Abstract
We report potent radiosensitization of prostate cancers in vitro and in vivo using goserelin-conjugated gold nanorods. Progressive receptor-mediated internalization of conjugated nanorods over time increases the radiation interaction cross-section of cells and contributes to the effects observed in vitro. The low concentrations of gold required, the long interval between injection of nanoparticles and radiation, and the use of megavoltage radiation to generate radiosensitization in vivo foretell the possibility of eventual clinical translation of this approach.
Keywords: megavoltage radiation therapy, gold nanoparticles, tumor targeted delivery, conjugated, prostate cancer, goserelin acetate
I. Background
The ability of gold nanoparticles (AuNPs) to enhance the effect of physical radiation dose on tumor cells has been previously shown. (1-12) Systemic injection of nanoparticles results in a preferential accumulation in the typically “leaky” tumor vasculature (the “enhanced permeability and retention” [EPR]. (6, 13, 14) The radiosensitization effect of AuNPs is thought to result from an increased number of photoelectric absorption events and low-range electron showers that follow the interaction of photons with the increased number of electrons present in gold. Hainfield et al. demonstrated that administration of AuNPs followed by kilovolt (kV) radiation substantially improved the 1-year survival rate of mice bearing subcutaneous mammary carcinoma (86%) over radiation alone (20%). (1) The aforementioned curative effect of combined radiotherapy and AuNPs was observed in mice following intravenous administration of approximately 1.35 mg of gold per gram of body weight. Immediate radiation – 2 min after AuNPs injection – guaranteed that the transient gold content encountered in the tumor, primarily within the vasculature, increased interaction probability with ionizing radiation, leading to enhanced biological effect. The photoelectric absorption is most prominent at kV energies near the binding energies of the lower shells of electrons in gold – a fact that has hindered the translation of this technology to the clinic due to the inherently shallow penetration of kV energies. Attempts at using the clinically-utilized megavoltage (MV) energies and clinically non-prohibitive amounts of gold have shown only modest dose enhancement from AuNPs, <17% in vitro as reported by Chitrani et al. (4)
We hypothesized that the MV radiation dose-enhancing effects of AuNPs could be amplified if more particles were taken up by the tumor cells, leading to greater cellular toxicity from the short-range secondary electron cascade. Majority of the successful studies combining AuNPs and ionizing radiation utilize a polyethelene glycol coating (PEGylation) – or similar coating molecules – to prevent nanoparticles from being rapidly eliminated from the body while still staying in the blood stream, ultimately reducing uptake of these particles by the reticuloendothelial cells, consequently enhancing their accumulation within tumors. (6, 10, 12, 15, 16) Modest radiosensitization has been reported with megavoltage irradiation of pegylated AuNPs in vitro with widely varying results based on the cell lines being treated and the treatment conditions where high concentrations of particles remain in the media (are not washed off) during irradiation. (3, 4, 6) In vivo, most of these PEGylated nanoparticles tend to accumulate in the perivascular space, however, with limited uptake by cells. We sought to further increase the amount and specificity of gold accumulation inside cancper cells by conjugating the nanoparticles to a small peptide targeted to a receptor preferentially overexpressed by tumors.
Radiation dose escalation has been demonstrated to be of clinical benefit in some cancers but not all. Prostate cancer is one instance where there is demonstrable therapeutic value to escalated doses of radiation to the primary tumor while sparing adjacent normal tissues. Recent clinical trial results have shown that overall survival is directly correlated to cumulative tumor dose due to more efficient elimination of radioresistent clones at the primary site. In addition, a cross-sectional analysis of these three major clinical trials revealed a linear correlation between total tumor dose and improvements in biochemical control. Moreover, modest local dose enhancements (between 11-24%) result in great improvements in the overall survival time (from 10% to 200%). (17-19)
Our search for a suitable targeting ligand for prostate cancer led us to choose goserelin acetate, a synthetic luteinizing hormone–releasing hormone (LHRH) analogue that binds to the LHRH receptor overexpressed in the vast majority of prostate cancers(20) and works by reducing the secretion of gonadotropins, which in turn reduces the testicular secretion of testosterone. (21) Concurrent administration of doses of goserelin acetate that result in sustained testosterone suppression to near-castrate levels improves local control and survival of locally advanced prostate cancer patients treated with radiation therapy. (22) However, no supra-additive radiosensitization was observed in vitro in human prostate cancer cells treated with low concentrations of goserelin (23) – the concentrations we evaluate in the present study – consistent with the conclusion that testosterone suppression does not sensitize prostate cancers to radiation therapy but the combination causes additive cytotoxicity and growth inhibitory effects that are clinically meaningful.(24) We then reasoned that the affinity of goserelin for prostate cancer cells, given the plentiful expression of type I and type II gonadotropin receptors on the membranes of such cells (20), could be exploited to enhance the accumulation of gold nanoparticles in prostate cancer cells for radiation therapy, and thus enhance the biological effects of radiation compared with untargeted AuNPs that accumulate in the extracellular and perivascular compartments. Here we describe our successful conjugation of goserelin acetate to the surface of AuNPs (gAuNRs) at a ratio of approximately 60 molecules per nanoparticle. We thereafter report our in vitro and in vivo investigations into the radiosensitizing effects of targeted gold nanoparticles when applying radiation in the MV energy range.
II. Methods
II.A Construction of Goserelin Conjugated AuNPs
Bare rod-shaped gold nanoparticles, gold nanorods (herein denoted AuNRs), were synthesized as described in the supplementary information. Further, these nanoparticles were reacted with thiol-terminated methoxy polyethylene glycol (PEG) to generate PEGylated AuNRs (pAuNRs). AuNRs were conjugated to goserelin via PEG as outlined in the cartoon (Figure. 1A). Briefly, goserelin acetate (Sigma) stock solutions were made by dissolving the peptide powder in deionized water at 5 mg/mL (0.8 mM), aliquot and kept at -80°C. 355 μL of ethyl (dimethylaminopropyl) carbodiimide, EDC (Thermo Scientific), solution at 2 mM was added to 100 μL of 1 mM thiol-PEG-carboxylate (SH-PEG-COOH) solution, immediately followed by the addition of 326 μL of 5 mM sulfo-N-hydroxysuccinimide, NHS (Thermo Scientific), solution resulting in a semi-stable amino-reactive SH-PEG-NHS-ester. Subsequently, 382 μL of 0.8 mM goserelin peptide solution was reacted with this mixture in glass vials gently agitated on ice and then allowed to equilibrate under constant gentle shaking for 30 min at 4°C. A molar ratio of goserelin to PEG of 1:1 was determined to be optimal for maximizing the number of goserelin molecules linked to the surface of each AuNRs in the next step. After creation of the SH-PEG-goserelin, the vial was again placed on ice and 3 mL of AuNRs solution (at OD=3) was added drop-wise to the vial. The entire solution was then kept overnight at 4°C under constant gentle mixing to allow the thiol end of the SH-PEG-goserelin to link to the surface of the gold, forming AuNR-PEG-goserelin (gAuNR) in solution. The particles were then centrifuged at 13,000 rpm for 10 min at 4°C followed by collection of the pellet and the supernatant was re-centrifuged until no pellet was observed – this resulted in elimination of reagents not bound to the AuNR and an AuNR recovery efficiency of >70%. Phosphate-buffered saline (PBS) (HyClone) was used as the conjugation buffer to maintain pH close to neutral at all steps. Conjugated particles were stored at 4°C. Spectrophotometry noted an absorbance peak of 264 nm for goserelin (within the range of absorbance of most proteins) and distinct from the AuNR peak (Figure. 1B from supplementary information shows this data), allowing quantification of the relative concentration of each in a conjugate using standard calibration curves. PEG with a straight carbon chain [lacking any benzene ring containing amino acids (tryptophan and tyrosine)] shows a very small peak near the 200 nm limit of detection of our spectrophotometer. Hence, we disregarded the contribution of PEG to the final spectrum in our calculations.
Figure 1.
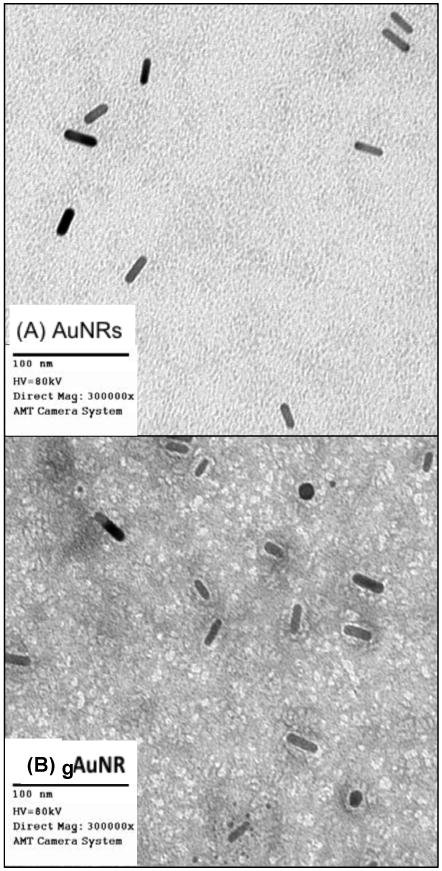
Architecture of goserelin-conjugated gold nanorods (gAuNR). Transmission electron microscopy images of gold nanorods (AuNRs) before conjugation (A), and (B) after conjugation (gAuNR). Detailed information for manufacture of gAuNR can be found in the supplementary material.
II.B Intracellular Localization and Uptake of AuNRs
The conjugated nanoparticles (gAuNRs) were then incubated with PC3 cells, a non-androgen-dependent prostate cancer cell line obtained from the American Type Culture Collection. Standard cell culture conditions were used and are detailed in the supplementary information. The following treatment conditions were used to evaluate uptake in vitro and radiosensitization effects of the conjugated gAuNRs: (I) cells treated with a 0.3 optical density (OD) solution of pAuNRs in the cell culture medium; (II) cells treated with a 0.3 OD solution of gAuNRs in the medium; and (III) cells treated with regular medium only, without nanoparticles. The incubation period ranged from 15 min to 72 h. The amount of gold retained in the cells after incubation was quantitatively assessed via inductively coupled mass spectrometry (ICP-MS). Over time, cells from each sample were subjected to TEM for visualization (described further in supplementary information).
II.C Clonogenic Assessment
Clonogenic assays were done as follows. Briefly, after 24 h of incubation, non-internalized nanoparticles were washed out of the medium and the cells were irradiated with a 6 MV radiation beam (Varian Clinac 6EX). Details on cell cultures and treatment conditions are shown in the supplementary information. Radiosensitization was evaluated by comparing the dose necessary to reduce the surviving fraction (SF) to 20%, and a radiosensitization enhancement factor (REF) was calculated as described in equation 1, where D20%(control) is the dose necessary to reduce the fraction of viable cells after radiation alone to 20% of that when treated without radiation, and D20%(AuNR) represents the dose necessary to reduce the fraction of viable cells after radiation and nanoparticles to 20% of that when treated with nanoparticles in the absence of radiation:
| (Eq. 1) |
Clonogenicity was assessed after irradiation with 0 to 6 Gy. Additionally, we measured the survival fraction of cells treated with goserelin acetate at two concentrations (0.1 μM and 40 μM) for 24 h prior to radiation.
II.D Biodistribution of Targeted gAuNRs
Next, thirty-six Foxn1nu mice received tail-vein injections of gAuNRs or pAuNRs (300 μg Au per mouse), with the biodistribution assessed 6 h or 24 h later. The designed strategy aimed to preserve the translational potential of this treatment combination, assessing intravenous administration of small gold contents (equivalent to approximately 10 μg of Au per g of body weight). For the biodistribution study, gold content was quantified by ICP-MS in brain, lung, heart, liver, spleen, gastrointestinal tract, kidney, muscle, and tumor specimens. Additional experimental details are given in the supplementary information.
II.E In Vivo Assessment of Nanoparticle-Mediated Tumor-Growth Delay
Using the targeted AuNPs, we studied tumor regrowth kinetics in xenograft subcutaneous models of prostate cancer. Sixty-fourmice with PC3 cells subcutaneously implanted in the thighs were treated with intravenously administered gAuNRs or pAuNRs, and subjected to local irradiation with a single 5 Gy dose. Megavoltage radiation therapy was delivered with the aid of a clinical linear accelerator (Varian Clinac 6EX) 24 h after nanoparticles' injection. Tumors in all mice were allowed to reach 7-8 mm in longest axis before receiving (i) no treatment (control), (ii) RT alone, (iii) gAuNR plus RT, or (iv) pAuNR plus RT. A negative control group was additionally considered in the study with mice receiving 100 μL of goserelin acetate solution in PBS at 40 μM 24 h prior to RT; no radiosensitization was observed in this group (see supplementary information). Radiosensitization was assessed comparing the time for tumors to triple in volume.
III. Results
After conjugation, nanoparticles were subjected to transmission electron microscopy (TEM) to verify their dispersion and coating. TEM images of these bare AuNRs and the gAuNRs, showed in Figure 1, reveal halos that form around the rods after they are coated.
We next assessed the zeta potential (surface charge) of the AuNRs in different configurations. The zeta potential is an alternative method of conceptualizing the conjugation process in terms of the net charge at the surface of the nanoparticles. As shown in the inset table on Figure 1B of the supplementary information, the strong positive charge on bare AuNRs due to the presence of the surfactant on the surface, cetyl trimethylammonium bromide (CTAB), was neutralized with thiol-terminated methoxy polyethylene glycol (PEG) to create PEGylated AuNRs (herein referred to as pAuNRs) where reactive PEG displaces CTAB on the AuNR surface. The zeta potential of these nanoparticles was -1.1±0.8 mV. To create goserelin-conjugated AuNRs (gAuNRs), heterobifunctional (thiol and carboxyl terminated) PEG was first linked to the zwitterionic goserelin peptide via classical ethyl (dimethylaminopropyl) carbodiimide (EDC)-N-hydroxysuccinimide (NHS) coupling and then reacted with bare AuNRs (as described in the supplementary information). The resulting targeted AuNR has a mildly negative zeta potential of -2.2±0.5 mV.
Both nanoparticles, gAuNRs or pAuNRs, were incubated with prostate cancer cells to investigate cellular internalization over time. The uptake of these particles is illustrated in Figure 2. Cellular uptake was strongly improved when goserelin was conjugated to AuNRs (Figure 2A). We qualitatively observed from the series of TEM images in Figure 2 that the location of the particles within the cells depended on the type of particle: gAuNRs accumulated at higher concentrations inside the cells, preferentially within endosome vesicles (big clusters with several nanoparticles) distributed across the cytoplasm. The pAuNRs, by contrast, tended to collect singly in the juxta-membrane region intracellularly. The mechanisms by which the conjugated nanoparticles and cells interact require more extensive investigation and are beyond the scope of this manuscript. Quantitative ICP-MS data (Figure 2B) demonstrates that gAuNRs were retained within cells over time, with concentrations peaking at 24 h at levels 5-fold higher than pAuNRs.
Figure 2.
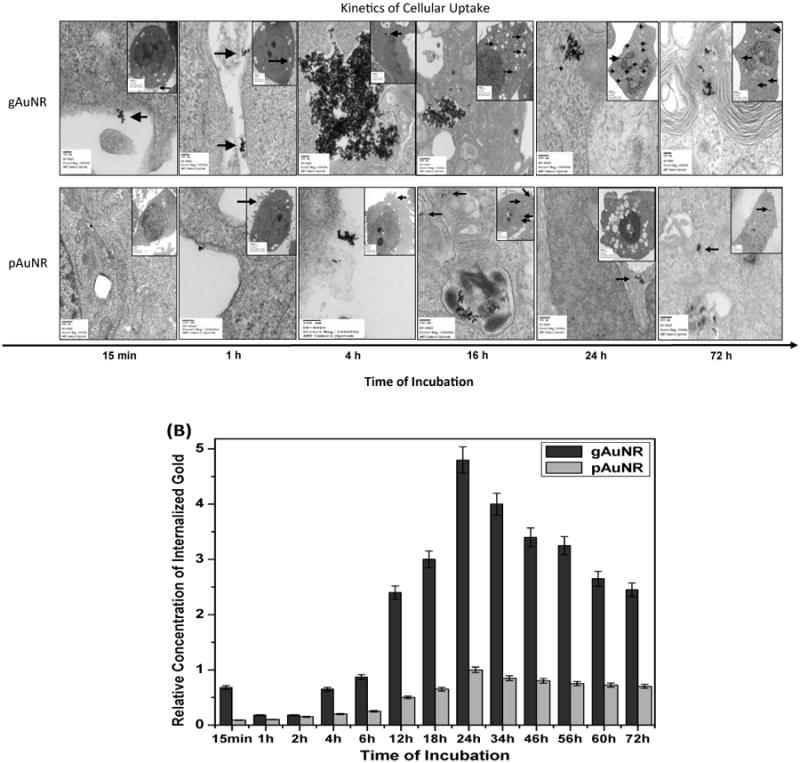
Kinetics of cellular internalization of gold nanoparticles: (A) Transmission electron microscopy images of PC3 cells treated with goserelin-conjugated gold nanorods (gAuNRs) and pegylated gold nanorods (pAuNR) for different times of incubation between 15 min and 72 h, demonstrating cell-surface binding, internalization, entrapment within endosomes, and persistence within cells even beyond 24 h. Arrows point to AuNRs. Note greater accumulation of AuNRs intracellular in the gAuNR group compared to the pAuNR group. (B) Quantitative analysis of cellular uptake by ion coupled plasma mass-spectroscopy.
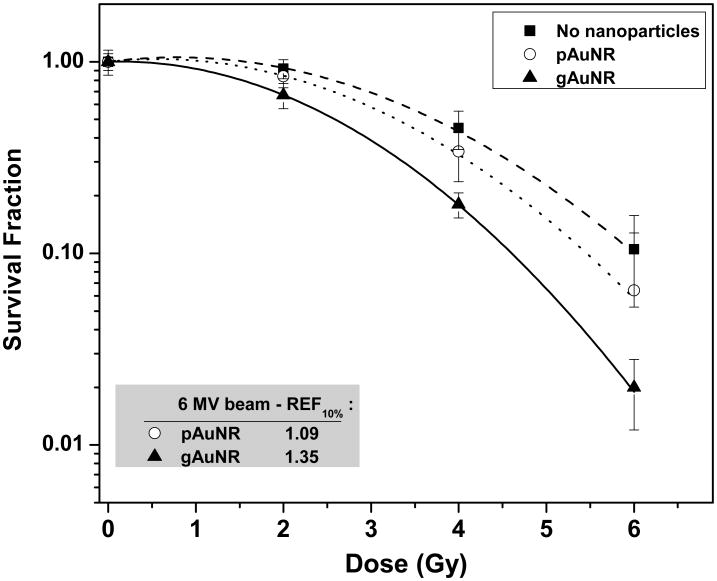
Next, radiosensitization was quantified in vitro, after incubation with nanoparticles for 24 h. Clonogenicity was assessed after irradiation with 0 to 6 Gy, and the results are shown in Figure 3. The inset table lists the REF20% calculated for curves fitted according to the linear-quadratic (L-Q) model for the response of these cells to radiation. Treatment with either type of nanoparticle led to radiosensitization, but this effect was more significant in cells treated with gAuNR than with pAuNR (1.36± 0.06 vs. 1.19± 0.04). No radiosensitization was observed in groups of cells treated with goserelin acetate solution alone and radiation. These negative control results are presented in Figure 2A of the supplementary information. The statistical coefficients of determination (R2) obtained for the L-Q fitting applied to the clonogenic curves were >0.996 for modeling curves of cells treated with gAuNR and >0.993 for cells treated with pAuNR.
Figure 3.
Clonogenic survival fraction of PC3 cells treated with goserelin-conjugated gold nanorods (gAuNRs) and pegylated gold nanorods (pAuNRs) for 24h prior to irradiation with 6MV beam. Inset table shows radiosensitization enhancement fraction (REF) obtained at 10% survival fraction. Conjugated nanoparticles, gAuNRs, were found to radiosensitize cancer cells significantly more than pegylated gold nanoparticles (p<0.0001).
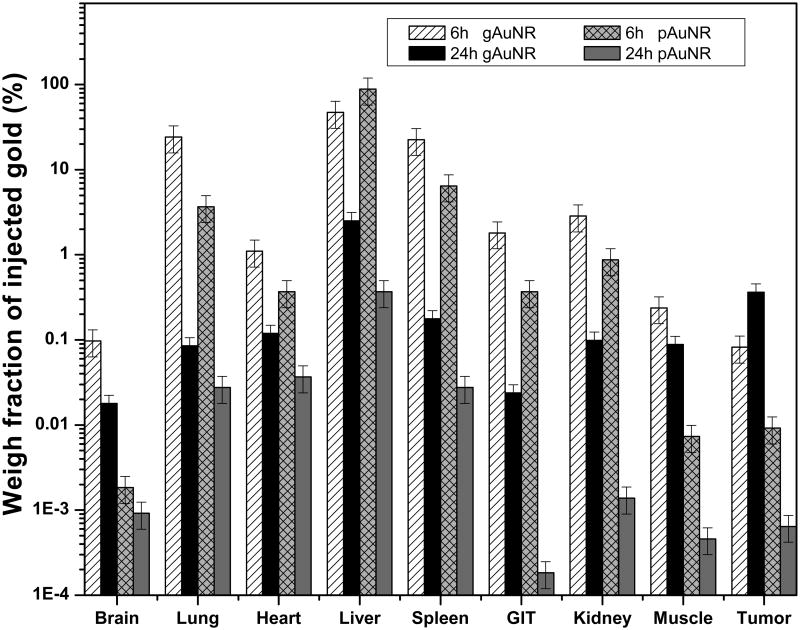
The translation to a preclinical level, however, required a complete biodistribution study. Metallic nanoparticles are typically cleared from the body quickly by the reticuloendothelial system, which results in large amounts of nanoparticles accumulating in the liver/spleen. To address the question of whether AuNRs circulate in the bloodstream long enough to allow tumor accumulation, we assessed the kinetics of biodistribution of gAuNRs and pAuNRs in mice. Results are shown in Figure 4. Both types of nanoparticles were eliminated by the reticuloendothelial system. The targeted gAuNRs, although also cleared via the liver and spleen, were still retained in the tumor at 24 h at concentrations 3 times higher than the non-targeted pAuNRs (Figure 4). Finally, combining our findings of favorable in vivo biodistribution of the gAuNRs and the enhanced sensitization to megavoltage radiation firstly observed in vitro, we assessed tumor tumor-growth in vivoto MV RT in combination with our targeted nanoparticles.
Figure 4.
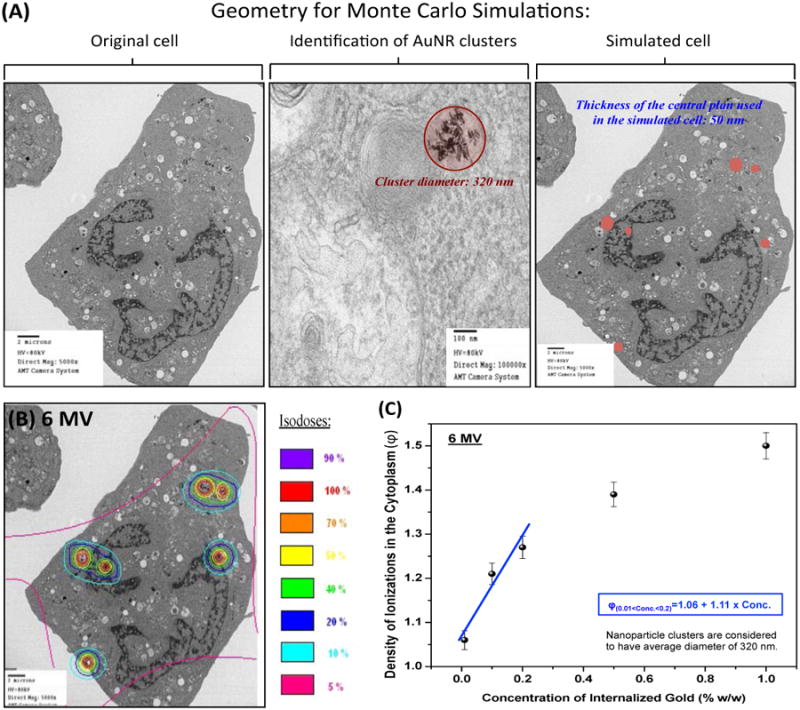
Monte Carlo simulation of gold nanoparticles internalized in a single cell: (A) Virtual geometry, including clusters, obtained from transmission electron microscopy image of a cell incubated with goserelin-conjugated gold nanoparticles (gAuNPs) for 24 h. (B) Isodoses formed by secondary electrons in the cytoplasm; Color scale indicates the normalized dose clouds around nanoparticle clusters. (C) Ratio of density of ionizations (Φ) in the cytoplasm of a cell with or without nanoparticles as a function of total gold concentration taken up by the cell.
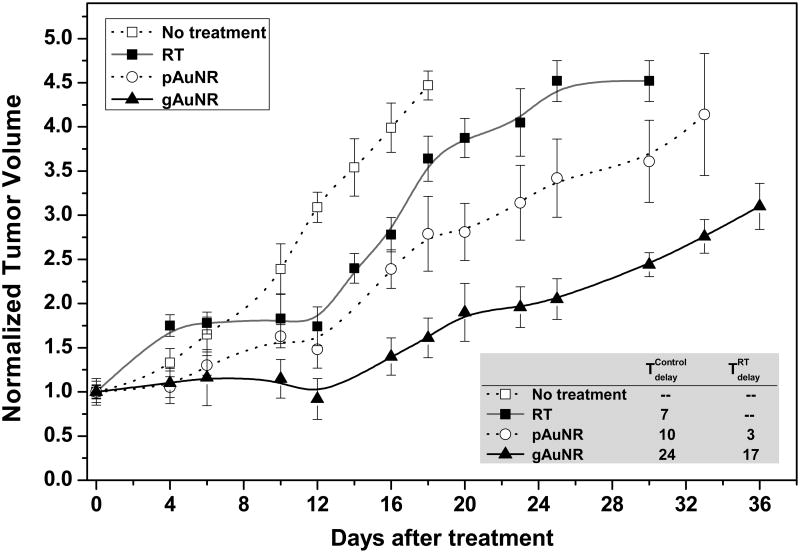
Tumor-growth delays were significantly enhanced in the mice given gAuNR and RT (Figure 5). The inset table in Figure 5 shows that treatment with gAuNR + RT delayed tumor regrowth by 17±1 days (p<0.001) compared to RT alone. Treatment with pAuNR + RT, however, did not delay tumor regrowth (3±2 days; p=0.78) compared to RT alone.
Figure 5.
Biodistribution of goserelin-conjugated gold nanorods (gAuNRs) and pegylated gold nanorods (pAuNR) in vivo over time. Total amount of gold accumulated in the organs was detected by inductively coupled plasma mass spectrometry at 6 h and 24 h time points after intravenous injection of 300 μg of gold. GIT: gastrointestinal tract.
IV. Discussion
Our results demonstrate that megavoltage radiosensitization is achievable in vivo using tumor targeted AuNPs administered intravenously at a low concentration of 10 mg/Kg body weight of gold. We surmise that this is due to the clusters of nanoparticles formed within the cytoplasm (see Figure 2A) ultimately increased the interaction cross-section of radiation with the cell, resulting in increased yield of secondary electrons. (4, 10, 11, 26-33) Previous studies have also found reductions in the SF of a variety of cell types irradiated in the presence of AuNPs, often at high concentrations that are not washed off from the media prior to irradiation. In this study, the gAuNRs produced greater dose enhancement than the pAuNRs because they accumulate in the cells at concentrations approximately 5 times higher than simple PEGylated nanoparticles, corroborating our hypothesis that the total amount of gold retained within the cells (i.e., concentration of nanoparticles accumulated in endosomes dispersed in the cytoplasm) is a significant contributor to radiosensitization, especially for megavoltage radiation.
The in vitro results suggest that once gAuNPs are confined in prostate tumor cells, they locally increase the yield of short-range secondary electrons generated after irradiation, enhancing the probability of oxidative stress and cellular damage. The yield of secondary electrons from the nanoparticles is proportional to the radiation quality of the incident primary beam, as demonstrated previously (34), and is driven by parameters as such gold concentration in the tumor and organization inside the cells (26) (i.e. targeted vs. non-tumor-specific nanoparticles).
Collectively, these results suggest that constructing gold nanoparticles with tumor-specific antibodies or other molecules with high affinity for cancer cells can lead to retention within the cells sufficient to lead to local dose enhancement at MV energy range.
The biodistribution data shows only a slight increase in absolute quantity of gold within tumors after conjugation with goserelin, and the clonogenic survival data demonstrated only conjugated AuNPs (that are actively internalized and stored in the cell over time) resulted in radiosensitization.
In summary, we found that goserelin-targeted gAuNRs engineered to exploit two independent mechanisms of tumor accumulation in vivo (EPR effect as a consequence of leaky tumor vasculature and active uptake by target cells) which, in combination with megavoltage RT, led to tumor-growth delays in mice with heterotopic prostate cancers. These in vivo effects were observed with a dose of gold within tumors that is considerably less than that reported by other groups,(1, 5, 35-37) using megavoltage radiation therapy, and at a time point beyond when particles are largely confined to the vascular compartment or immediate perivascular space only,(5) all of which suggest that this strategy is of clinical translational relevance. More importantly, significant radiosensitization to MV radiation was not observed with unconjugated AuNRs despite their intratumoral concentration being only 3-fold less than that of conjugated AuNRs. This suggests that radiosensitization is improved by active targeting that leads to cellular internalization of nanoparticles and the consequent increase in ionization density within the cytoplasm, rather than merely passive accumulation of nanoparticles in the perivascular space by EPR.
Taken together, these findings suggest that conjugation of nanoparticles to tumor-specific antigens that promote internalization within cancer cells causes radiosensitization and that goserelin-conjugated AuNRs can be effective prostate cancer radiosensitizers when used with megavoltage radiation.
Supplementary Material
Figure 6.
Tumor-growth in nude mouse xenograft models with and without treatment with gold nanoparticles prior to radiation therapy with 6 MV beam. Inside: Table showing tumor-growth delays comparing groups that received either goserelin-conjugated gold nanorods (gAuNR) and radiation therapy (RT) (
 ) or pegylated gold nanorods (pAuNR) and RT (
) or pegylated gold nanorods (pAuNR) and RT (
 ) with the groups that received only radiation for treatment (
) with the groups that received only radiation for treatment (
 ) and no treatment (
) and no treatment (
 ).
).
Acknowledgments
We thank Ms. Christine Wogan (Program Manager, Divisional Publications, Radiation Oncology Department, MD Anderson Cancer Center) for carefully editing the manuscript and Kenneth Dunner Jr. for assistance with transmission electron microscopy.
This work was supported in part by NIH grant CA155446 and DoD/PCRP grant W81XWH-12-1-0198 to S.K., and CAPES/REDE NANOBIOTEC, FAPESP and CNPq to T.W and P.N. This work was also partially supported by the MD Anderson Cancer Center support grant P30 CA16672 and the John E. and Dorothy J. Harris Endowed Professorship to SK.
References
- 1.Hainfeld JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Physics in Medicine and Biology. 2004;49(18):N309–N15. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 2.Rahman WN, Bishara N, Ackerly T, He CF, Jackson P, Wong C, et al. Enhancement of radiation effects by gold nanoparticles for superficial radiation therapy. Nanomedicine-Nanotechnology Biology and Medicine. 2009;5(2):136–42. doi: 10.1016/j.nano.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Roa W, Zhang XJ, Guo LH, Shaw A, Hu XY, Xiong YP, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20(37) doi: 10.1088/0957-4484/20/37/375101. [DOI] [PubMed] [Google Scholar]
- 4.Chithrani DB, Jelveh S, Jalali F, van Prooijen M, Allen C, Bristow RG, et al. Gold Nanoparticles as Radiation Sensitizers in Cancer Therapy. Radiation Research. 2010;173(6):719–28. doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 5.Hainfeld JF, Dilmanian FA, Zhong Z, Slatkin DN, Kalef-Ezra JA, Smilowitz HM. Gold nanoparticles enhance the radiation therapy of a murine squamous cell carcinoma. Physics in Medicine and Biology. 2010;55(11):3045–59. doi: 10.1088/0031-9155/55/11/004. [DOI] [PubMed] [Google Scholar]
- 6.Liu CJ, Wang CH, Chen ST, Chen HH, Leng WH, Chien CC, et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Physics in Medicine and Biology. 2010;55(4):931–45. doi: 10.1088/0031-9155/55/4/002. [DOI] [PubMed] [Google Scholar]
- 7.Hirn S, Semmler-Behnke M, Schleh C, Wenk A, Lipka J, Schaffler M, et al. Particle size-dependent and surface charge-dependent biodistribution of gold nanoparticles after intravenous administration. Eur J Pharm Biopharm. 2011;77(3):407–16. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polf JC, Bronk LF, Driessen WHP, Arap W, Pasqualini R, Gillin M. Enhanced relative biological effectiveness of proton radiotherapy in tumor cells with internalized gold nanoparticles. Applied Physics Letters. 2011:193702–3. doi: 10.1063/1.3589914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng F, Song K, Xing JZ, Yuan CZ, Yan S, Yang QF, et al. Thio-glucose bound gold nanoparticles enhance radio-cytotoxic targeting of ovarian cancer. Nanotechnology. 2011;22(28) doi: 10.1088/0957-4484/22/28/285101. [DOI] [PubMed] [Google Scholar]
- 10.Yasui H, Takeuchi R, Nagane M, Meike S, Nakamura Y, Yamamori T, et al. Radiosensitization of tumor cells through endoplasmic reticulum stress induced by PEGylated nanogel containing gold nanoparticles. Cancer Lett. 2014;347(1):151–8. doi: 10.1016/j.canlet.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Khoshgard K, Hashemi B, Arbabi A, Rasaee MJ, Soleimani M. Radiosensitization effect of folate-conjugated gold nanoparticles on HeLa cancer cells under orthovoltage superficial radiotherapy techniques. Physics in Medicine and Biology. 2014;59(9):2249–63. doi: 10.1088/0031-9155/59/9/2249. [DOI] [PubMed] [Google Scholar]
- 12.Geng F, Xing JZ, Chen J, Yang R, Hao Y, Song K, et al. Pegylated Glucose Gold Nanoparticles for Improved In-Vivo Bio-Distribution and Enhanced Radiotherapy on Cervical Cancer. Journal of Biomedical Nanotechnology. 2014;10(7):1205–16. doi: 10.1166/jbn.2014.1855. [DOI] [PubMed] [Google Scholar]
- 13.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. Journal of Controlled Release. 2000;65(1-2):271–84. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 14.Sancey L, Barbier E, Hirsjarvi S, Dufort S, Benoit JP, Remy C, et al. Enhanced Permeability and Retention (EPR) effect in tumors: characterization by MRI and fluorescence imaging. Bulletin Du Cancer. 2011;98:89. [Google Scholar]
- 15.Lipka J, Semmler-Behnke M, Sperling RA, Wenk A, Takenaka S, Schleh C, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31(25):6574–81. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Arnida, Janat-Amsbury MM, Ray A, Peterson CM, Ghandehari H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm. 2011;77(3):417–23. doi: 10.1016/j.ejpb.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuban DA, Tucker SL, Dong L, Starkschall G, Huang EE, Cheung MR, et al. Long-term results of the M. D. Anderson randomized dose-escalation trial for prostate cancer. International Journal of Radiation Oncology Biology Physics. 2008;70(1):67–74. doi: 10.1016/j.ijrobp.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 18.Zietman AL, Bae K, Slater JD, Shipley WU, Efstathiou JA, Coen JJ, et al. Randomized Trial Comparing Conventional-Dose With High-Dose Conformal Radiation Therapy in Early-Stage Adenocarcinoma of the Prostate: Long-Term Results From Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106–11. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spratt DE, Pei X, Yamada J, Kollmeier MA, Cox B, Zelefsky MJ. Long-term Survival and Toxicity in Patients Treated With High-Dose Intensity Modulated Radiation Therapy for Localized Prostate Cancer. International journal of radiation oncology, biology, physics. 2013;85(3):686–92. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halmos G, Arencibia JM, Schally AV, Davis R, Bostwick DG. High incidence of receptors for luteinizing hormone-releasing hormone (LHRH) and LHRH receptor gene expression in human prostate cancers. Journal of Urology. 2000;163(2):623–9. [PubMed] [Google Scholar]
- 21.Montagnani Marelli M, Moretti RM, Mai S, Januszkiewicz-Caulier J, Motta M, Limonta P. Type I gonadotropin-releasing hormone receptor mediates the antiproliferative effects of GnRH-II on prostate cancer cells. The Journal of clinical endocrinology and metabolism. 2009;94(5):1761–7. doi: 10.1210/jc.2008-1741. [DOI] [PubMed] [Google Scholar]
- 22.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 23.Hermann RM, Schwarten D, Fister S, Grundker C, Rave-Frank M, Nitsche M, et al. No supra-additive effects of goserelin and radiotherapy on clonogenic survival of prostate carcinoma cells in vitro. Radiation Oncology. 2007;2 doi: 10.1186/1748-717X-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollack A, Salem N, Ashoori F, Hachem P, Sangha M, von Eschenbach AC, et al. Lack of prostate cancer radiosensitization by androgen deprivation. International Journal of Radiation Oncology Biology Physics. 2001;51(4):1002–7. doi: 10.1016/s0360-3016(01)01750-3. [DOI] [PubMed] [Google Scholar]
- 25.Salvat F, Fernández-Varea JM, Sempau J. PENELOPE: a code system for Monte Carlo simulation of electron and photon transport: Universitat de Barcelona. 2008 [Google Scholar]
- 26.Lechtman E, Chattopadhyay N, Cai Z, Mashouf S, Reilly R, Pignol JP. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Physics in Medicine and Biology. 2011;56(15):4631–47. doi: 10.1088/0031-9155/56/15/001. [DOI] [PubMed] [Google Scholar]
- 27.Butterworth KT, McMahon SJ, Currell FJ, Prise KM. Physical basis and biological mechanisms of gold nanoparticle radiosensitization. Nanoscale. 2012;4(16):4830–8. doi: 10.1039/c2nr31227a. [DOI] [PubMed] [Google Scholar]
- 28.Lechtman E, Mashouf S, Chattopadhyay N, Keller BM, Lai P, Cai Z, et al. A Monte Carlo-based model of gold nanoparticle radiosensitization accounting for increased radiobiological effectiveness. Physics in Medicine and Biology. 2013;58(10):3075–87. doi: 10.1088/0031-9155/58/10/3075. [DOI] [PubMed] [Google Scholar]
- 29.Cho SH. Estimation of tumor dose enhancement due to gold nanoparticles during typical radiation treatments: A preliminary Monte Carlo study. Medical Physics. 2005;32(6):2162. doi: 10.1088/0031-9155/50/15/N01. [DOI] [PubMed] [Google Scholar]
- 30.Cho S, Vassiliev O, Jang S, Krishnan S. Modifications of megavoltage photon beams for gold nanoparticle-aided radiation therapy (GNRT): A Monte Carlo study. Medical Physics. 2006;33(6):2121. [Google Scholar]
- 31.Cho SH, Jones BL, Krishnan S. The dosimetric feasibility of gold nanoparticle-aided radiation therapy (GNRT) via brachytherapy using low-energy gamma-/x-ray sources. Physics in Medicine and Biology. 2009;54(16):4889–905. doi: 10.1088/0031-9155/54/16/004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang SX, Gao JF, Buchholz TA, Wang ZL, Salehpour MR, Drezek RA, et al. Quantifying tumor-selective radiation dose enhancements using gold nanoparticles: a monte carlo simulation study. Biomedical Microdevices. 2009;11(4):925–33. doi: 10.1007/s10544-009-9309-5. [DOI] [PubMed] [Google Scholar]
- 33.Amato E, Italiano A, Pergolizzi S. Gold nanoparticles as a sensitising agent in external beam radiotherapy and brachytherapy: a feasibility study through Monte Carlo simulation. International Journal of Nanotechnology. 2013;10(12):1045–54. [Google Scholar]
- 34.Jones BL, Krishnan S, Cho SH. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Medical Physics. 2010;37(7):3809–16. doi: 10.1118/1.3455703. [DOI] [PubMed] [Google Scholar]
- 35.Joh DY, Sun L, Stangl M, Al Zaki A, Murty S, Santoiemma PP, et al. Selective Targeting of Brain Tumors with Gold Nanoparticle-Induced Radiosensitization. Plos One. 2013;8(4) doi: 10.1371/journal.pone.0062425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al Zaki A, Joh D, Cheng Z, De Barros ALB, Kao G, Dorsey J, et al. Gold-Loaded Polymeric Micelles for Computed Tomography-Guided Radiation Therapy Treatment and Radiosensitization. Acs Nano. 2014;8(1):104–12. doi: 10.1021/nn405701q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hainfeld JF, Smilowitz HM, O'Connor MJ, Dilmanian FA, Slatkin DN. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine. 2013;8(10):1601–9. doi: 10.2217/nnm.12.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berbeco RI, Ngwa W, Makrigiorgos GM. Localized Dose Enhancement to Tumor Blood Vessel Endothelial Cells via Megavoltage X-rays and Targeted Gold Nanoparticles: New Potential for External Beam Radiotherapy. International Journal of Radiation Oncology Biology Physics. 2011;81(1):270–6. doi: 10.1016/j.ijrobp.2010.10.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.