Abstract
We used sex, observed parenting quality at 18 months, and three variants of the catechol-O-methyltransferase gene (Val158Met [rs4680], intron1 [rs737865], and 3′-untranslated region [rs165599]) to predict mothers’ reports of inhibitory and attentional control (assessed at 42, 54, 72, and 84 months) and internalizing symptoms (assessed at 24, 30, 42, 48, and 54 months) in a sample of 146 children (79 male). Although the pattern for all three variants was very similar, Val158Met explained more variance in both outcomes than did intron1, the 3′-untranslated region, or a haplotype that combined all three catechol-O-methyltransferase variants. In separate models, there were significant three-way interactions among each of the variants, parenting, and sex, predicting the intercepts of inhibitory control and internalizing symptoms. Results suggested that Val158Met indexes plasticity, although this effect was moderated by sex. Parenting was positively associated with inhibitory control for methionine–methionine boys and for valine–valine/valine–methionine girls, and was negatively associated with internalizing symptoms for methionine–methionine boys. Using the “regions of significance” technique, genetic differences in inhibitory control were found for children exposed to high-quality parenting, whereas genetic differences in internalizing were found for children exposed to low-quality parenting. These findings provide evidence in support of testing for differential susceptibility across multiple outcomes.
The differential susceptibility perspective provides a framework for investigating individual differences in reactivity to environments (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011; see also Boyce & Ellis, 2005). According to this theory, some individuals are relatively insensitive or resilient to their environment, whereas others are more receptive or vulnerable to their environment. Although this thinking is compatible with the diathesis-stress perspective (Monroe & Simons, 1991; Zuckerman, 1999), the differential susceptibility position further asserts that susceptible individuals will differ in a “for better and for worse” pattern (Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007, p. 300); that is, from an evolutionary perspective, these individuals’ increased vulnerability in unsupportive environments is countered by better functioning in highly supportive environments.
A number of genetic polymorphisms have been suggested as influencing susceptibility to environmental influences. Research efforts have primarily focused on variation in genes that influence neurotransmitter activity, with intense interest in the serotonin transporter (SLC6A4), and dopaminergic genes such as the dopamine transporter (SLC6A3), dopamine receptors (e.g., DRD4), and mono-amine oxidase A (MAOA). Although other reports using the same sample have investigated other genetic variants (Sulik et al., 2012; Taylor et al., 2013), in this manuscript we focus uniquely on the catechol-O-methyltransferase gene (COMT). According to one recent meta-analysis, genes known to affect dopamine can be considered measures of susceptibility to environmental influences (Bakermans-Kranenburg & van IJzendoorn, 2011), and there is evidence that COMT (which affects dopamine and other catecholamines) indexes responsiveness to environmental influences (Voelker, Sheese, Rothbart, & Posner, 2009) or plasticity (e.g., Belsky & Beaver, 2011).
The manner in which the relations between individual differences, environmental effects, and psychopathology are conceptualized differs dramatically between the diathesis–stress and differential susceptibility perspectives. In the former, individual differences in susceptibility to environmental influences are viewed as a weakness that is only revealed in the presence of negative or toxic environments. In contrast, the differential susceptibility framework is an evolutionary developmental theory (Ellis et al., 2011) in which individual differences in susceptibility to environmental influences cannot be interpreted straightforwardly as vulnerability because the same characteristic that gives rise to vulnerability in some environments will yield advantage in other environments. Given the dominance of the diathesis–stress perspective in conceptualizing person–environment interactions in the prediction of psychopathology, it can be difficult for researchers to shift their conceptualization of individual difference variables from a focus on risk and resilience to a focus on developmental plasticity. Nonetheless, the differential susceptibility framework, which has been receiving increasing empirical support, is changing how researchers view the joint role of the environment and individual differences in susceptibility as predictors of psychopathology.
To date, most investigators have focused on a single dependent variable when testing differential susceptibility (e.g., Kochanska, Kim, Barry, & Philibert, 2011; Laucht et al., 2012; Sulik et al., 2012; for an exception, see Obradović, Bush, Stampterdahl, Adler, & Boyce, 2010). This might be because formal criteria for testing this theory have been limited to a single dependent variable (see Belsky et al., 2007). There is, however, little reason to expect that differential susceptibility should apply to a single outcome. Instead, consistent with the evolutionary basis of differential susceptibility theory, we might expect tradeoffs: susceptible individuals could plausibly demonstrate different behavioral responses depending on the relative advantage and disadvantage of these responses across different environments. In this study, we test whether COMT polymorphisms are associated with children’s susceptibility to the influence of parenting across multiple outcomes: mothers’ reports of their children’s effortful control (i.e., inhibitory and attentional control) and internalizing symptoms. Consequently, our analyses address the issue of domain specificity in differential susceptibility (Ellis et al., 2011) by testing (a) whether COMT polymorphisms contribute to plasticity across a range of positive and negative parenting environments, or whether COMT primarily indexes receptivity to highly unsupportive environments (diathesis–stress; Monroe & Simons, 1991) or receptivity to highly supportive environments (vantage sensitivity; Pluess & Belsky, 2012); and (b) whether COMT moderates the association between parenting and multiple outcomes in a similar way, or whether the pattern of associations differs across outcomes. The two outcomes examined in this study (effortful control and internalizing symptoms) were specifically selected for analysis because both have been examined in COMT association studies, with inconsistent results across studies, and both are related to the quality of parenting behavior, which is positively related to effortful control and negatively related to internalizing symptoms. Because COMT has been theorized to act as a “plasticity gene,” we were interested in examining whether it would moderate the effects of parenting in a similar way across these two outcomes. To our knowledge, this is the first study to test the interaction of COMT and parenting as a predictor of internalizing symptoms.
Relations Between Parenting and Effortful Control and Internalizing Symptoms
Effortful control is an aspect of temperament involved in behavioral and attentional self-regulation. According to Rothbart and Bates (2006, p. 129), it includes “the efficiency of executive attention—including the ability to inhibit a dominant response and/or to activate a subdominant response, to plan, and to detect errors.” There is substantial evidence that high effortful control is protective against the development of psychological symptoms, particularly for children high in negative emotions such as anger and frustration (Eisenberg, Spinrad, & Eggum, 2010). Although temperament is typically conceptualized as having a biological basis, high-quality laboratory measures of parenting consistently predict the development of effortful control. For example, increases in effortful control have been predicted by aspects of positive parenting such as maternal responsiveness, warmth, respect for autonomy, and limit setting in early childhood (Kochanska, Murray, & Harlan, 2000; Lengua, Honorado, & Bush, 2007) and by parental warmth in middle childhood (Eisenberg et al., 2005).
Meta-analyses indicate that parenting quality is negatively related to anxiety and depression in childhood and adolescence, although the effect size of this relation tends to be modest (McLeod, Weisz, & Wood, 2007; McLeod, Wood, & Weisz, 2007). Perhaps because the validity of internalizing disorders such as depression in early childhood has only recently been established (Luby et al., 2003), few investigators have examined parenting as a predictor of internalizing symptoms in young children. The best evidence for such a relation comes from two large population cohort studies, in which maternal sensitivity observed during parent–child interactions was negatively, albeit modestly, associated with mothers’ reports of their children’s internalizing symptomatology (Kok et al., 2013). In another study using observed measures of parenting quality and mothers’ reports of early childhood internalizing symptoms, negative and intrusive parenting was positively associated with symptomatology, whereas parental warmth was negatively associated with symptomatology for African American children, but was unrelated to symptomatology for European American children (Propper, Willoughby, Halpern, Carbone, & Cox, 2007). In addition to findings for observed parenting, investigators have also reported relations between questionnaire measures of parenting and internalizing symptoms in early childhood (Bayer, Sanson, & Hemphill, 2006). In this study, we contribute to this limited body of literature using a reliable composite measure of early observed parenting quality and a longitudinal design in which symptomatology was assessed multiple times.
COMT
The COMT gene encodes the COMT enzyme that degrades catecholamines, including dopamine, epinephrine, and nor-epinephrine, and is particularly relevant in brain synapses (Tenhunen et al., 1994). Functioning of the prefrontal cortex (PFC) of the brain, using animal models, has been shown to be sensitive to small changes in dopamine and norepinephrine levels (Arnsten & Pliszka, 2011). In mouse models, the COMT enzyme has been demonstrated to be responsible for approximately half of the dopamine degradation that occurs in the PFC (Yavich, Forsberg, Karayiorgou, Gogos, & Männistö, 2007). One of the more widely studied variants in this gene is a single nucleotide polymorphism (SNP) that results in a valine (Val) to methionine (Met) amino acid substitution to the protein and is commonly referred to as COMT Val158-Met (rs4680; Lachman et al., 1996). The methionine allele results in an estimated three- to fourfold reduction in overall enzyme activity, that is, lower COMT efficiency, compared to that of the valine allele (Lachman et al., 1996; Lotta et al., 1995).
However, two other common polymorphisms, a promoter SNP in intron1 (rs737865) and a 3′-untranslated region (3′ UTR) SNP just downstream of the last exon (rs165599), show effects on COMT gene expression (i.e., variation in the relative amount of the enzyme that is made, rather than the activity or efficiency of the enzyme). Specifically, the in-tron1-G and 3′ UTR-G alleles are associated with low COMT expression, and a haplotype constructed of these two alleles along with the valine allele results in the lowest COMT expression in human PFC assays (Bray et al., 2003; Meyer-Lindenberg et al., 2006). These combinations may create a “tradeoff” scenario of “efficiency” versus “abundance” that is controlled by protein function and its expression, respectively. For example, having high COMT enzyme activity/efficiency in degrading catecholamines may not be adaptive if an individual has a low enzyme concentration, in contrast to having lower COMT activity but relatively higher concentration of it available when needed. Thus, individuals with COMT-Met enzyme might have lower enzyme activity than those with COMT-Val, but only when we examine the promoter SNPs in conjunction might we know how much of this enzyme is actually available and what side of the trade-off may be adaptive (Bray et al., 2003; Lipska et al., 2006; Shifman et al., 2002; Tunbridge et al., 2007). In this respect, evaluation of each of these SNPs and their haplotype combinations in association analyses is necessary to testing these hypotheses, especially in different environments. Analyses of COMT haplotypes often have shown greater associations with various clinical behaviors related to cognitive stability and functioning relative to individual SNPs, and have sometimes been found to interact with sex (e.g., Burdick et al., 2007; Kocabas et al., 2010; Liu et al., 2010; Shifman et al., 2002).
Associations Between COMT and Executive Function/Effortful Control
COMT Val158Met has been examined as a predictor of performance on executive function and attentional control tasks in adulthood. In these studies, the methionine allele has been consistently associated with greater executive function and attentional control (Bishop, Cohen, Fossella, Casey, & Farah, 2006; Blasi et al., 2005; Egan et al., 2001; Lipsky et al., 2005; Malhotra et al., 2002). There is little evidence for moderation of this effect by sex (Barnett, Jones, Robbins, & Müller, 2007), even though there are sex differences in effortful control favoring girls (Kochanska et al., 2000; Kochanska, Murray, Jacques, Koenig, & Vandegeest, 1996). The genetic differences in task performance are consistent with genetic differences in PFC and anterior cingulate activity, two brain regions that are involved in executive function and effortful control and in which dopamine and COMT are thought to play an important role (Bishop et al., 2006; Blasi et al., 2005; Egan et al., 2001; Mier, Kirsch, & Meyer-Lindenberg, 2009). Although a number of studies have examined the association between COMT Val158Met and executive function in adult samples, these associations have not been extensively examined in child samples. In one such study, investigators reported that children’s performance on an executive function task was higher for methionine homozygotes (Diamond, Briand, Fossella, & Gehlbach, 2004).
Val158Met has been found to interact with environmental variables to predict outcomes related to executive function, which provides evidence that this SNP may index plasticity to environmental influences. For example, Val158Met interacted with educational attainment to predict intelligence test scores, which included assessments of working memory and attention (Enoch, Waheed, Harris, Albaugh, & Goldman, 2009). In this study, educational attainment was positively related to intelligence scores for methionine carriers and unrelated to scores for valine homozygotes. Val158Met also interacted with socioeconomic status (SES) to predict attention-deficit/hyperactivity disorder symptoms (Nobile et al., 2009); SES was negatively related to attention-deficit/hyperactivity disorder symptoms for methionine homozygotes but was unrelated for valine carriers.
Associations Between COMT and Internalizing Symptoms
COMT Val158Met has also been related to internalizing symptoms, primarily in adult women, although there is some inconsistency in the direction of the effects. For example, the valine allele predicted relatively high levels of phobic anxiety in a sample of adult women (McGrath et al., 2004), whereas in another study, the methionine allele predicted panic and phobic anxiety symptoms in women, but not in men (Olsson et al., 2005). The methionine allele has also been associated with correlates of internalizing problems, such as high neuroticism, in women, but not in men (Stein, Fallin, Schork, & Gelernter, 2005). Finally, the valine allele (Mier et al., 2009) and the methionine allele (Montag et al., 2008; Smolka et al., 2007) have been related to greater peripheral and central nervous system responses to affective stimuli that are thought to underlie internalizing disorders. In addition to uncertainty about the direction of these effects, the relation between COMT and internalizing symptoms has not been examined in samples of children.
Research on COMT has not been limited to the Val158Met SNP, and combinations of multiple COMT SNPs have been found to relate to internalizing symptoms and related phenotypes in adults. For example, the combination of Val158Met-A (i.e., the methionine allele), intron1-A, and 3′ UTR-A alleles as a haplotype has been found to predict neuroticism in women, but not men (Stein et al., 2005). Furthermore, although the valine allele was only marginally associated with internalizing symptoms in women (but not in men), in one study, a haplotype that combined the Val158Met-G and 3′ UTR-A alleles was more predictive of women’s internalizing symptoms than the Val158Met alone (Hettema et al., 2008). These studies provide evidence that COMT haplotypes that incorporate multiple variants may provide better prediction than the frequently examined Val158Met SNP alone.
The Present Investigation
Genetic variation at COMT has been found to predict both executive function and internalizing symptoms. However, in factor-analytic studies, the temperament dimensions of effortful control and negative emotionality, as well as the personality dimensions of constraint/conscientiousness and neuroticism/emotional stability, have been found to be largely orthogonal (McCrae & Costa, 1987; Rothbart, Ahadi, Hershey, & Fisher, 2001). In addition, although effortful control may moderate the relation between negative emotionality and internalizing symptomatology (Oldehinkel, Hartman, Ferdinand, Verhulst, & Ormel, 2007), direct relations between effortful control and internalizing symptomatology appear to be modest (Eisenberg, Spinrad, & Eggum, 2010; White, McDermott, Degnan, Henderson, & Fox, 2011). Because COMT is related to both of these constructs, but they are not strongly related to one another, it makes sense to examine moderators to determine the conditions under which COMT is related to effortful control and the conditions under which it is related to internalizing symptomatology. Moreover, because attentional control overlaps nearly entirely with skills included in measures of executive control, whereas inhibition of behavior (in contrast to inhibition of cognition) is less consistently central to measures of executive functioning, it made sense to explore if these two aspects of effortful control were similarly predicted by parenting and COMT.
A large majority of investigators studying the association of COMT with phenotypes has only examined the Val158Met SNP; however, it is also clear from previous work that high-and low-functioning COMT can be highly dependent on the combination of multiple variants. In this study, we tested the hypothesis that COMT allelic and haplotype variation indexes responsivity to early parenting as a predictor of children’s effortful control and internalizing symptoms. At present, there is evidence that haplotypes may improve prediction relative to individual variants alone, although the degree of overlap between individual variants and haplotypes may limit the magnitude of these differences (Voelker et al., 2009). With respect to the specific SNPs chosen for analysis, we have chosen to analyze variation in Val158Met because it is known to affect the efficiency of the COMT enzyme and the intron1 and 3′ UTR SNPs because they are known to affect the quantity of enzyme produced. We tentatively predicted that supportive parenting would have a stronger effect on children with the COMT SNP and haplotype variation associated with low COMT function (i.e., the methionine allele and the GGG haplotype). In contrast, consistent with the differential susceptibility hypothesis, we would predict that this interaction is complex and that both low and high COMT SNP and haplotype functioning have beneficial effects, but these effects likely vary with the parenting environments and, based on prior work, with the sex of the child. Based on prior work in adult samples (described above) and functional evidence from animal studies that COMT is sexually dimorphic (for a review, see Harrison & Tunbridge, 2008), we expected COMT to play a stronger role in the prediction of internalizing symptoms for girls than for boys. Given the lack of moderation by sex for the relation between COMT and executive function in adults, we included COMT as a moderator in analyses predicting inhibitory control and attention focusing in a more exploratory framework. A major strength of this study was the use of a longitudinal design to examine COMT as a predictor of development in childhood; most studies have used cross-sectional adult samples.
Method
Participants
Participants were 153 children with genetic information who were drawn from a larger study of children’s social and emotional development. Mothers and their infants were recruited shortly after birth at three hospitals in a large metropolitan area in the southwestern United States. All infants were healthy, full term, and from adult parents who were able to read English. For details about the larger study, refer to Eisenberg, Spinrad, Eggum, et al. (2010) and Spinrad et al. (2007).
Of the children with genetic data, 8 did not have observed parenting data at the 18-month visit and were therefore excluded from the analyses. We report sample characteristics at the initial (18-month) assessment for the remaining 146 participants (79 male). All participants had mother questionnaire data at one or more assessments (Ns = 114 at 24 months, 143 at 30 months, 143 at 42 months, 126 at 48 months, 139 at 54 months, 139 at 72 months, and 126 at 84 months). Children ranged in age from 17.00 to 19.97 (M = 17.72, SD = 0.52) months at the initial assessment. The median annual family income in 2002 was $45,000–$60,000. Racial composition for children in our sample, as reported by parents, was as follows: 86% Caucasian, 5% Native American, 5% African American, 2% Asian, 1% mixed between two minorities, and 1% other; 21% reported Hispanic/Latino ethnicity. Five percent of mothers did not complete high school, 10% graduated from high school, 41% attended some college, 32% graduated from college, and 12% had a graduate or professional degree.
We examined mean differences on study variables between children included in our analyses and children who were not included due to missing parenting or genetic data. Children included in analyses were from families with higher SES (a composite formed by standardizing and averaging household income and mothers’ and fathers’ education; M = 0.08) compared to those who were not included due to missing data (M = −0.13), t (241) = −1.98, p < .05. No mean differences were found for the 18-month measure of parenting or for internalizing symptoms or effortful control at any time point.
Procedures
Laboratory visits were conducted when children were age 18, 30, 42, and 54 months, and a home visit was conducted when children were age 72 months. At these visits, children completed a battery of tasks presented as games, and parents completed questionnaires (as well as returned or completed other questionnaires sent a few weeks earlier). In addition, questionnaires were mailed to mothers when their children were age 24, 48, and 84 months. Mothers were paid between $20 (at the 18-month assessment) and $35 (at the 84-month assessment) for filling out questionnaires. Quality of parenting was observed at the 18-month laboratory visit, and cell tissue samples for genetic analyses were collected at the 72-month visit.
Measures
Effortful control
At 42, 54, 72, and 84 months, mothers reported on the attention focusing (14 items; e.g., “When drawing or coloring in a book, shows strong concentration”) and inhibitory control (13 items; e.g., “Is usually able to resist temptation when told s/he is not supposed to do something”) scales from the Children’s Behavior Questionnaire (Rothbart et al., 2001). One item on the attention focusing scale, “Will ignore others when playing with an interesting toy,” was negatively correlated with the scale score at all four time points and was therefore dropped. Cronbach α for attention focusing and inhibitory control ranged from 0.78 to 0.83 across all time points. At 42, 54, 72, and 84 months, these two scales were substantially correlated, rs = .48, .56, .60, and .66, respectively, so we initially averaged the two scales and analyzed the outcome as a composite measure of effortful control. Although differences were not hypothesized for the two scales, supplemental analyses indicated that COMT was differentially related to attention focusing and inhibitory control. We therefore report results separately for each of these scales (in addition to the composite of both scales). Effortful control was assessed with a different measure (i.e., the Early Children’s Behavior Questionnaire) prior to 42 months, so earlier assessments could not be used in the growth curve analyses.
Internalizing symptomatology
At the 24-, 30-, 42-, 48-, and 54-month assessments, mothers reported on children’s general anxiety (five items, e.g., “Seems nervous, tense, or fearful”), depression (nine items, e.g., “Seems very unhappy, sad, or depressed”), and anxiety/obsessive–compulsive (five items, e.g., “Is very worried about getting dirty”) symptoms using scales from the Infant–Toddler Social and Emotional Assessment (ITSEA; Briggs-Gowan & Carter, 1998); the ITSEA was not administered at the 72- and 84-month assessments because it is only appropriate for young children. We averaged the ITSEA items, which were rated on a scale ranging from 0 (not true/rarely) to 2 (very true/often), to create a composite measure of internalizing symptomatology. One item from the depression scale (“Laughs easily or a lot,” reverse scored) lowered reliability of the internalizing composite at multiple visits and was therefore dropped from the composite, resulting in an 18-item internalizing scale. The Cronbach α values for this scale at 24, 30, 42, 48, and 54 months were 0.72, 0.64, 0.72, 0.74, and 0.70, respectively. To reduce the influence of outliers, scores that were more than 3 SD above the mean for internalizing at each assessment (n = 1 at 24 months, n = 3 at 30 months, n = 1 at 42 months, n = 2 at 48 months, n = 1 at 54 months) were recoded so that they were only 3 SD above the mean (Tabachnik & Fidell, 2006). ITSEA scores ranged from 0.00 to 1.06 before recoding outliers, and from 0.00 to 0.82 after recoding outliers. Recoding outliers did not substantively affect the reported results.
Parenting quality
At the 18-month visit, mothers and children were video recorded during free play, a challenging teaching task, and a clean-up task. Maternal sensitivity and intrusiveness were coded during the free-play interaction and teaching task. During a 3-min free-play session, a basket of toys was given to mothers with instructions to play with their child as they would at home. In the teaching task, mothers had 3 min to teach their child to complete a difficult puzzle. For the clean-up task, mothers were told to ask their child to pick up toys and place them in a basket. The task lasted 3 min or until all the toys were cleaned up (Braungart-Rieker, Garwood, & Stifter, 1997; Kochanska, Aksan, & Nichols, 2003; Stifter, Spinrad, & Braungart-Rieker, 1999). Ratings of sensitivity and intrusiveness were made at 15-s intervals during the free-play interaction and at 30-s intervals during the teaching task (Fish, Stifter, & Belsky, 1991). Sensitivity was coded based on maternal behaviors that indicated attention toward the child and responsiveness to the child’s emotions, interests, and level of ability (1 = no evidence of sensitivity; 4 = mother was very aware of the toddler, contingently responsive to his/her interests and affect, and had an appropriate level of response/stimulation (interrater reliabilities [interclass correlations (ICC)] = 0.81 and 0.82, for free-play and teaching task, respectively). Intrusiveness was assessed by coding maternal behaviors that evidenced overstimulating the child, physically intruding, or providing help to the child when not needed (1 = no overcontrolling behavior observed; 4 =extreme intrusive or overcontrolling behaviors; ICC = 0.82 and 0.81 for free-play and teaching task, respectively). Ratings of maternal warmth were also made every 30 s during the teaching task. Warmth was assessed using mother’s expressions of friendliness and closeness, physical affection, positive affect toward child, supportiveness, and the quality of their tone/conversation (1 = no evidence of warmth; 5 = very engaged with the child, positive affect was predominant, and the mother was physically affectionate; ICC = 0.83). Mothers’ verbal control, assertive commands, and prohibitions given without force or threat (e.g., “We have to clean up NOW”) were rated every 15 s during the clean-up task (1 = present, 0 = not present; κ = 0.70). After reverse scoring maternal control and intrusiveness, all measures of parenting were significantly positively correlated, with rs ranging from .19 to .85 (all ps <.05). A composite of parenting quality was created by standardizing these six scores and computing the mean. The standardized α for the composite measure of parenting was 0.72.
Genotyping
After collecting a buccal oral cheek sample from each individual, DNA extractions were conducted using a standard isolation protocol (Sambrook & Russell, 2001). Three pairs of polymerase chain reaction primers were designed based on the GenBank human genome sequence draft to amplify three DNA fragments that included three COMT SNPs, respectively, for each individual. Primers included the intron1 G/A SNP (rs737865) as ATG TGT GGT GTG CAG GAC C (forward) and CAA ATC AGC ATG GAG CCA G (reverse), for a 267 base pair (bp) fragment; the Val (G)/Met (A) SNP (rs4680) as CTG TGG CTA CTC AGC TGT GC (forward) and TGG TGT GAA CAC CTG GTG G (Reverse), for a 227-bp fragment; the 3′ UTR G/A SNP (rs165599) as GAC GGA CGC TAA CGC TAA G (Forward) and GAT GCT TCC ACT CTG TGC C (reverse), for a 276-bp fragment. These fragments were cleaned, and full nucleotide sequences were generated and analyzed, following our previous protocol (Claw, Tito, Stone, & Verrelli, 2010).
Haplotypes and population structure
Allele frequencies for each of the three SNPs are shown in Table 1. Chi-squared tests of independence confirmed that genotype frequencies for each of the three SNPs were consistent with Hardy–Weinberg equilibrium (intron1: χ2 = 2.94, p = .09; Val158Met: χ2 = 0.05, p = .82; 3′ UTR: χ2 = 0.36, p = .55), which is an important assumption of subsequent genotype–phenotype association analyses. In addition to association tests with independent SNPs, analyses were conducted with combinations of the SNPs as haplotypes. Thus, we used the PHASE v. 2.1.1 program (Stephens, Smith, & Donnelly, 2001) to statistically infer and construct haplotypes that included the in-tron1, Val158Met, and 3′ UTR SNPs. These samples were previously genotyped for 10 unlinked variable number tandem repeats (VNTRs) distributed across the genome to identify and account for any population stratification (Sulik et al., 2012). The VNTRs were D1S1612, D2S1356, D4S1280, D5S1471, D6S1006, D7S2847, D17S1308, D18S535, D19S714, and D20S604 (protocol previously in Egan et al., 2001; Straub et al., 1993). In an analysis of these VNTR markers using the STRUCTURE program (Pritchard, Stephens, & Donnelly, 2000), genetic variation among individuals was statistically consistent with a single population sample with no significant effects of population admixture. Thus, no correction for population stratification was needed in subsequent analyses of the full sample.
Table 1.
Frequencies and correlations among genetic variables
| Frequencies
| |||
|---|---|---|---|
| Val158Met | 3′ UTR | Intron1 | |
| AA | 39 | 61 | 78 |
| AG | 72 | 70 | 52 |
| GG | 35 | 15 | 16 |
| Correlationsa
| ||||
|---|---|---|---|---|
| Val158Met | 3′ UTR | Intron1 | GGG | |
| Val158Met | — | .56 | .47 | .44 |
| 3′ UTR | — | .32 | .62 | |
| Intron1 | — | .78 | ||
| GGG | — | |||
Note: 3′ UTR, 3′ Untranslated region.
Correlations are for dominantly coded variables (0 =AA, 1 =AG/GG). The GGG haplotype was coded as follows: 0 = not present, 1 = at least one GGG haplotype present. All correlations are significant at p < .001.
Allele frequencies for each variant are shown in Table 1. Based on the literature on the function of our COMT variants and evidence from association studies previously noted, we also examined a haplotype that combined intron1-G, Val158Met-G, and 3′ UTR-G alleles (henceforth referred to as the GGG haplotype) as a predictor in our analyses. The GGG haplotype has been associated with the lowest COMT enzyme expression, and thus, like the Met (A) allele that has been associated with low COMT enzyme activity, it also reflects reduced COMT and related PFC functioning (Shifman et al., 2002). For the GGG haplotype, 95 children had zero copies, 49 children had one copy, and 2 children had two copies. COMT expression appears to be additive with respect to this haplotype combination; that is, the more copies of the GGG haplotype, the lower the COMT expression (Shifman et al., 2002). In all analyses involving this haplotype, we collapsed the latter two groups to compare children with and without at least one copy of this haplotype, essentially contrasting groups of lower and higher COMT expression, respectively.
Results
Descriptive analyses
When the genetic variables were coded dominantly (AA = 0, AG/GG = 1, G is dominant), there was considerable overlap between Val158Met, intron1, and 3′ UTR, with correlations ranging from .32 to .56 (see Table 1). The relations between the individual variants (especially intron1) and the GGG haplotype were also substantial, with correlations ranging from .44 to .78. Correlations between other study variables are presented in Table 2. Rank-order stabilities for attention focusing, inhibitory control, and internalizing were high, with rs across time ranging from .52 to .74 for attention focusing, from .65 to .79 for inhibitory control, and from .48 to .71 for internalizing. Internalizing was largely unrelated to attention focusing and inhibitory control; the only significant correlation between these measures was between internalizing at 48 months and inhibitory control at 84 months, r (111) = −.21, p < .05. Sex was significantly correlated with intron1 (coded dominantly), r (143) = −.19, p < .05, but was unrelated to all other study variables; girls were more likely than boys to have AA for intron1. Val158Met, intron1, 3′ UTR, and the GGG haplotype were not significantly correlated with any other study variables. Observed parenting quality, which was assessed at the 18-month visit, was negatively related to internalizing at 48 months, r (124) = −.23, p < .05, and was positively related to attention focusing and inhibitory control across all assessments, with rs ranging from .22 to .32, ps < .05.
Table 2.
Correlations and descriptive statistics
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Val158Met | — | −.06 | −.03 | −.08 | −.07 | −.04 | −.02 | .03 | .18* | −.07 | −.04 | .09 | .03 | −.04 | −.01 | .11 |
| 2. Sex | — | .02 | −.00 | .04 | −.05 | −.02 | −.09 | −.06 | −.16† | −.13 | −.17* | −.13 | −.13 | −.03 | .02 | |
| 3. Parenting | — | −.01 | −.05 | −.11 | −.23* | −.16† | .31* | .32* | .30* | .25* | .22* | .23* | .30* | .22* | ||
| 4. 24-month INT | — | .71* | .54* | .51* | .52* | −.10 | .05 | .04 | −.02 | .03 | .02 | .06 | .11 | |||
| 5. 30-month INT | — | .56* | .53* | .48* | .03 | .15 | .13 | −.02 | .13 | .09 | .07 | .08 | ||||
| 6. 42-month INT | — | .63* | .62* | .02 | .12 | −.01 | −.11 | .09 | .06 | −.04 | −.04 | |||||
| 7. 48-month INT | — | .66* | −.23* | −.10 | −.10 | −.21* | −.07 | −.05 | −.12 | −.15 | ||||||
| 8. 54-month INT | — | −.06 | .03 | .02 | −.16† | .05 | .07 | .05 | .02 | |||||||
| 9. 42-month IC | — | .75* | .67* | .65* | .48* | .48* | .40* | .41* | ||||||||
| 10. 54-month IC | — | .76* | .69* | .46* | .56* | .46* | .41* | |||||||||
| 11. 72-month IC | — | .79* | .37* | .48* | .60* | .54* | ||||||||||
| 12. 84-month IC | — | .34* | .41* | .48* | .66* | |||||||||||
| 13. 42-month AF | — | .74* | .61* | .52* | ||||||||||||
| 14. 54-month AF | — | .73* | .60* | |||||||||||||
| 15. 72-month AF | — | .70* | ||||||||||||||
| 16. 84-month AF | — | |||||||||||||||
| Mean | — | — | 0.00 | 0.20 | 0.23 | 0.26 | 0.24 | 0.25 | 4.38 | 4.68 | 5.02 | 5.09 | 4.57 | 4.66 | 4.79 | 4.89 |
| SD | — | — | 1.00 | 0.15 | 0.15 | 0.18 | 0.18 | 0.17 | 0.73 | 0.78 | 0.75 | 0.85 | 0.75 | 0.76 | 0.80 | 0.78 |
Note: Val, Valine; Met, methionine; INT, internalizing; IC, inhibitory control; AF, attention focusing. Val158Met is coded so that 0 = Met-Met and 1 =Met-Val/Val-Val; sex is coded so that 0 =female and 1 =male.
p < .10.
p < .05.
Random effects model
Using SAS 9.3 (PROC MIXED), we tested a series of unconditional models of increasing complexity to establish the best-fitting unconditional growth model prior to adding substantive predictors (Singer & Willett, 2003). These models use maximum likelihood estimation as a missing data treatment (Schafer & Graham, 2002). Likelihood ratio tests were used to compare nested models, and the best-fitting model for each dependent variable was used as the basis for subsequent analyses. Pseudo r2 statistics are reported as a measure of effect size and were computed by calculating the squared correlation between the actual scores and the model-predicted scores (Singer & Willett, 2003).
Attention focusing
The best-fitting unconditional model included fixed and random effects for the intercept and linear slope. With time centered at the first measurement occasion (42 months), the estimates for the fixed effects were as follows: intercept = 4.57; linear slope = 0.09, t = 5.21, p < .001. The significant, positive linear slope indicates that attention focusing was increasing at 42 months. The random effects were as follows: intercept = 0.427, z = 6.73, p < .001; linear slope = 0.019, z = 3.62, p <.001, indicating that there was significant between-person variability in both the intercept and linear slope. The covariance between the intercept and linear slope was nonsignificant (z = −1.84, ns), indicating that initial levels of attention focusing were uncorrelated with rates of growth. The residual (within-person) variance was 0.159 (z = 11.59, p < .001). This model explained 2.4% of the variance in attention focusing.
Inhibitory control
The best-fitting unconditional model included fixed effects for the intercept, linear slope, and quadratic slope, and random effects for the intercept and linear slope. With time centered at the first measurement occasion (42 months), the estimates for the fixed effects were as follows: intercept = 4.36; linear slope = 0.36, t = 7.74, p < .001; quadratic slope =−0.04, t =−3.35, p <.001. The significant, positive linear slope indicates that inhibitory control was increasing at 42 months, but the significant negative quadratic term indicates that this upward trend decelerated over time. The random effects were as follows: intercept = 0.415, z = 6.88, p < .001; linear slope = 0.015, z = 3.35, p < .001, indicating that there was significant between-person variability in both the intercept and the linear slope. The covariance between the intercept and the linear slope was nonsignificant, z = −0.58, ns, indicating that the initial level of inhibitory control was uncorrelated with rate of growth. The residual (within-person) variance was 0.136, z = 11.45, p < .001. This model explained 12.0% of the variance in inhibitory control.
Internalizing symptomatology
The best-fitting unconditional model included fixed effects for the intercept, linear slope, and quadratic slope, and random effects for the intercept and linear slope. With time centered at the first measurement occasion (24 months), the estimates for the fixed effects were as follows: intercept = 0.21; linear slope = 0.05, t = 3.10, p <.01; quadratic slope =−0.02, t =−2.37, p <.05. The significant, positive linear slope indicates that internalizing symptoms were increasing at 24 months, but the significant negative quadratic term indicates that the rate of change decelerated (i.e., became less positive) over time. The random effects were as follows: intercept = 0.014, z = 5.59, p < .001; linear slope = 0.002, z = 2.83, p < .01; this finding indicates that there was significant between-person variability in both the intercept and the linear slope. The covariance between the intercept and linear slope was nonsignificant (z = −0.38, ns), indicating that initial levels of internalizing symptoms were uncorrelated with rates of growth. The residual (within-person) variance was 0.010 (z = 13.79, p < .001). This model explained 1.2% of the variance in internalizing symptomatology.
Substantive models
For each of the three dependent variables (attentional control, inhibitory control, and internalizing problems), we estimated models containing all lower order terms, and the three-way interaction among Val158Met1 (dummy coded: 0 = AA [Met-Met] and 1 = AG/GG [Met-Val/Val-Val], sex (dummy coded: 0 = female, 1 = male), and parenting quality as predictors of the intercept and linear slope. For all three dependent variables, despite significant between-person variability in the linear slopes, there was no prediction of the slope from parenting, COMT genotype, sex, or any two- or three-way interactions between these variables. Consequently, prediction of the slope was dropped from all models to simplify the results.2 In addition to examining the Val158Met SNP, we estimated similar models for the intron1 and 3′ UTR SNPs, as well as for the GGG haplotype. None of the genetic predictors, alone or in two- or three-way interactions with sex and parenting, predicted attention focusing. Although there were significant three-way interactions among parenting, sex, and each of the three individual variants (coded dominantly) for internalizing and inhibitory control, the Val158Met SNP explained more variance in these outcomes, and therefore we focus our presentation of results on Val158Met. Consistent with the moderate to high correlations among the genetic variables, when three-way interactions among sex, parenting, and the other genetic variables (intron1, 3′ UTR, and the GGG haplotype) were found, they were highly similar to the results for Val158Met. Consequently, results for these other genetic predictors are reported in abbreviated detail.
For each significant three-way interaction involving Val158Met, we probed the two-way interaction between Val158Met and parenting quality for boys and for girls, and then calculated the simple effects of parenting on the outcome for each combination of genetic group and sex (Aiken & West, 1991), resulting in four simple effects for each model. To determine whether genetic differences were primarily found in highly supportive or unsupportive environmental contexts, we also examined the region of significance (Johnson & Neyman, 1936) for the genetic effects.3 The region of significance corresponds to the boundaries of the significant effect (i.e., the areas in which the simple effect is significant vs. nonsignificant) and has been used to test for differential susceptibility in other studies (e.g., Kochanska et al., 2011; see also Roisman et al., 2012).
Attention focusing
There were no main effects of any individual COMT SNPs or the GGG haplotype when predicting attention focusing; nor were there any two- or three-way interactions with sex or parenting for any individual COMT SNPs or for the GGG haplotype.
Inhibitory control
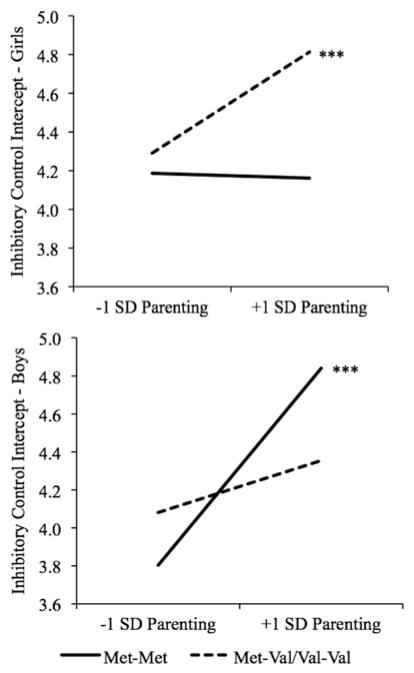
There was a significant three-way interaction among sex, Val158Met, and parenting quality4 (see Table 3; b = −0.66, t = −2.75, p < .01). This model explained 26.7% of the variance in inhibitory control, an increase of 14.7% over the unconditional model. The two-way interaction between Val158Met and parenting quality was significant for boys (b = −0.38, t = −2.34, p < .05), but not for girls (b = 0.27, t = 1.58, ns). Despite a nonsignificant two-way interaction term, there was no effect of parenting on inhibitory control for girls with Met-Met (see Figure 1; b = −0.01, t = −0.08, ns), but there was a positive effect of parenting on inhibitory control for girls with Met-Val/Val-Val (b = 0.26, t = 3.38, p < .001). Conversely, there was a positive effect of parenting quality on inhibitory control for boys with Met-Met (b = 0.52, t = 3.76, p < .001), but there was no effect of parenting on inhibitory control for boys with Met-Val/Val-Val (b = 0.14, t = 1.57, ns). Examining the regions of significance revealed that significant genetic differences were detected at values of parenting ≥0.12 SD below the mean for girls and at values of parenting ≥0.62 SD above the mean for boys.
Table 3.
Substantive models predicting mother-reported internalizing symptomatology and inhibitory control
| Fixed Effects | Internalizing
|
Inhibitory Control
|
||
|---|---|---|---|---|
| b | t | b | t | |
| Intercepta | 0.20 | 4.17 | ||
| Sex | 0.06 | 1.45 | 0.15 | 0.74 |
| Val158Met | 0.03 | 0.75 | 0.38 | 2.16* |
| Parenting Quality | 0.05 | 1.56 | −0.01 | −0.08 |
| Sex ×Val158Met | −0.10 | −2.12* | −0.48 | −2.08* |
| Sex ×Parenting Quality | −0.14 | −3.25** | 0.53 | 2.56* |
| Val158Met ×Parenting Quality | −0.07 | −1.98* | 0.27 | 1.58 |
| Sex×Val158Met×Parenting Quality | 0.14 | 2.94** | −0.66 | −2.75** |
| Linear slope | 0.05 | 3.11** | 0.36 | 7.73*** |
| Quadratic slope | −0.02 | −2.37* | −0.04 | −3.34** |
|
| ||||
| Random Effectsa,b | σ | z | σ | z |
|
| ||||
| Intercept variance | 0.013 | 5.28*** | 0.325 | 6.48*** |
| Linear slope variance | 0.002 | 2.80** | 0.016 | 3.36*** |
| Covariance: intercept/linear slope | −0.001 | −0.67 | −0.010 | −0.87 |
| Residual variance | 0.010 | 13.78*** | 0.136 | 11.46*** |
| Pseudo r2 | 9.2% | 26.7% | ||
Note: Val, Valine; Met, methionine; sex was dummy coded: 0 = female, 1 = male. Val158Met was coded dominantly: 0 = Met-Met, 1 = Met-Val/Val-Val. Parenting quality is standardized (M = 0, SD = 1).
Time is centered at 24 months for internalizing symptomatology and at 42 months for effortful control.
The random effects differ as a function of time.
p < .05.
p < .01.
p < .001.
Figure 1.
Simple effect of parenting quality on mothers’ reports of inhibitory control for children with Val-Val and Met-Val/Met-Met genotype. Val, Valine; Met, methionine. ***p < .001.
There were also significant three-way interactions among parenting, sex, and intron1 (t = −2.63, p < .01, pseudo r2 = 20.9), 3′ UTR (t = −2.75, p < .01, r2 = 20.1%), and the GGG haplotype (t = −2.23, p < .05, pseudo r2 = 25.1%). Consistent with the positive correlations among the various genetic variables, an examination of the simple effects revealed that the pattern of results for intron1, 3′ UTR, and the GGG haplotype were all very similar to the results for Val158Met. Parenting was positively related to inhibitory control for boys with the intron1 AA genotype, for boys with the 3′ UTR AA genotype, and for boys without the GGG haplotype, and for girls with the intron1 GA/GG genotype, girls with the 3′ UTR GA/GG genotype, and for girls with the GGG haplotype. Parenting was not significantly related to inhibitory control for boys with the intron1 GA/GG genotype, boys with the 3′ UTR GA/GG genotype, or boys with the GGG haplotype, or for girls with the intron1 AA genotype, girls with the 3′ UTR AA genotype, or girls without the GGG haplotype.
Internalizing symptomatology
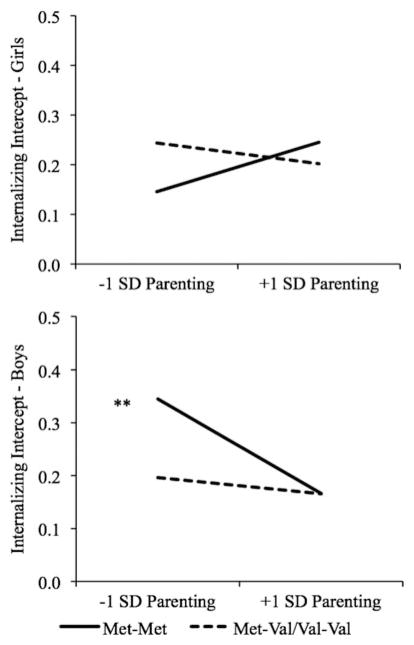
There was a significant three-way interaction among sex, Val158Met, and parenting quality (see Table 3; b = −0.07, t = −1.98, p < .05). This model explained 9.2% of the variance in internalizing, an increase of 8.0% over the unconditional model. The two-way interaction between Val158Met and parenting quality was significant for boys (b = 0.07, t = 2.18, p < .05) and for girls (b = −0.07, t = −1.98, p < .05). For boys with Met-Met, parenting was negatively related to internalizing (see Figure 2; b = −0.09, t =−3.13, p <.01), but for boys with Met-Val/Val-Val, parenting was unrelated to internalizing (b = −0.02, t = −0.84, ns). Despite a significant Val158Met×Parenting Quality interaction, parenting was unrelated to internalizing for girls with Met-Met and with Met-Val/Val-Val (bs =0.05 and −0.02, ts =1.56 and −1.30, ns). Examining the regions of significance indicated that significant genetic differences were detected at values of parenting ≤ 0.86 SD below the mean for girls (Val-Val/Val-Met > Met-Met) and at values of parenting ≤ 0.17 SD above the mean for boys (Met-Met > Val-Val/Val-Met).
Figure 2.
Simple effect of parenting quality on mothers’ reports of internalizing symptomatology for children with Val-Val and Met-Val/Met-Met genotype. Val, Valine; Met, methionine. **p < 01.
There were no main effects or two- or three-way interactions with sex or parenting for the GGG haplotype or for intron1. There was a three-way interaction among 3′ UTR, parenting, and sex, explaining 5.2% of the variance in internalizing symptomatology (b =0.11, t =2.29, p <.05). The two-way Parenting×3′ UTR interactions were not significant for girls or for boys (bs = −0.05 and 0.05, ts = −1.74 and 1.53). Examining the simple effects revealed that parenting was unrelated to internalizing for girls with AA, girls with AG/GG, and boys with AG/GG (bs = 0.03, −0.03, and −0.02; ts = 1.16, −1.35, and −0.92; all ns). Parenting quality was, however, negatively related to internalizing for boys with AA (b = −0.07, t = −2.43, p < .05).
Discussion
In this study, we present evidence that COMT is related to inhibitory control and internalizing symptomatology relatively early in childhood. The longitudinal nature of the data allowed us to determine that this relation was stable over the time period studied (see footnote 2). The pattern of findings in this study was relatively complex, with significant three-way interactions among COMT SNPs (and a haplotype incorporating information from all three SNPs), parenting quality, and sex for internalizing symptoms and inhibitory control, but not for attention focusing. Within males and within females, the interaction between parenting quality and Val158-Met was consistent across internalizing and inhibitory control outcomes, although this pattern was reversed across sex: relatively greater internalizing symptomatology in unsupportive environments and greater inhibitory control in supportive environments was predicted by Met-Val/Val-Val in girls and by Met-Met in boys.
Few researchers have attempted to examine differential susceptibility across multiple outcomes; it has typically been evaluated in the context of only a single dependent variable (although see Obradović et al., 2010). Consequently, this study provides evidence that a genetic variant can serve as a vulnerability factor for one phenotype (in this case, internalizing symptomatology) and a promotive factor for a different phenotype (in this case, inhibitory control). Consistent with most studies of psychopathology, differences were most pronounced for internalizing in unsupportive environmental contexts. This result, considered alone, is consistent with the diathesis–stress framework. However, this pattern was reversed for an aspect of effortful control, with differences emerging in more supportive environmental contexts (consistent with vantage sensitivity; Pluess & Belsky, 2012). Taken together, our results for inhibitory control and internalizing symptoms provide evidence supporting the differential susceptibility perspective (Ellis et al., 2011). Of specific interest is the pattern of for better and for worse responding in particularly supportive and unsupportive environments. Although extreme reductions in dopamine functioning have been tied to behavioral disorders, subtle differences in dopamine at intermediate levels may be “better” in one environment but “worse” in another. These results suggest that the level of COMT activity may have a differential and resulting beneficial impact on boys and girls depending on their environments. Assuming these phenotypes are not the only ones moderated by COMT, this adaptive gene by environment interaction could maintain COMT genetic variation (i.e., multiple SNPs and haplotype combinations) over evolutionary time.
These results highlight the contributions of evolutionary theory that underpin the differential susceptibility perspective. We speculate that population variation in COMT alleles is maintained because each of two alleles is more adaptive in a specific type of environment: in unsupportive environments, vigilant behavior might be more adaptive, whereas in supportive environments, good self-regulation may be more adaptive. Although self-regulatory ability is generally viewed positively, we do see environmental calibration of self-regulatory capacity over time (unsupportive environments are associated with poor self-regulation), which would be consistent with the adaptive calibration and/or biological sensitivity to context theories (Boyce & Ellis, 2005; Del Giudice, Ellis, & Shirtcliff, 2011). Furthermore, high levels of self-regulation may be mal-adaptive in unsupportive contexts, in which behaviors such as delay of gratification can cause individuals to forgo current benefits in favor of future benefits. Under conditions of high uncertainty, this may not represent an optimal strategy, and the development of self-regulation may actually impose high costs (e.g., consumption of metabolic resources for the creation and maintenance of the cortical structures underlying these abilities). Within males and within females, we see a pattern of results that is consistent with this theorizing: within sex, the same allele is associated with greater plasticity to environmental influences, although the nature of these differences depends on the type of environment.
We found evidence that the association between Val158Met and internalizing symptoms was present primarily in the context of unsupportive parenting, whereas the association between Val158Met and inhibitory control was present primarily in the context of supportive parenting. Consequently, although COMT was related to both of these outcomes, it was not related to both outcomes within the same individuals. Within males and within females, there appears to be a trade-off between the possibility of both greater executive function and greater internalizing symptomatology. This finding supports moving away from defining genetic alleles as indicators of risk or resilience based on a medical model, toward a deeper understanding of gene–environment interplay and development (Lemery-Chalfant, 2010).
The Val158Met SNP has been the most studied variant on the COMT gene; however, as previously noted, there are other COMT SNPs that also may affect expression of the COMT enzyme. For the outcomes examined in this study, neither the intron1 or 3′ UTR SNPs nor the GGG haplotype explained more variance in the outcomes relative to Val158Met, although the three-way interactions for intron1 and the GGG haplotype were significant for inhibitory control, and the three-way interaction for 3′ UTR was significant across inhibitory control and internalizing symptoms. Consistent with the moderate to high positive correlations among the genetic variables, results for Val158Met and the other genetic predictors were largely similar. In addition, our results supported coding Val158Met (as well as the intron1 and 3′ UTR SNPs) dominantly for the outcomes examined in this study. We did not find support for better prediction by the GGG haplotype relative to the individual SNPs, although levels of prediction were comparable for inhibitory control, with 25.1% of the variance explained for the GGG haplotype versus 25.7% for Val158Met. Because the Val158Met SNP seemed to provide better resolution than the intron1 and 3′ UTR SNPs and the GGG haplotype, COMT enzyme activity may be relatively more important than levels of COMT expression. That is, catecholamine degradation (i.e., “availability” of dopamine and other neurotransmitters in the brain over time) and its relation to balancing internalizing and inhibitory behavior may be better regulated by COMT efficiency (i.e., high or low enzyme function) rather than COMT expression (i.e., high or low concentrations of the enzyme regardless of its efficiency). Thus, our findings contribute to understanding of an adaptive trade-off between “efficiency” and “abundance” with respect to the COMT enzyme in different environments. Our study and that by Voelker et al. (2009) both examined the Val158Met SNP; however, the additional COMT SNPs used to compose haplotypes were different in the two studies. Nonetheless, both studies found that haplotypes provided no significantly greater resolution or increase in variance than the Val158Met SNP alone, partly because there can be a great deal of correlation (i.e., linkage disequilibrium) among some SNPs, and thus, we may expect similar results across studies for haplotype analyses even when different SNPs are used.
Our findings for effortful control were specific to the inhibitory control subscale and not the attentional focusing sub-scale. Other studies have reported differential patterns of relations with respect to these different aspects of effortful control (e.g., White et al., 2011), and our finding is in line with specificity reported in Diamond et al. (2004), the first study to consider COMT in relation to executive functioning in children. In that study, typically developing Met/Met children (i.e., those with predicted higher PFC dopamine) performed better on a directional Stroop “dots-mixed” task than did Val/Val children (i.e., those with predicted lower PFC dopamine). This task requires working memory and inhibitory control, such that participants must remember two rules and sometimes inhibit the prepotent tendency to respond on the same side as the stimulus. In contrast, COMT did not predict performance on mental rotation, recall memory, or self-ordered pointing tasks that do not require inhibitory control. Different executive functioning and cognitive tasks are differentially sensitive to dopamine in the PFC, and dopamine in the dorsolateral PFC, in particular, appears to affect inhibitory control. Studies with adults that utilize tasks that require working memory and inhibition and recruit the dorsolateral PFC also find associations with COMT (e.g., Wisconsin Card Sorting Test; Bruder et al., 2005; Egan et al., 2001), whereas studies that assess storage, updating of information in working memory, or maintenance of temporal order are not associated (Bruder et al., 2005). Thus, specificity in our findings is in line with existing literature, although too few studies in children have been conducted to draw sweeping conclusions.
Previous work has associated the Val158Met SNP with executive function and with internalizing symptoms. For the latter outcome, however, the direction of this effect has appeared to vary from study to study and has also been moderated by other COMT variants (e.g., Voelker et al., 2009) and by other genes such as the dopamine transporter gene (Holmboe et al., 2010). We provide evidence that this association also depends on measures of environmental quality such as parenting. Our results are similarly in line with the evidence that COMT is sexually dimorphic (Harrison & Tunbridge, 2008), although our findings with respect to sex differences are not entirely consistent with previous studies using adult samples. We found that parenting was differentially related to our outcomes within each sex but the direction of this effect differed for boys and girls. Gene function can be modified by other genes as well as by nongenetic environmental effects. In this case, being male or female (which is genetically determined) influences an individual’s genetic and environmental contexts, either of which could potentially affect COMT, catecholamine levels, or the interplay between COMT and catecholamines. Currently, the mechanism underlying sexual dimorphism in humans for COMT is poorly understood. Nonetheless, it might be the case that, as is the case for any gene by sex relationships, a dosage effect also moderates COMT interactions here. For example, while one allele is more adaptive for females (on average), the other allele is more adaptive for males (on average), thereby contributing to the maintenance of genetic variation in the population. Considering that few sex differences in relations with COMT are found for executive function and that investigators who document sex differences for COMT and internalizing symptoms find effects for women but not for men, the sex differences in this study, as a novel Gene×Environment interaction, should be interpreted with caution (Duncan & Keller, 2011). This is especially true because the precision of estimates is positively related to sample size (Cohen, 1988), which was relatively modest in our study.
The relatively low-risk nature of the sample was a limitation, especially for the measure of internalizing symptoms. The mean level of problems was low and well within the normative range, and therefore it is unclear whether the findings in this study would generalize to clinical levels of symptomatology. However, restriction of range tends to attenuate measures of association, so the relation between COMT and internalizing may be stronger than indicated by our results. In our sample, there was considerable variability in parenting quality, but extremely poor parenting quality (e.g., abuse or maltreatment) was not observed. This could be considered a limitation insofar as some theorists have argued that the full range of environmental variation is important for testing differential susceptibility (Ellis et al., 2011). Another limitation was that our measures were unable to differentiate between different types of internalizing problems (e.g., depression vs. anxiety). Although the reliability of individual ITSEA scales has been demonstrated elsewhere (Briggs-Gowan & Carter, 1998), these scales are short and would not have sufficient reliability to examine separately in this sample. Finally, although the stability of our findings across time contributes to confidence in our results, there is a clear need for replication of these relatively complex three-way interactions among COMT, sex, and parenting in samples with similarly high-quality measures of environment.
Acknowledgments
This research was supported by Grant R01 MH060838 from the National Institute of Mental Health (to N.E. and T.L.S., Principal Investigators, K.L.-C., and B.C.V.). We express our appreciation to the parents and children who participated in the study and to the many research assistants who contributed to this project.
Footnotes
When Val158Met was dummy coded so that AA, AG, and GG were three distinct groups, the results for the AG and GG groups were very similar. Thus, this variant was coded dominantly (AA vs. AG/GG), as in some previous work (e.g., de Frias et al., 2005).
We conducted supplemental analyses in which we included substantive predictors of the slope. In these analyses, the three-way interaction among Val158Met, parenting, and sex was a significant predictor of the intercept when time was centered at the first and the last assessments for internalizing (24 and 54 months) and inhibitory control (42 and 84 months). Thus, prediction of the intercept was equally good across the entire period of data collection. We also tested the simple effect of parenting quality on the dependent variables in these models and found a high degree of consistency in the significance of the simple effects between models with prediction of the slope and models without prediction of the slope.
Due to moderate negative skewness for the parenting quality distribution, parenting scores ranged from −2.91 SD below the sample mean to 1.67 SD above the sample mean. We did not implement the recommendation to plot the simple effects up to ±2 SD from the sample mean (Roisman et al., 2012) because the standard errors are large at such extreme values, indicating considerable uncertainty about the parameter estimates, and it is generally considered undesirable to attempt to generalize beyond the range of the observed data.
Substantive results for the effortful control composite that included attention focusing and inhibitory control were similar to the results for inhibitory control alone: there was a significant three-way interaction among sex, Val158Met, and parenting quality (b = −0.43, t = −2.06, p < .05). This model explained 21.2% of the variance in effortful control, an increase of 13.3% over the unconditional model. The two-way interactions between Val158Met and parenting quality were not significant for girls or for boys (bs = 0.22 and −0.22, ts = 1.40 and −1.51, ns). Examination of the simple effects indicated that there was no effect of parenting on effortful control for girls with Met-Met (b = 0.01, t = 0.08, ns), but a positive effect of parenting on effortful control for girls with Met-Val/Val-Val (b = 0.23, t = 3.30, p < .01). Conversely, there was a positive effect of parenting on effortful control for boys with Met-Met (b = 0.38, t = 3.03, p <.01), but only a marginal positive effect of parenting on effortful control for boys with Met-Val/Val-Val (b = 0.15, t = 1.96, p < .06). Significant genetic differences were detected at values of parenting ≥ 0.39 SD above the mean for girls and but not for any values of parenting for boys.
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: Relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacology Biochemistry and Behavior. 2011;99:211–216. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Barnett JH, Jones PB, Robbins TW, Müller U. Effects of the catechol-O-methyltransferase Val158Met polymorphism on executive function: A meta-analysis of the Wisconsin Card Sort Test in schizophrenia and healthy controls. Molecular Psychiatry. 2007;12:502–509. doi: 10.1038/sj.mp.4001973. [DOI] [PubMed] [Google Scholar]
- Bayer JK, Sanson AV, Hemphill SA. Parent influences on early childhood internalizing difficulties. Journal of Applied Developmental Psychology. 2006;27:542–559. [Google Scholar]
- Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science. 2007;16:300–304. [Google Scholar]
- Belsky J, Beaver KM. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology and Psychiatry. 2011;52:619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, Cohen JD, Fossella J, Casey BJ, Farah MJ. COMT genotype influences prefrontal response to emotional distraction. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:62–70. doi: 10.3758/cabn.6.1.62. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino E, Callicott JH, Das S, Kolachana BS, et al. Effect of catechol-O-methyltransferase Val158Met genotype on attentional control. Journal of Neuroscience. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary–developmental theory of the origins and functions of stress reactivity. Development and Psychopathology. 2005;17:271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Braungart-Rieker J, Garwood MM, Stifter CA. Compliance and noncompliance: The roles of maternal control and child temperament. Journal of Applied Developmental Psychology. 1997;18:411–428. [Google Scholar]
- Bray NJ, Buckland PR, Williams NM, Williams HJ, Norton N, Owen MJ, et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. American Journal of Human Genetics. 2003;73:152–161. doi: 10.1086/376578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs-Gowan MJ, Carter AS. Preliminary acceptability and psychometrics of the Infant–Toddler Social and Emotional Assessment (ITSEA): A new adult-report questionnaire. Infant Mental Health Journal. 1998;19:422–445. [Google Scholar]
- Bruder GE, Keilp JG, Xu H, Shikhman M, Schori E, Gorman JM, et al. Catechol-O-methyltransferase (COMT) genotypes and working memory: Associations with differing cognitive operations. Biological Psychiatry. 2005;58:901–907. doi: 10.1016/j.biopsych.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Burdick KE, Funke B, Goldberg JF, Bates JA, Jaeger J, Kucherlapati R, et al. COMT genotype increases risk for bipolar I disorder and influences neurocognitive performance. Bipolar Disorders. 2007;9:370–376. doi: 10.1111/j.1399-5618.2007.00384.x. [DOI] [PubMed] [Google Scholar]
- Claw KG, Tito RY, Stone AC, Verrelli BC. Haplotype structure and divergence at human and chimpanzee serotonin transporter and receptor genes: Implications for behavioral disorder association analyses. Molecular Biology and Evolution. 2010;27:1518–1529. doi: 10.1093/molbev/msq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- De Frias CM, Annerbrink K, Westberg L, Eriksson E, Adolfsson R, Nilsson L. Catechol-O-methyltransferase Val158Met polymorphism is associated with cognitive performance in nondemented adults. Journal of Cognitive Neuroscience. 2005;17:1018–1025. doi: 10.1162/0898929054475136. [DOI] [PubMed] [Google Scholar]
- Del Giudice M, Ellis BJ, Shirtcliff EA. The adaptive calibration model of stress responsivity. Neuroscience & Biobehavioral Reviews. 2011;35:1562–1592. doi: 10.1016/j.neubiorev.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Briand L, Fossella J, Gehlbach L. Genetic and neurochemical modulation of prefrontal cognitive functions in children. American Journal of Psychiatry. 2004;161:125–132. doi: 10.1176/appi.ajp.161.1.125. [DOI] [PubMed] [Google Scholar]
- Duncan LE, Keller MC. A critical review of the first 10 years of candidate gene-by-environment interaction research in psychiatry. American Journal of Psychiatry. 2011;168:1041–1049. doi: 10.1176/appi.ajp.2011.11020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences. 2001;98:6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Eggum ND. Emotion-related self-regulation and its relation to children’s maladjustment. Annual Review of Clinical Psychology. 2010;6:495–525. doi: 10.1146/annurev.clinpsy.121208.131208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL, Eggum NM, Silva KM, Reiser M, Hofer C, et al. Relations among maternal socialization, effortful control, and maladjustment in early childhood. Development and Psychopathology. 2010;22:507–525. doi: 10.1017/S0954579410000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Zhou Q, Spinrad TL, Valiente C, Fabes RA, Liew J. Relations among positive parenting, children’s effortful control, and externalizing problems: A three-wave longitudinal study. Child Development. 2005;76:1055–1071. doi: 10.1111/j.1467-8624.2005.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. Differential susceptibility to the environment: An evolutionary–neurodevelopmental theory. Development and Psychopathology. 2011;23:7–23. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Enoch MA, Waheed JF, Harris CR, Albaugh B, Goldman D. COMT Val158Met and cognition: Main effects and interaction with educational attainment. Genes, Brain and Behavior. 2009;8:36–42. doi: 10.1111/j.1601-183X.2008.00441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish M, Stifter CA, Belsky J. Conditions of continuity and discontinuity in infant negative emotionality: Newborn to five months. Child Development. 1991;62:1525–1537. [PubMed] [Google Scholar]
- Harrison PJ, Tunbridge EM. Catechol-O-methyltransferase (COMT): A gene contributing to sex differences in brain function, and to sexual dimorphism in the predisposition to psychiatric disorders. Neuropsychopharmacology. 2008;33:3037–3045. doi: 10.1038/sj.npp.1301543. [DOI] [PubMed] [Google Scholar]
- Hettema JM, An S, Bukszar J, van den Oord EJ, Neale MC, Kendler KS, et al. Catechol-O-methyltransferase contributes to genetic susceptibility shared among anxiety spectrum phenotypes. Biological Psychiatry. 2008;64:302–310. doi: 10.1016/j.biopsych.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmboe K, Nemoda Z, Fearon RM, Csibra G, Sasvari-Szekely M, Johnson MH. Polymorphisms in dopamine system genes are associated with individual differences in attention in infancy. Developmental Psychology. 2010;46:404–416. doi: 10.1037/a0018180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PO, Neyman J. Tests of certain linear hypotheses and their application to some educational problems. Statistical Research Memoirs. 1936;1:57–93. [Google Scholar]
- Kocabas NA, Faghel C, Barreto M, Kasper S, Linotte S, Mendlewicz J, et al. The impact of catechol-O-methyltransferase SNPs and haplotypes on treatment response phenotypes in major depressive disorder: A case–control association study. International Clinical Psychopharmacology. 2010;25:218–227. doi: 10.1097/YIC.0b013e328338b884. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N, Nichols KE. Maternal power assertion in discipline and moral discourse contexts: Commonalities, differences, and implications for children’s moral conduct and cognition. Developmental Psychology. 2003;39:949–963. doi: 10.1037/0012-1649.39.6.949. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Kim S, Barry RA, Philibert RA. Children’s genotypes interact with maternal responsive care in predicting children’s competence: Diathesis–stress or differential susceptibility? Development and Psychopathology. 2011;23:605–616. doi: 10.1017/S0954579411000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanska G, Murray KT, Harlan ET. Effortful control in early childhood: Continuity and change, antecedents, and implications for social development. Developmental Psychology. 2000;36:220–232. [PubMed] [Google Scholar]
- Kochanska G, Murray K, Jacques TY, Koenig AL, Vandegeest KA. Inhibitory control in young children and its role in emerging internalization. Child Development. 1996;67:490–507. [PubMed] [Google Scholar]
- Kok R, Linting M, Bakermans-Kranenburg M, IJzendoorn MH, Jaddoe VW, Hofman A, et al. Maternal sensitivity and internalizing problems: Evidence from two longitudinal studies in early childhood. Child Psychiatry and Human Development. 2013;44:751–765. doi: 10.1007/s10578-013-0369-7. [DOI] [PubMed] [Google Scholar]
- Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6:243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
- Laucht M, Blomeyer D, Buchmann AF, Treutlein J, Schmidt MH, Esser G, et al. Catechol-O-methyltransferase Val158Met genotype, parenting practices and adolescent alcohol use: Testing the differential susceptibility hypothesis. Journal of Child Psychology and Psychiatry. 2012;53:351–359. doi: 10.1111/j.1469-7610.2011.02408.x. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K. Genes and environments: How they work together to promote resilience. In: Reich JW, Zautra AJ, Hall JS, editors. Handbook of adult resilience. New York: Guilford Press; 2010. pp. 55–78. [Google Scholar]
- Lengua LJ, Honorado E, Bush NR. Contextual risk and parenting as predictors of effortful control and social competence in pre-school children. Journal of Applied Developmental Psychology. 2007;28:40–55. doi: 10.1016/j.appdev.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipska BK, Mitkus S, Caruso M, Hyde TM, Chen J, Vakkalanka R, et al. RGS4 mRNA expression in postmortem human cortex is associated with COMT Val158Met genotype and COMT enzyme activity. Human Molecular Genetics. 2006;15:2804–2812. doi: 10.1093/hmg/ddl222. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Sparling MB, Ryan LM, Xu K, Salazar AM, Goldman D, et al. Association of COMT Val158Met genotype with executive functioning following traumatic brain injury. Journal of Neuropsychiatry. 2005;17:465–471. doi: 10.1176/jnp.17.4.465. [DOI] [PubMed] [Google Scholar]
- Liu B, Song M, Li J, Liu Y, Li K, Yu C, et al. Prefrontal-related functional connectivities within the default network are modulated by COMT Val158Met in healthy young adults. Journal of Neuroscience. 2010;30:64–69. doi: 10.1523/JNEUROSCI.3941-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, et al. Kinetics of human soluble and membrane-bound catechol-O-methyltransferase: A revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry. 1995;34:4202–4210. doi: 10.1021/bi00013a008. [DOI] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger AK, Mrakotsky C, Brown KM, Hessler MJ, Wallis JM, et al. The clinical picture of depression in preschool children. Journal of the American Academy of Child & Adolescent Psychiatry. 2003;42:340–348. doi: 10.1097/00004583-200303000-00015. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg TE, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. American Journal of Psychiatry. 2002;159:652–654. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Validation of the five-factor model of personality across instruments and observers. Journal of Personality and Social Psychology. 1987;85:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- McGrath M, Kawachi I, Ascherio A, Colditz GA, Hunter DJ, De Vivo I. Association between catechol-O-methyltransferase and phobic anxiety. American Journal of Psychiatry. 2004;161:1703–1705. doi: 10.1176/appi.ajp.161.9.1703. [DOI] [PubMed] [Google Scholar]
- McLeod BD, Weisz JR, Wood JJ. Examining the association between parenting and childhood depression: A meta-analysis. Clinical Psychology Review. 2007;27:986–1003. doi: 10.1016/j.cpr.2007.03.001. [DOI] [PubMed] [Google Scholar]
- McLeod BD, Wood JJ, Weisz JR. Examining the association between parenting and childhood anxiety: A meta-analysis. Clinical Psychology Review. 2007;27:155–172. doi: 10.1016/j.cpr.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Nichols T, Callicott JH, Ding J, Kolachana B, Buckholtz J, et al. Impact of complex genetic variation in COMT on human brain function. Molecular Psychiatry. 2006;11:867–877. doi: 10.1038/sj.mp.4001860. [DOI] [PubMed] [Google Scholar]
- Mier D, Kirsch P, Meyer-Lindenberg A. Neural substrates of pleiotropic action of genetic variation in COMT: A meta-analysis. Molecular Psychiatry. 2009;15:918–927. doi: 10.1038/mp.2009.36. [DOI] [PubMed] [Google Scholar]
- Monroe SM, Simons AD. Diathesis–stress theories in the context of life stress research: Implications for the depressive disorders. Psychological Bulletin. 1991;110:406–425. doi: 10.1037/0033-2909.110.3.406. [DOI] [PubMed] [Google Scholar]
- Montag C, Buckholtz JW, Hartmann P, Merz M, Burk C, Hennig J, et al. COMT genetic variation affects fear processing: Psychophysiological evidence. Behavioral Neuroscience. 2008;122:901–909. doi: 10.1037/0735-7044.122.4.901. [DOI] [PubMed] [Google Scholar]
- Nobile M, Rusconi M, Bellina M, Marino C, Giorda R, Carlet O, et al. COMT Val158Met polymorphism and socioeconomic status interact to predict attention deficit/hyperactivity problems in children aged 10–14. European Child and Adolescent Psychiatry. 2009;19:549–557. doi: 10.1007/s00787-009-0080-1. [DOI] [PubMed] [Google Scholar]
- Obradović J, Bush NR, Stampterdahl J, Adler NE, Boyce WT. Biological sensitivity to context: The interactive effects of stress reactivity and family adversity on socioemotional behavior and school readiness. Child Development. 2010;81:270–289. doi: 10.1111/j.1467-8624.2009.01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, Ferdinand RF, Verhulst FC, Ormel J. Effortful control as modifier of the association between negative emotionality and adolescents’ mental health problems. Development and Psychopathology. 2007;19:523–539. doi: 10.1017/S0954579407070253. [DOI] [PubMed] [Google Scholar]
- Olsson CA, Anney RJ, Lotfi-Miri M, Byrnes GB, Williamson R, Patton GC. Association between the COMT Val158Met polymorphism and propensity to anxiety in an Australian population-based longitudinal study of adolescent health. Psychiatric Genetics. 2005;15:109–115. doi: 10.1097/00041444-200506000-00007. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J. Vantage sensitivity: Individual differences in response to positive experiences. Psychological Bulletin. 2012 doi: 10.1037/a0030196. Advance online publication. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Propper C, Willoughby M, Halpern CT, Carbone MA, Cox M. Parenting quality, DRD4, and the prediction of externalizing and internalizing behaviors in early childhood. Developmental Psychobiology. 2007;49:619–632. doi: 10.1002/dev.20249. [DOI] [PubMed] [Google Scholar]
- Roisman GI, Newman DA, Fraley RC, Haltigan JD, Groh AM, Haydon KC. Distinguishing differential susceptibility from diathesis–stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24:389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey K, Fisher P. Investigations of temperament at three to seven years: The Children’s Behavior Questionnaire. Child Development. 2001;72:1394–1408. doi: 10.1111/1467-8624.00355. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Bates JE. Temperament. In: Damon W, Lerner RM, Eisenberg N, editors. Handbook of child psychology: Vol. 3. Social, emotional, and personality development. 6. Hoboken, NJ: Wiley; 2006. pp. 99–166. [Google Scholar]
- Sambrook J, Russell DW. Molecular cloning: A laboratory manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Shifman S, Bronstein M, Sternfeld M, Pisanté-Shalom A, Lev-Lehman E, Weizman A, et al. A highly significant association between a COMT haplotype and schizophrenia. American Journal of Human Genetics. 2002;71:1296–1302. doi: 10.1086/344514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JA, Willett JB. Applied longitudinal data analysis. New York: Oxford University Press; 2003. [Google Scholar]
- Smolka MN, Bühler M, Schumann G, Klein S, Hu X, Moayer M, et al. Gene–gene effects on central processing of aversive stimuli. Molecular Psychiatry. 2007;12:307–317. doi: 10.1038/sj.mp.4001946. [DOI] [PubMed] [Google Scholar]
- Spinrad TL, Eisenberg N, Gaertner B, Popp T, Smith CL, Kupfer A, et al. Relations of maternal socialization and toddlers’ effortful control to children’s adjustment and social competence. Developmental Psychology. 2007;43:1170–1186. doi: 10.1037/0012-1649.43.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stifter CA, Spinrad TL, Braungart-Rieker JM. Toward a developmental model of child compliance: The role of emotion regulation in infancy. Child Development. 1999;70:21–32. doi: 10.1111/1467-8624.00003. [DOI] [PubMed] [Google Scholar]
- Straub RE, Speer MC, Luo Y, Rojas K, Overhauser J, Ott J, et al. A microsatellite genetic linkage map of human chromosome 18. Genomics. 1993;15:48–56. doi: 10.1006/geno.1993.1008. [DOI] [PubMed] [Google Scholar]
- Sulik MJ, Eisenberg N, Lemery-Chalfant K, Spinrad TL, Silva KM, Eggum ND, et al. Interactions between serotonin transporter gene haplotypes and quality of mothers’ parenting predict the development of children’s noncompliance. Developmental Psychology. 2012;48:740–754. doi: 10.1037/a0025938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabachnik BG, Fidell LS. Using multivariate statistics. 5. Needham Heights, MA: Pearson/Allyn & Bacon; 2006. [Google Scholar]
- Taylor ZE, Sulik MJ, Eisenberg N, Spinrad TL, Silva KM, Lemery-Chalfant K, et al. Development of ego-resiliency: Relations to observed parenting and polymorphisms in the serotonin transporter gene during early childhood. Social Development. 2013 doi: 10.1111/sode.12041. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol-O-methyltransferase gene and its expression from two distinct promoters. European Journal of Biochemistry. 1994;223:1049–1059. doi: 10.1111/j.1432-1033.1994.tb19083.x. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Weickert CS, Kleinman JE, Herman MM, Chen J, Kolachana BS, et al. Catechol-O-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the post-natal lifespan. Cerebral Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- Voelker P, Sheese BE, Rothbart MK, Posner MI. Variations in catechol-O-methyltransferase gene interact with parenting to influence attention in early development. Neuroscience. 2009;164:121–130. doi: 10.1016/j.neuroscience.2009.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral inhibition and anxiety: The moderating roles of inhibitory control and attention shifting. Journal of Abnormal Child Psychology. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavich L, Forsberg MM, Karayiorgou M, Gogos JA, Männistö PT. Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum. Journal of Neuroscience. 2007;27:10196–10209. doi: 10.1523/JNEUROSCI.0665-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. Vulnerability to psychopathology: A biosocial model. Washington, DC: American Psychological Association; 1999. [Google Scholar]