Abstract
We report a case of a 42-year-old man with a rare disorder known as primary intestinal lymphangiectasia, which is characterized by dilated intestinal lymphatics that lead to the development of protein-losing enteropathy. The patient presented with a grand mal seizure caused by malabsorption-derived electrolytes and a protein disorder. Signs of the disease, including chronic diarrhea and peripheral edema, manifested 10 years ago, but a diagnosis was never made. The diagnosis was suspected because of the clinical manifestations, laboratory tests, imaging and endoscopic findings. Hyperemic and edematous mucosa of the small intestine corresponded to scattered white spots with dilated intestinal lymphatics and whitish villi in the histological specimen of the biopsied jejunal mucosa. Although numerous therapeutic strategies are available, only octreotide therapy proved to be an effective means of therapeutic resolution in this patient. Although the patient had a partial remission following the use of a slow release formula of octreotide, his prognosis, clinical course, and future treatment challenges are yet to be determined.
Keywords: Primary intestinal lymphangiectasia, Malabsorption, Seizure, Treatment, Diet, Octreotide
Core tip: This is a case of a 42-year-old man who presented with a grand mal seizure, malabsorption-derived electrolytes and a protein disorder caused by primary intestinal lymphangiectasia. After an extensive management and exclusion of secondary intestinal lymphangiectasia, a diagnosis was suspected because of the clinical manifestations, laboratory tests, imaging and endoscopic findings. Although numerous therapeutic strategies are available, only octreotide therapy proved to be a potentially effective means of therapeutic resolution; however, a longer follow-up period is needed. More case reports and/or studies on similar cases are needed to determine the best possible treatment strategies for this rare disorder.
INTRODUCTION
Defined in 1961[1], primary intestinal lymphangiectasia (PIL) is a rare, undiagnosed, clinical entity. It has a diverse clinical presentation, diagnostic and treatment management, which may result in various outcomes. The present recommendations are derived from no more than 200 cases; there are an insufficient numbers of cases to make concrete statements and create diagnostic algorithms, which are much needed. PIL, or Waldman disease, is a disorder with an unknown underlying etiology; its histopathological findings are characterized by dilated and tortuous lymphatics in the small bowel mucosa and submucosa, resulting in protein-losing enteropathy and its sequela. Although mostly affects children, it is not exclusively a childhood disease. Intermittent diarrhea and edema, accompanied by other signs of malnutrition and hypoalbuminemia, are the most common symptoms of PIL[2]. The principles of treatment are similar to the treatment of other forms of protein-losing enteropathy, with no exact recommendations on dosage and duration of therapy. We present a case report based on our experience in PIL treatment; we also present both past and present challenges and concerns regarding this rare disease.
CASE REPORT
A 42-year-old male patient presented with an isolated episode of a grand mal seizure. The patient was admitted to the Department of Neurology at the Clinical Hospital “Sveti Duh”, Zagreb, Croatia. The neuroradiological assessment excluded any neurological system pathology. The patient presented with signs of severe malabsorption (anemia, hypocalcaemia, hypomagnesaemia and hypoproteinemia) and was transferred to the Department of Gastroenterology and Hepatology within the same hospital. On physical examination, only peripheral edema was present. The patient’s previous medical history was unremarkable. The patient had noticed occasional bilateral perimaleolar edema over the previous ten years, which was predominant during the summer and also had one episode of scrotal edema a few months ago. He had a history of large-volume non-bloody diarrhea for six months, three times a day, associated with cramps in the lower part of the abdomen; the patient had also lost six kilos of weight. Two months before admission, he started having nocturnal diarrhea. On admission, laboratory findings were as follows: decrease in total proteins 28 g/L, albumins 14 g/L, globulins 14 g/L, serum calcium 1.26 mmol/L, magnesium 0.46 mmol/L, immunoglobulins (IgG < 3.8 g/L, IgM 0.47 g/L, IgA 0.47 g/L), and fibrinogen 1.6 g/L. Although leukocytes were within the referral range, a diagnosis of lymphocytopenia was made; lymphocytes comprised 13% of the white blood cell count. Direct stool examination was negative for occult blood and culture showed no pathogens. Secondary hyperparathyroidism and subsequent osteoporosis were diagnosed. Immunological and thyroid disorders, renal and hepatic impairment, tuberculosis, and human immunodeficiency virus were excluded. Additional tests were made to exclude possible secondary intestinal lymphangiectasia. Levels of serum chromogranin A and 5-Hydroxyindoleacetic acid in 24-h urine were within the referral range. Multislice computed tomography of the chest and abdomen showed no signs of lymphoma, lymphadenopathy or tumors, and an abdominal Doppler ultrasound showed no signs of portal hypertension or compromised mesenteric venous circulation. An X-ray examination of the small intestine revealed thickened folds, as well as a dilated lumen of the jejunum and ileum (Figure 1). Upper endoscopy showed mucosal changes characteristic of chronic gastritis (H. pylori negative) and duodenitis; colonoscopy demonstrated terminal ileitis. Histological examinations of biopsied mucosa excluded Whipple’s and celiac disease. Double balloon enteroscopy was highly suggestive of intestinal lymphangiectasia, which demonstrated white spots overlying edematous and hyperemic duodenal and jejunal mucosa (Figure 2). Histology of the intestinal biopsy specimens demonstrated dilated lymphatic vessels in the tips of whitish jejunal villi (Figure 3). Scintigraphy with Technetium 99 m labeled human serum albumin showed extravasation of radiopharmaceutical throughout small intestine (Figure 4). After an extensive management and exclusion of secondary intestinal lymphangiectasia, a diagnosis of PIL was suspected. A low-fat diet supplemented with medium-chain triglyceride (MCT), human albumin transfusion, vitamins, and electrolyte supplements were introduced, but neither clinical nor laboratory improvement was observed. Although there was no concomitant autoimmune disease established in this patient, we prescribed corticosteroid therapy for two months, without any improvement. Finally, octreotide (Sandostatin) 200 mcg subcutaneously twice daily for two weeks resulted in clinical and laboratory improvement. The patient later recovered, showing no signs of malabsorption and was discharged from the hospital with dietary modifications as a life-long therapy. Two months later, the patient relapsed with more severe signs of malabsorption and catabolic syndrome [creatine kinase 5818 U/L, aspartate aminotransferase 274 U/L, alanine aminotransferase 108 U/L, lactate dehydrogenase (LDH) 2109 U/L, total proteins 29 g/L, albumins 16 g/L, serum calcium 1.17 mmol/L, ionized calcium 0.83 mmol/L, magnesium 0.38 mmol/L]; the patient also showed signs of minor pleural effusion and ascites. We decided to treat the patient with the same therapy regime (octreotide a 200 mcg sc, twice a day), together with infusions of human albumins. Although the aforementioned laboratory results and endoscopic findings improved, the patient still had significant hypoproteinemia persistent at discharge. Considering the patient’s clinical course, we decided to continue with a slow-release formula of octreotide (Sandostatin LAR® 20 mg) once every 4 wk, in the outpatient clinic. Surgical resection was not an option because of the extent of the disease.
Figure 1.
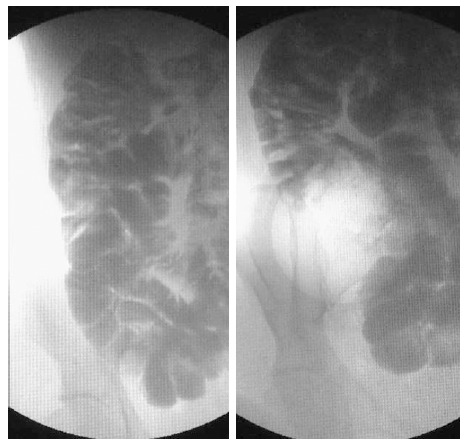
X-ray examination of the small intestine. X-ray examination of the small intestine revealing thickened folds as well as a dilated lumen of the jejunum and ileum.
Figure 2.
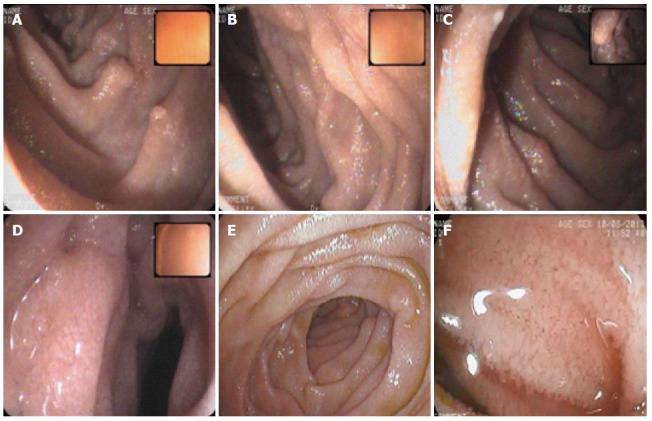
Double balloon enteroscopy. Double balloon enteroscopy demonstrating edematous distal duodenum and jejunal mucosa scattered with white spots. A-C: duodenum; D-F: Jejunum.
Figure 3.
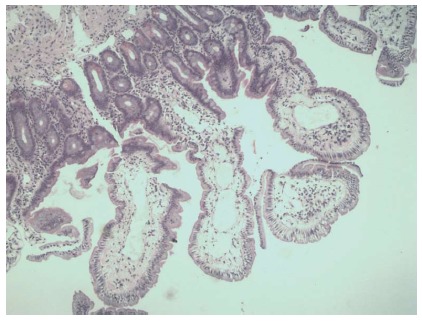
Histopathological findings. Histopathological findings: dilated lymphatic vessels in the tips of whitish jejunal villi (hematoxylin-eosin stain, magnification × 40).
Figure 4.
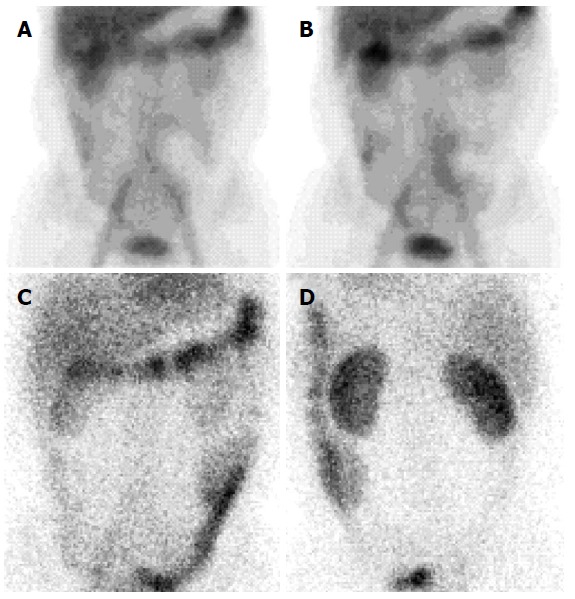
Technetium 99m human serum albumin scintigraphy. Scintigraphy showing Technetium 99m labeled human serum albumin extravasation throughout small intestine after 6 h of injection (A and B) and presence in the large intestine after 24 h of injection (C and D).
DISCUSSION
PIL is a rare condition, which results in malabsorption and can be fatal. Although it occurs predominantly in children, this patient experienced his first symptoms as an adult. The most frequent clinical manifestations of PIL are intermittent diarrhea and peripheral edema; there may also occasionally be steatorrhea, accompanied by fat-soluble vitamin deficiencies, and pleural or pericardial effusions. We report a patient who presented with a grand mal seizer as a consequence of a malabsorption-derived electrolyte imbalance, predominantly hypocalcemia; to the best of our knowledge, there has been only one other similar published case[3].
Although several therapeutic management strategies for PIL have been proposed, no standardized algorithms exist.
A high-protein, low-fat diet supplemented with medium chain triglycerides (MCT) has been proven to be the most effective and widely prescribed treatment, with minimal side effects. The benefit of this treatment is accomplished through several mechanisms: the exclusion of long-chain fatty acids prevents congestion and rupture of dilated lymphatics; and MCTs directly absorb into the portal venous circulation, which avoids lymphatic vessel overload[4]. Desai et al[5] showed that this type of diet not only improves symptoms of the disease, but also reduces mortality. Based on the published study results, it was suggested that diet supplemented with MCTs should be continued as a lifelong therapy, regardless of the inadequate patient response. We also tried dietary modification, combined with albumin infusions, calcium salts, and water soluble forms of fat soluble vitamins, without any improvement. The reason for the introduction of the concomitant albumin infusion was the previously proven short transit effects in improving complications of hypoproteinemia, which were present in this case.
In case of poor response to the above treatment, partial or total parenteral nutrition (TPN) should be considered. In addition, gamma globulin infusions should be considered in the presence of recurrent infections, preceded by low serum IgG. We did not apply either therapy, as Aoyagi et al[6] have shown that enteral nutrition is an effective substitute for TPN in patients with PIL. Gamma globulin therapy was not used because there was no obvious infection.
Instead, we started with corticosteroid therapy, even though there was no confirmed underlying inflammatory or autoimmune disease. The efficacy of corticosteroids for PIL remains unclear. In our case, they had no effect; therefore, we tapered and discontinued therapy after two months.
Other treatment modalities described in the literature, with variable efficacy, include antiplasmin therapy, octreotide, small bowel resection, peritoneovenous (Levine) shunt, and intestinal transplant.
The antiplasmin therapy affects fibrinolysis locally and improves lymphatic permeability for proteins[7]. Previous studies have confirmed its use (trans-4-aminothylcyclohexamine carboxylic acid (tranexamic acid, Cyklokapron, Transamin, Cyclo-F and Femstrual)) in partially improving serum albumin, immunoglobulins, endoscopically observed duodenal lesions, and gastrointestinal bleeding. Our patient did not have signs of gastrointestinal bleeding; therefore, we did not consider using antiplasmin therapy.
Surgery is reserved for palliation of large ascites or resection of segmental and localized intestinal lymphangiectasia[8,9].
Octreotide, a somatostatin analog, is a treatment of choice in patients in which treatment with MCT has failed; however, it is expensive and requires parenteral administration, which usually decreases patients’ compliance. The proposed mechanism of action of octreotide in PIL includes the decreased intestinal absorption of fats, inhibition of gastrointestinal vasoactive peptides, and stimulation of the autonomic nervous system[10]. Long-term therapy with octreotide showed good results in PIL by improving histopathological and endoscopic findings, decreasing stool frequency, and maintaining serum albumin at normal levels, thus reducing requirements for albumin infusions. Among the severe adverse side effects, only acute pancreatitis has been reported[11]. In published case reports, octreotide at dosage of 150-200 mcg subcutaneously two times daily showed good results; however, there is no standardized recommended dose or duration of therapy[10,12-14]. Therapy with octreotide 200 mcg subcutaneously two times daily in this case led to the resolution of almost all symptoms. The discontinuation of therapy in the majority of cases resulted in recurrence of the disease; restarting the therapy resulted in remission[15]. Although regular administration is desirable, depending on symptoms the short-term use of octreotide may be considered. The slow release formation of octreotide (Sandostatin LAR® 20 mg) has been shown to be effective as a regular form, but the high cost is an obstacle for its long-term use. To confirm whether octreotide is an adequate and effective long-term therapy in this patient, a longer follow-up period is needed. Unfortunately, cost considerations meant that we cannot confirm for how long we will be able to provide this therapy. The patient continues to show signs of significant hypoalbuminemia.
This case report demonstrated that PIL is a serious chronic disease. The frequent recurrence has had a significant impact on the patient’s quality of life. More case reports and/or studies on similar cases are needed to determine the best possible treatment strategies for this rare disorder.
COMMENTS
Case characteristics
A 42-year-old male with a history of chronic diarrhea and peripheral edema presented with a grand mal seizure caused by malabsorption-derived electrolytes and a protein disorder.
Clinical diagnosis
Hyperemic and edematous mucosa of small intestine corresponded to scattered white spots with dilated intestinal lymphatics and whitish villi in the histological specimen of the biopsied jejunal mucosa.
Differential diagnosis
Mediastinal and abdominal lymphoma as well as sarcoma, intestinal infection, inflammatory bowel disease, neuroendocrine tumors, portal hypertension, compromised mesenteric venous circulation, immunological and thyroid disorders, renal and hepatic impairment, tuberculosis, and human immunodeficiency virus.
Laboratory diagnosis
Decreased total proteins 28 g/L; albumins 14 g/L; globulins 14 g/L; serum calcium 1.26 mmol/L; magnesium 0.46 mmol/L; immunoglobulins (IgG < 3.8 g/L, IgM 0.47 g/L, IgA 0.47 g/L); fibrinogen 1.6 g/L; lymphocyte 13%; secondary hyperparathyroidism; CBC was within referral range, direct stool examination was negative for occult blood and culture showed no pathogens.
Imaging diagnosis
An X-ray examination of the small intestine revealed thickened folds as well as a dilated lumen of the jejunum and ileum. Double balloon enteroscopy showed white spots overlying edematous as well as hyperemic duodenal and jejunal mucosa. Scintigraphy with Technetium 99 m labeled human serum albumin showed extravasation of radiopharmaceutical throughout small intestine.
Pathological diagnosis
Histology of the intestinal biopsy specimens demonstrated dilated lymphatic vessels in the tips of whitish jejunal villia and diagnosis of primary intestinal lymphangiectasia (PIL) was established.
Treatment
A low-fat diet supplemented with medium-chain triglyceride, human albumin transfusion, vitamins, electrolyte supplements, corticosteroids and octerotide (Sandostatin 200 mcg subcutaneously twice a day and later Sandostatin LAR® 20 mg once every 4 wk) were applied.
Related reports
PIL is rare chronic clinical entity without exact recommendations on dosage and duration of therapy; therefore, we presented a case report based on our experience in PIL treatment. We also present both past and present challenges and concerns regarding this rare disease.
Experiences and lessons
This case report demonstrated that PIL is a serious chronic disease characterized by challenging treatment strategies and only octreotide therapy was proved to be an effective means of therapy resolution in this patient.
Peer-review
Although treatment strategies are the focus of this case report, it is also highly important to exclude secondary intestinal lymphangiectasia and take into account numerous diseases and disorders that are mentioned in the text.
Footnotes
Ethics approval: This case report was reviewed and approved by the Clinical Hospital “Sveti Duh” Ethics Committee.
Informed consent: The patient signed the informed consent form approved by the local Ethics Committee.
Conflict-of-interest: No potential conflicts of interest relevant to this article were reported.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: January 10, 2015
First decision: February 10, 2015
Article in press: March 31, 2015
P- Reviewer: Wittmann T S- Editor: Qi Y L- Editor: Stewart G E- Editor: Liu XM
References
- 1.Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS. The role of the gastrointestinal system in “idiopathic hypoproteinemia”. Gastroenterology. 1961;41:197–207. [PubMed] [Google Scholar]
- 2.Lee KH, Chung JK, Lee DS, Lee MC, Song IS, Koh CS. Intestinal leakage of technetium-99m-MDP in primary intestinal lymphangiectasia. J Nucl Med. 1996;37:639–641. [PubMed] [Google Scholar]
- 3.Katou N, Tsukada H, Ohno T, Hayashi R, Tomono S, Suzuki T, Murata K, Imanari T, Kogure S, Tsuchiya Y. [Primary intestinal lymphangiectasia with epileptic episodes as the initial symptom of the disease] Nihon Naika Gakkai Zasshi. 1988;77:1037–1040. doi: 10.2169/naika.77.1037. [DOI] [PubMed] [Google Scholar]
- 4.Suresh N, Ganesh R, Sankar J, Sathiyasekaran M. Primary intestinal lymphangiectasia. Indian Pediatr. 2009;46:903–906. [PubMed] [Google Scholar]
- 5.Desai AP, Guvenc BH, Carachi R. Evidence for medium chain triglycerides in the treatment of primary intestinal lymphangiectasia. Eur J Pediatr Surg. 2009;19:241–245. doi: 10.1055/s-0029-1216389. [DOI] [PubMed] [Google Scholar]
- 6.Aoyagi K, Iida M, Matsumoto T, Sakisaka S. Enteral nutrition as a primary therapy for intestinal lymphangiectasia: value of elemental diet and polymeric diet compared with total parenteral nutrition. Dig Dis Sci. 2005;50:1467–1470. doi: 10.1007/s10620-005-2863-7. [DOI] [PubMed] [Google Scholar]
- 7.MacLean JE, Cohen E, Weinstein M. Primary intestinal and thoracic lymphangiectasia: a response to antiplasmin therapy. Pediatrics. 2002;109:1177–1180. doi: 10.1542/peds.109.6.1177. [DOI] [PubMed] [Google Scholar]
- 8.Kneist W, Drescher DG, Hansen T, Kreitner KF, Lang H. [Surgical therapy of segmental jejunal, primary intestinal lymphangiectasia] Z Gastroenterol. 2013;51:576–579. doi: 10.1055/s-0031-1273473. [DOI] [PubMed] [Google Scholar]
- 9.Kim NR, Lee SK, Suh YL. Primary intestinal lymphangiectasia successfully treated by segmental resections of small bowel. J Pediatr Surg. 2009;44:e13–e17. doi: 10.1016/j.jpedsurg.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 10.Suehiro K, Morikage N, Murakami M, Yamashita O, Hamano K. Late-onset primary intestinal lymphangiectasia successfully managed with octreotide: a case report. Ann Vasc Dis. 2012;5:96–99. doi: 10.3400/avd.cr.11.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sari S, Baris Z, Dalgic B. Primary intestinal lymphangiectasia in children: is octreotide an effective and safe option in the treatment? J Pediatr Gastroenterol Nutr. 2010;51:454–457. doi: 10.1097/MPG.0b013e3181d1b162. [DOI] [PubMed] [Google Scholar]
- 12.Lee HL, Han DS, Kim JB, Jeon YC, Sohn JH, Hahm JS. Successful treatment of protein-losing enteropathy induced by intestinal lymphangiectasia in a liver cirrhosis patient with octreotide: a case report. J Korean Med Sci. 2004;19:466–469. doi: 10.3346/jkms.2004.19.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ballinger AB, Farthing MJ. Octreotide in the treatment of intestinal lymphangiectasia. Eur J Gastroenterol Hepatol. 1998;10:699–702. [PubMed] [Google Scholar]
- 14.Al Sinani S, Rawahi YA, Abdoon H. Octreotide in Hennekam syndrome-associated intestinal lymphangiectasia. World J Gastroenterol. 2012;18:6333–6337. doi: 10.3748/wjg.v18.i43.6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroiwa G, Takayama T, Sato Y, Takahashi Y, Fujita T, Nobuoka A, Kukitsu T, Kato J, Sakamaki S, Niitsu Y. Primary intestinal lymphangiectasia successfully treated with octreotide. J Gastroenterol. 2001;36:129–132. doi: 10.1007/s005350170142. [DOI] [PubMed] [Google Scholar]


