Abstract
Botulinum neurotoxins (BoNTs) are extremely poisonous protein toxins that cause the fatal paralytic disease botulism. They are naturally produced in bacteria with several nontoxic neurotoxin-associated proteins (NAPs) and together they form a progenitor toxin complex (PTC), the largest bacterial toxin complex known. In foodborne botulism, the PTC functions as a molecular machine that helps BoNT breach the host defense in the gut. Here, we discuss the substantial recent advance in elucidating the atomic structures and assembly of the 14-subunit PTC, including structures of BoNT and four NAPs. These structural studies shed light on the molecular mechanisms by which BoNT is protected against the acidic environment and proteolytic destruction in the gastrointestinal tract, and how it is delivered across the intestinal epithelial barrier.
Introduction
Botulinum neurotoxins (BoNTs) are secreted by the bacterium Clostridium botulinum and less frequently, by Clostridium butyricum and Clostridium baratii. There are seven serotypes of BoNTs, designated type A through G (BoNT/A–G), which include at least 40 different subtypes (for a recent review, see [1]). An eighth serotype, BoNT/H, has been reported recently, but is pending further validation [2,3]. BoNT/A, B, E and F are known to cause botulism in both human and other animals, while BoNT/C and D mainly affect cattle, poultry, and wild birds (for a recent review, see [4]). All BoNTs carry out their damage as potent blockers of neurotransmission in the peripheral cholinergic nerve terminals [5].
BoNTs are tripartite proteins consisting of a ∼50 kDa light chain (LC) and a ∼100 kDa heavy chain (HC). HC can be further divided into an N-terminal translocation domain (HN) and a C-terminal receptor-binding domain (HC or RBD) (Figure 1A). Upon arriving at neuromuscular junctions, HC helps BoNT attach to the neuronal membrane by binding to gangliosides and specific synaptic vesicle proteins (e.g., synaptotagmin or synaptic vesicle glycoprotein 2) [6-8]. The toxin is then endocytosed with its receptors, followed by HN-mediated translocation of LC across the vesicle membrane to the cytosol. LC is a Zn2+-endopeptidase that cleaves SNARE (soluble N-ethylmaleimide sensitive factor attachment protein receptor) components and arrests the synaptic recycling. The resulting blockade of cholinergic neurons subsequently leads to fatal muscle paralysis [9].
Figure 1.
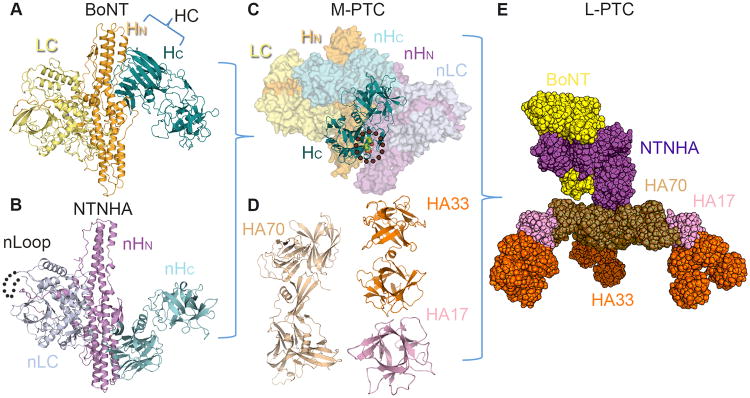
Macromolecular assembly of the L-PTC. (A) BoNT is composed of the N-terminal LC (pale yellow), HN (bright orange), and the C-terminal HC (deep teal) (PDB: 3BTA). (B) NTNHA displays a similar domain organization as BoNT, which contains nLC (blue white), nHC (cyan), and nHN (violet). The nLoop likely mediates interaction with HAs (dotted line) (PDB: 3V0A). (C) The assembly of the interlocked M-PTC is regulated by environmental pH, and two pH sensing residues on BoNT/A have been identified (green sphere and circled). (D–E) The M-PTC (BoNT/yellow–NTNHA/purple) further assembles with three HA70 (sand) (PDB: 4LO4), three HA17 (pink), and six HA33 (orange) (PDB: 4LO0) to form the bimodular L-PTC.
By contrast to the well-studied BoNT–neuron interaction, it is not known how BoNTs in foodborne botulism manage to achieve efficient absorption through the gastrointestinal (GI) tract, which is possibly the most challenging route of entry into the systemic circulation. After ingestion with toxin-contaminated food, BoNTs have to tolerate the extremely acidic (pH < 3) and protease-rich environment of the stomach, and the tightly regulated intestinal barrier. We now know that BoNTs overcome the host defense in the form of a large multi-protein complex, the progenitor toxin complex (PTC). The PTC of some BoNT serotypes exhibits ∼360–16,000-fold greater oral toxicity than the free BoNT [10-13]. In this review, we summarize recent progress in understanding the structure and assembly of the PTC, emphasizing the structural determinants that guard the toxin when circumventing the primary host defense in the gut.
The progenitor toxin complex, BoNT's landing gear
BoNTs are naturally produced as PTCs, which are composed of BoNT and several auxiliary components termed nontoxic neurotoxin-associated proteins (NAPs). The NAPs are encoded together with the bont gene in one of two different gene clusters, the HA cluster or the orfX cluster [14]. Both clusters encode the non-toxic non-hemagglutinin (NTNHA) protein (Figure 1B), which assembles with BoNT to form the minimally functional PTC (M-PTC, also termed the 12S toxin) (Figure 1C). The HA gene cluster, as observed in BoNT/A–D and G, encodes three hemagglutinins (HA70, HA17, and HA33; also called HA3, HA2, and HA1, respectively) (Figure 1D), which together with BoNT and NTNHA constitute the large PTC (L-PTC or the 16S toxin) (Figure 1E) [15]. By contrast, some BoNTs, such as BoNT/A2–4, E, and F, are encoded in the orfX gene cluster, which contains several orfX genes but not the HA genes. The function of the corresponding orfX proteins remains elusive.
Atomic models of the L-PTC of BoNT/A (L-PTC/A) and BoNT/B (L-PTC/B) have been recently elucidated using a combination of X-crystallography and electron microscopy (EM) [16••,17••]. They display a similar structural organization, which is composed of 14 protein subunits including BoNT, NTNHA, HA70, HA17 and HA33 in a 1:1:3:3:6 stoichiometry. The overall architecture of the L-PTC consists of two structurally and functionally independent entities, an ovoid-shaped M-PTC and a triskelion-shaped HA complex (Figure 1E). The M-PTC protects BoNT from destruction in the GI tract and the HA complex allows BoNT to dock onto the receptors located on the lumen of the small intestine. Based on an earlier EM study, the L-PTC of BoNT/D (L-PTC/D) likely adopts a similar structure [18], suggesting that the L-PTC structure may be conserved across HA-containing BoNT serotypes.
Structure and function of the M-PTC
In the absence of M-PTC formation, free BoNT/A is easily inactivated by digestive proteases or by incubation under an acidic environment. Its oral median lethal dose (LD50) is reduced 10-20-fold when it forms the M-PTC with NTNHA. The crystal structure of the M-PTC of BoNT/A offers the first molecular insight into the protection mechanism (Figure 1C) [19••,20]. NTNHA-A has a strikingly similar tripartite architecture as BoNT/A, despite their low amino acid sequence identity. The three domains of NTNHA-A are therefore named as nLC, nHN, and nHC, because they resemble LC, HN, and HC of BoNT/A, respectively. However, BoNT/A residues that are important for its Zn2+-dependent endopeptidase activity and receptor binding are lost in NTNHA-A, which therefore lacks the neurotoxicity. BoNT/A and NTNHA-A form an inter-locked complex that buries a large solvent-accessible area of ∼3200 Å per subunit. Interestingly, all three domains of NTNHA-A bind to the HC fragment of BoNT/A, leaving LC largely exposed (Figure 1C), which is consistent with the biochemical finding that HC is more susceptible to proteolytic cleavage than LC and HN [21,22]. Mechanistically, HC-mediated receptor binding is the earliest step during neuron invasion and likely one of the most crucial, because damage to HC would otherwise jeopardize the enrichment of BoNT/A on the neuron membrane [23]. Therefore, the apparently biased molecular safeguard for HC, as opposed to the other toxin domains, is likely the most efficient strategy to protect such a large protein [19••].
Interestingly, BoNT is released from the PTC upon transition from acidic to neutral pH, as occurs during absorption from the intestine into the general circulation [24]. This is achieved by the presence of pH-dependent interactions between BoNT and NTNHA [19••]. Recent small-angle X-ray scattering studies indicate that NTNHA-A is able to sense the change of environmental pH, and that acidic conditions induce NTNHA-A to adopt a specific conformation that initiates a mutual induced fit between NTNHA-A and BoNT/A [25•,26]. At the same time, pH-sensing residues on BoNT/A (e.g., Glu982 and Asp1037) and NTNHA-A are protonated to allow favorable local electrostatic interactions between them to strengthen the binding (Figure 1C) [19••]. The inherent pH sensing feature of the M-PTC is crucial to ensure stable binding to protect BoNT in the GI tract and release it in systemic circulation.
Currently, a high-resolution structure of an M-PTC is only available for BoNT/A. The structures of the M-PTC of BoNT/B and BoNT/E revealed by negative stained EM and 3D reconstruction closely resemble that of BoNT/A [17••]. The crystal structure of the free-form of NTNHA-D is highly similar to NTNHA-A [26]. Therefore, the BoNT–NTNHA binding module is likely conserved across different BoNTs serotypes. It is worth noting that a unique peptide fragment in nLC of NTNHA, termed the nLoop, is conserved in HA-containing BoNT serotypes, and likely mediates the interaction between the M-PTC and the HA complex [19••,27]. But the molecular details of this interaction have yet to be determined.
Structure and function of the HA complex
Architecture of the HA complex
Atomic models of the fully assembled HA complexes of BoNT/A (HA/A) and BoNT/B (HA/B) have been determined recently [16••,17••,28•]. In addition, the subcomponent structures of HAs are available for BoNT/C (HA33 and HA70) [29-32] and BoNT/D (HA17–HA33 complex) [18]. The HA complex features three prominent triangular blades (Figure 2). The center of the complex is the trimeric HA70 hub. Each HA70 contains three domains (named D1–3): D1 and D2 participate in homo-trimerization and D3 sits at the periphery of the trimer and interacts with HA17. HA17 contains a compact β-trefoil fold and simultaneously binds two HA33 molecules. Each HA33 is composed of two β-trefoil domains linked by an α helix (Figure 2). Although the N-terminal domain of HA33 is restrained by docking to HA17, its C-terminal domain is exposed and exhibits significant conformational plasticity [16••,28•]. The overall structure of the HA complex is likely to be flexible and its three arms may adopt different conformations [17••].
Figure 2.
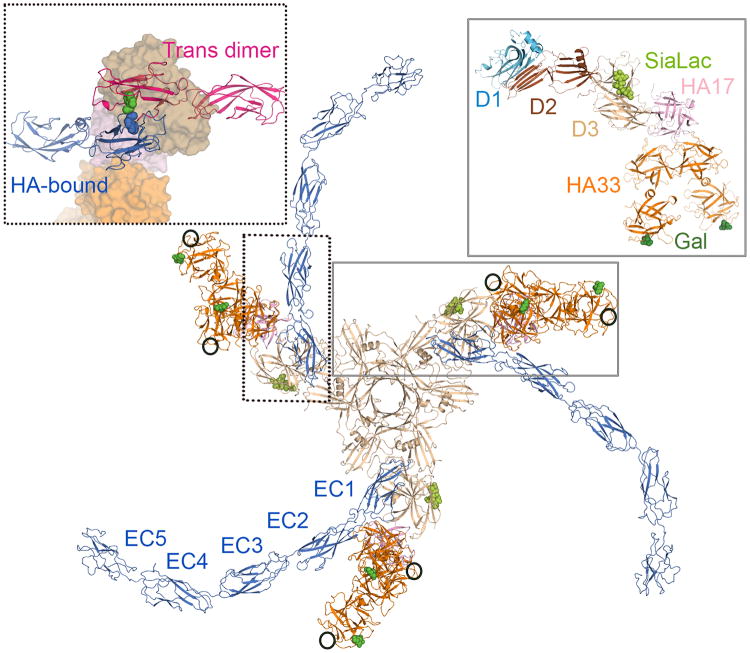
The triskelion-shaped HA complex in complex with carbohydrates and E-cadherin. As highlighted in the right box, each arm of the HA complex contains one HA70 (sand), one HA17 (pink), and two HA33 (orange). HA70 D1 (light blue) and D2 (brown) domains are crucial for the trimer formation of HA70. HA/A contains nine glycan-binding sites with three Neu5Ac-binding sites on HA70 (lime) and six galactose-binding sites on HA33 (forest) (PDB: 4LO1 and 4LO5). HA/C may contain one additional Neu5Ac-binding site on each HA33 (circled). E-cadherin has five extracellular domains (EC1–EC5). To form a trans dimer, residue Trp2 (green sphere) of an E-cadherin (blue molecule) needs to dock into the complementary Trp-binding pocket on the partner E-cadherin (pink molecule). As shown in the left box, the HA complex (surface representation) sequesters E-cadherin (blue molecule) in the monomeric state with the Trp2 (blue sphere) resting in its own Trp-binding pocket, and also blocks the access of its potential dimer partner (PDB: 4QD2).
The HA complex binds to cell-surface carbohydrates
The intestinal microvilli are covered by a stratified layer of mucus. The HA complex is believed to anchor the L-PTC on the intestinal surface through its multiple carbohydrate-binding sites. HA70 binds one Neu5Ac-containing carbohydrate [16••]. HA33, on the other hand, binds one galactose-containing carbohydrate through its C-terminal β-trefoil domain in serotypes A and B [16••,33•]. HA33 serotype C, however, displays a lower affinity for galactose, but carries an extra Neu5Ac-binding site near the Gal-binding pocket [30,34]. These serotype-specific HA–glycan interactions may partially contribute to the different oral toxicity and host susceptibility among different BoNT serotypes [35-38]. Altogether, each HA complex likely displays multivalent carbohydrate binding, involving up to 9 carbohydrates in L-PTC/A and B and up to 15 carbohydrates in L-PTC/C (Figure 2) [16••,30,34]. Moreover, the carbohydrate-binding domain of HA33 is located at the very tip of the HA complex and displays significant conformational flexibility, which may allow the complex to adjust itself on the intestinal surface to achieve optimal multivalent glycan binding [35,39]. The physiological importance of the HA– carbohydrate interactions has been further validated by in vivo studies using a mouse oral toxicity assay. Carbohydrate receptor mimics (for instance, IPTG) extended survival of mice following lethal BoNT/A oral intoxication [16••], and a mutated L-PTC/A that is unable to bind carbohydrate displayed drastically reduced oral toxicity [40••].
The HA complex disrupts the E-cadherin-mediated cell-cell junctions
The intestinal epithelial cells are tightly regulated by adhesion proteins that physically separate the lumen from the lamina propria. Therefore, it is fascinating that the HAs can hijack the adhesion protein E-cadherin to disrupt the intestinal epithelial barrier [41,42]. E-cadherin contains five tandem extracellular cadherin (EC) domains (EC1–EC5), which share a common seven-stranded β-barrel fold. They are typically located below tight junctions and mediate cell-cell recognition and adhesion via trans-dimerization between their N-terminal EC1 domains, which extend from the apposed cells, and cis-dimerization between molecules on the same cell [43,44]. In the trans-dimer, the EC1 domains from apposed cells form a “strand-swapped” conformation in which residue Trp2 acts as an anchor by docking into a complementary Trp-binding pocket in the partner E-cadherin molecule (Figure 2, left panel). This occurs via a two step-binding process that involves a fast-binding intermediate conformation named the “X-dimer” [43,45]. The dimeric E-cadherin is energetically more favorable than a monomeric conformation that places a conformational strain on its N-terminal 10 amino acids (termed the A*/A strand) [46].
A major advance was provided by the crystal structure of an HA/A subcomplex bound with EC1–EC2 of E-cadherin [40••]. This structure showed that the HA complex stabilizes the A*/A strand of E-cadherin in its monomeric conformation with Trp2 binding intra-molecularly into its own Trp-binding pocket, therefore relieving the driving force for trans-dimerization (Figure 2, left panel). Furthermore, the HA complex occupies the E-cadherin dimer interface that is required to form the trans-dimer and the X-dimer. Consistent with this finding, HA/A binds the monomeric EC1–EC2 domains with an affinity that is much stronger than the affinity of E-cadherin homo-dimerization [40••,44,47]. The model that disruption of the adherens junctions of epithelial cells by the HA complex opens up a paracellular route to facilitate BoNT absorption has been supported by extensive in vitro and ex vivo studies [33•,40••,42] (Figure 3), and was further confirmed by an in vivo study showing that an E-cadherin binding deficient L-PTC/A has markedly decreased oral toxicity in mouse [40••].
Figure 3.
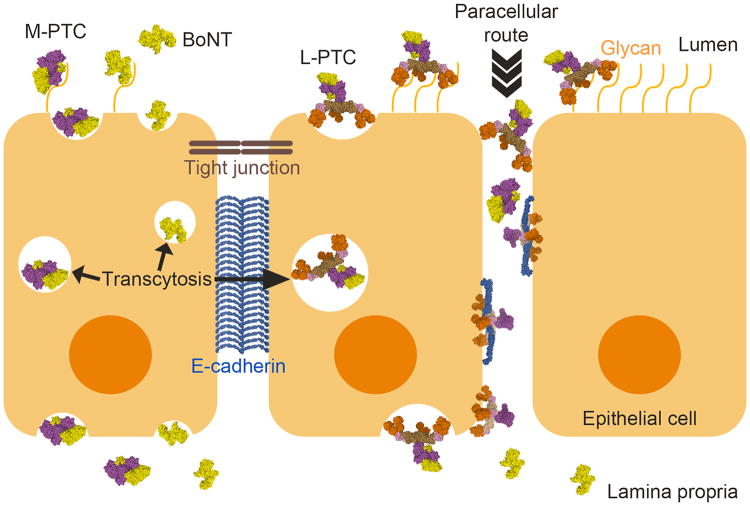
Models for the intestinal absorption of the BoNT complexes. L-PTC, M-PTC or BoNT could be adsorbed by transcytosis that is mediated by carbohydrates and/or unknown protein receptors on the cell surface. When the L-PTC gains access to E-cadherin, the HA complex could disrupt the epithelial barrier, leading to efficient absorption of BoNTs and PTCs through the paracellular route. After crossing the epithelial barrier, BoNT dissociates from the complex and enters into the systemic circulation.
Interestingly, the complete triskelion-shape of the HA complex is crucial for its function, because a sub-complex representing one arm of the HA complex failed to disrupt cell-cell junctions [40••]. The fully assembled HA complex might simultaneously bind three E-cadherins, which would greatly strengthen binding through avidity effects and is likely necessary to achieve potent binding in vivo. Furthermore, the bulky triskelion-shaped HA complex (∼260 Å wide and ∼100 Å height) might disrupt the condensed arrays of E-cadherin dimers that normally stabilize adherens junctions. Additionally, sequestration of E-cadherin by the HA complex might destabilize adherens junctions by affecting the turnover of E-cadherin at adherens junctions [48].
Most of the E-cadherin-binding residues are conserved between the HAs of BoNT/A and BoNT/B, suggesting that the HA–E-cadherin binding mode is likely conserved between these human toxins. By contrast, the HAs of BoNT/C and D, which predominantly cause botulism in birds and cattle, do not bind E-cadherin [33•,40••]. Future studies should aim to understand whether or how HA/C and HA/D disrupt the intestinal epithelial barrier and the functional role of HAs in determining host tropism of various BoNTs [49,50].
Current models for trans-epithelial delivery of the L-PTC
Many structural and functional studies suggest that BoNTs have two different routes of passing through the intestinal epithelial cells (Figure 3). In one scenario, BoNTs in the forms of L-PTC, M-PTC or the free form may cross the epithelial cells by transcytosis without interfering with the epithelial barrier. Once the HA complexes gain access to the basolateral surface, they disrupt E-cadherin-mediated cell-cell adhesion, thereby opening up the paracellular route for BoNT absorption. However, many fundamental questions remain unanswered. For example, the mechanism of BoNT transcytosis is largely unknown. Some data suggest that BoNT might directly recognize specific receptors on intestinal cell surface that mediate transcytosis [51,52]. Alternatively, it is possible that there are transcytosis hot spots on intestinal epithelia, for instance microfold cells (M cells) and neuroendocrine crypt cells, which could mediate BoNT transcytosis [42,53]. Notably, E-cadherin is luminally accessible around mucus-expelling goblet cells, around extruding enterocytes at the tip and lateral sides of villi, and in villus epithelial folds [54]. Hence, the HA complex might access E-cadherin in the intestinal lumen to mediate transcytosis and/or paracellular crossing.
Conclusion and future perspectives
Structures of the 760 kDa L-PTC have revealed a sophisticated macromolecular machine of bacterial toxins that evades host defense systems. Besides stabilizing BoNT in the harsh environment of the GI tract, the L-PTC efficiently delivers BoNT into the general circulation through up to 15 binding sites for cell surface carbohydrates and 3 binding sites for the crucial host adhesion protein E-cadherin. It is worth noting that BoNTs use a dual-receptor mechanism to recognize nerve terminals by interacting with both a protein receptor and gangliosides to mediate cell entry at neuromuscular junctions [23]. It is remarkable that BoNTs use the “same” strategy twice, targeting different host receptors at different times and locations, to ensure its extreme toxicity.
The advances in understanding the structure and function of the L-PTC will promote the development of novel chemical inhibitors or antibody/peptide inhibitors that block the L-PTC from recognizing intestinal glycan and protein receptors, thereby preventing toxin invasion. The L-PTC could also be exploited for alternative applications. For example, coupling of protein-based therapeutics to a modified non-toxic L-PTC or the HA complex might facilitate drug delivery by enhancing permeability of the intestinal epithelium. Thus, the improved structural understanding of these fascinating macromolecular assemblies informs efforts to treat a deadly toxin and opens opportunities to develop novel therapeutics.
Highlights.
Botulinum neurotoxins are highly potent oral toxins.
The large progenitor complex of BoNT is a bimodular 14-subunit complex.
NTNHA protects BoNT in the acidic and protease-rich gastrointestinal tract.
The HA complex displays multivalent binding with the host glycans in the intestine.
The HA complex hijacks E-cadherin to cross epithelial cell junctions.
Acknowledgments
This work was supported in part by National Institute of Allergy and Infectious Diseases (NIAID) grant R01AI091823 to R.J..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smith TJ, Hill KK, Raphael BH. Historical and Current Perspectives on Clostridium botulinum Diversity. Res Microbiol. 2014 doi: 10.1016/j.resmic.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barash JR, Arnon SS. A novel strain of Clostridium botulinum that produces type B and type H botulinum toxins. J Infect Dis. 2014;209:183–191. doi: 10.1093/infdis/jit449. [DOI] [PubMed] [Google Scholar]
- 3.Dover N, Barash JR, Hill KK, Xie G, Arnon SS. Molecular characterization of a novel botulinum neurotoxin type H gene. J Infect Dis. 2014;209:192–202. doi: 10.1093/infdis/jit450. [DOI] [PubMed] [Google Scholar]
- 4.Rossetto O, Pirazzini M, Montecucco C. Botulinum neurotoxins: genetic, structural and mechanistic insights. Nat Rev Microbiol. 2014;12:535–549. doi: 10.1038/nrmicro3295. [DOI] [PubMed] [Google Scholar]
- 5.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 6.Jin R, Rummel A, Binz T, Brunger AT. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 2006;444:1092–1095. doi: 10.1038/nature05387. [DOI] [PubMed] [Google Scholar]
- 7.Chai Q, Arndt JW, Dong M, Tepp WH, Johnson EA, Chapman ER, Stevens RC. Structural basis of cell surface receptor recognition by botulinum neurotoxin B. Nature. 2006;444:1096–1100. doi: 10.1038/nature05411. [DOI] [PubMed] [Google Scholar]
- 8.Benoit RM, Frey D, Hilbert M, Kevenaar JT, Wieser MM, Stirnimann CU, McMillan D, Ceska T, Lebon F, Jaussi R, et al. Structural basis for recognition of synaptic vesicle protein 2C by botulinum neurotoxin A. Nature. 2014;505:108–111. doi: 10.1038/nature12732. [DOI] [PubMed] [Google Scholar]
- 9.Schiavo G, Rossetto O, Benfenati F, Poulain B, Montecucco C. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann N Y Acad Sci. 1994;710:65–75. doi: 10.1111/j.1749-6632.1994.tb26614.x. [DOI] [PubMed] [Google Scholar]
- 10.Ohishi I, Sugii S, Sakaguchi G. Oral toxicities of Clostridium botulinum toxins in response to molecular size. Infect Immun. 1977;16:107–109. doi: 10.1128/iai.16.1.107-109.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng LW, Onisko B, Johnson EA, Reader JR, Griffey SM, Larson AE, Tepp WH, Stanker LH, Brandon DL, Carter JM. Effects of purification on the bioavailability of botulinum neurotoxin type A. Toxicology. 2008;249:123–129. doi: 10.1016/j.tox.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 12.Ohishi I. Oral toxicities of Clostridium botulinum type A and B toxins from different strains. Infect Immun. 1984;43:487–490. doi: 10.1128/iai.43.2.487-490.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakaguchi G. Clostridium botulinum toxins. Pharmacol Ther. 1982;19:165–194. doi: 10.1016/0163-7258(82)90061-4. [DOI] [PubMed] [Google Scholar]
- 14.Hill KK, Smith TJ. Genetic Diversity Within Clostridium botulinum Serotypes, Botulinum Neurotoxin Gene Clusters and Toxin Subtypes. Curr Top Microbiol Immunol. 2013;364:1–20. doi: 10.1007/978-3-642-33570-9_1. [DOI] [PubMed] [Google Scholar]
- 15.Somers E, DasGupta BR. Clostridium botulinum types A, B, C1, and E produce proteins with or without hemagglutinating activity: do they share common amino acid sequences and genes? J Protein Chem. 1991;10:415–425. doi: 10.1007/BF01025256. [DOI] [PubMed] [Google Scholar]
- 16••.Lee K, Gu S, Jin L, Le TT, Cheng LW, Strotmeier J, Kruel AM, Yao G, Perry K, Rummel A, et al. Structure of a bimodular botulinum neurotoxin complex provides insights into its oral toxicity. PLoS Pathog. 2013;9:e1003690. doi: 10.1371/journal.ppat.1003690. This is a comprehensive study describing the structure and function of the L-PTC/A. The authors present an atomic model of the L-PTC/A using a combination of X-ray crystallography and single particle EM and 3D reconstruction. Together with biochemical studies, these findings reveal that the HA complex mediates BoNT absorption by binding to host carbohydrates. Furthermore, the authors suggest that carbohydrate receptor mimics could be developed into novel oral inhibitors as preventive countermeasures aganist BoNTs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Benefield DA, Dessain SK, Shine N, Ohi MD, Lacy DB. Molecular assembly of botulinum neurotoxin progenitor complexes. Proc Natl Acad Sci U S A. 2013;110:5630–5635. doi: 10.1073/pnas.1222139110. Using EM 3D reconstruction and the known component structures, the authors present the structural models of the fully assembled L-PTC/A and B. They also show that the M-PTC of BoNT/E closely resembles the M-PTC of BoNT/A and BoNT/B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasegawa K, Watanabe T, Suzuki T, Yamano A, Oikawa T, Sato Y, Kouguchi H, Yoneyama T, Niwa K, Ikeda T, et al. A novel subunit structure of clostridium botulinum serotype D toxin complex with three extended arms. J Biol Chem. 2007;282:24777–24783. doi: 10.1074/jbc.M703446200. [DOI] [PubMed] [Google Scholar]
- 19••.Gu S, Rumpel S, Zhou J, Strotmeier J, Bigalke H, Perry K, Shoemaker CB, Rummel A, Jin R. Botulinum neurotoxin is shielded by NTNHA in an interlocked complex. Science. 2012;335:977–981. doi: 10.1126/science.1214270. The paper describes the first crystal structure of the M-PTC of BoNT/A, which reveals the molecular mechanism of how NTNHA-A protects BoNT/A through extensive protein-protein interactions. Furthermore, some key residues on BoNT/A that regulate the pH-dependent assembly of the M-PTC were identified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu S, Jin R. Assembly and function of the botulinum neurotoxin progenitor complex. Curr Top Microbiol Immunol. 2013;364:21–44. doi: 10.1007/978-3-642-33570-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen F, Kuziemko GM, Amersdorfer P, Wong C, Marks JD, Stevens RC. Antibody mapping to domains of botulinum neurotoxin serotype A in the complexed and uncomplexed forms. Infect Immun. 1997;65:1626–1630. doi: 10.1128/iai.65.5.1626-1630.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shone CC, Hambleton P, Melling J. Inactivation of Clostridium botulinum type A neurotoxin by trypsin and purification of two tryptic fragments. Proteolytic action near the COOH-terminus of the heavy subunit destroys toxin-binding activity Eur J Biochem. 1985;151:75–82. doi: 10.1111/j.1432-1033.1985.tb09070.x. [DOI] [PubMed] [Google Scholar]
- 23.Montecucco C. How do tetanus and botulinum neurotoxins bind to neuronal membranes? Trends Biochem Sci. 1986;11:314–317. [Google Scholar]
- 24.Eisele KH, Fink K, Vey M, Taylor HV. Studies on the dissociation of botulinum neurotoxin type A complexes. Toxicon. 2011;57:555–565. doi: 10.1016/j.toxicon.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 25•.Matsui T, Gu S, Lam KH, Carter LG, Rummel A, Mathews II, Jin R. Structural Basis of the pH-Dependent Assembly of a Botulinum Neurotoxin Complex. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.09.009. This paper reports small-angle X-ray scattering studies on BoNT/A, NTNHA-A, and the M-PTC, which is complementary to the previous X-ray crystallographic studies [19]. It suggests that the assembly of the M-PTC depends on the environmental pH and that BoNT/A adopts a large conformational change that is induced by interacting with NTNHA-A at acidic pH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sagane Y, Miyashita S, Miyata K, Matsumoto T, Inui K, Hayashi S, Suzuki T, Hasegawa K, Yajima S, Yamano A, et al. Small-angle X-ray scattering reveals structural dynamics of the botulinum neurotoxin associating protein, nontoxic nonhemagglutinin. Biochem Biophys Res Commun. 2012;425:256–260. doi: 10.1016/j.bbrc.2012.07.077. [DOI] [PubMed] [Google Scholar]
- 27.Kouguchi H, Watanabe T, Sagane Y, Sunagawa H, Ohyama T. In vitro reconstitution of the Clostridium botulinum type D progenitor toxin. J Biol Chem. 2002;277:2650–2656. doi: 10.1074/jbc.M106762200. [DOI] [PubMed] [Google Scholar]
- 28•.Amatsu S, Sugawara Y, Matsumura T, Kitadokoro K, Fujinaga Y. Crystal structure of Clostridium botulinum whole hemagglutinin reveals a huge triskelion-shaped molecular complex. J Biol Chem. 2013;288:35617–35625. doi: 10.1074/jbc.M113.521179. The authors present the structure of the complete HA complex of BoNT/B (HA/B) that was determined by X-ray crystallography. The overall structure of the HA/B is highly similar to the HA/A reported by Lee et al.[16] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue K, Sobhany M, Transue TR, Oguma K, Pedersen LC, Negishi M. Structural analysis by X-ray crystallography and calorimetry of a haemagglutinin component (HA1) of the progenitor toxin from Clostridium botulinum. Microbiology. 2003;149:3361–3370. doi: 10.1099/mic.0.26586-0. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura T, Tonozuka T, Ito S, Takeda Y, Sato R, Matsuo I, Ito Y, Oguma K, Nishikawa A. Molecular diversity of the two sugar-binding sites of the beta-trefoil lectin HA33/C (HA1) from Clostridium botulinum type C neurotoxin. Arch Biochem Biophys. 2011;512:69–77. doi: 10.1016/j.abb.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura T, Kotani M, Tonozuka T, Ide A, Oguma K, Nishikawa A. Crystal structure of the HA3 subcomponent of Clostridium botulinum type C progenitor toxin. J Mol Biol. 2009;385:1193–1206. doi: 10.1016/j.jmb.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Yamashita S, Yoshida H, Uchiyama N, Nakakita Y, Nakakita S, Tonozuka T, Oguma K, Nishikawa A, Kamitori S. Carbohydrate recognition mechanism of HA70 from Clostridium botulinum deduced from X-ray structures in complexes with sialylated oligosaccharides. FEBS Lett. 2012;586:2404–2410. doi: 10.1016/j.febslet.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 33•.Sugawara Y, Yutani M, Amatsu S, Matsumura T, Fujinaga Y. Functional Dissection of the Clostridium botulinum Type B Hemagglutinin Complex: Identification of the Carbohydrate and E-Cadherin Binding Sites. PLoS One. 2014;9:e111170. doi: 10.1371/journal.pone.0111170. This paper reports the carbohydrate and E-cadherin binding sites on the L-PTC/B, which are similar to that of the L-PTC/A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T, Tonozuka T, Ide A, Yuzawa T, Oguma K, Nishikawa A. Sugar-binding sites of the HA1 subcomponent of Clostridium botulinum type C progenitor toxin. J Mol Biol. 2008;376:854–867. doi: 10.1016/j.jmb.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 35.Lee K, Lam KH, Kruel AM, Perry K, Rummel A, Jin R. High-resolution crystal structure of HA33 of botulinum neurotoxin type B progenitor toxin complex. Biochem Biophys Res Commun. 2014;446:568–573. doi: 10.1016/j.bbrc.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inoue K, Fujinaga Y, Honke K, Arimitsu H, Mahmut N, Sakaguchi Y, Ohyama T, Watanabe T, Inoue K, Oguma K. Clostridium botulinum type A haemagglutinin-positive progenitor toxin (HA(+)-PTX) binds to oligosaccharides containing Gal beta1-4GlcNAc through one subcomponent of haemagglutinin (HA1) Microbiology. 2001;147:811–819. doi: 10.1099/00221287-147-4-811. [DOI] [PubMed] [Google Scholar]
- 37.Kojima S, Eguchi H, Ookawara T, Fujiwara N, Yasuda J, Nakagawa K, Yamamura T, Suzuki K. Clostridium botulinum type A progenitor toxin binds to Intestine-407 cells via N-acetyllactosamine moiety. Biochem Biophys Res Commun. 2005;331:571–576. doi: 10.1016/j.bbrc.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Yoneyama T, Miyata K, Chikai T, Mikami A, Suzuki T, Hasegawa K, Ikeda T, Watanabe T, Ohyama T, Niwa K. Clostridium botulinum serotype D neurotoxin and toxin complex bind to bovine aortic endothelial cells via sialic acid. FEMS Immunol Med Microbiol. 2008;54:290–298. doi: 10.1111/j.1574-695X.2008.00475.x. [DOI] [PubMed] [Google Scholar]
- 39.Sagane Y, Hayashi S, Matsumoto T, Miyashita S, Inui K, Miyata K, Yajima S, Suzuki T, Hasegawa K, Yamano A, et al. Sugar-induced conformational change found in the HA-33/HA-17 trimer of the botulinum toxin complex. Biochem Biophys Res Commun. 2013;438:483–487. doi: 10.1016/j.bbrc.2013.07.112. [DOI] [PubMed] [Google Scholar]
- 40••.Lee K, Zhong X, Gu S, Kruel AM, Dorner MB, Perry K, Rummel A, Dong M, Jin R. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science. 2014;344:1405–1410. doi: 10.1126/science.1253823. This is the first crystal structure that reveals how the HA complex specifically binds to E-cadherin and disrupts the E-cadherin-mediated intercellular barrier. Furthermore, the authors successfully reconstituted the entire 14-subunit L-PTC/A using recombinant components, which allowed them to perform in vivo studies that directly addressed the physiological roles of host carbohydrates and E-cadherin in oral BoNT intoxication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsumura T, Jin Y, Kabumoto Y, Takegahara Y, Oguma K, Lencer WI, Fujinaga Y. The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol. 2008;10:355–364. doi: 10.1111/j.1462-5822.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 42.Sugawara Y, Matsumura T, Takegahara Y, Jin Y, Tsukasaki Y, Takeichi M, Fujinaga Y. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J Cell Biol. 2010;189:691–700. doi: 10.1083/jcb.200910119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends Cell Biol. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harrison OJ, Bahna F, Katsamba PS, Jin X, Brasch J, Vendome J, Ahlsen G, Carroll KJ, Price SR, Honig B, et al. Two-step adhesive binding by classical cadherins. Nat Struct Mol Biol. 2010;17:348–357. doi: 10.1038/nsmb.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vendome J, Posy S, Jin X, Bahna F, Ahlsen G, Shapiro L, Honig B. Molecular design principles underlying beta-strand swapping in the adhesive dimerization of cadherins. Nat Struct Mol Biol. 2011;18:693–700. doi: 10.1038/nsmb.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koch AW, Pokutta S, Lustig A, Engel J. Calcium binding and homoassociation of E-cadherin domains. Biochemistry. 1997;36:7697–7705. doi: 10.1021/bi9705624. [DOI] [PubMed] [Google Scholar]
- 48.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jin Y, Takegahara Y, Sugawara Y, Matsumura T, Fujinaga Y. Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins - differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type C. Microbiology. 2009;155:35–45. doi: 10.1099/mic.0.021246-0. [DOI] [PubMed] [Google Scholar]
- 50.Miyashita S, Niwa K, Watanabe T, Sagane Y. Host-cell specificity and transcytosis of nontoxic nonhemagglutinin protein of botulinum neurotoxin serotype D. FEMS Microbiol Lett. 2014;357:115–122. doi: 10.1111/1574-6968.12527. [DOI] [PubMed] [Google Scholar]
- 51.Couesnon A, Pereira Y, Popoff MR. Receptor-mediated transcytosis of botulinum neurotoxin A through intestinal cell monolayers. Cell Microbiol. 2008;10:375–387. doi: 10.1111/j.1462-5822.2007.01051.x. [DOI] [PubMed] [Google Scholar]
- 52.Maksymowych AB, Simpson LL. Structural features of the botulinum neurotoxin molecule that govern binding and transcytosis across polarized human intestinal epithelial cells. J Pharmacol Exp Ther. 2004;310:633–641. doi: 10.1124/jpet.104.066845. [DOI] [PubMed] [Google Scholar]
- 53.Mabbott NA, Donaldson DS, Ohno H, Williams IR, Mahajan A. Microfold (M) cells: important immunosurveillance posts in the intestinal epithelium. Mucosal Immunol. 2013;6:666–677. doi: 10.1038/mi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nikitas G, Deschamps C, Disson O, Niault T, Cossart P, Lecuit M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J Exp Med. 2011;208:2263–2277. doi: 10.1084/jem.20110560. [DOI] [PMC free article] [PubMed] [Google Scholar]