Abstract
Background
Infections due to methicillin-resistant Staphylococcus aureus (MRSA) are associated with significant morbidity and mortality and are typically treated with intravenous vancomycin. Given vancomycin’s time dependent mechanism of action, it is unlikely that vancomycin administration in the ED prior to disposition home could be beneficial.
Study Objectives
To characterize the indications, dosing, and appropriateness of vancomycin use in patients discharged from the Emergency Department (ED).
Methods
This is a single-center retrospective observational cohort study of patients who received vancomycin in an urban, academic, tertiary care ED. The subjects were consecutive adult patients administered intravenous vancomycin in the ED and then discharged home over an 18-month period. Outcomes were measured 1) to characterize patients receiving vancomycin prior to discharge home from the ED; and 2) to identify patients that did not meet indications for appropriate use based on the 2011 ISDA guidelines for treating MRSA infections
Results
526 patients received vancomycin in the ED prior to discharge during the study period. In this cohort, 368 (70%) patients were diagnosed with skin and soft tissue infections. A MRSA risk factor was present in 396 (75%) patients. Prior to discharge, one dose of vancomycin was administered to 357 (68%) patients. Underdosing of vancomycin occurred in 239 (73%) patients.
Conclusions
Vancomycin was given frequently to patients discharged home from the ED, most commonly for conditions where vancomycin was not indicated, such as skin and soft tissue infections. The majority of these patients received a vancomycin dosing strategy that is not only unlikely to lead to clinical improvement, but also has the potential to contribute adversely to the development of antibiotic resistance. Further investigation is needed into the impact of vancomycin use on the emergence vancomycin resistance and the role of ED based antibiotic stewardship.
Keywords: Antibiotic Stewardship, Emergency Department, Methicillin-resistant Staphylococcus aureus (MRSA), Skin and soft tissue infections (SSTI), Vancomycin
Introduction
Antibiotic resistance is a major public health concern, and is developing at a rate that outpaces new antimicrobial therapies (1, 2). The emergence of multidrug resistant (MDR) pathogens is frequently related to inappropriate antimicrobial therapy, and is associated with worse outcomes in a variety of infectious conditions (3–9). Methicillin-resistant Staphylococcus aureus (MRSA) is a major problem in both community and in-hospital settings, and causes significant morbidity, mortality and financial burden in the United States (10–15). Additionally, the emergence of vancomycin intermediate Staphylococcus aureus (VISA) and vancomycin resistant Staphylococcus aureus (VRSA) is invariably associated with previous vancomycin exposure and threatens the efficacy of vancomycin in the treatment of severe MRSA infections (16–22).
There is an increased interest in the potential role of the ED in antibiotic stewardship (23, 24). It is appropriate to administer vancomycin in the setting of a known or suspected MRSA infection, or in the setting of a severe systemic illness with a high risk of mortality (6, 22, 28). In 2011 the Infectious Diseases Society of America (ISDA) specifically recommended the use of vancomycin in complicated skin and soft tissue infections (SSTI), bacteremia, infective endocarditis, pneumonia, osteomyelitis, septic arthritis, meningitis, and intracranial abscesses (29). Our previous work indicate that vancomycin is commonly administered in the ED, but that the correct weight based dose was only given in the ED 22% of the time, and that the majority of patients (83.8%) were given inpatients dose of vancomycin unchanged from the dose administered in the ED (30). Vancomycin use in patients discharged from the ED has not been studied, however. The bactericidal activity of vancomycin utilizes a time-dependent mechanism of action (22, 31). Increased mean inhibitory concentration (MIC) of vancomycin needed to treat MRSA infections is a mechanism of action of VISA (32). For this reason, a single use dosing scheme is unlikely to yield significant clinical improvement prior to discharge home from the ED, and may be a patient safety issue with respect to the development of MDR pathogens and unnecessary drug exposure. For example, prior in vitro work indicates that any exposure to vancomycin in the previous 30 days increases the MIC of vancomycin needed to treat MRSA, potentially leading to the development of VISA (19).
We therefore decided to investigate vancomycin use in patients discharged from the ED. This study was designed to achieve the following objectives: 1) to characterize patients receiving vancomycin prior to discharge home from the ED; and 2) to identify patients that did not meet indications for appropriate use based on the 2011 ISDA guidelines for treating MRSA infections (29). Based on the known pharmacokinetic properties of vancomycin, ISDA guidelines, and our previous work on vancomycin dosing in the ED, we hypothesized that vancomycin administration would be common in patients discharged from the ED, and inappropriate based on indication and dosing strategy.
Methods
This analysis was a single-center, retrospective, observational cohort study conducted in the ED of an urban, academic, tertiary care institution with an annual census of > 90,000 patients. This observational study is reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies (33). The subjects were consecutive adult patients administered intravenous vancomycin in the ED and then discharged home over an 18-month period (December 2008 to June 2010). The protocol was approved by the Human Research Protection Office at the principal investigator’s institution.
Data were collected on patients identified by query of the ED electronic medical record. The medical record was queried for all patients who received IV vancomycin in the ED and were subsequently discharged home during the study period. Variables were defined before data extraction and placed in a standardized format during the data collection process. All data was collected by the principal investigator and cross checked for accuracy prior to data analysis.
Data included patient demographics, chief complaint, diagnosis, dose of vancomycin administered in the ED, other antibiotics administered in the ED, antibiotics prescribed on discharge home from the ED, MRSA risk factors, and appropriateness of vancomycin use. In accordance with the 2011 ISDA guidelines for the treatment of MRSA, we defined appropriate use as vancomycin used in complicated skin and soft tissue infections (SSTI), bacteremia, infective endocarditis, pneumonia, osteomyelitis, septic arthritis, meningitis, and intracranial abscesses (29). For the purposes of this retrospective study, patients who presented with SSTI and subsequently discharged home were defined as not having a complicated SSTI, with the assumption that complicated SSTIs would be admitted for further management and treatment. We defined the “correct” dose of vancomycin as 15–20 mg/kg of the actual body weight based on guideline recommendations (22). MRSA risk factors considered appropriate for empiric IV vancomycin therapy included the following as identified in a recent multi-center investigation in the ED setting: diagnosis of abscess, antibiotic use in the last 30 days, reported spider or insect bite, a personal history of MRSA, and close contacts with a similar infection (28). SSTIs were defined as an ED diagnosis of abscess, abscess plus cellulitis, or cellulitis. Outcomes of interest included subsequent return to the ED with the same medical complaint and/or need for admission for the same medical complaint, and resolution of symptoms by follow up office or ED visit. All outcomes of interest were assessed for the 12-month period following the initial ED visit.
Descriptive statistics were used to further characterize this data. The data was generated using SAS software, version 9.1of the SAS System for Linux (SAS Institute Inc., Cary, NC). Statistical analysis was completed in consultation with a biostatistician.
Results
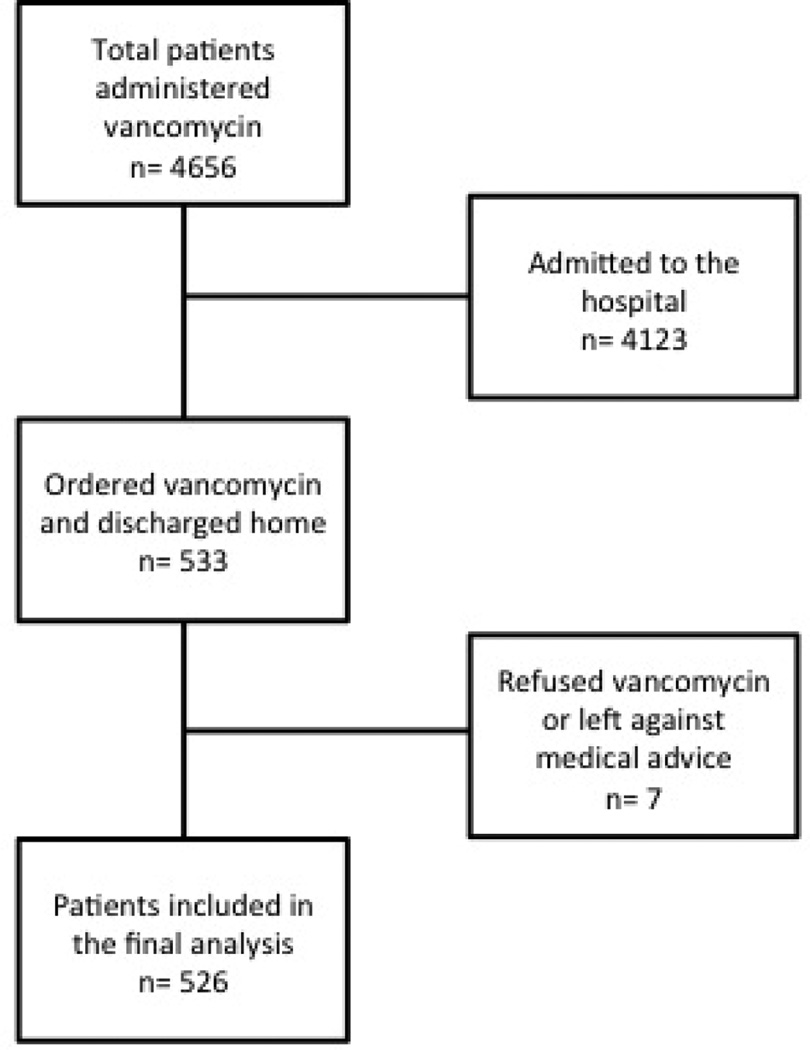
A total of 526 patients were included in the study (Figure 1). Patient characteristics are shown in Table 1. Table 2 shows that the majority of patients had risk factors for MRSA infection; the most common risk factor was ED diagnosis of abscess.
Figure 1.
Flow diagram of patients administered vancomycin in the emergency department and subsequently discharged home
Table 1.
Characteristics of patients given vancomycin in the ED
| n (%) | |
|---|---|
| Subjects, n | 526 (100) |
| Male, n (%) | 269 (51) |
| Age (years), median (IQR) | 43 (30–52) |
| Race, n (%) | |
| Black | 312 (59) |
| White | 190 (36) |
| Other | 24 (5) |
| Weight (kg), mean (SD) | 85.4 (26) |
| Location prior to ED, n (%) | |
| Home | 484 (92) |
| Nursing home or extended care facility | 25 (5) |
| Other hospital | 17 (3) |
| ED visit last 3 months, n (%) | 200 (38) |
| Hospitalization last 3 months, n (%) | 106 (20) |
| Allergy, n (%) | |
| No known drug allergies | 400 (76) |
| Penicillins | 64 (12) |
| Antibiotics in the past 90 days, n (%) | 177 (34) |
| Vancomycin | 62 (12) |
| Comorbidities, n (%) | |
| Hypertension | 165 (31) |
| Cardiac/Congestive heart failure | 57 (11) |
| Diabetes | 87 (17) |
| Chronic obstructive pulmonary disease/asthma | 59 (11) |
| Immunosuppression | 19 (4) |
| End stage renal disease on hemodialysis | 23 (4) |
| Malignancy | 30 (6) |
| Cirrhosis | 20 (4) |
| Temperature > 38°C | 33 (6) |
| Systolic blood pressure < 90 mmHg | 21 (4) |
| Heart rate > 100 beats per minute | 262 (50) |
ED: emergency department; IQR: interquartile range; SD: standard deviation
Table 2.
Risk factors for methicillin-resistant Staphylococcus aureus (MRSA) based on the largest study of MRSA risk factors to date (28).
| n (%) | |
|---|---|
| Diagnosis of abscess | 281 (53) |
| Antibiotics in last 30 days | 152 (29) |
| History of MRSA | 102 (19) |
| Reported insect bite or spider bite | 69 (13) |
| Close contact with a person with a similar infection | 16 (3) |
| No MRSA Risk Factor | 130 (25) |
In addition to these data, 368 (70%) patients were diagnosed with a skin and soft tissue infection. Diagnoses in the other 158 (30%) patients in this cohort included fever, urinary tract infection, laceration, altered mental status, headache, device complication, meningitis, hypotension, bacteremia, osteomyelitis and pneumonia. Culture data was obtained in 289 (55%) patients, with wound cultures performed in 195 (37%) patients. 99 (19%) patients were culture positive for MRSA.
Abscess incision and drainage was performed on 219 (42%) patients. Sixty-two (12%) patients were diagnosed with abscess or abscess plus cellulitis and did not have an incision and drainage preformed.
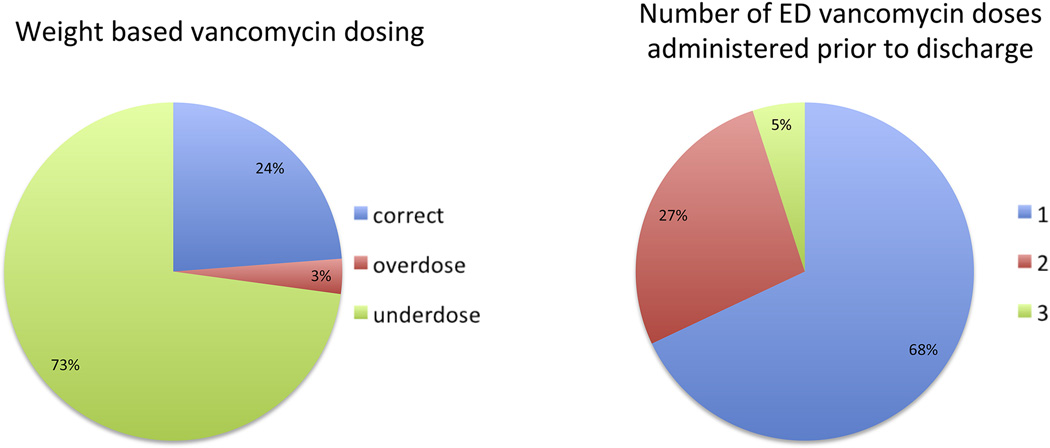
Figure 2 shows vancomycin exposure in the ED, with respect to dose received and number of doses. Underdosing (<15mg/kg) occurred in 239 (73%) patients and 357 (68%) patients received only one dose of vancomycin in the ED prior to discharge. In patients that did receive more than one dose of vancomycin in the ED (n= 169), all but one received the same dose on subsequent dosing.
Figure 2.
Vancomycin dosing practices for the cohort in which an ED weight was available (n = 328). Correct dose was defined at 15–20 mg/kg actual body weight based on guideline recommendations [27].
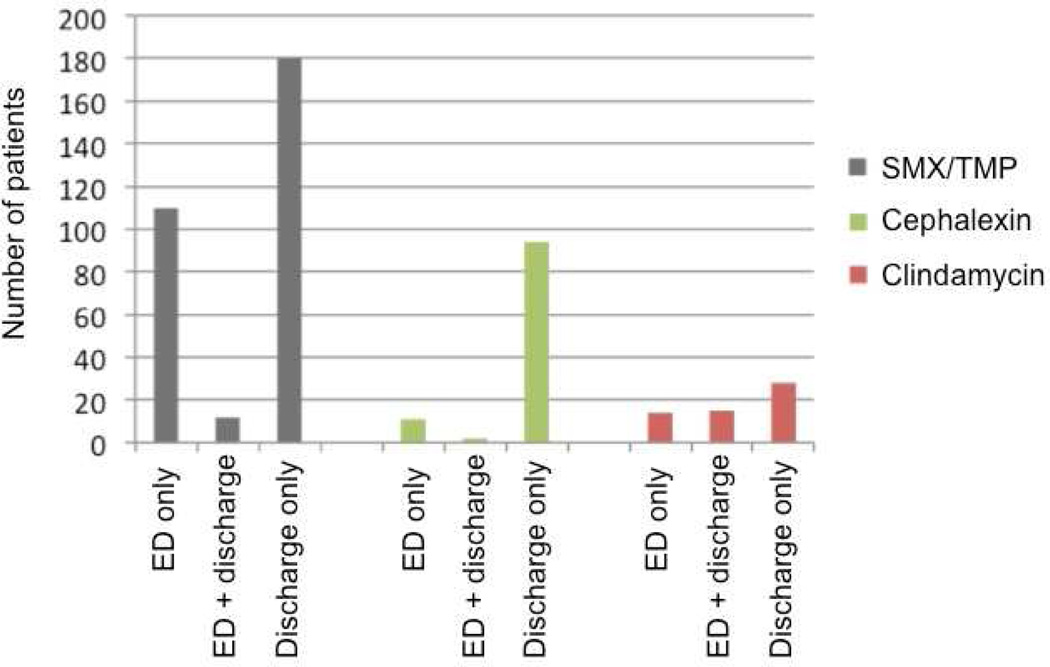
Table 3 shows the co-administration of other antibiotics in the ED. Along with vancomycin, 217 (41%) patients received one additional antibiotic prior to discharge from the ED, and 96 (18%) received two additional antibiotics. The majority of additional antibiotics were given for potential MRSA and gram-negative bacterial coverage. Two hundred-thirteen (41%) patients did not receive any additional antibiotics other than vancomycin in the ED. Outpatient antibiotics were prescribed to 384 (73%) patients. Figure 3 shows the prescribing patterns of the three most common antibiotics given upon discharge from the ED. The majority of patients treated with outpatient antibiotics were not dosed with these antibiotics in the ED in lieu of intravenous vancomycin.
Table 3.
Antibiotics administered in the ED
| n (%) | |
|---|---|
| Vancomycin | 526 (100) |
| Sulfamethoxazole/trimethoprim (SMX/TMP) | 122 (23) |
| Ceftriaxone | 54 (10) |
| Cefepime | 36 (7) |
| Pipericillin/tazobactam | 34 (7) |
| Clindamycin | 29 (6) |
| Cefazolin | 22 (4) |
| Ciprofloxacin | 24 (5) |
| Ampicillin/sublactam | 22 (5) |
| Cephalexin | 13 (3) |
| Other | 41 (8) |
Figure 3.
Prescribing practices of the three most common antibiotics prescribed on discharge.
When 2011 ISDA guidelines for the treatment of MRSA were retrospectively applied to this cohort based on ED diagnosis, 49 (9%) met criteria for vancomycin use in the setting of suspected MRSA infection (table 4).
Table 4.
Appropriateness of Vancomycin Use according to 2011 ISDA guidelines for the Treatment of MRSA infections.
| n (%) | |
|---|---|
| Approved uses of IV vanc | |
| Complicated skin and soft tissue infection | 0 (0) |
| Bacteremia | 1 (<1) |
| Endocarditis | 0 (0) |
| Pneumonia | 4 (<1) |
| Septic arthritis | 0 (0) |
| Osteomyelitis | 7 (1) |
| Meningitis (including empiric treatment for suspected meningitis) | 37 (7) |
| Other Intracranial abscess or infection | 0 (0) |
There was follow up data available for 332 (63%) patients. After receiving vancomycin in the ED, 139 (42%) of these patients presented to the ED with the same problem within one month, and 42 (12% of n = 332) patients required hospital admission for the same diagnosis within one month. Symptom resolution occurred in 197 (60% of n = 332) patients on follow up within 12 months of initial ED visit.
Discussion
While there is increased interest in antibiotic stewardship for ED patients, information regarding current practice patterns is vital before performance improvement initiatives can occur. The findings from this study provide new information regarding vancomycin use in the ED, build on previous work in this area, and provide potential targets for future improvements.
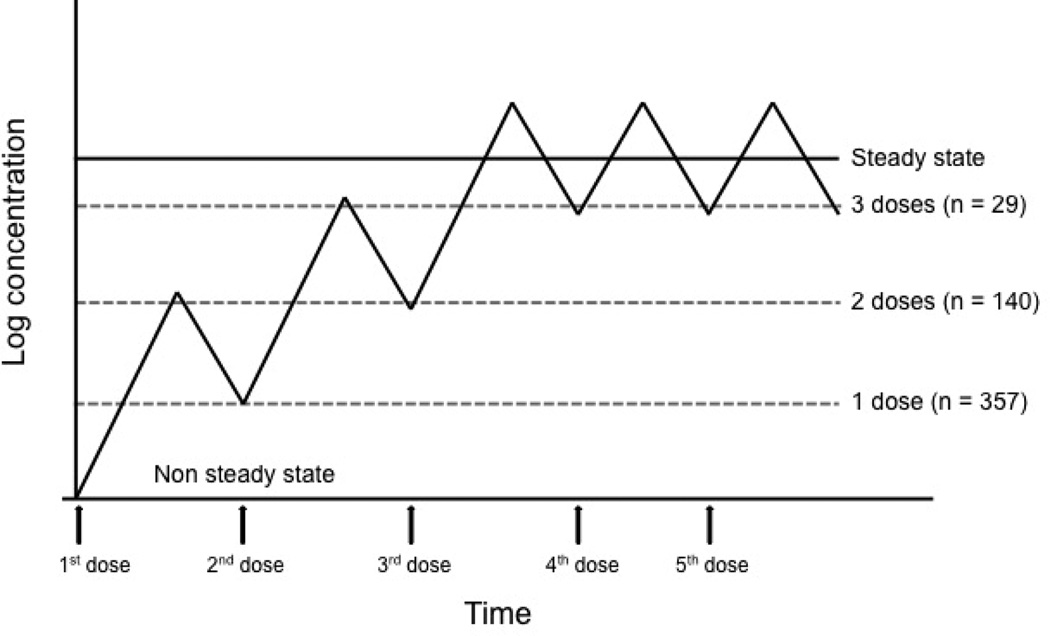
Approximately ten percent of vancomycin administered in our ED is given to patients subsequently discharged to home (30). Vancomycin exposure such as this, both in terms of dose administered (e.g. underdosed) and number of doses (e.g. one dose typically), serves little purpose in the eradication of infection. The majority of patients received only 1 (68%) or 2 (27%) doses. It is possible that vancomycin is given in this fashion in effort to get antibiotics “on board”, which could theoretically assist in treatment of infection after discharge. However, given vancomycin’s time dependent mechanism of action, this dosing strategy is unlikely to yield clinical improvement (figure 4). Approximately one third of patients treated in this fashion re-presented to the ED with same problem within one month. While outcome data is limited by the retrospective nature of this study, this further supports the low likelihood that ED vancomycin use contributed significantly to resolution of symptoms in this patient cohort.
Figure 4.
Number of vancomycin doses administered in the ED plotted over a vancomycin steady state concentration graph. Vancomycin utilizes time-dependent killing, therefore administration of a limited number of doses in the ED is highly unlikely to achieve a therapeutic killing effect.
Inappropriate antibiotic use is the most modifiable cause of MDR pathogen emergence, and the sub-optimal dosing strategy seen in this study could contribute to further emergence of VISA and VRSA in the future (19, 27, 32). Eleven cases of VRSA have been reported in the US since 2002 (16). The first European case of VRSA was reported in 2014, with no shared epidemiologic link with the US strains, indicating potential independent acquisition of vancomycin resistance in the setting of previous vancomycin exposure (34). Additionally, our investigation into other co-administered antibiotics revealed that a significant minority of this cohort was dosed with antibiotics such as ceftriaxone, cefepime and pipericillin/tazobactam that may also contribute to the emergence of resistant gram-negative pathogens (table 3).
While the majority of the patients in this cohort had MRSA risk factors, based on a 2006 study investigating MRSA infections in ED patients (28), of great concern is the fact that in our cohort 91% of patients who received vancomycin in the ED prior to discharge did not have an indication for use supported by the 2011 ISDA guidelines for the treatment of MRSA infections (29). For example, vancomycin was most commonly administered to treat uncomplicated SSTIs, despite a lack of literature to support this practice (23, 29). Based on these recommendations, if an infection is deemed serious enough to merit vancomycin therapy, these patients should be admitted to the hospital for further observation and treatment as opposed to discharged home (23, 29).
While vancomycin is indicated for serious MRSA infections, it is concerning that nearly 25% of patients in this cohort had no risk factors for MRSA and still received vancomycin prior to discharge from the ED. Equally concerning, of patients in this cohort diagnosed with abscess and abscess plus cellulitis, 12% of patients did not receive a documented incision and drainage of their abscess. Source control is a mainstay of treatment in infectious processes, and there is substantial evidence that indicate adequate incision and drainage alone is sufficient therapy for uncomplicated abscess, without the need for any antibiotics much less vancomycin (11, 29, 35–37). Additionally, an important factor in antibiotic stewardship is culture-driven antibiotic dosing. Recent SSTI guidelines recommend culture data on all purulent SSTIs treated with antibiotic therapy (29). In this SSTI heavy cohort, only 195 (37%) patients had a wound culture obtained. It is vital, both for ED-based antibiograms and de-escalation of therapy, to obtain appropriate culture data to guide future antibiotic use.
Additionally, there is discordance between ED antibiotic dosing and the antibiotics prescribed for outpatient therapy. Only 60% of patients received any antibiotics beyond vancomycin during their ED visit. When patients were discharged with additional antibiotics for SSTIs, they most commonly received sulfamethoxazole/trimethoprim, cephalexin or clindamycin. However, as shown in figure 3, only a minority of these patients received oral antibiotics both in ED and on discharge; most patients received these additional antibiotics only on discharge. This dosing strategy not only fails to take into account vancomycin’s time dependent mechanism of action, but also delays the administration of the potentially therapeutic discharge oral antibiotic. As these oral antibiotics utilize different mechanisms of action from vancomycin, delay in administration of the anticipated discharge antibiotic leads to a delay in attaining a clinically effective therapeutic steady state concentration of the discharge antibiotic. Additionally, when the oral antibiotic is not given in the ED, the opportunity is lost to observe a patient’s response to the antibiotic, and to monitor for a potential adverse reaction.
These data further support a need for antibiotic stewardship initiatives in the ED (23). In our previous work on vancomycin, ED vancomycin use strongly influenced inpatient use, both in terms of subsequent dose administered and decision to continue vancomycin therapy (30). The current work extends those findings, and further suggests that the ED, as the link between the inpatient and outpatient setting, could play a vital role in limiting inappropriate vancomycin exposure.
Limitations
This study is limited by its retrospective design. It is impossible to account for all of the influential factors associated with the decision to administer vancomycin, and this study is limited by a lack of information regarding why vancomycin was given. The fact that all of these patients were discharged home does provide further support and face validity to the supposition that vancomycin was likely not indicated. This is a single center study in an academic, urban ED. In areas where MRSA risk is low, and vancomycin administration is limited, these results are less significant. The majority of these data are descriptive in nature. While these data are limited, it is a transparent reflection of how vancomycin is being used in the ED. Finally, while we suspect that vancomycin dosed in this fashion is unlikely to lead to clinical improvement, without a control group of patients that did not receive vancomycin, there remains uncertainty regarding the efficacy of this dosing strategy.
Conclusion
Vancomycin is administered with relative frequency in patients discharged from the ED, and typically for conditions where vancomycin is not indicated. As previous work indicates, this practice is unlikely to yield clinical improvement, and has potential to contribute to antimicrobial resistance. The role of ED-based antibiotic stewardship needs to be further investigated, and ED providers must exercise vigilance in the selection and use of antibiotics, especially in the treatment of skin and soft tissue infections. Through coordinated initiatives that begin in the ED, there is the potential to appropriately tailor antibiotic therapy and promote antimicrobial stewardship practices.
Article Summary.
1. Why is this topic important?
Antibiotic stewardship is essential to promote the long term clinical efficacy of antibiotics like vancomycin.
2. What does this study attempt to show?
This study characterizes how vancomycin is used in patients discharged from the emergency department.
3. What are the key findings?
Vancomycin was given relatively frequently to patients discharged home from the ED, and most commonly for conditions where vancomycin was not indicated (e.g. uncomplicated SSTIs).
The majority of patients in this cohort received only one subtheraputic dose of vancomycin prior to discharge form the ED.
4. How is patient care impacted?
Patients who receive a subtheraputic dose of vancomycin coupled with an inadequate number of total vancomycin doses administered are unlikely to benefit clinically from this antibiotic regimen.
Inappropriate use of vancomycin has the potential to contribute adversely to the development of antimicrobial resistance.
Acknowledgements
The authors acknowledge Karen Steger-May, MA, from the Division of Biostatistics for assistance with the statistical analysis of these data.
Acknowledgments of grant support and of individuals who were of direct help in the preparation of the study
Financial support for this project was provided to Kristen Mueller, MD by the Emergency Medicine Residents’ Association Research Grant, and Brian M. Fuller, MD was supported by a Grant-in-Aid from the Division of Emergency Medicine at Washington University in St. Louis, as well as the Postdoctoral Mentored Training Program in Clinical Investigation. This publication was made possible, in part, by the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant UL1 TR000448. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Statement whether Institutional Review Board approval or exemption was obtained
The protocol was approved by the Human Research Protection Office at the principal investigator’s institution.
The authors have no conflicts of interest or disclosures to declare.
References
- 1.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(1):1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 2.Norrby SR, Nord CE, Finch R, European Society of Clinical M, Infectious D. Lack of development of new antimicrobial drugs: a potential serious threat to public health. Lancet Infect Dis. 2005;5(2):115–119. doi: 10.1016/S1473-3099(05)01283-1. [DOI] [PubMed] [Google Scholar]
- 3.Alvarez-Lerma F. Modification of empiric antibiotic treatment in patients with pneumonia acquired in the intensive care unitICU-Acquired Pneumonia Study Group. Intensive Care Med. 1996;22(5):387–394. doi: 10.1007/BF01712153. [DOI] [PubMed] [Google Scholar]
- 4.Ibrahim EH, Sherman G, Ward S, Fraser VJ, Kollef MH. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest. 2000;118(1):146–155. doi: 10.1378/chest.118.1.146. [DOI] [PubMed] [Google Scholar]
- 5.Kollef MH. Inadequate antimicrobial treatment: an important determinant of outcome for hospitalized patients. Clin Infect Dis. 2000;31(Suppl 4):S131–S138. doi: 10.1086/314079. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 7.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med. 1998;244(5):379–386. doi: 10.1046/j.1365-2796.1998.00379.x. [DOI] [PubMed] [Google Scholar]
- 8.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 2003;36(11):1418–1423. doi: 10.1086/375057. [DOI] [PubMed] [Google Scholar]
- 9.Mueller EW, Hanes SD, Croce MA, Wood GC, Boucher BA, Fabian TC. Effect from multiple episodes of inadequate empiric antibiotic therapy for ventilator-associated pneumonia on morbidity and mortality among critically ill trauma patients. J Trauma. 2005;58(1):94–101. doi: 10.1097/01.ta.0000141890.29032.9a. [DOI] [PubMed] [Google Scholar]
- 10.Achiam CC, Fernandes CM, McLeod SL, Salvadori MI, John M, Seabrook JA, et al. Methicillin-resistant Staphylococcus aureus in skin and soft tissue infections presenting to the Emergency Department of a Canadian Academic Health Care Center. Eur J Emerg Med. 2010 doi: 10.1097/MEJ.0b013e328337901a. [DOI] [PubMed] [Google Scholar]
- 11.May L, Harter K, Yadav K, Strauss R, Abualenain J, Keim A, et al. Practice patterns and management strategies for purulent skin and soft-tissue infections in an urban academic ED. Am J Emerg Med. 2012;30(2):302–310. doi: 10.1016/j.ajem.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Odell CA. Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) skin infections. Curr Opin Pediatr. 2010;22(3):273–277. doi: 10.1097/MOP.0b013e328339421b. [DOI] [PubMed] [Google Scholar]
- 13.Pallin DJ, Egan DJ, Pelletier AJ, Espinola JA, Hooper DC, Camargo CA., Jr Increased US emergency department visits for skin and soft tissue infections, and changes in antibiotic choices, during the emergence of community-associated methicillin-resistant Staphylococcus aureus. Ann Emerg Med. 2008;51(3):291–298. doi: 10.1016/j.annemergmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Whitby M, McLaws ML, Berry G. Risk of death from methicillin-resistant Staphylococcus aureus bacteraemia: a meta-analysis. Med J Aust. 2001;175(5):264–267. doi: 10.5694/j.1326-5377.2001.tb143562.x. [DOI] [PubMed] [Google Scholar]
- 15.Liu ERaL. Estimating the annual hospital excess cost of methicillin-resistant Staphylococcus aureus infections in the United States. International Society for Pharmacoeconomics and Outcomes Research (IPSOR) Tenth Annual International Meeting. 2005 [Google Scholar]
- 16.CDC Reminds Clinical Laboratories and Healthcare Infection Preventionists of their Role in the Search and Containment of Vancomycin-Resistant Staphylococcus aureus (VRSA) [Google Scholar]
- 17.Fridkin SK, Hageman J, McDougal LK, Mohammed J, Jarvis WR, Perl TM, et al. Epidemiological and microbiological characterization of infections caused by Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997–2001. Clin Infect Dis. 2003;36(4):429–439. doi: 10.1086/346207. [DOI] [PubMed] [Google Scholar]
- 18.Hidayat LK, Hsu DI, Quist R, Shriner KA, Wong-Beringer A. High-dose vancomycin therapy for methicillin-resistant Staphylococcus aureus infections: efficacy and toxicity. Arch Intern Med. 2006;166(19):2138–2144. doi: 10.1001/archinte.166.19.2138. [DOI] [PubMed] [Google Scholar]
- 19.Moise PA, Smyth DS, El-Fawal N, Robinson DA, Holden PN, Forrest A, et al. Microbiological effects of prior vancomycin use in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother. 2008;61(1):85–90. doi: 10.1093/jac/dkm445. [DOI] [PubMed] [Google Scholar]
- 20.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci U S A. 2007;104(22):9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastagia M, Kleinman LC, Lacerda de la Cruz EG, Jenkins SG. Predicting risk for death from MRSA bacteremia. Emerg Infect Dis. 2012;18(7):1072–1080. doi: 10.3201/eid1807.101371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–S39. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 23.May L, Cosgrove S, L’Archeveque M, Talan DA, Payne P, Jordan J, et al. A call to action for antimicrobial stewardship in the emergency department: approaches and strategies. Ann Emerg Med. 2013;62(1):69–77. doi: 10.1016/j.annemergmed.2012.09.002. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Acquisto NM, Baker SN. Antimicrobial stewardship in the emergency department. J Pharm Pract. 2011;24(2):196–202. doi: 10.1177/0897190011400555. [DOI] [PubMed] [Google Scholar]
- 25.Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) MMWR Recomm Rep. 1995;44(RR-12):1–13. [PubMed] [Google Scholar]
- 26.Infectious Diseases Society of A. The 10 × '20 Initiative: pursuing a global commitment to develop 10 new antibacterial drugs by 2020. Clin Infect Dis. 2010;50(8):1081–1083. doi: 10.1086/652237. [DOI] [PubMed] [Google Scholar]
- 27.Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Craig WA, Billeter M, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis. 2009;49(3):325–327. doi: 10.1086/600877. [DOI] [PubMed] [Google Scholar]
- 28.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Methicillin-resistant S, et al. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 29.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 30.Fuller BM, Mohr N, Skrupky L, Mueller K, McCammon C. Emergency Department vancomycin use: dosing practices and associated outcomes. J Emerg Med. 2013;44(5):910–918. doi: 10.1016/j.jemermed.2012.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauro Santos Filho JLK, David P. Nicolau. Employing Pharmacokinetic and Pharmacodynamic Principles to Optimize Antimicrobial Treatment in the Face of Emerging Resistance. Brazilian Journal of Microbiology. 2007;38:183–193. [Google Scholar]
- 32.Gardete S, Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J Clin Invest. 2014;124(7):2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Melo-Cristino J, Resina C, Manuel V, Lito L, Ramirez M. First case of infection with vancomycin-resistant Staphylococcus aureus in Europe. The Lancet. 2013;382(9888):9205. doi: 10.1016/S0140-6736(13)61219-2. [DOI] [PubMed] [Google Scholar]
- 35.Duong M, Markwell S, Peter J, Barenkamp S. Randomized, controlled trial of antibiotics in the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55(5):401–407. doi: 10.1016/j.annemergmed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 36.Mistry RD. Skin and soft tissue infections. Pediatr Clin North Am. 2013;60(5):1063–1082. doi: 10.1016/j.pcl.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz GR, Bruner D, Pitotti R, Olderog C, Livengood T, Williams J, et al. Randomized controlled trial of trimethoprim-sulfamethoxazole for uncomplicated skin abscesses in patients at risk for community-associated methicillin-resistant Staphylococcus aureus infection. Ann Emerg Med. 2010;56(3):283–287. doi: 10.1016/j.annemergmed.2010.03.002. [DOI] [PubMed] [Google Scholar]