Abstract
Nanotechnology represents a major frontier with potential to significantly advance the field of bone tissue engineering. Current limitations in regenerative strategies include impaired cellular proliferation and differentiation, insufficient mechanical strength of scaffolds, and inadequate production of extrinsic factors necessary for efficient osteogenesis. Here we review several major areas of research in nanotechnology with potential implications in bone regeneration: 1) nanoparticle-based methods for delivery of bioactive molecules, growth factors, and genetic material, 2) nanoparticle-mediated cell labeling and targeting, and 3) nano-based scaffold construction and modification to enhance physicochemical interactions, biocompatibility, mechanical stability, and cellular attachment/survival. As these technologies continue to evolve, ultimate translation to the clinical environment may allow for improved therapeutic outcomes in patients with large bone deficits and osteodegenerative diseases.
Keywords: Bone, SPIONs, Nanoparticle, Osteogenesis, Scaffold, Nanotechnology
Graphical Abstract
Nanotherapeutic strategies to promote bone regeneration have seen recent progress on several fronts. Nanoparticles may be employed for drug, growth factor, and gene delivery. Similarly, nanoparticle-mediated stem cell labeling and targeting to specific anatomic sites is an exciting area of research. Finally, incorporation of nanomaterials into scaffolds may enhance mechanical stability, biocompatibility, and cellular survival for implanted constructs.
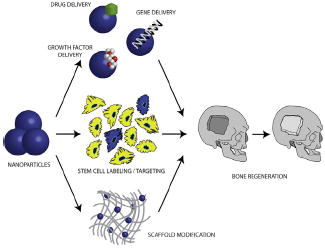
Introduction
Bone grafts represent one of the most common tissue transplants, with over 2.2 million performed annually worldwide.1 While autologous bone grafting for the reconstruction of skeletal defects is the current gold standard, this technique is hindered by variable resorption, limited supply, donor site morbidity, and high failure rates (up to 50%) in certain sites.2-4 These limitations lead to the development of synthetic biomaterials for the replacement of bone tissue. However, these synthetic materials are hindered/limited by their potential for both foreign-body reactions and infection. In recent years, nano-engineered particles and porous 3D scaffolds that facilitate growth of new bone have garnered significant attention.
There are several critical considerations which must be made to successfully guide bone regeneration. Importantly, natural bone is comprised of 30% w/v organic collagen fibrils and 70% inorganic calcium phosphate crystals. This composition has served as a model to mimic bone structure on a macro- and nanoscale level.5,6 Polymeric matrices combining calcium phosphates with materials such as chitosan have been studied to treat various bone defects.7 Advances in nanotherapeutic approaches, however, have allowed for further manipulation of the extracellular matrix to provide a more appropriate surface chemistry and interconnected porosity for cellular proliferation and angiogenesis. Another important factor is the need for controlled spatial and temporal delivery of signaling molecules to guide cellular survival and differentiation. Finally, biocom-patibility is key, as synthetic nanomaterials should remain inert or ideally resorb in a predictable and controlled manner to allow for remodeling.
Nanoparticles exist in the nanosize range, usually <100 nm, and due to their size and surface area, they can be exploited as vectors for delivery of drugs, growth factors, and genetic material.8 Importantly, the size of nanoparticles can determine their half-life and distribution. While particles <10 nm are cleared by the kidney, those larger than 200 nm are typically phagocytosed and removed by the spleen.9-11 Most therapeutic nanoparticles therefore range from 10 to 100 nm where they can be distributed throughout the circulatory system and penetrate through small capillaries.11 Surface properties may also affect stability and localization in the body, and charge has been shown to be a large determinant impacting internalization of nanopar-ticles into various target cells.8 For example, superparamagnetic iron oxide nanoparticles (SPIONs) have been employed to convey drugs or genetic material to target sites/cells in the body under the influence of a magnetic field. Similarly, hydrophobic surfaces have been found to promote engulfment by circulating macrophages whereas surface-engineered hydrophilic polymers (e.g. polyethylene glycol with hydroxyl or amino functional groups) allows for escape of nanoparticles from reticuloendothelial cells.12,13 Importantly, the physical properties of nanovectors should allow for loading that does not compromise functionality of the package, distribution to desired sites, and finally release at a desired rate.
In this review, we discuss past and current advances in nanoparticle-based therapies for bone tissue engineering. These include developments in nanotherapeutic strategies to deliver drugs and growth factors promoting bone formation, as well as gene therapy reagents (i.e. siRNAs or plasmid DNA). Nanoma-terials have also allowed for significant advances in imaging and stem cell targeting and these applications will be elaborated. Lastly, recent discoveries in nano-composite designs and scaffold modifications will be highlighted aiding mechanical stability, biocompatibility, and cellular survival for implanted constructs.
Nanoparticle-based delivery
In general, nanoparticles can be applied locally in bone tissue engineering (BTE) to augment tissue regeneration, enhance osseointegration of implants, and to prevent infections.14 Given unsatisfactory outcomes with many contemporary biomaterials alone for bone replacement, increasing interest has thus developed in the use of bioactive molecules aimed to promote bone formation. Direct administration of therapeutic agents suffers from the intrinsic limitations of these small molecules including poor physiological stability, non-specific targeting and low cell membrane permeability.15 In many cases, supraphysiological doses are necessary to combat the poor pharmacokinetics of these compounds, thereby increasing the potential risk of adverse effects.16 Nanomaterial carriers can overcome these limitations by stabilizing the bioactive molecules through encapsulation or surface attachment,16 facilitating entry into cells, targeting cellular delivery,17 and providing controlled drug release at the designated target18 (Figure 1).
Figure 1.
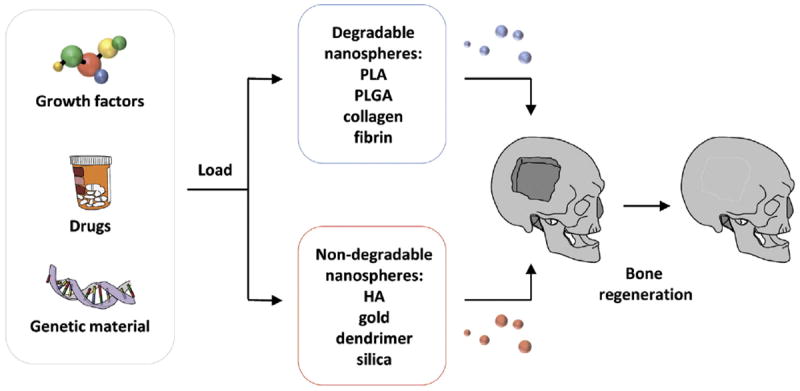
Nanoparticle-based strategies to promote bone regeneration. Nanoparticles may be classified as either degradable (poly(l-lactide) (PLA), poly(l-lactide-co-glycolic) (PLGA), collagen, fibrin) or non-degradable (hydroxyapatite (HA), gold, dendrimer, silica), each with their own range of benefits and drawbacks. Loading of drugs, growth, factors, or genes can be employed to enhance bone formation at sites of pathology and fracture.
Nanospheres have been widely accepted as a useful tool for controlled drug delivery due to their inherently small size and corresponding large specific surface area, a high drug loading efficiency, a high reactivity towards surrounding tissues in vivo, and an ease of diffusion of drug-loaded particles.19 A goal of modern clinical therapeutics is the targeted delivery of drugs. To this end, the small size of nanospheres allows them to quickly respond to stimuli from the surrounding environment (for example pH, magnetic fields, ultrasounds, and irradiation) and thus, these spheres can serve as stimulus-driven delivery for biologically or chemically active agents, and subsequently, establish triggered release by responding to external stimulation.19-23
Delivery of drugs, growth factors, or genetic material may be accomplished following encapsulation in, either, degradable or non-degradable nano-spheres. Examples of non-degradable nanoparticles include hydroxyapatite, gold, dendrimer, and silica24-27, while degradable nanoparticles include poly(l-lactide) (PLA) or poly(l-lactide-co-glycolic) (PLGA).28,29 The selection of the base biomaterial for nanosphere construction depends on the desired end application criteria. It depends on many factors such as (i) size of the desired nanoparticles, (ii) properties of the drug (aqueous solubility, stability, etc.) to be encapsulated in the polymer, (iii) surface characteristics and functionality, (iv) degree of biodegradability and biocompatibility, and (v) drug release profile of the final product.30 Frequently, nanoparticles can be combined with scaffolds such as proteinaceous hydrogels or biodegradable polymeric matrices to facilitate application in bone. Osteoblasts and osteoclasts have an intricate relationship and their respective activity is key to bone homeostasis.14 Osteoblasts can be supported by nanoparticle-based drug/growth factor (GF) delivery or alternatively osteoclasts can be modulated by nanoparticles locally releasing specific inhibitors.14
Biodegradable nanospheres can be prepared from a variety of materials such as natural polymers (proteins and polysaccharides) and synthetic polymers. In contrast to injected proteins, which are usually rapidly cleared from the body, locally adsorbed proteins are released by desorption or diffusion and thus, can be retained longer.31 Towards this end, nanospheres are being explored as finely adjustable delivery systems with regard to the location and time period of drug release. Local drug delivery is favorable to systemic application to minimize adverse effects. Moreover, adequate tuning of the nanoparticles allows for a temporally-controlled, sustained delivery according to requirements. Furthermore, unstable biological activity of growth factors, genes and drugs can result in inefficient delivery of these bioactive molecules.32 Compared to direct adsorption of a bioactive molecule on the surface of an implanted scaffold, a carrier delivery system provides controlled, long-term release with adequate efficacy.33 Delivery vectors require materials that are biocompatible, biodegradable as well as suitable for encapsulation of bioactive molecules. In particular, encapsulated growth factors may be released as the polymer degrades following a controlled and predetermined profile, a key factor of biodegradable nanosphere design. Thus, nanospheres are being increasingly explored as finely adjustable delivery systems with regard to the location and time period of drug release, while also simultaneously protecting the therapeutic agent from the biological milieu.
Natural polymers include collagen, fibrin, gelatin, alginate, and chitosan and are of interest due to their intrinsic biocompatibility and biodegradability. For instance, a great advantage of collagen in BTE is that the extracellular matrix (ECM) of bone is composed mainly of collagen in its organic phase, thus it possesses great biocompatibility in addition to biodegradability, and negligible immunogenicity. Loading of an appropriate amount of recombinant human morphogens and avoiding its “burst-release” represent critical challenges for upgrading the biological performance of thin bioactive coatings on metal implants used in BTE. In an effort to overcome this challenge, Wang and colleagues34 recently reported that nanostruc-tured colloidal gelatin gels show great potential for sustained delivery of therapeutic osteogenic proteins: bone morphogenetic protein-2 (BMP-2) and alkaline phosphatase.
Natural polymers have plentiful molecular chain side groups, which allow for further manipulation and functionalization.19 Therefore, natural polymers such as collagen and gelatin contain motifs such as arginine–glycine–aspartic acid (RGD) sequences, which can modulate cell adhesion, thereby improving the cellular behavior compared with the polymers that lack these cell recognition sites. Chitosan, a linear polysaccharide produced from crustacean shells, has additional benefits in nanosphere design as it is soluble in aqueous media, avoids the use of organic solvents, and does not require further purification of nanoparticles.35 With the presence of free amine groups in its linear structure, chitosan has a cationic nature and can interact with various crosslinkers to form nanoparticles. While chemical crosslinkers such as glutaraldehyde can be toxic for biological systems, chitosan can also be ionically crosslinked with multivalent anions like tripolyphosphate. This process, known as ionic gelation, has some advantages since it is a mild process resulting in nanoparticles with sizes less than 200 nm and has been proven to encapsulate different biological and active compounds.35-37 Kong and colleagues38 recently adopted incorporation of chitosan nanospheres into thin mineralized collagen coatings to enhance rhBMP-2 loading and improve morphogen release kinetics based on the good affinity of chitosan for proteins and the large surface area of nanospheres. A drawback of other natural polymers is that their biological activity can be lost during processing, which may then result in induction of an immune response. While developments in BTE are focusing on producing recombinant collagen, which is a safe and predictable alternative and can be tailor-made chemically to meet clinical requirements (i.e., various chemical formulations promote maintenance of a spherical shape), synthetic polymers constitute a viable alternative which bring other advantages to the BTE platform.
Typically, synthetic biodegradable polymers, including poly(l-lactide) (PLA) or poly(l-lactide-co-glycolic) (PLGA), offer advantages such as inherent ease of manufacture and modification, and cost efficiency. Importantly, polymer degradation profiles can be tailor-made to achieve optimal bioactive molecule release profile. The most widely used polymers for nanospheres and nanoparticle generation have been PLA, poly(glycolic acid) (PGA), and their co-polymers, poly(lactide-co-glycolide) (PLGA). These polymers are known for both their biocompatibility and resorbability through natural pathways. Encapsulation of material in a shell comprised of a phospholipid bilayer can enhance tissue specific targeting, protection from degradation, and delivery of a large amount of drug.8,39 Surface conjugation may be achieved through cleavable covalent linkages using amino or hydroxyl functional groups on surface polymers or by physical interactions such as electrostatic, hydrophobic/hydrophilic, and affinity interactions. Additionally, the degradation rate and accordingly the drug release rate can be tailored according to the clinical process by varying the ratio of PLA, which offers increased hydrophobicity, to PGA, which offers increased hydrophilicity.40
Commonly used in the treatment of osteoporosis, bispho-sphonates such as alendronate are well known to have poor bioavailability, and thus, administration of high doses is usually required to be clinically effective, leading to systemic toxicity. Thus, a local sustained method of alendronate delivery is preferable and promising results have been seen with alendronate loading onto PLGA nanoparticles. This approach has been shown to be more effective at inducing osteoclast apoptosis and impairing osteoclast function than unbound drug alone, a phenomenon which has also been reproduced with non-degradable nanoparticles.25,41,42 Similarly, in vitro studies using poly(l-lysine) (PLL) nanoparticles to deliver BMP-2 within a fibrin hydrogel showed enhanced osteogenic differentiation of bone marrow-derived mesenchymal cells43In vivo studies using this strategy have also revealed BMP-2-coated PLGA nanoparticles within a fibrin hydrogel complex to be capable of significantly enhancing bone regeneration in a critical-sized rat calvarial defect.44,45 And like BMP-2, BMP-7 has been encapsulated in PLGA nanospheres, resulting in temporally controlled release and ectopic bone formation following subcutaneous implantation on nano-fibrous PLA scaffolds in rats.46 These findings thus underscore the applicability of nanoparticles for delivery of growth factors in novel bone regenerative strategies.
Non-degradable nanoparticles are typically composed of ceramic nanoparticles (silica, alumina), metals, metal oxides, and metal sulfides, which can be used to produce a myriad of nanostructures with varying size and shape. In general, inorganic nanoparticles can be designed to evade the reticuloendothelial system by varying sizes and surface compositions.
Bioactive glasses are increasingly described for use in BTE. First developed in 1969, bioactive glasses represent a group of surface reactive materials that are able to bond to bone in a physiological environment.47 Bioactive glasses most commonly used in BTE consist of a silicate network incorporating sodium, calcium, and phosphorous, but modifications with additional elements such as fluorine, magnesium, strontium, iron, silver, boron, potassium, or zinc have been described in the literature.48-51 In particular, mesoporous silica nanoparticles are widely used as a delivery reagent because silica possesses favorable chemical properties, thermal stability and biocompatibility. Currently, sol–gel-derived mesoporous silica nanoparti-cles in soft conditions are of great interest due to simplicity in production and modification and the capacity to maintain function of bioactive agents. The unique mesoporous structure of silica facilitates effective loading of drugs and their subsequent controlled release. The properties of mesopores, including pore size and porosity as well as the surface properties, can be altered depending on additives used to fabricate mesoporous silica nanoparticles. Hollow silica nanoparticles have been prepared, such as calcium phosphate-based nano-shells, with surface pores leading to a central reservoir.51
The unique surface of silica enables functionalization to modify surface properties and link therapeutic molecules. The tunable mesopore structure and modifiable surface of mesopo-rous silica nanoparticles allow for incorporation of various classes of drug molecules and controlled delivery to the target sites.52 For example, mesoporous silica materials containing a complex ‘worm-like’ network of channels throughout the interior of the solid nanoparticles can be utilized as vectors for controlled delivery of therapeutic agents. Looking to the oncological community for further advances, pH-responsive charge-reversal, polymer-coated mesoporous silica nanoparticles were recently described as an effective, cell-specific targeted chemotherapeutic agent delivery method.53 The described pH-controlled smart-release platform holds promise for targeted drug/morphogen delivery with impact in diffuse fields such as BTE.53
Delivery of growth factor (GF) genes can be more effective than the delivery of GFs alone due to sustained production and secretion of GFs achieved by gene transfection.54 Gene therapy targeting downregulation of undesirable genes or upregulation of pro-osteogenic genes represents two approaches which may be employed, but delivery of constructs efficiently while maintaining integrity and stability remains a major concern. Transfer of genetic material poses specific challenges in a sequential manner. To effectively transfer exogenous DNA encoding GF, several steps need to be followed. For example, the following process must be completed successfully: (i) internalization of the DNA–nanoparticle complex through the cell membrane, (ii) intracellular endosome uptake, (iii) release into the cytoplasm, (iv) nuclear uptake of the complex, (v) dissociation from the vector, (vi) protein expression and finally, and (vii) secretion of the GF protein.54,55 For successful gene transfection, the carrier vector should be small enough to be internalized into the cell and capable of escaping recognition by the endosome lysosome processing so as to protect the DNA until it reaches the target cell. Thus, one can easily identify the role for nanoparticle-based delivery of genetic material. Sub-cellular sized nanoparticles can penetrate into targeted tissues and cells and easily deliver a therapeutic through endocytosis, avoiding the inherent complications of viral-based vector systems.56 Cationic polymer-coated nanoparticles have been developed which interact with negatively charged DNA and can help to stabilize and traffic constructs to desired sites.57
Many nanoparticle gene delivery systems have been studied, including polymeric nanoparticle DNA encapsulation, DNA PEGylation, micelles, liposomes, dendrimers, and nanosized inorganic material systems.54 One of the most commonly employed strategies has incorporated polyethylenimine (PEI), a cationic polymer, which can interact with negatively charged DNA. In similar fashion, dextran-coated nanoparticles, with a negatively charged functional group, can couple with positively charged peptide oligomers.58-60 Nanostructured polymers and PEI-coated superparamagnetic iron oxide nanoparticles (SPIONs) have been found to be effective at delivering siRNAs and microRNAs to human mesenchymal cells (hMSCs) and MC3T3-E1 preosteoblasts with minimal to no cytotoxic effects.61,62 Studies have also suggested nanoparticle-based transfection of MSCs to be comparable or more efficient than lipofectamine.63-65 Through the proton-sponge effect, positively charged nanoparticles can facilitate DNA uptake through endocytosis and evade the lysosome for delivery to the nucleus.8 Furthermore, enhanced transfection can be accomplished through introduction of magnetic fields to target SPION–DNA complexes to desired sites throughout the body, and once there, reduce free diffusion of these particles.66,67
PEI-coated nanoparticles have already been described for delivery of plasmids containing the BMP-2 gene to enhance bone formation. In vitro studies by Lu et al.68 found MSCs transfected with this approach to demonstrate increased expression of osteogenic markers, as well as alkaline phosphatase and alizarin red staining. Subcutaneous implantation of these cells on a calcium phosphate cement scaffold into immunocompromised mice also led to significantly greater formation of ectopic new bone.68,69 Nanoparticle delivery of the BMP-4 gene has likewise been reported, and use of PLGA nanoparticles to enhance transfection of adipose-derived stromal cells (ASCs) was found to promote osteo-chondrogenic differentiation.70 Finally, PEI-conjugated gold nanoparticles complexed with plasmids containing the BMP-7 gene have been administered in rabbits to alter wound healing and fibrosis.71
Collectively, nanoparticles have been found to be quite adaptable for delivery of drugs, growth factors, or genetic material. Due to their physical characteristics, nanoparticles can interact with their payload to not only enhance stability, but efficiently traffic complexed material to targeted cells/sites throughout the body. Through such a nanotherapeutic approach, modifications can thus be made influencing cell survival, proliferation, and differentiation to promote bone regeneration.
Nanoparticle-mediated stem cell labeling/targeting
In contrast to delivery of compounds to specific sites of need, nanoparticles can also be used for labeling and guiding of stem cells to various target locations. The utility of magnetic nanoparticles, in particular, has long been established, with use as a contrast agent in magnetic resonance imaging (MRI) for over two decades.8 Along these lines, recent investigations have evaluated the ability for nanoparticles to label and track stem cells noninvasively and follow the localization of transplanted cells in vivo.72,73 With respect to bone regeneration, multiple reports have looked at the ability for nanoparticles to specifically label MSCs74 (Figure 2).
Figure 2.
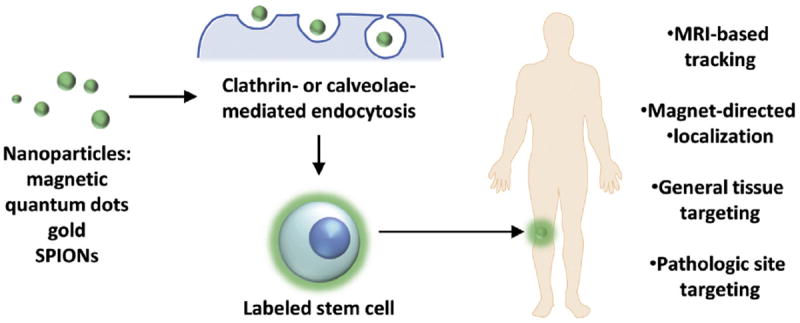
Nanoparticle-mediated stem cell labeling/targeting. A variety of nanoparticles including quantum dots, mesoporous silica, gold, and SPIONs have been used to tag cells, with uptake through clathrin- or caveolae-mediated endocytosis and macropinocytosis. In addition to MRI-based tracking, nanoparticles may be used to chaperon cells to desired sites through magnet- and antibody-based targeting.
A variety of nanoparticles including quantum dots, mesoporous silica, gold, and SPIONs have been used to tag MSCs, with uptake through clathrin- or caveolae-mediated endocytosis and macropinocytosis.75-80 In contrast to cells with inherent phagocytic activity, though, MSCs do not take up particles efficiently. To promote internationalization, Babic et al.81 and Kwoh et al.82 both modified the surface of nanoparticles with positively charged PLL. Other positive-charged polymeric-complexed nanoparticles have similarly been noted to be taken up by MSCs.83,84 Alternatively, negatively charged polymeric modifications to nanoparticles have also been found to enhance uptake efficiency, suggesting either type of charge to be capable of facilitating interaction with the cell surface.85,86 Aside from these modifications, manipulation of nanoparticles to enhance MSC uptake has been accomplished through attachment of a neural ganglioside GD2 antibody. As this marker has been found to be expressed on the surface of MSCs, GD2 antibodies conjugated to the distal ends of PEG-g-PEI SPIONs increased labeling of human MSCs and delivery of plasmid DNA.65 Importantly, while nanoparticles have been shown to be suitable for efficient in vivo tacking of cells through MRI, ongoing investigations continue to evaluate whether these tags may interfere with cellular function and osteogenic differentiation capacity. In vitro studies have shown relative innocuousness of SPION and gold nanoparticles, however other reports suggest certain nanoparticles to interfere with bone forming capacity of MSCs in vivo.80,87,88
In addition to labeling of MSCs, nanoparticles also possess the potential to chaperon cells to desired sites. SPIONs, in particular, have been shown to be capable of delivering stem cells to specific locations through surface modifications or their own inherent paramagnetic property. By conjugating dextrancoated magnetic nanoparticles with anti-CK-MB or anti-troponin I antibodies, Yang et al.89 demonstrated localization of stem cells to infarcted myocardium. MSCs have also been magnetized following uptake of SPIONs, and this has allowed for localization of intravenously injected cells in rats through application of a surface magnet.90 Furthermore, progress has been made in the development of magnetic fields which can be focused at a distance from the pole.91 This has allowed for targeting of magnetically loaded MSCs to deeper targets previously limited by magnetic attenuation. Such a strategy may thus facilitate homing of MSCs to sites such as bone for engraftment and cellular therapy. In the field of bone tissue engineering where targeted and site-specific tissue regeneration is required, this technology holds enormous potential.
Nano-based scaffold construction and modification
Scaffolds are mechanical constructs that act as carriers for cells and/or growth factors. An ideal scaffold should be both biocompatible and biodegradable, allowing for ultimate replacement with functional tissue. Many scaffolds have been designed to mimic the extracellular matrix, which typically provides support, tensile strength, and serves as a lattice for cell adhesion, movement, and tissue ingrowth.92 Simple biodegradable polymeric materials or ceramics have been investigated as bone tissue engineering scaffolds, however these materials have significant limitations, including insufficient mechanical strength. In contrast, nanoparticle-modified composite scaffolds offer significant promise to facilitate bone regeneration. Nanoscale organic and inorganic materials incorporated into polymeric scaffolds may provide the proper surface and mechanical properties necessary for support, as well as cellular adhesion, differentiation, and integration into the surrounding environment54 (Figure 3).
Figure 3.
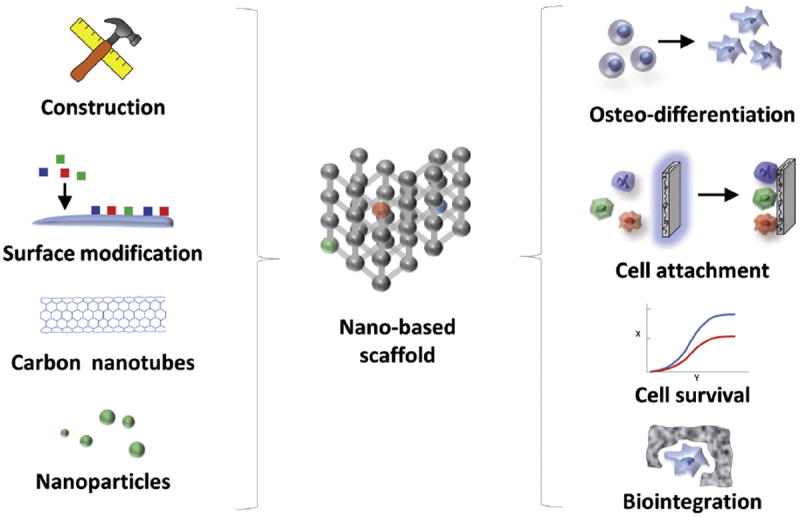
Nano-based scaffold construction and modification. The material used for construction of a scaffold impacts many aspects of bone tissue engineering including cell survival, attachment, differentiation, and integration. Following construction, modification of scaffold surface topography to improve the physicochemical interaction between implanted materials and the native in vivo environment allows for enhancement of mechanical stability, biocompatibility, and cellular survival of implanted constructs. For example, metallic nanoparticles have also been incorporated into scaffolds to increase mechanical strength, cellular adhesion, and bone forming capacity.
Nano-scale surface modification of scaffolds allows for modulation of biological activity, enhanced cell survival, and improved regenerative outcomes. Attachment of cells to the underlying substrate has been found to be critical for survival and retention at implanted sites. Detachment of anchorage-dependent cells from the surrounding matrix may result in loss of normal cell–matrix interactions leading to anoikis.93 The well-known arginine–glycine–aspartate (RGD) cell adhesion ligand has been incorporated into scaffolds to enhance attachment of cells. Whether through formation of polymer-RGD peptide hybrid molecules or by surface modification of prefabricated polymers, addition of the RGD ligand enhances the ability for scaffolds to mimic the extracellular matrix and guide behavior and development of implanted cells.94 From the perspective of bone engineering, incorporation of carbon nanotubes and micro-hydroxyapatite (HA) particles with PLA-based scaffolds to create nanocomposites has been shown to increase attachment of MSCs and differentiation to osteoprogenitors.95 Incorporation of a biomimetic HA surface was also found to significantly alter the microenvironment of adherent preosteoblasts and promote survival and extension of cellular projections along textured surfaces.96
As HA reflects the native mineral structure of bone, it is also not surprising that biomimetic apatite nanoparticles may enhance the osteogenic capacity of progenitor cells. HA-coated PLGA scaffolds alone have been found to facilitate bone regeneration in rat calvarial defects, and greater exposure of HA nanoparticles on the scaffold surface was noted to result in accelerated bone deposition by local progenitors.97 In addition, apatite-coated PLGA scaffolds have been known to promote differentiation of seeded preosteoblasts. Chou et al.98 found significantly elevated expression levels for markers of bone differentiation from MC3T3-E1 cells cultured on three-dimensional apatite-coated PLGA. How these cells interact with apatite nanoparticles has recently been shown to be regulated by adsorbed protein layers which may alter the surface potential/charge or modulate phase transformation of calcium phosphate.99 In similar fashion, PLA scaffolds coated with HA nanoparticles can enhance the bone forming capacity of MSCs. Guo and colleagues100 noted that HA-PLA scaffolds stimulated expression of osteogenic proteins (i.e. BMP-2, osteopontin, collagen type I, and osteocalcin) in rabbit bone marrow-derived MSCs and facilitated bone regeneration of critical-sized mandible defects. Therefore, while HA alone is a poor scaffold for bone reconstruction secondary to its brittleness and slow degradation rate, incorporation of this nanoparticle into surface modifications of other polymeric materials has created more promising scaffolds for bone tissue engineering.
Modification of scaffold surface topography to improve the physicochemical interaction between implanted materials and the native in vivo environment continues to be an exciting area of research. Aside from HA, metallic nanoparticles have also been incorporated into scaffolds to increase mechanical strength, cellular adhesion, and bone forming capacity. Studies have shown that the addition of various materials including titanium, alumoxane, and iron can also enhance collagen synthesis, alkaline phosphatase activity, and calcium deposition by osteoblasts, leading to enhanced tensile strength.101-103 It has been hypothesized that the phenomenon of enhanced osteoblast adhesion and function with metallic nanoparticle-coated scaffolds is secondary to nanoscale surface roughness.54 Nano-structural topographical properties of a scaffold may be critical for osteoinduction, as bone cells naturally grow, adhere, proliferate, and differentiate within a complex extracellular matrix through interaction with diverse adhesion proteins.104 Incorporation of metallic nanoparticles thus not only enhances the mechanical properties of the composite scaffolds, but also augments their ability to alter the biologic activity of adjacent bone progenitor cells.
The importance of surface topography in dictating the response of native cells to scaffold implantation has also been documented outside the field of bone tissue engineering. Lapointe et al.105 were the first to demonstrate that 4.5 nm particles created a surface topography that affected embryonic stem cell (ESC) differentiation and concluded that nanoscale chemistry and topography influence stem cell differentiation, particularly the early differentiation markers Fgf5 and Foxa2. The implications of this finding for bone tissue engineering are enormous considering that ESCs can be directed to efficiently regenerate bone. Another recent study showed that the topography created by silicone nanopillars can hasten neuronal elongation, diminish neurites, and encourage axonal differentiation.106 Acute topographical changes induced by forming small wrinkles in a hydrogel surface were sufficient to alter the morphology of vascular smooth cells in one study,107 while bacterial cells of microbes such as Escherichia coli, Listeria innocua, and Pseudomonas fluorescens altered the number and type of their cellular appendages in response to the nanoscale surface topography of the material to which they attached.108 These findings in E. coli are reinforced by evidence that even minute changes in surface topography can alter the expression of stress response genes in this bacteria.109
In addition to modification, the process of scaffold construction itself has seen advances in recent years. Electrospinning involves converting a polymer into a viscous solution with the addition of solvent, charging the polymer with a high voltage source to create a Taylor cone, extending the polymer into a thin jet stream across an electrostatic field, and then collecting the produced nanofibers on a grounded collector.111 The process of electrospinning biocomposite scaffolds offers numerous advantages for tissue engineering. Alignment of polyaniline or poly(ε-caprolactone) nanofibers at the surface through electro-spinning was found to reduce fibrous capsule formation, as well as improve cellular guidance cues to modulate cell behavior.112 Highly aligned and electrically conductive nanofibers may thus provide topographical cues that regulate scaffold degradation while simultaneously guiding skeletal tissue engineering. These scaffolds can stimulate osteoconduction and osteoinduction, provide a fertile ground for stem cell proliferation, and be made out of biodegradable synthetic polymers that trigger little immunogenic response.113,114 Indeed, inflammation and adhesion of macrophages, with formation/release of bioreactive agents such as reactive oxygen intermediates, digestive enzymes, and acidic phagolysosomes, may result in premature degradation of scaffolds. Control and manipulation of this response to enhance bone repair and regeneration has therefore been a goal of immunobioengineering, and some progress has been made through electrospun nanofibers which may minimize host response.110
The process of fabricating scaffolds with electrospinning has seen numerous innovations to improve the field. There have been new techniques to overcome the hydrophobic nature of polymeric materials using surfactants,113 new scaffolds tailored to specific application such as aligned nanoyarn reinforcement for tendon repair,115 new structural formations like that of cotton wool,116 new composite combinations like chitosan (CS) and silk fibroin (SF),117 and new confirmatory studies of the bone stimulating effects of known pro-osteogenic proteins like BMP-2 within the context of electrospun scaffolds.118 Electrospun scaffolds are typically limited for bone tissue replacement due to low mechanical strength. Recent progress on this front has been made with electrospun silk fibroin scaffolds through uniform dispersion of hydroxyapatite nanoparticles to increase tensile strength.119 Likewise, a functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate (BCP) had markedly increased tensile strength over scaffolds without BCP.120
Recently, Raghavendran et al.114 explored the benefits of combining different biomaterials into a single scaffold to balance the advantages and disadvantages of each material in isolation in order to obtain the ideal biocompatible scaffold for stimulating osseous union using stem cell based therapy. They discovered that integrating PLA, HA, and collagen into a single scaffold improved the viability of engrafted hMSCs, increased the osteogenic gene expression as shown by significantly elevated levels of osteocalcin, improved matrix mineralization as demonstrated by elevated calcium and Alizarin Red staining, and augmented osteoinduction.114 Expanding the clinical potential of electrospun scaffolds, Xue et al.121 offered a promising solution to the clinical problem of failure of guided tissues regeneration/guided bone regeneration due to infections when they showed that electrospinning metronidazole into membranes could significantly decrease anaerobic colonization. Overcoming the relatively tight structure of electrospun nanoscaffolds that can prevent 3D cell penetration, airbrushed composite nanofiber scaffolds present an arguably simpler alternative that produce a more open network allowing, in one study, more than double the depth of cell penetration.122
Conclusion
Reconstruction of large bone defects remains challenging and development of novel strategies improving on the limited currently available techniques will require a marriage of material science, biology, and tissue engineering. As a template for three dimensional tissue growth, scaffolds must emulate native extracellular matrix. Nanoparticle modifications of scaffolds enhance this capacity to mimic complex properties of the natural bone environment and provide a more favorable milieu for cellular attachment, ingrowth, and bone formation. Whether through promotion of cellular survival, osteoblastic differentiation, or modulation of immunological response, nanostructural changes to polymer surfaces may provide a more favorable environment for bone regenerative strategies. Notwithstanding the formidable advances within the field, the problem persists to better understand the interactions between nanoscale surface topography and the biological system into which it is introduced. We know that this interface plays a significant role in osseointegration of implants, but evidence based nanoscaled surfaced design is an elusive technique.123 Further understanding of surface characteristics such as wettability, surface energy and roughness, surface curvature and nanoscale features, organic and inorganic coatings effect cell signaling, proliferation, integration, and viability will be required. In the foreseeable future, nanotechnology may allow for specifically tailored treatment of various disease states with complex skeletal defects at distinct anatomical sites.
Acknowledgments
M.T.L. was supported by NIH grants U01 HL099776, R01 DE021683-01, RC2 and DE020771; the Oak Foundation; the Gunn/Olivier Fund; and Hagey Laboratory for Pediatric Regenerative Medicine, D.C.W. was supported by NIH grant K08 DE024269, the ACS Franklin H. Martin Faculty Research Fellowship, the Hagey Laboratory for Pediatric Regenerative Medicine, and the Stanford University Child Health Research Institute Faculty Scholar Award. G.G.W. was supported by the Stanford School of Medicine, the Stanford Medical Scientist Training Program, and NIGMS training grant GM07365. R.T. was supported by the Plastic Surgery Foundation/Plastic Surgery Research Council Pilot Grant and the Stanford University Transplant and Tissue Engineering Center of Excellence Fellowship.
References
- 1.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: an update. Injury. 2005;36(Suppl 3):S20–7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj AK, Wongworawat AA, Punjabi A. Management of alveolar clefts. J Craniofac Surg. 2003;14(6):840–6. doi: 10.1097/00001665-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Clavero J, Lundgren S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin Implant Dent Relat Res. 2003;5(3):154–60. doi: 10.1111/j.1708-8208.2003.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 4.Brighton CT, et al. Tibial nonunion treated with direct current, capacitive coupling, or bone graft. Clin Orthop Relat Res. 1995;321:223–34. [PubMed] [Google Scholar]
- 5.Ryu J, et al. Mineralization of self-assembled peptide nanofibers for rechargeable lithium ion batteries. Adv Mater. 2010;22(48):5537–41. doi: 10.1002/adma.201000669. [DOI] [PubMed] [Google Scholar]
- 6.Zietz C, et al. Third-body abrasive wear of tibial polyethylene inserts combined with metallic and ceramic femoral components in a knee simulator study. Int J Artif Organs. 2013;36(1):47–55. doi: 10.5301/ijao.5000189. [DOI] [PubMed] [Google Scholar]
- 7.Liu H, et al. Novel injectable calcium phosphate/chitosan composites for bone substitute materials. Acta Biomater. 2006;2(5):557–65. doi: 10.1016/j.actbio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 8.Wahajuddin Arora S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomedicine. 2012;7:3445–71. doi: 10.2147/IJN.S30320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez-Urrusuno R, et al. Effect of polymeric nanoparticle administration on the clearance activity of the mononuclear phagocyte system in mice. J Biomed Mater Res. 1996;31(3):401–8. doi: 10.1002/(SICI)1097-4636(199607)31:3<401::AID-JBM15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 10.Verdun C, et al. Tissue distribution of doxorubicin associated with polyisohexylcyanoacrylate nanoparticles. Cancer Chemother Pharmacol. 1990;26(1):13–8. doi: 10.1007/BF02940287. [DOI] [PubMed] [Google Scholar]
- 11.Rolland A, et al. Blood clearance and organ distribution of intravenously administered polymethacrylic nanoparticles in mice. J Pharm Sci. 1989;78(6):481–4. doi: 10.1002/jps.2600780613. [DOI] [PubMed] [Google Scholar]
- 12.Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010;62(3):284–304. doi: 10.1016/j.addr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2(3):214–21. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 14.Tautzenberger A, Kovtun A, Ignatius A. Nanoparticles and their potential for application in bone. Int J Nanomedicine. 2012;7:4545–57. doi: 10.2147/IJN.S34127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhuang J, et al. Optimized metal-organic-framework nanospheres for drug delivery: evaluation of small-molecule encapsulation. ACS Nano. 2014;8(3):2812–9. doi: 10.1021/nn406590q. [DOI] [PubMed] [Google Scholar]
- 16.Faraji AH, Wipf P. Nanoparticles in cellular drug delivery. Bioorg Med Chem. 2009;17(8):2950–62. doi: 10.1016/j.bmc.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 17.Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60(16):4440–5. [PubMed] [Google Scholar]
- 18.Trewyn BG, et al. Synthesis and functionalization of a mesoporous silica nanoparticle based on the sol–gel process and applications in controlled release. Acc Chem Res. 2007;40(9):846–53. doi: 10.1021/ar600032u. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, et al. The use of micro- and nanospheres as functional components for bone tissue regeneration. Tissue Eng Part B Rev. 2012;18(1):24–39. doi: 10.1089/ten.teb.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collier JH, et al. Thermally and photochemically triggered self-assembly of peptide hydrogels. J Am Chem Soc. 2001;123(38):9463–4. doi: 10.1021/ja011535a. [DOI] [PubMed] [Google Scholar]
- 21.Fundueanu G, et al. pH- and temperature-sensitive polymeric microspheres for drug delivery: the dissolution of copolymers modulates drug release. J Mater Sci Mater Med. 2009;20(12):2465–75. doi: 10.1007/s10856-009-3807-0. [DOI] [PubMed] [Google Scholar]
- 22.Ino K, Ito A, Honda H. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol Bioeng. 2007;97(5):1309–17. doi: 10.1002/bit.21322. [DOI] [PubMed] [Google Scholar]
- 23.Ito A, et al. Magnetic force-based cell patterning using Arg–Gly–Asp (RGD) peptide-conjugated magnetite cationic liposomes. J Biosci Bioeng. 2007;104(4):288–93. doi: 10.1263/jbb.104.288. [DOI] [PubMed] [Google Scholar]
- 24.Shah DA, et al. Regulation of stem cell signaling by nanoparticle-mediated intracellular protein delivery. Biomaterials. 2011;32(12):3210–9. doi: 10.1016/j.biomaterials.2010.11.077. [DOI] [PubMed] [Google Scholar]
- 25.Fanord F, et al. Bisphosphonate-modified gold nanoparticles: a useful vehicle to study the treatment of osteonecrosis of the femoral head. Nanotechnology. 2011;22(3):035102. doi: 10.1088/0957-4484/22/3/035102. [DOI] [PubMed] [Google Scholar]
- 26.Jensen T, et al. Osteopontin functionalization of hydroxyapatite nanoparticles in a PDLLA matrix promotes bone formation. J Biomed Mater Res A. 2011;99(1):94–101. doi: 10.1002/jbm.a.33166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira JM, et al. The osteogenic differentiation of rat bone marrow stromal cells cultured with dexamethasone-loaded carboxymethylchitosan/poly(amidoamine) dendrimer nanoparticles. Biomaterials. 2009;30(5):804–13. doi: 10.1016/j.biomaterials.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 28.Chen L, et al. A new growth factor controlled drug release system to promote healing of bone fractures: nanospheres of recombinant human bone morphogenetic-2 and polylactic acid. J Nanosci Nanotechnol. 2011;11(4):3107–14. doi: 10.1166/jnn.2011.3820. [DOI] [PubMed] [Google Scholar]
- 29.Mercado AE, et al. Release characteristics and osteogenic activity of recombinant human bone morphogenetic protein-2 grafted to novel self-assembled poly(lactide-co-glycolide fumarate) nanoparticles. J Control Release. 2009;140(2):148–56. doi: 10.1016/j.jconrel.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahapatro A, Singh DK. Biodegradable nanoparticles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J Nanobiotechnol. 2011;9:55. doi: 10.1186/1477-3155-9-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kofron MD, Li X, Laurencin CT. Protein- and gene-based tissue engineering in bone repair. Curr Opin Biotechnol. 2004;15(5):399–405. doi: 10.1016/j.copbio.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17(5):497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 33.Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002;8(4):529–39. doi: 10.1089/107632702760240454. [DOI] [PubMed] [Google Scholar]
- 34.Wang H, et al. Comparison of micro- vs. nanostructured colloidal gelatin gels for sustained delivery of osteogenic proteins: bone morphogenetic protein-2 and alkaline phosphatase. Biomaterials. 2012;33(33):8695–703. doi: 10.1016/j.biomaterials.2012.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H, et al. Chitosan-pluronic nanoparticles as oral delivery of anticancer gemcitabine: preparation and in vitro study. Int J Nanomedicine. 2012;7:1851–63. doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvo P, et al. Novel hydrophilic chitosan polyethylene oxide nanoparticles as protein carriers. J Appl Polym Sci. 1997;63(1):125–32. [Google Scholar]
- 37.Shah S, et al. Preparation and characterization of venlafaxine hydrochloride loaded chitosan nanoparticles and in vitro release of drug. J Appl Polym Sci. 2009;112(5):2876–87. [Google Scholar]
- 38.Kong Z, et al. Enhanced loading and controlled release of rhBMP-2 in thin mineralized collagen coatings with the aid of chitosan nanospheres and its biological evaluations. J Mater Chem B. 2014;2:4572–82. doi: 10.1039/c4tb00404c. [DOI] [PubMed] [Google Scholar]
- 39.Skouras A, et al. Magnetoliposomes with high USPIO entrapping efficiency, stability and magnetic properties. Nanomedicine. 2011;7(5):572–9. doi: 10.1016/j.nano.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 40.Hans ML, Lowman A. Biodegradable nanoparticles for drug delivery and targeting. Curr Opinion Solid State Mater Sci. 2002;6(4):319–27. [Google Scholar]
- 41.Cohen-Sela E, et al. A new double emulsion solvent diffusion technique for encapsulating hydrophilic molecules in PLGA nanoparticles. J Control Release. 2009;133(2):90–5. doi: 10.1016/j.jconrel.2008.09.073. [DOI] [PubMed] [Google Scholar]
- 42.Zhu X, et al. Inherent anchorages in UiO-66 nanoparticles for efficient capture of alendronate and its mediated release. Chem Commun (Camb) 2014;50(63):8779–82. doi: 10.1039/c4cc02570a. [DOI] [PubMed] [Google Scholar]
- 43.Park KH, et al. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. J Biosci Bioeng. 2009;108(6):530–7. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 44.Qiao C, et al. Using poly(lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo. Int J Nanomedicine. 2013;8:2985–95. doi: 10.2147/IJN.S45184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodriguez-Evora M, et al. Osteogenic effect of local, long versus short term BMP-2 delivery from a novel SPU-PLGA-betaTCP concentric system in a critical size defect in rats. Eur J Pharm Sci. 2013;49(5):873–84. doi: 10.1016/j.ejps.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Wei G, et al. The enhancement of osteogenesis by nano-fibrous scaffolds incorporating rhBMP-7 nanospheres. Biomaterials. 2007;28(12):2087–96. doi: 10.1016/j.biomaterials.2006.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17(11):967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 48.Sepulveda P, Jones JR, Hench LL. Characterization of melt-derived 45S5 and sol–gel-derived 58S bioactive glasses. J Biomed Mater Res. 2001;58(6):734–40. doi: 10.1002/jbm.10026. [DOI] [PubMed] [Google Scholar]
- 49.Oki A, et al. Preparation and in vitro bioactivity of zinc containing sol–gel-derived bioglass materials. J Biomed Mater Res A. 2004;69(2):216–21. doi: 10.1002/jbm.a.20070. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, et al. Bioactive borosilicate glass scaffolds: in vitro degradation and bioactivity behaviors. J Mater Sci Mater Med. 2009;20(6):1237–43. doi: 10.1007/s10856-009-3691-7. [DOI] [PubMed] [Google Scholar]
- 51.Boccaccini A, et al. Polymer/bioactive glass nanocomposites for biomedical applications: a review. Compos Sci Technol. 2010;70:1764–76. [Google Scholar]
- 52.Kwon S, et al. Silica-based mesoporous nanoparticles for controlled drug delivery. J Tissue Eng. 2013;4 doi: 10.1177/2041731413503357. 2041731413503357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Wu T, Kong J. In situ monitoring of intracellular controlled drug release from mesoporous silica nanoparticles coated with pH-responsive charge-reversal polymer. ACS Appl Mater Interfaces. 2014 doi: 10.1021/am5059519. [DOI] [PubMed] [Google Scholar]
- 54.Kim K, Fisher JP. Nanoparticle technology in bone tissue engineering. J Drug Target. 2007;15(4):241–52. doi: 10.1080/10611860701289818. [DOI] [PubMed] [Google Scholar]
- 55.Mansouri S, et al. Chitosan–DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 2004;57(1):1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto M, Tabata Y. Tissue engineering by modulated gene delivery. Adv Drug Deliv Rev. 2006;58(4):535–54. doi: 10.1016/j.addr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 57.Lungwitz U, et al. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60(2):247–66. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Petri-Fink A, et al. Effect of cell media on polymer coated superparamagnetic iron oxide nanoparticles (SPIONs): colloidal stability, cytotoxicity, and cellular uptake studies. Eur J Pharm Biopharm. 2008;68(1):129–37. doi: 10.1016/j.ejpb.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 59.Bankura KP, et al. Synthesis, characterization and antimicrobial activity of dextran stabilized silver nanoparticles in aqueous medium. Carbohydr Polym. 2012;89(4):1159–65. doi: 10.1016/j.carbpol.2012.03.089. [DOI] [PubMed] [Google Scholar]
- 60.Zhang S, et al. Polyethylenimine-PEG coated albumin nanoparticles for BMP-2 delivery. Biomaterials. 2010;31(5):952–63. doi: 10.1016/j.biomaterials.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 61.Schade A, et al. Magnetic nanoparticle based nonviral microRNA delivery into freshly isolated CD105(+) hMSCs. Stem Cells Int. 2014;2014:197154. doi: 10.1155/2014/197154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu EW, et al. Cationic nanostructured polymers for siRNA delivery in murine calvarial pre-osteoblasts. J Biomed Nanotechnol. 2014;10(6):1130–6. doi: 10.1166/jbn.2014.1823. [DOI] [PubMed] [Google Scholar]
- 63.Ding LF, et al. Study on bone mesenchymal stem cells transfected by polyethylene glycol/bone morphogenetic protein-2. Zhongguo Gu Shang. 2014;27(1):48–53. [PubMed] [Google Scholar]
- 64.Wang W, et al. Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. J Cell Mol Med. 2011;15(9):1989–98. doi: 10.1111/j.1582-4934.2010.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang P, et al. An MRI-visible non-viral vector bearing GD2 single chain antibody for targeted gene delivery to human bone marrow mesenchymal stem cells. PLoS One. 2013;8(10):e76612. doi: 10.1371/journal.pone.0076612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petri-Fink A, Hofmann H. Superparamagnetic iron oxide nanoparticles (SPIONs): from synthesis to in vivo studies—a summary of the synthesis, characterization, in vitro, and in vivo investigations of SPIONs with particular focus on surface and colloidal properties. IEEE Trans Nanobioscience. 2007;6(4):289–97. doi: 10.1109/tnb.2007.908987. [DOI] [PubMed] [Google Scholar]
- 67.Kamau SW, et al. Enhancement of the efficiency of non-viral gene delivery by application of pulsed magnetic field. Nucleic Acids Res. 2006;34(5):e40. doi: 10.1093/nar/gkl035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu K, et al. Ectopic study of calcium phosphate cement seeded with pBMP-2 modified canine bMSCs mediated by a non-viral PEI derivative. Cell Biol Int. 2012;36(2):119–28. doi: 10.1042/CBI20100848. [DOI] [PubMed] [Google Scholar]
- 69.Lu K, et al. An ectopic study of apatite-coated silk fibroin scaffolds seeded with AdBMP-2-modified canine bMSCs. J Biomater Sci Polym Ed. 2012;23(1–4):509–26. doi: 10.1163/092050610X552861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi J, et al. Nanoparticle delivery of the bone morphogenetic protein 4 gene to adipose-derived stem cells promotes articular cartilage repair in vitro and in vivo. Arthroscopy. 2013;29(12):2001–2011.e2. doi: 10.1016/j.arthro.2013.09.076. [DOI] [PubMed] [Google Scholar]
- 71.Tandon A, et al. BMP7 gene transfer via gold nanoparticles into stroma inhibits corneal fibrosis in vivo. PLoS One. 2013;8(6):e66434. doi: 10.1371/journal.pone.0066434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bulte JW, et al. Magnetodendrimers allow endosomal magnetic labeling and in vivo tracking of stem cells. Nat Biotechnol. 2001;19(12):1141–7. doi: 10.1038/nbt1201-1141. [DOI] [PubMed] [Google Scholar]
- 73.Jendelova P, et al. Imaging the fate of implanted bone marrow stromal cells labeled with superparamagnetic nanoparticles. Magn Reson Med. 2003;50(4):767–76. doi: 10.1002/mrm.10585. [DOI] [PubMed] [Google Scholar]
- 74.Edmundson M, Thanh NT, Song B. Nanoparticles based stem cell tracking in regenerative medicine. Theranostics. 2013;3(8):573–82. doi: 10.7150/thno.5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–96. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byers RJ, Hitchman ER. Quantum dots brighten biological imaging. Prog Histochem Cytochem. 2011;45(4):201–37. doi: 10.1016/j.proghi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 77.Muller-Borer BJ, et al. Quantum dot labeling of mesenchymal stem cells. J Nanobiotechnol. 2007;5:9. doi: 10.1186/1477-3155-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.He H, et al. Synthesis of mesoporous silica nanoparticle-oxaliplatin conjugates for improved anticancer drug delivery. Colloids Surf B: Biointerfaces. 2014;117:75–81. doi: 10.1016/j.colsurfb.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y, et al. A general strategy for dual-triggered combined tumor therapy based on template semi-graphitized mesoporous silica nanoparticles. Adv Healthc Mater. 2014;3(4):485–9. doi: 10.1002/adhm.201300324. [DOI] [PubMed] [Google Scholar]
- 80.Ricles LM, et al. Function of mesenchymal stem cells following loading of gold nanotracers. Int J Nanomedicine. 2011;6:407–16. doi: 10.2147/IJN.S16354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Babic M, et al. Poly(l-lysine)-modified iron oxide nanoparticles for stem cell labeling. Bioconjug Chem. 2008;19(3):740–50. doi: 10.1021/bc700410z. [DOI] [PubMed] [Google Scholar]
- 82.Kwoh DY, et al. Stabilization of poly-l-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta. 1999;1444(2):171–90. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 83.Jiang X, et al. Specific effects of surface amines on polystyrene nanoparticles in their interactions with mesenchymal stem cells. Biomacromolecules. 2010;11(3):748–53. doi: 10.1021/bm901348z. [DOI] [PubMed] [Google Scholar]
- 84.Lorenz MR, et al. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials. 2006;27(14):2820–8. doi: 10.1016/j.biomaterials.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 85.Jiang X, et al. Specific effects of surface carboxyl groups on anionic polystyrene particles in their interactions with mesenchymal stem cells. Nanoscale. 2011;3(5):2028–35. doi: 10.1039/c0nr00944j. [DOI] [PubMed] [Google Scholar]
- 86.Tautzenberger A, et al. Effect of functionalised fluorescence-labelled nanoparticles on mesenchymal stem cell differentiation. Biomaterials. 2010;31(8):2064–71. doi: 10.1016/j.biomaterials.2009.11.099. [DOI] [PubMed] [Google Scholar]
- 87.Chen YC, et al. The inhibitory effect of superparamagnetic iron oxide nanoparticle (Ferucarbotran) on osteogenic differentiation and its signaling mechanism in human mesenchymal stem cells. Toxicol Appl Pharmacol. 2010;245(2):272–9. doi: 10.1016/j.taap.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 88.Jasmin, et al. Optimized labeling of bone marrow mesenchymal cells with superparamagnetic iron oxide nanoparticles and in vivo visualization by magnetic resonance imaging. J Nanobiotechnol. 2011;9:4. doi: 10.1186/1477-3155-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang SY, J ZF, H HE, Hong CY, Yang HC, Wu CC, Lee YH. Dual immobilization and magnetic manipulation of magnetic nanoparticles. J Magn Magn Mater. 2008;320:2688–91. [Google Scholar]
- 90.Yanai A, et al. Focused magnetic stem cell targeting to the retina using superparamagnetic iron oxide nanoparticles. Cell Transplant. 2012;21(6):1137–48. doi: 10.3727/096368911X627435. [DOI] [PubMed] [Google Scholar]
- 91.Huang Z, et al. Deep magnetic capture of magnetically loaded cells for spatially targeted therapeutics. Biomaterials. 2010;31(8):2130–40. doi: 10.1016/j.biomaterials.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 92.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526–32. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 93.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13(5):555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 94.Shakesheff K, Cannizzaro S, Langer R. Creating biomimetic micro-environments with synthetic polymer–peptide hybrid molecules. J Biomater Sci Polym Ed. 1998;9(5):507–18. doi: 10.1163/156856298x00596. [DOI] [PubMed] [Google Scholar]
- 95.Ciapetti G, et al. Enhancing osteoconduction of PLLA-based nanocomposite scaffolds for bone regeneration using different biomimetic signals to MSCs. Int J Mol Sci. 2012;13(2):2439–58. doi: 10.3390/ijms13022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chou YF, et al. The effect of biomimetic apatite structure on osteoblast viability, proliferation, and gene expression. Biomaterials. 2005;26(3):285–95. doi: 10.1016/j.biomaterials.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 97.Kim SS, et al. A poly(lactide-co-glycolide)/hydroxyapatite composite scaffold with enhanced osteoconductivity. J Biomed Mater Res A. 2007;80(1):206–15. doi: 10.1002/jbm.a.30836. [DOI] [PubMed] [Google Scholar]
- 98.Chou YF, Dunn JC, Wu BM. In vitro response of MC3T3-E1 pre-osteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomed Mater Res B Appl Biomater. 2005;75(1):81–90. doi: 10.1002/jbm.b.30261. [DOI] [PubMed] [Google Scholar]
- 99.Tsang EJ, et al. Osteoblast interactions within a biomimetic apatite microenvironment. Ann Biomed Eng. 2011;39(4):1186–200. doi: 10.1007/s10439-010-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo J, et al. Restoration of critical-size defects in the rabbit mandible using porous nanohydroxyapatite–polyamide scaffolds. Tissue Eng Part A. 2012;18(11–12):1239–52. doi: 10.1089/ten.TEA.2011.0503. [DOI] [PubMed] [Google Scholar]
- 101.Tran N, Webster TJ. Increased osteoblast functions in the presence of hydroxyapatite-coated iron oxide nanoparticles. Acta Biomater. 2011;7(3):1298–306. doi: 10.1016/j.actbio.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 102.Goto K, et al. Bioactive bone cements containing nano-sized titania particles for use as bone substitutes. Biomaterials. 2005;26(33):6496–505. doi: 10.1016/j.biomaterials.2005.04.044. [DOI] [PubMed] [Google Scholar]
- 103.Horch RA, et al. Nanoreinforcement of poly(propylene fumarate)-based networks with surface modified alumoxane nanoparticles for bone tissue engineering. Biomacromolecules. 2004;5(5):1990–8. doi: 10.1021/bm049768s. [DOI] [PubMed] [Google Scholar]
- 104.Demais V, et al. Diversity of bone matrix adhesion proteins modulates osteoblast attachment and organization of actin cytoskeleton. Morphologie. 2014 doi: 10.1016/j.morpho.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 105.Lapointe VL, et al. Nanoscale topography and chemistry affect embryonic stem cell self-renewal and early differentiation. Adv Healthc Mater. 2013;2(12):1644–50. doi: 10.1002/adhm.201200382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bugnicourt G, et al. Nanoscale surface topography reshapes neuronal growth in culture. Langmuir. 2014;30(15):4441–9. doi: 10.1021/la5001683. [DOI] [PubMed] [Google Scholar]
- 107.Kiang JD, et al. Dynamic and reversible surface topography influences cell morphology. J Biomed Mater Res A. 2013;101(8):2313–21. doi: 10.1002/jbm.a.34543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hsu LC, et al. Effect of micro- and nanoscale topography on the adhesion of bacterial cells to solid surfaces. Appl Environ Microbiol. 2013;79(8):2703–12. doi: 10.1128/AEM.03436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rizzello L, et al. Molecular response of Escherichia coli adhering onto nanoscale topography. Nanoscale Res Lett. 2012;7(1):575. doi: 10.1186/1556-276X-7-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007;59(4–5):339–59. doi: 10.1016/j.addr.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 111.Leach MK, et al. Electrospinning fundamentals: optimizing solution and apparatus parameters. J Vis Exp. 2011(47) doi: 10.3791/2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen MC, Sun YC, Chen YH. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013;9(3):5562–72. doi: 10.1016/j.actbio.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 113.Kuo YC, Hung SC, Hsu SH. The effect of elastic biodegradable polyurethane electrospun nanofibers on the differentiation of mesenchymal stem cells. Colloids Surf B: Biointerfaces. 2014;122:414–22. doi: 10.1016/j.colsurfb.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 114.Balaji Raghavendran HR, et al. A comparative study on in vitro osteogenic priming potential of electron spun scaffold PLLA/HA/Col, PLLA/HA, and PLLA/Col for tissue engineering application. PLoS One. 2014;9(8):e104389. doi: 10.1371/journal.pone.0104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang C, et al. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloids Surf B: Biointerfaces. 2014;122:270–6. doi: 10.1016/j.colsurfb.2014.06.061. [DOI] [PubMed] [Google Scholar]
- 116.Poologasundarampillai G, et al. Cotton-wool-like bioactive glasses for bone regeneration. Acta Biomater. 2014;10(8):3733–46. doi: 10.1016/j.actbio.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 117.Lai GJ, et al. Composite chitosan/silk fibroin nanofibers for modulation of osteogenic differentiation and proliferation of human mesenchymal stem cells. Carbohydr Polym. 2014;111:288–97. doi: 10.1016/j.carbpol.2014.04.094. [DOI] [PubMed] [Google Scholar]
- 118.Kim BR, et al. In vitro and in vivo studies of BMP-2-loaded PCL–gelatin–BCP electrospun scaffolds. Tissue Eng Part A. 2014 doi: 10.1089/ten.tea.2014.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim H, et al. Mechanically-reinforced electrospun composite silk fibroin nanofibers containing hydroxyapatite nanoparticles. Mater Sci Eng C Mater Biol Appl. 2014;40:324–35. doi: 10.1016/j.msec.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 120.Linh NT, Lee KH, Lee BT. Functional nanofiber mat of polyvinyl alcohol/gelatin containing nanoparticles of biphasic calcium phosphate for bone regeneration in rat calvaria defects. J Biomed Mater Res A. 2013;101(8):2412–23. doi: 10.1002/jbm.a.34533. [DOI] [PubMed] [Google Scholar]
- 121.Xue J, et al. Drug loaded homogeneous electrospun PCL/gelatin hybrid nanofiber structures for anti-infective tissue regeneration membranes. Biomaterials. 2014;35(34):9395–405. doi: 10.1016/j.biomaterials.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 122.Hoffman K, et al. Airbrushed composite polymer Zr-ACP nanofiber scaffolds with improved cell penetration for bone tissue regeneration. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2014.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bruinink A, et al. Addition of nanoscaled bioinspired surface features: a revolution for bone-related implants and scaffolds? J Biomed Mater Res A. 2013 doi: 10.1002/jbm.a.34691. [DOI] [PubMed] [Google Scholar]


