholar]
- 32.Tanno S, Nakano Y, Sugiyama Y, Nakamura K, Sasajima J, Koizumi K, Yamazaki M, Nishikawa T, Mizukami Y, Yanagawa N, et al. Incidence of synchronous and metachronous pancreatic carcinoma in 168 patients with branch duct intraductal papillary mucinous neoplasm. Pancreatology. 2010;10:173–178. doi: 10.1159/000231982. [DOI] [PubMed] [Google Scholar]
- 33.Larghi A, Panic N, Capurso G, Leoncini E, Arzani D, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, et al. Prevalence and risk factors of extrapancreatic malignancies in a large cohort of patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Oncol. 2013;24:1907–1911. doi: 10.1093/annonc/mdt184. [DOI] [PubMed] [Google Scholar]
- 34.Tewari N, Zaitoun AM, Lindsay D, Abbas A, Ilyas M, Lobo DN. Three cases of concomitant intraductal papillary mucinous neoplasm and pancreatic neuroendocrine tumour. JOP. 2013;14:423–427. doi: 10.6092/1590-8577/1491. [DOI] [PubMed] [Google Scholar]
- 35.Uehara H, Nakaizumi A, Ishikawa O, Iishi H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S, Takenaka A. Development of ductal carcinoma of the pancreas during follow-up of branch duct intraductal papillary mucinous neoplasm of the pancreas. Gut. 2008;57:1561–1565. doi: 10.1136/gut.2007.145631. [DOI] [PubMed] [Google Scholar]
- 36.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, Centeno BA, Szydlo T, Regan S, del Castillo CF, Warshaw AL. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–1336. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Baiocchi GL, Bertagna F, Gheza F, Grazioli L, Calanducci D, Giubbini R, Portolani N, Giulini SM. Searching for indicators of malignancy in pancreatic intraductal papillary mucinous neoplasms: the value of 18FDG-PET confirmed. Ann Surg Oncol. 2012;19:3574–3580. doi: 10.1245/s10434-012-2234-5. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17–32. doi: 10.1159/000090023. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183–197. doi: 10.1016/j.pan.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Pedrazzoli S, Sperti C, Pasquali C, Bissoli S, Chierichetti F. Comparison of International Consensus Guidelines versus 18-FDG PET in detecting malignancy of intraductal papillary mucinous neoplasms of the pancreas. Ann Surg. 2011;254:971–976. doi: 10.1097/SLA.0b013e3182383137. [DOI] [PubMed] [Google Scholar]
- 41.Takanami K, Hiraide T, Tsuda M, Nakamura Y, Kaneta T, Takase K, Fukuda H, Takahashi S. Additional value of FDG PET/CT to contrast-enhanced CT in the differentiation between benign and malignant intraductal papillary mucinous neoplasms of the pancreas with mural nodules. Ann Nucl Med. 2011;25:501–510. doi: 10.1007/s12149-011-0494-y. [DOI] [PubMed] [Google Scholar]
- 42.Bertagna F, Treglia G, Baiocchi GL, Giubbini R. F18-FDG-PET/CT for evaluation of intraductal papillary mucinous neoplasms (IPMN): a review of the literature. Jpn J Radiol. 2013;31:229–236. doi: 10.1007/s11604-012-0176-2. [DOI] [PubMed] [Google Scholar]
- 43.Hong HS, Yun M, Cho A, Choi JY, Kim MJ, Kim KW, Choi YJ, Lee JD. The utility of F-18 FDG PET/CT in the evaluation of pancreatic intraductal papillary mucinous neoplasm. Clin Nucl Med. 2010;35:776–779. doi: 10.1097/RLU.0b013e3181e4da32. [DOI] [PubMed] [Google Scholar]
- 44.Baiocchi GL, Portolani N, Bertagna F, Gheza F, Pizzocaro C, Giubbini R, Giulini SM. Possible additional value of 18FDG-PET in managing pancreas intraductal papillary mucinous neoplasms: preliminary results. J Exp Clin Cancer Res. 2008;27:10. doi: 10.1186/1756-9966-27-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SY, Choi DW, Jang KT, Lee KT, Choi SH, Heo JS, Lee JK, Paik SW, Rhee JC. High expression of intestinal-type mucin (MUC2) in intraductal papillary mucinous neoplasms coexisting with extrapancreatic gastrointestinal cancers. Pancreas. 2006;32:186–189. doi: 10.1097/01.mpa.0000202939.40213.fd. [DOI] [PubMed] [Google Scholar]
- 46.Baiocchi GL, Portolani N, Grazioli L, Mazza G, Gheza F, Bartoli M, Vanzetti E, Giulini SM. Management of pancreatic intraductal papillary mucinous neoplasm in an academic hospital (2005-2010): what follow-up for unoperated patients? Pancreas. 2013;42:696–700. doi: 10.1097/MPA.0b013e318270b98b. [DOI] [PubMed] [Google Scholar]


