Abstract
Primary liver carcinosarcoma is rare. Here we report an unusual case of liver carcinosarcoma containing combined hepatocellular cholangiocarcinoma. A mass in the right liver lobe of a 45-year-old man was accidentally discovered by ultrasonic inspection and computed tomography (CT) scan. Surgical resection was performed following a diagnosis of primary liver cancer. Micropathologically, both carcinomatous and sarcomatous elements were present, and diagnosis of liver carcinosarcoma was confirmed. The carcinomatous element consisted of hepatocellular carcinoma and foci of cholangiocellular carcinoma. The sarcomatous element was composed of spindle cells and bizarre cells, as well as foci of osteosarcoma and chondrosarcoma. Hepatocellular carcinoma cells diffusely expressed both hepatocyte specific markers cytokeratin (CK) 8/18 and cholangiocyte specific markers CK19, and sarcoma cells were positive for vimentin. Interestingly, both carcinomatous and sarcomatous cells expressed epithelial membrane antigen. CD117-positive ductular reactions and small undifferentiated cells were observed. A liver progenitor cell origin of the liver carcinosarcoma was proposed.
Keywords: Carcinosarcoma, Cholangiocellular carcinoma, Hepatocellular carcinoma, Liver neoplasm, Stem cells
Core tip: Primary mixed-type hepatocellular-cholangiocellular sarcoma is extremely rare. The histogenetic origin of this tumor is controversial. We report a case with special characteristics and propose a new hypothesis combining stem cell theory and “conversion” theory, which may be more appropriately applied to this case.
INTRODUCTION
Primary liver carcinosarcoma is a very rare liver malignant tumor. According to the World Health Organization definition, liver carcinosarcoma should be composed of both carcinomatous and sarcomatous elements. Carcinomatous elements may be hepatocellular or cholangiocellular components or both[1]. The incidence of liver carcinosarcoma with carcinomatous elements containing both hepatocellular and cholangiocellular is extremely rare, and only three cases have been reported to date in the English literature (Table 1)[1-3]. Here we report a case of liver carcinosarcoma containing combined hepatocellular cholangiocarcinoma.
Table 1.
Summary of reported cases of liver carcinosarcoma with combined hepatocellular cholangiocarcinoma
| Ref. | Age (yr) | Sex | Positive tumor marker | HBV | HCV | Cirrhosis | Size (cm) | Sarcoma element | Treatment | Metastasis | Outcome |
| Nakajima et al[2] | 74 | M | - | - | NA | Precirrhotic fibrosis | 15 × 13 | Pleomorphic cells, spindle shaped cells, and osteoplastic cells | Mitomycin C | Liver, lung, lymph nodes, stomach, peritoneum | Died within 5 mo |
| Papotti et al[3] | 59 | M | - | - | - | - | 4.0 × 5.2 | Spindle cells | Right hepatectomy | PVTT | Died within 4 mo |
| Goto et al[1] | 73 | M | AFP | + | NA | + | 3 × 2.5 × 2 | Chondrosarcoma, osteosarcoma | Segmentectomy | - | 19 mo, alive |
| CEA | |||||||||||
| CA19-9 | |||||||||||
| DCP | |||||||||||
| Present case | 45 | M | AFP | + | - | + | 7 × 5 | Chondrosarcoma, osteosarcoma, spindle cells, and bizarre cells | Segmentectomy | Diaphragm | 7 mo, alive |
| CA 12-5 |
AFP: Alfa-fetoprotein; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; DCP: Des-γ-carboxy prothrombin; HBV: Hepatitis B virus; HCV: Hepatitis C virus; NA: Not available; PVTT: Portal vein tumor thrombus.
CASE REPORT
A mass was accidentally discovered by ultrasonic inspection in the right liver lobe of a 45-year-old Chinese man with a ten-year history of hepatitis B virus infection during a routine follow-up examination in a local hospital. The patient was transferred to our hospital. He had experienced mild, right upper abdominal pain for more than two months, but did not pay special attention to it. No positive signs were present upon physical examination. Abdominal ultrasonography showed a well-defined hypoechoic mass in liver segments V and VIII. The mass was 5 cm × 7 cm in size, and blood flow signals were present on the edge. An enhanced computed tomography (CT) scan of the tumor revealed heterogeneous enhancement during the arterial phase with delayed washout in the portal and delayed phases (Figure 1). The tumor was growing exophytic ally, and there was no imaging evidence of tumor metastasis. The main findings of laboratory examinations were: normal liver function, elevated serum alpha-fetoprotein level of 24.55 ng/mL (normal range: < 8.78 ng/mL) and carbohydrate antigen 12-5 level of 141 U/mL (normal range: 1-35 U/mL), and hepatitis B virus DNA copies of 7.42 × 104. Both carbohydrate antigen 19-9 and carcinoembryonic antigen levels were within normal ranges. Under a diagnosis of primary liver cancer, segmentectomy of liver segments V and VIII was performed. A 0.5-cm white nodule on the peritoneum of the diaphragm was suspected to be a metastasis during the operation and thus was resected.
Figure 1.
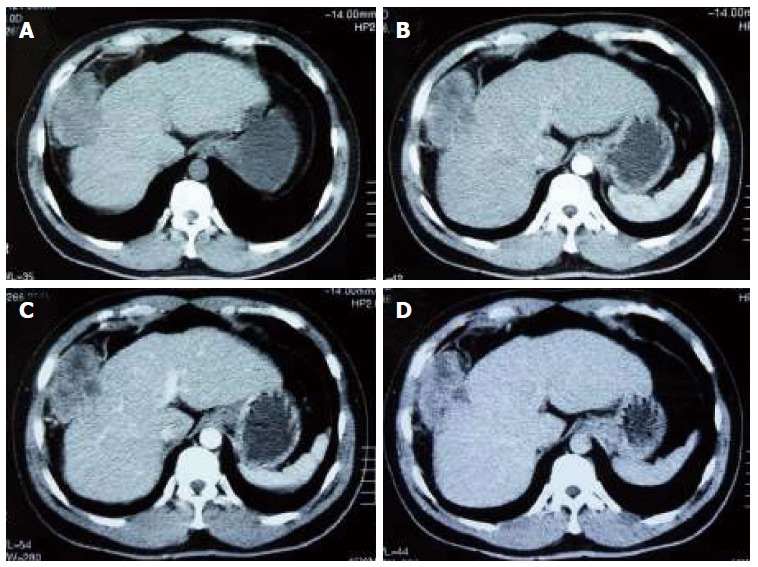
Contrast-enhanced computed tomography showing a 5 cm × 7 cm mass in segments V-VIII of the liver. A: Baseline scan; B: Arterial phase; C: Portal venous phase; D: Delayed phase.
Macropathology of the tumor showed that it measured 8 cm in the largest dimension. The exophytic portion of the tumor was 8 cm × 4 cm in size, and the cut surface was grayish-yellow and grayish-red with several firmer areas. The intrahepatic portion of the tumor was 5 cm × 3 cm, and the cut surface was grayish-white. The tumor was not surrounded by a fibrous capsule. The diameter of the junction between the two portions was about 2 cm.
Micropathologically, the tumor-adjacent liver parenchyma showed obvious portal cirrhosis (Figure 2A). The intrahepatic portion of the tumor was comprised mainly of a carcinomatous element, consisting of hepatocellular carcinoma (> 90%) and foci of cholangiocellular carcinoma (Figure 2C). Each of the two types of carcinoma showed various differentiation degrees, and there was no clear boundary between the two elements. The exophytic portion of the tumor mainly contained a sarcomatous element. At the boundary area between the carcinomatous and sarcomatous components, there was a transitional zone with intermingled cells of the two elements (Figure 2B). The sarcomatous element was composed of spindle cells and bizarre cells, as well as foci of osteosarcoma and chondrosarcoma (Figure 2D). A metastasis nodule was composed of undifferentiated sarcoma cells with deeply stained nuclei (Figure 2E and F).
Figure 2.
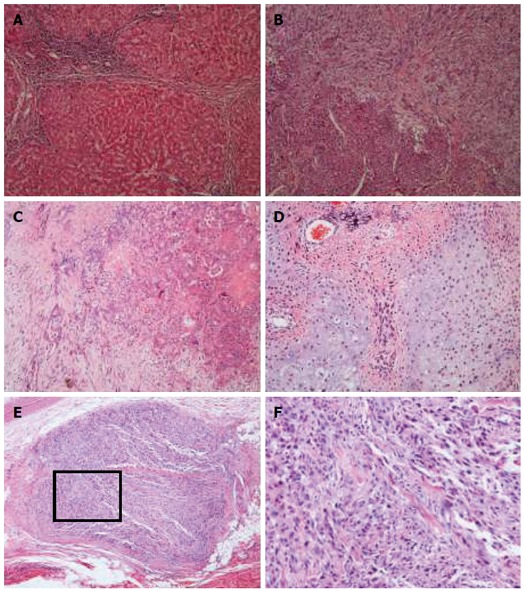
Microscopic appearance of the specimen (hematoxylin and eosin staining). A: Tumor-adjacent liver parenchyma showed portal cirrhosis (magnificaton × 100); B: A transitional zone could be defined between the carcinomatous (lower left) and sarcomatous components (upper right). There were intermingled cells of the two elements (magnificaton × 100); C: Foci of cholangiocellular carcinoma with various grades of differentiation (magnificaton × 100); D: Neoplastic osteoid and chondroid formation was observed in the sarcoma element (magnificaton × 100); E: Metastasis nodule was composed of undifferentiated sarcoma cells with deeply stained nuclei (magnificaton × 40); F: Higher-magnification view of the squared part in panel E.
The results of immunohistochemical staining are summarized in Table 2. A hepatocellular carcinoma (HCC) component was positive for various epithelial markers. Being strongly positive for hepatocyte-specific markers such as cytokeratin (CK)8/18 and hepatocyte, the HCC component diffusely expressed cholangiocyte-specific markers, such as Pan-CK and CK19 (Figure 3A and B). The CK19 immunostaining signal in HCC was not uniform in either intensity or staining pattern. Some CK19-positive HCC cells showed an intermediate hepatobiliary cell-like pattern (Figure 3B), similar to the report by Durnez et al[4]. Carcinomatous elements were strongly positive for epithelial membrane antigen (EMA) (Figure 3C). Interestingly, sarcomatous elements, such as spindle cells and chondrosarcoma cells, were also positive for EMA (Figure 3D). Small foci of HCC cells were weekly positive for CD117, and showed small ductular reactions adjacent to the HCC. The immunostaining signals for CD117 were strong in some of the nearby small, undifferentiated cells (Figure 3E). Sarcoma cells were positive for CD117 (Figure 3F) and diffusely positive for vimentin and smooth muscle actin (Figure 3G and H).
Table 2.
Results of immunohistochemistry
| Marker | Carcinomatous component | Sarcomatous component |
| EMA | ++ | + |
| CK19 | ++ | - |
| CK8/18 | ++ | - |
| Hepatocyte | ++ | - |
| Pan-CK | ++ | - |
| Galectin-3 | + | + |
| CD117 | + | + |
| VIM | - | ++ |
| SMA | - | ++ |
| S-100 | - | + |
| DES | - | - |
| Ki-67 | + | ++ |
-: Negative; +: < 50% cells are positive or mildly immunostained; ++: Diffusely positive or strongly immunostained. CK: Cytokeratin; EMA: Epithelial membrane antigen; SMA: Smooth muscle actin; VIM: Vimentin; DES: Desmin.
Figure 3.
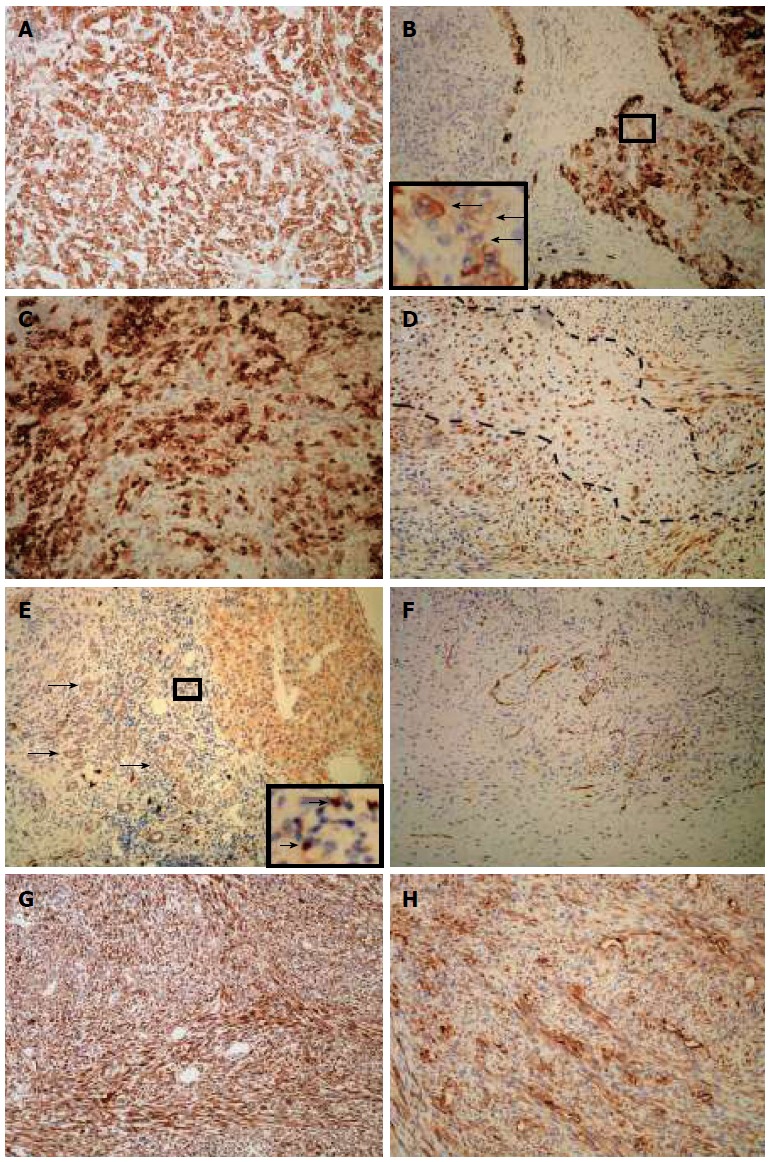
Immunohistochemical expression of the carcinosarcoma. A: Pan-cytokeratin staining showed diffuse and strong signals for HCC cells; B: HCC elements showed various staining pattern for cytokeratin 19 with intermediate hepatobiliary cell-like tumor cells (arrows); C: Carcinomatous elements were strongly positive for epithelial membrane antigen; D: Spindle cells and chondrosarcoma cells (area fenced by dotted line) were positive for epithelial membrane antigen; E: Carcinoma cells were positive for CD117, as were ductular reactions (arrows) and undifferentiated cells (arrows, inset); F: Sarcoma cells were positive for CD117; G: Sarcomatous elements strongly stained for vimentin; H: Diffuse staining for smooth muscle actin in sarcoma cells; All magnifications are × 100.
The tumor recurred on the peritoneum two months after the operation, and the patient received chemotherapy for four courses. The patient was alive at the last follow-up on November 9, 2014.
DISCUSSION
Primary liver carcinosarcoma is very rare. No more than 56 cases have been reported in the English literature[5-12]. Nearly half of the cases were reported after 2010, and the largest series report of 14 cases was by Wang et al[9] in 2012. Liver carcinosarcoma exhibits no specific clinical presentation. In the early stage, the tumor develops no obvious symptoms and usually is found during routine health examinations[2,10,11,13]. In the middle and advanced stages, symptoms are similar to HCC. Upon imaging, liver carcinosarcoma presents no specific features, and cannot be precisely diagnosed preoperatively. Liu et al[5] reported the characterization of a case of liver carcinosarcoma by contrast-enhanced ultrasonography. Contrast-enhanced ultrasonography is more informative for diagnosis than conventional ultrasonography, but is not much more valuable than CT. Although preoperative percutaneous transhepatic biopsy can be done and provide pathologic findings, diagnosis is usually uncertain or inaccurate because of insufficient tissue or ample necrotic tissues[14,15]. Diagnosis of primary liver carcinosarcoma is usually established through extensive sampling from surgical specimens or autopsy, and should be differentiated from liver sarcomatoid carcinoma via immunohistochemical studies. In our case, foci of sarcomatous elements were typically differentiated, such as chondroid and osteoid components, and the spindle cells diffusely expressed mesenchymal markers such as smooth muscle actin and vimentin. Thus, this case of neoplasm met the criteria for primary liver carcinosarcoma.
The present case has several characteristics. First, the tumor growth was exophytic, and the exophytic portion was mainly composed of a sarcomatous element, while the intrahepatic portion consisted of a carcinomatous element with transitional zones between the two elements. These findings suggest that the sarcomatous element might have transformed from the pre-existing combined carcinoma, and grew more rapidly. Second, the carcinomatous element was a combined hepatocellular cholangiocarcinoma, and HCC occupied the majority of the tumor. Third, the sarcomatous element consisted of various types of mesenchymal cells, including chondroid and osteoid cells.
The histogenetic origin of liver carcinosarcoma is still controversial. One theory is that both carcinomatous and sarcomatous neoplasms arise from differentiated multipotent stem cells[7,8], and the other is that the sarcomatous element is transformed or dedifferentiated from pre-existing carcinoma based on the observation of a transitional zone[13,14,16,17]. Goto et al[2] reported a case with c-kit-positive tumor cells and the presence of transitional zones, advocating the possibilities of the two hypotheses. We propose a hypothesis combining these two theories, which may be more appropriately applied to our case of liver carcinosarcoma. Liver progenitor cells malignantly transformed to cancer stem cells and developed into liver cancer. Microenvironmental change and genetic mutation might promote epithelial-mesenchymal transition (EMT) of these cancer stem cells or their progeny, and result in formation of the sarcomatous elements.
In the cancer stem cell theory, cancer stem cells are thought to be the “root” of a tumor, and they possess the ability of indefinite self-renewal that drives tumorigenesis[18]. Liver progenitor cells expressing both hepatocellular and cholangiocellular lineage markers can be activated and mediate liver regeneration when mature hepatocytes are suppressed in the diseased liver[19]. They also exist in more than half of the foci of small-cell dysplasia, which are considered to be genuine preneoplastic lesions[20]. Meanwhile, human HCCs can express liver progenitor markers such as CK7 and CK19[19], and these cancers tend to be poorly differentiated[21]. Liver progenitor cells might transform into cancer stem cells that promote liver cancer development. Our previous study showed that combined hepatocellular cholangiocarcinoma originates from liver progenitor cells[22]. In our case, the carcinomatous element consisted of both HCC and cholangiocellular carcinoma, and a large number of HCC cells expressed CK19, suggesting liver progenitor cell origin of this cancer.
Our previous study found that LE/6 cells could form mesenchymal tumor tissue through EMT after subcutaneous transplantation, suggesting that liver progenitor cells are not determined stem cells, which have limited differentiation potential[23]. In another study, we observed that intrahepatic transplantation of HBx-transfected oval cells could generate tumors with combined histologic features of HCC and mesenchymal tumors[24]. These results suggest that liver progenitor cells could be the cell origin of liver tumors containing both carcinomatous and sarcomatous elements. CD117 is one of the liver progenitor cell markers[25]. In the present case, HCC cells and sarcoma cells were immunostained with CD117. More interestingly, small ductular reactions that represent liver progenitor cells in diseased liver could also be clearly identified as positive for CD117. These findings further suggest that the present case was generated from liver progenitor cells. Recently, geographic mutational analysis indicated the origin of a carcinosarcoma from a common progenitor cell that underwent divergent clonal evolution[10,26].
Many authors favoring the theory of transformation of carcinoma into mesenchymal elements show evidence of transitional zones between carcinoma and sarcoma[13,14,16,17]. In the present case, not only are there transitional zones, but also the sarcoma mainly occupied the exophytic portion of the tumor. Thus, the “transformation” theory could be employed in this case. The idea of epithelial origin also is also based on sarcoma cells positive for epithelial markers[1]. Similarly, besides diffusely expressing mesenchymal markers, most sarcoma cells in the present case also expressed EMA, suggesting an obvious bi-directionally differentiated feature of the sarcoma (epithelial and mesenchymal), which could have originated from transformed epithelial cells.
In summary, we report an unusual case of liver carcinosarcoma with combined hepatocellular cholangiocarcinoma and many differentiated heterologous sarcomatous elements, and propose that liver progenitor cells could be the cell origin of the liver carcinosarcoma, while the sarcomatous element is generated from aberrant liver progenitor cells that undergo EMT.
COMMENTS
Case characteristics
Case of primary liver carcinosarcoma combined with hepatocelluar cholangiocarcinoma.
Clinical diagnosis
A mass was found in the right liver lobe by ultrasonic inspection, and the patient had a history of ten-year hepatitis B virus hepatitis. Primary liver cancer was diagnosed.
Differential diagnosis
Primary liver cancer should be differentially diagnosed from liver sarcomatoid carcinoma.
Laboratory diagnosis
Laboratory examination showed an elevated serum alpha-fetoprotein level of 24.55 ng/mL and carbohydrate antigen 12-5 level of 141 U/mL, and hepatitis B virus DNA copies of 7.42 × 104.
Imaging diagnosis
Abdominal ultrasonography showed a well-defined hypoechoic mass in liver segments V and VIII, and enhanced CT of the tumor revealed heterogeneous enhancement during the arterial phase with delayed washout in the portal and delayed phases.
Pathological diagnosis
Liver carcinosarcoma was diagnosed based on the presence of both carcinomatous and sarcomatous elements in the tumor.
Treatment
Surgical resection was performed.
Related reports
No more than 56 cases of liver carcinosarcoma have been reported in the English literature, and only three cases were reported to contain both hepatocellular and cholangiocellular carcinoma.
Experiences and lessons
The authors reported an unusual case of liver carcinosarcoma with combined hepatocellular cholangiocarcinoma and many differentiated heterologous sarcomatous elements, and propose that liver progenitor cells could be the cell origin of the liver carcinosarcoma, while the sarcomatous element is generated from aberrant liver progenitor cells that undergo EMT.
Peer-review
The authors present a nice, unusual case of a primary carcinosarcoma-a mixed type hepatocellular-cholangiocellular sarcoma.
Footnotes
Supported by Grants from the Hepatic Surgery Clinical Study Center of Hubei Province, China, No. 2014BKB089.
Ethics approval: The study was reviewed and approved by the Institutional Review Board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
Informed consent: The patient and his wife provided informed written consent prior to study enrollment.
Conflict-of-interest: The authors declare no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 15, 2014
First decision: January 22, 2015
Article in press: April 3, 2015
P- Reviewer: Desiderio J, Kollmar O S- Editor: Yu J L- Editor: AmEditor E- Editor: Wang CH
References
- 1.Nakajima T, Kubosawa H, Kondo Y, Konno A, Iwama S. Combined hepatocellular-cholangiocarcinoma with variable sarcomatous transformation. Am J Clin Pathol. 1988;90:309–312. doi: 10.1093/ajcp/90.3.309. [DOI] [PubMed] [Google Scholar]
- 2.Goto H, Tanaka A, Kondo F, Takeshita K, Nagashima I, Hanawa N, Aiso M, Takamori Y, Kato K, Takahashi Y, et al. Carcinosarcoma of the liver. Intern Med. 2010;49:2577–2582. doi: 10.2169/internalmedicine.49.3581. [DOI] [PubMed] [Google Scholar]
- 3.Papotti M, Sambataro D, Marchesa P, Negro F. A combined hepatocellular/cholangiocellular carcinoma with sarcomatoid features. Liver. 1997;17:47–52. doi: 10.1111/j.1600-0676.1997.tb00778.x. [DOI] [PubMed] [Google Scholar]
- 4.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu LP, Yu XL, Liang P, Dong BW. Characterization of primary hepatic carcinosarcoma by contrast-enhanced ultrasonography: a case report. World J Gastroenterol. 2014;20:1630–1634. doi: 10.3748/wjg.v20.i6.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdulrezzak U, Kula M, Erdoğan Z, Tutuş A. Imaging of primary liver carcinosarcoma scintigraphically; a case report. Mol Imaging Radionucl Ther. 2014;23:31–34. doi: 10.4274/Mirt.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto T, Kurashima Y, Ohata K, Hashiba R, Tanaka S, Uenishi T, Ohno K, Ikebe T, Miyaji K, Fukumoto N. Carcinosarcoma of the liver: report of a case. Surg Today. 2014;44:1161–1170. doi: 10.1007/s00595-013-0612-7. [DOI] [PubMed] [Google Scholar]
- 8.Lin YS, Wang TY, Lin JC, Wang HY, Chou KF, Shih SC, Chen MJ. Hepatic carcinosarcoma: clinicopathologic features and a review of the literature. Ann Hepatol. 2013;12:495–500. [PubMed] [Google Scholar]
- 9.Wang QB, Cui BK, Weng JM, Wu QL, Qiu JL, Lin XJ. Clinicopathological characteristics and outcome of primary sarcomatoid carcinoma and carcinosarcoma of the liver. J Gastrointest Surg. 2012;16:1715–1726. doi: 10.1007/s11605-012-1946-y. [DOI] [PubMed] [Google Scholar]
- 10.Schaefer IM, Schweyer S, Kuhlgatz J. Chromosomal imbalances in primary hepatic carcinosarcoma. Hum Pathol. 2012;43:1328–1333. doi: 10.1016/j.humpath.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Lai Q, Levi Sandri GB, Melandro F, Di Laudo M, Garofalo M, Guglielmo N, Grieco M, Di Tondo U, Rossi M, Berloco PB. An unusual case of hepatic carcinosarcoma. G Chir. 2011;32:372–373. [PubMed] [Google Scholar]
- 12.Aparicio MA, Esteban C, Bengoechea O, Muñoz-Bellvís L. Primary carcinosarcoma of the liver: an unusual case with clearly separated epithelial and mesenchymal components. Rev Esp Enferm Dig. 2011;103:336–338. doi: 10.4321/s1130-01082011000600014. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto Y, Ojima H, Shimada K, Onaya H, Hiraoka N, Mizuguchi Y, Kosuge T, Kanai Y. Long-term recurrence-free survival in a patient with primary hepatic carcinosarcoma: case report with a literature review. Jpn J Clin Oncol. 2010;40:166–173. doi: 10.1093/jjco/hyp123. [DOI] [PubMed] [Google Scholar]
- 14.Sumiyoshi S, Kikuyama M, Matsubayashi Y, Kageyama F, Ide Y, Kobayashi Y, Nakamura H. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809–812. doi: 10.3748/wjg.v13.i5.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aita K, Seki K. Carcinosarcoma of the liver producing granulocyte-colony stimulating factor. Pathol Int. 2006;56:413–419. doi: 10.1111/j.1440-1827.2006.01979.x. [DOI] [PubMed] [Google Scholar]
- 16.Lao XM, Chen DY, Zhang YQ, Xiang J, Guo RP, Lin XJ, Li JQ. Primary carcinosarcoma of the liver: clinicopathologic features of 5 cases and a review of the literature. Am J Surg Pathol. 2007;31:817–826. doi: 10.1097/01.pas.0000213431.07116.e0. [DOI] [PubMed] [Google Scholar]
- 17.She R, Szakacs J. Carcinosarcoma of the liver: a case report and review of the literature. Arch Pathol Lab Med. 2005;129:790–793. doi: 10.5858/2005-129-790-COTLAC. [DOI] [PubMed] [Google Scholar]
- 18.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 19.Roskams TA, Libbrecht L, Desmet VJ. Progenitor cells in diseased human liver. Semin Liver Dis. 2003;23:385–396. doi: 10.1055/s-2004-815564. [DOI] [PubMed] [Google Scholar]
- 20.Libbrecht L, Desmet V, Van Damme B, Roskams T. The immunohistochemical phenotype of dysplastic foci in human liver: correlation with putative progenitor cells. J Hepatol. 2000;33:76–84. doi: 10.1016/s0168-8278(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 21.Uenishi T, Kubo S, Yamamoto T, Shuto T, Ogawa M, Tanaka H, Tanaka S, Kaneda K, Hirohashi K. Cytokeratin 19 expression in hepatocellular carcinoma predicts early postoperative recurrence. Cancer Sci. 2003;94:851–857. doi: 10.1111/j.1349-7006.2003.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Chen XP, Zhang W, Dong HH, Xiang S, Zhang WG, Zhang BX. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–232. doi: 10.1111/j.1365-2559.2007.02929.x. [DOI] [PubMed] [Google Scholar]
- 23.Dong HH, Xiang S, Chen XP, Liang HF, Zhang W, Jing K, Zhang W, Zhang WG, Chen L. The epithelial-mesenchymal transition promotes transdifferentiation of subcutaneously implanted hepatic oval cells into mesenchymal tumor tissue. Stem Cells Dev. 2009;18:1293–1298. doi: 10.1089/scd.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li CH, Wang YJ, Dong W, Xiang S, Liang HF, Wang HY, Dong HH, Chen L, Chen XP. Hepatic oval cell lines generate hepatocellular carcinoma following transfection with HBx gene and treatment with aflatoxin B1 in vivo. Cancer Lett. 2011;311:1–10. doi: 10.1016/j.canlet.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 25.Baumann U, Crosby HA, Ramani P, Kelly DA, Strain AJ. Expression of the stem cell factor receptor c-kit in normal and diseased pediatric liver: identification of a human hepatic progenitor cell? Hepatology. 1999;30:112–117. doi: 10.1002/hep.510300140. [DOI] [PubMed] [Google Scholar]
- 26.Luchini C, Capelli P, Fassan M, Simbolo M, Mafficini A, Pedica F, Ruzzenente A, Guglielmi A, Corbo V, Scarpa A. Next-generation histopathologic diagnosis: a lesson from a hepatic carcinosarcoma. J Clin Oncol. 2014;32:e63–e66. doi: 10.1200/JCO.2012.47.5855. [DOI] [PubMed] [Google Scholar]


