Abstract
Antiviral restriction factors are an integral part of the host innate immune system that protects cells from viral pathogens, such as human immunodeficiency virus (HIV). Studies of the interactions between restriction factors and HIV have greatly advanced our understanding of both the viral life cycle and basic cell biology, as well as provided new opportunities for therapeutic intervention of viral infection. Here we review the recent developments towards establishing the structural and biochemical bases of HIV inhibition by, and viral countermeasures of, the restriction factors TRIM5, MxB, APOBEC3, SAMHD1, and BST2/tetherin.
Introduction
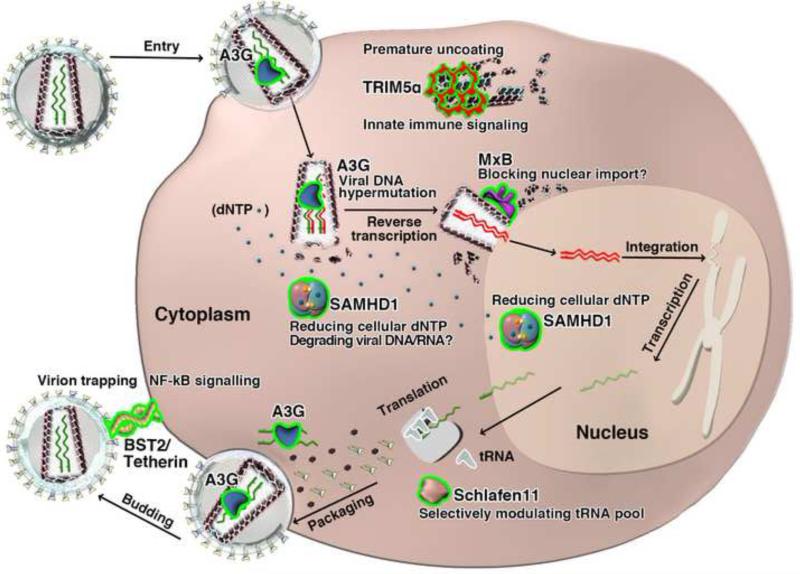
The host innate immune system has a diverse array of antiviral responses, including intrinsic antiviral proteins termed restriction factors that inhibit distinct stages of the viral life cycle. The last decade of HIV research has seen the discovery of many anti-HIV restriction factors that blocks the infection at steps including capsid uncoating (TRIM5), reverse transcription (APOBEC3 and SAMHD1), nuclear import and integration (MxB), translation (Schlafen 11), and budding (BST2/Tetherin) (Figure 1). Some of these restriction factors also trigger broad innate immune signaling for further viral suppression. The virus, in turn, has developed an arsenal of methods to evade the host defense. HIV counteracts or avoids some of these restriction factors either by using antagonistic accessory proteins (Vif, Vpu, Vpx/Vpr, Nef, etc.) or by mutating protein interfaces. Studying the ongoing arms race between HIV and the host greatly advances our understanding of the viral life cycle and the human immune system, while simultaneously providing new avenues for therapeutic intervention.
Figure 1. Host restriction factors that inhibit various stages of the HIV life cycle.
HIV particles are shown as gray circles with envelop glycoproteins coated on the outside and a cone (trapezoid)-shaped capsid inside. Capsid protein hexamers are shown as black hexagons. Green curvy lines represent viral RNA and red curvy lines viral DNA. Restriction factors are illustrated in various shapes with the periphery highlighted in yellow and with their functions labeled.
A first line of defense: Retrovirus capsid pattern sensing by TRIM5 proteins
The tripartite motif 5 (TRIM5) proteins, including TRIM5α and TRIMCyp, directly recognize the retroviral capsid in a species-specific manner [1,2]. TRIM5 proteins elicit premature disassembly of the capsid and activate cellular innate immune signaling pathways [3]. The conserved N-terminal tripartite motif of TRIM5 proteins consists of a RING domain (E3 ligase activity), a B-box domain (higher-order oligomerization), and a coiled-coil domain (dimerization) (Figure 2A). The C-terminal PRY/SPRY (TRIM5α) or CypA (TRIMCyp) domain confers capsid binding and specificity. Functioning as a viral capsid pattern sensor, TRIM5α binds only to the assembled hexameric capsid lattice [4]. Interestingly, although the global capsid pattern is the binding determinant, TRIM5α has an ability to interact with retroviral capsids of diverse shapes and curvatures.
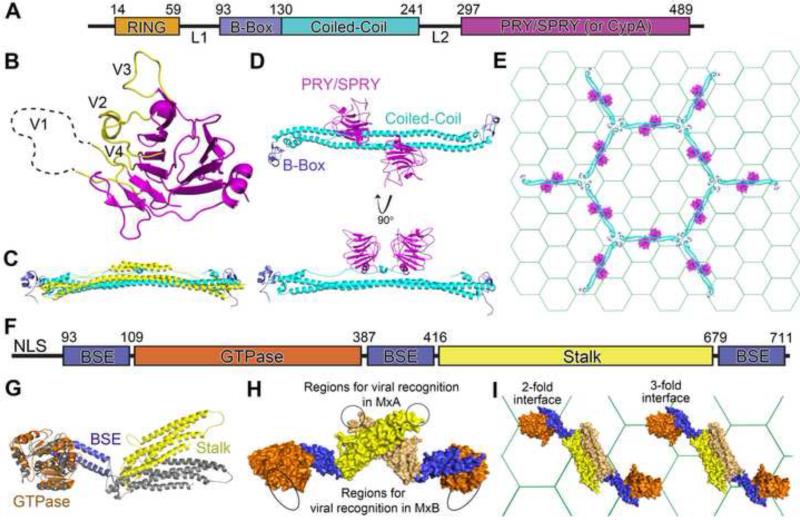
Figure 2. Structural insights for the antiviral mechanisms of TRIM5α and MxB.
(A) Domain organization of rhesus TRIM5α or TRIMCyp. (B) The crystal structure of the PRY/SPRY domain of rhesus TRIM5α (PDB ID: 4B3N) in ribbon presentation, with the variable loops in yellow. The unobserved portion of the V1 loop is illustrated as a dotted line. (C) Overlay of the crystal structures of the TRIM25 coiled-coil domain (yellow, PDB ID: 4LTB) and the rhesus TRIM5α B-box (blue) and coiled-coil (cyan) domain (PDB ID: 4TN3). (D) Two orthogonal views of a model of the rhesus TRIM5α structure containing the B-box, coiled-coil, and PRY/SPRY domains. The PRY/SPRY (PDB ID: 4B3N) and the B-box and coiled-coil (PDB ID: 4TN3) domains were positioned together by overlaying a six-amino acid region present in both structures. (E) Model of the lattice of rhesus TRIM5α (ribbon representation, without the ring domain) formed on top of the HIV capsid lattice (hexagonal grid). TRIM5α and the capsid lattice are drawn to the same scale based on the observed dimensions. (F) Domain organization of MxB. (G) The MxB (orang, blue, and yellow, PDB ID: 4WHJ) and MxA (gray, PDB ID: 3SZR) structures overlaid based on the BSE domains. (H) The MxB dimer in surface representation. Regions in MxA (circles) and MxB (ovals) responsible for viral recognitions are located at the opposite side of the dimer. (I) Model of MxB dimer binding to the capsid lattice (hexagonal grid). MxB and the capsid lattice are drawn to the same scale based on the observed dimensions.
Crystal structures of the rhesus macaque TRIM5α PRY/SPRY domain provided the first insight into the potential mode of interaction between TRIM5α and the viral capsid. The structures show a core of two antiparallel β sheets with one side of the molecule displaying flexible variable loops that are critical for capsid binding (Figure 2B) [5•,6•]. Rhesus TRIM5α, but not human TRIM5α, recognizes and restricts HIV-1, although a single mutation (R332P) in the variable loop 1 of the human PRY/SPRY domain confers HIV-1 capsid binding and restriction [7-9]. Modeling of the TRIM5α PRY/SPRY domain onto the capsid lattice suggests that the flexibility provided by the variable loops may enable TRIM5α to recognize retroviral capsids with a variety of curvatures [6•].
The TRIM5 B-box and coiled-coil domains mediate higher-order self-association that enables avid binding to retroviral capsid cores, as monomeric TRIM5 domains bind capsid weakly. A TRIM5-TRIM21 chimera (TRIM5-21R) has been observed by electron microscopy to form a two-dimensional hexagonal lattice on the surface of a preassembled HIV-1 capsid lattice [10]. The crystal structures of the coiled-coil region of TRIM25 and the B-box-coiled-coil region of TRIM5α provided insights into TRIM5 dimerization and lattice assembly [11•,12•] (Figure 2C). The coiled-coil region of TRIM proteins adopts an antiparallel conformation, with a downstream α-helix folding back in a hairpin-like manner to bring the capsid-binding modules near the midpoint of the coiled-coil (Figure 2D). The length of the coiled-coil (17 nm) matches the dimension of the observed TRIM5-21R hexagonal lattice. The TRIM5α B-box domains sit at opposite ends of the coiled-coil. This configuration suggests that within the TRIM5 lattice the B-box domains locate at three-fold symmetry axes, while each PRY/SPRY or CypA pair reside at the center of a hexagonal edge for viral capsid interaction (Figure 2E).
Structural studies of more complete TRIM5 constructs containing the coiled-coil and additional domains (RING, PRY/SPRY or CypA) are needed to further understand TRIM5 architecture. More importantly, the most intriguing and challenging questions remain: what is the structural basis of capsid lattice recognition and disruption by TRIM5α, and how does this recognition lead to downstream immune responses? Answering these questions will likely require innovative approaches to reconstitute the lattice interactions in a form amenable for biochemical and structural biology studies.
HIV restriction by myxovirus resistance protein 2 (MxB)
Human MxB is an interferon-induced restriction factor, which was recently discovered to target HIV-1 [13-15]. Evidence suggests it acts between reverse transcription and integration, and likely interacts with the viral capsid [13-17]. MxB is highly homologous to the well-studied MxA (63% identity) that inhibits influenza-like viruses. Both MxA and MxB are dynamin-like GTPases that contain three domains: GTPase, bundle signaling element (BSE), and stalk (Figure 2F). The recent crystal structure of MxB shows an MxA-like extended anti-parallel dimer [18••]. The relative orientations of the structurally homologous domains are different in the two proteins (Figure 2G), although the functional significance of this difference is currently unclear.
The antiviral mechanism of MxB is distinct from that of MxA. MxB, but not MxA, contains a nuclear localization signal (NLS), which is critical for HIV-1 restriction [14]. The anti-HIV-1 function of MxB is independent of the transfer of information between the GTPase and the stalk domains through hinge regions surrounding the BSE domain [18••]. This is consistent with data showing that MxB antiviral function is independent of GTPase activity [13,14]. Both BSE hinge communication and GTPase activity are critical for MxA antiviral functions. In addition, higher-order oligomerization is required for the antiviral activity of MxA but not MxB [18••]. Strikingly, the viral interaction modules of MxA and MxB appear to lie on the opposite faces of the dimer structures (Figure 2H).
MxB binds to HIV-1 capsid assemblies, but not to capsid protein (CA) hexamers, indicating that MxB may function as another viral capsid pattern sensor (Figure 2I) [18••]. The MxB regions critical for antiviral function, such as the N-terminus and the dimerization interface, are also important for capsid binding. Intriguingly, MxB also interacts with CA mutants that evade MxB restriction in vivo, indicating capsid binding by MxB may be necessary but not sufficient for HIV-1 restriction [18••]. The current data suggest that MxB may restrict HIV-1 infection by interfering with viral nuclear import. However, much remains to be uncovered to explain the detailed mechanism of this newly discovered restriction factor.
Mutation of viral DNA by APOBEC3 proteins
Apolipoprotein B mRNA-editing enzyme catalytic polypeptide-like 3G (APOBEC3G or A3G) is the first cellular protein discovered with intrinsic anti-HIV activity, which marks the beginning of research on HIV restriction factors [19]. A3G is packaged into assembling virions and upon infection of target cells causes dC to dU mutations on the newly reverse transcribed minus strand of viral DNA [20,21]. A3G also restricts HIV by residing on the viral RNA and thereby blocking reverse transcription [22,23]. Antiviral activity is observed for other A3 proteins (A3F/D/H), although to a lesser extent. To evade restriction by the A3 proteins, HIV expresses virion infectivity factor (Vif) to target A3F/G/C for degradation via the host ubiquitin-proteasome pathway [24]. Vif specifically interacts with the cellular cofactor CBFβ and a host E3 ubiquitin ligase that contains the scaffolding protein Cullin 5 (Cul5), the adaptor proteins ElonginB and ElonginC (EloBC), and the E2-linking protein Rbx2. By mimicking the substrate receptor in the multi-component E3 ligase complex, Vif hijacks the ligase to polyubiquitinate A3 proteins.
A3 proteins are cytidine deaminases that contain a primordial Zn2+-coordinating catalytic motif with conserved His-X-Glu and Pro-Cys-X-X-Cys residues (Figure 3A), yet they have different sequence specificity for DNA substrates. The A3G C-terminal domain (CTD) harbors the deaminase activity while the homologous N-terminal domain (NTD) binds DNA/RNA and HIV nucleocapsid for packaging into virions, and contains the target site of HIV Vif. In contrast, the CTD of A3F contains both the deamination and Vif target site. A3G preferentially recognizes a 5′-CCCA-3′ DNA sequence, while A3F prefers 5′–(T/C)TCA-3′ (the deaminated C is underlined). Multiple structures of A3 protein domains have been solved (Figure 3B). The structural findings on the A3 proteins, including the catalytic sites, the predicted DNA-binding regions, and the Vif sensitive regions, have been thoroughly summarized recently in an excellent review [25•] and will not be discussed here. Despite the abundant structural information of apo-proteins, how A3 proteins interact with DNA is still under debate (Figure 3C) [25•], and the molecular determinants that govern the DNA sequence-specific interactions are unknown. These questions can only be addressed unambiguously with structures of appropriate A3 protein-DNA complexes.
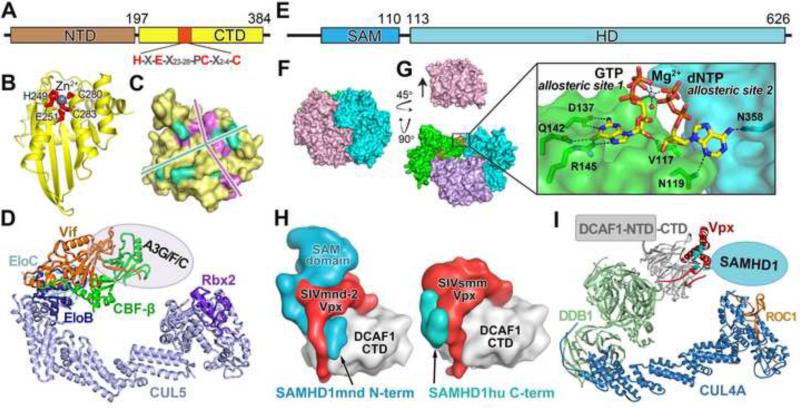
Figure 3. Structural bases of the APOBEC3 (deaminase) and SAMHD1 (dNTPase) activities and their antagonization by viral hijacking of host E3 ubiquitin ligase complexes.
(A) Domain organization of A3G with the catalytic center marked in red and the consensus sequence labeled. (B) Crystal structure of A3G-CTD in ribbon representation with the catalytic residues shown as red sticks and the Zn ion as a gray sphere. (C) Possible DNA binding paths (marked by the rods) on A3G (surface representation). The residues involved in DNA-binding are colored to match the rods. (D) Model of the Vif-E3 ligase based on the structure of the Vif/CBFβ/EloBC/Cul5NTD complex (PDBID: 4N9F). The bound A3 protein target is indicated by an oval. (E) Domain organization of SAMHD1. (F) Crystal structure of SAMHD1 catalytic core tetramer in surface representation (PDB ID: 4TO0) with protomers colored individually. (G) Binding of allosteric GTP/dNTP at a trimeric SAMHD1 interface (boxed), revealed by removing one protomer. Four such GTP/dNTP-binding sites exist in the SAMHD1 tetramer. The bound GTP/dATP and SAMHD1 side chains recognizing the nucleotide bases are shown as sticks and the phosphate-coordinating magnesium ion as a gray sphere (with a bound water molecule as a red sphere). (H) Left: Surface representation of the structure of SIVmnd-2 Vpx (red) in complex with DCAF1-CTD (gray) and the N-terminal region of mandrill SAMHD1 (residues 1-109, light blue) (PDB ID: 5AJA); Right: The structure of SIVsmm Vpx (red) in complex with DCAF1-CTD (gray), and human SAMHD1 C-terminus (residues 606-625, cyan) (PDB ID: 4CC9). (I) Model of the Vpx-E3 ubiqutin ligase targeting SAMHD1, based on the crystal structures of the complexes shown in (H, right structure), the DDB1-DDB2 complex (PDB ID: 3EI3), and the DDB1-Cul4A-Rbx1-SV5V complex (PDB ID: 2HYE).
The structural basis of how HIV Vif targets A3 proteins has not be established, although the recent crystal structure of HIV-1 Vif in complex with CBFβ, EloBC, and Cul5-NTD provided substantial insight into the interactions that enable Vif to hijack the E3 ligase (Figure 3D) [26••]. Notably, Vif engages in extensive contact with the E3 ligase components while keeping residues implicated in A3 binding solvent exposed adjacent to the CBFβ binding site. Interestingly, while HIV-1 Vif directs multiple A3 proteins to the E3 ligase, distinct binding surfaces of Vif are used to antagonize each A3. Accordingly, A3 proteins have different surface regions sensitive to Vif binding. Furthermore, evidence suggests that A3F binding may alter the interaction between CBFβ and Vif [27]. Future structural elucidation showing how A3 proteins are recruited to the Vif-E3 ligase complex will be required to clarify the underlying mechanisms by which Vif antagonizes the APOBEC3 proteins.
Suppression of HIV reverse transcription and regulation of cellular dNTPs by SAMHD1
SAMHD1, a deoxyribonucleoside triphosphate triphosphohydrolase (dNTPase), prevents the infection of blood cells by retroviruses, including HIV, likely by depleting the cellular dNTP pool available for viral reverse transcription [28-30]. In addition, SAMHD1 is ubiquitously expressed in cells of various human tissues [31,32], where it functions in the regulation of cellular dNTP levels, DNA damage signaling, and proper activation of the innate immune response [31,33,34]. Mutations in SAMHD1 are associated with chronic lymphocytic leukemia (CLL) and the autoimmune disease Aicardi-Goutieres syndrome (AGS).
A series of structural and biochemical studies firmly established the mechanism of the dNTPase activity of SAMHD1. The C-terminal catalytic core of SAMHD1 is sufficient to hydrolyze dNTPs and inhibit HIV (Figure 3E). The first crystal structure of the SAMHD1 catalytic core was determined as a dimer without nucleotide bound [30], although its dNTPase activity was found to require a dGTP cofactor. The molecular basis of SAMHD1 activation was subsequently elucidated by determination of crystal structures of the catalytic core in the active, tetrameric conformation (Figure 3F) [35••,36•]. Strikingly, a total of 12 dGTPs bind to the SAMHD1 tetramer, two at each of the four allosteric sites and one at each of the four catalytic sites. The two dGTPs at each allosteric site are non-equivalent (designated as allosteric sites 1 and 2). Binding of the allosteric nucleotides promotes SAMHD1 tetramer formation, which induces a conformational change in the substrate-binding pocket to yield the catalytically active enzyme.
Recently, comprehensive structural and enzymatic studies revealed the complete regulatory mechanism of the dNTPase activity of SAMHD1 via combined action of both GTP and dNTPs [37••,38•]. Ji and Tang el al. used 26 GTP/dNTP-bound crystal structures to determine the full spectrum of allosteric and substrate nucleotides bound to SAMHD1 [37••]. Allosteric site 1 strictly selects for GTP (slightly favored) or dGTP, with GTP likely occupying the site in cells due to its much higher cellular concentration (Figure 3G). In allosteric site 2, any type of dNTP, but only a dNTP, can bind with distinct binding affinities. Nonetheless, the rate of catalysis remains relatively constant regardless of which dNTP is bound at allosteric site 2, demonstrating a robust regulatory mechanism of SAMHD1 activity regardless of the identity of the bound dNTPs [37••].
SAMHD1 is counteracted in HIV-2 and related SIVs by the viral proteins Vpx and Vpr [39,40]. Vpx links SAMHD1 to a DDB1/DCAF1-dependent E3 ubiquitin ligase for ubiquitination and proteasomal degradation. Intriguingly, this viral antagonization comes in different flavors as Vpx proteins from different SIV lineages target distinct regions of SAMHD1 and recruit it to the same E3 ligase [41]. The multifaceted targeting mechanisms were elucidated by two recent crystal structures that demonstrated how Vpx molecules target different (N- or C-terminal) tails of SAMHD1 [42••,43••]. One of the two structures consists of a protein complex comprising the human SAMHD1 C-terminus (residues 582-626), the C-terminal domain of DCAF1 (DCAF1-CTD), and the SIVsmm (infecting sooty mangabey monkey) Vpx (Figure 3H,I) [42••]. The second structure illustrated the interactions between the N-terminal region (residues 1-109) of mandrill SAMHD1, DCAF1-CTD, and SIVmnd-2 (SIV infecting mandrill) Vpx (Figure 3H) [43••]. As revealed by the two structures, both of the Vpx lineages co-opt DCAF1 to form a structurally conserved, predefined substrate receptor that has the flexibility to bind either the N- or C-terminal tail of SAMHD1 in a species-specific manner (Figure 3H). DCAF1 and the core of Vpx associate in the same manner in both cases, while the flexible N-terminus of Vpx enables the C-terminal tail of SAMHD1hu to bind to the SIVsmm Vpx-DCAF1 interface, or allows for the N-terminal tail of SAMHD1mnd to be sandwiched between SIVmnd-2 Vpx and DCAF1 (Figure 3H)
Despite the rapid progress, much remains to be learned about SAMHD1. The antiviral activity of SAMHD1 is negatively regulated by phosphorylation at residue T592 [32]. However, the phosphorylation-dependent regulation mechanism is currently unknown and it is under debate whether the phosphorylation-induced loss of the antiviral function of SAMHD1 is due to the reduction of its dNTPase activity. In addition to the dNTPase activity, SAMHD1 also possesses exonuclease activities on single-stranded DNA or RNA substrates [44-46], although the functional relevance of these activities is under investigation. A recent report suggested that the RNase activity of SAMHD1, but not the dNTPase activity, is responsible for HIV restriction [47]. Future studies, including structures of phosphorylated SAMHD1 and SAMHD1 in complex with DNA or RNA, are needed to completely elucidate the mechanisms of antiviral and cellular functions of SAMHD1.
The last line of defense: Enveloped virus tethering by BST2/tetherin
Bone marrow stromal cell antigen 2 (BST2, also named tetherin or CD317) inhibits the release of nascent HIV particles [48,49] and other enveloped viruses by retaining the budding virions at the cell surface. In addition, viral tethering by BST2 triggers NF-κB signaling to activate the innate immune responses against infections [50,51]. To evade this host antiviral response, an assortment of viral proteins evolved to antagonize BST2 by hijacking cellular ubiquitin-proteosome or endosome-lysosome pathways for the degradation or mistrafficking of BST2.
The presence of two membrane anchors on BST2 allows it to function as a direct tether between enveloped viruses and host cells. BST2 consists of an N-terminal cytoplasmic tail, a transmembrane helix, a coiled-coil ectodomain, and a C-terminal glycosylphosphatidylinositol (GPI) membrane anchor (Figure 4A). The coiled-coil ectodomain forms a long parallel dimer, which is stabilized by intermolecular disulfide bonds [52-55]. Protease and epitope mapping experiments suggest that the “axial” orientation, with the coiled-coil ectodomain perpendicular to the membranes, is likely the dominant configuration of functional BST2 (Figure 2B) [56•]. However, the results do not rule out the possibility that the tetrameric form of BST2, observed in the crystal structures [53-55], also contributes to virion tethering (Figure 2B). Interestingly, the C-terminus of BST2 was found incorporated in the virion more often than the N-terminus.
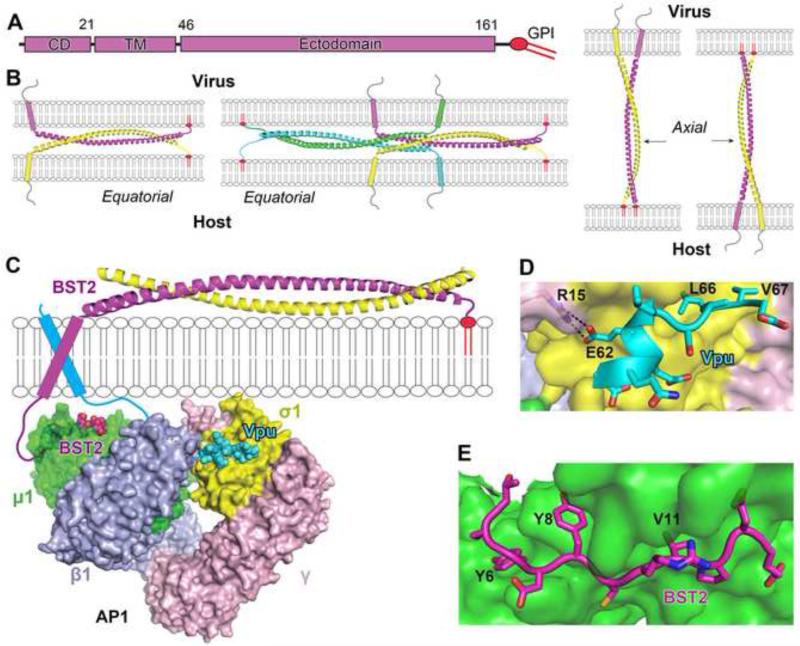
Figure 4. Mechanism of virion trapping by BST2 and its antagonization by HIV-1 Vpu-mediated mistrafficking.
(A) Domain organization of BST2. (B) Models for possible configurations of BST2 in trapping of HIV virions, based on the crystal structure of the BST2 ectodomain (PDB ID: 3MQ7). BST2 is in cylinder and ribbon representation with the GPI anchors shown as red insertions into the membranes. (C) Model of Vpu-mediated mistrafficking of BST2, based on the crystal structure of BST2 and Vpu cytoplasmic domains in complex with the clathrin AP1 core (PDB ID: 4P6Z). The overall binding is achieved by a combination of binary associations between each pair of components (Vpu, AP1, and BST2). (D) Binding of the ExxxLV motif of Vpu (cyan ribbon and sticks) to the acidic dileucine motif-binding pocket in AP1 (yellow and pink surface). (E) Binding of BST2 cytoplasmic domain (magenta ribbon and sticks) to the tyrosine motif-binding pockect of AP1 (green surface) via the YxYxxV motif in BST2.
Retaining the N-terminus of BST2 in the cytoplasm may be advantageous for the host, as determinants in the N-terminal tail control the internalization and trafficking of trapped virions for lysosomal degradation. The BST2-mediated activation of NF-κb-associated immune responses also depends on the BST2 N-terminal tail.
BST2 is antagonized by HIV-1 Vpu and diverse viral proteins from other viruses [57]. In fact, prior to the discovery of its anti-HIV activity, BST2 was found to be counteracted by the K5 protein from the Karposi sarcoma-associated herpesvirus (KSHV) [58]. The great variety of BST2-antogonizing strategies highlights the convergent evolution of viruses for targeting this broad-spectrum antiviral factor.
HIV-1 Vpu utilizes two possible methods to decrease BST2 at the cell surface: i) the β-TrCP-dependent ubiquitination and lysosomal degradation of BST2 and ii) the mistrafficking of BST2 in the clathrin-dependent membrane trafficking pathways. While the effects of the β-TrCP-associated pathways remain under debate, our understanding of the Vpu-mediated BST2 mistrafficking was greatly advanced by the crystal structure of a protein complex containing Vpu and BST2 cytoplasmic domains and the core of the clathrin adaptor protein complex 1 (AP1) (Figure 4C) [59••]. Vpu mimics a membrane cargo to associate with AP1 at its acidic dileucine cargo-binding site (Figure 4D), while simultaneously forming a tight transmembrane interaction with BST2. This ternary association substantially increases the weak binary binding between BST2 and AP1, causing mistrafficking. Furthermore, this structure provided insight into how endogenous BST2 traffics within the endosomal system via the clathrin dependent pathways. BST2 binds to the tyrosine motif-binding site of AP1 via a unique di-tyrosine motif (Figure 4E). In the absence of Vpu, such an interaction may facilitate the endocytosis of BST2-trapped virions for eventual degradation in the lysosome. Further work is needed to structurally address how the transmembrane helices of Vpu and BST2 interact and how other viruses antagonize BST2.
Concluding Remarks
Structural studies of the HIV restriction factors have brought unparalleled insight into HIV biology and the innate immunity of the host. Information gained regarding viral evasion of the host restriction is particularly valuable as it offers new opportunities for antiviral drug discovery. In addition, as the factors pose significant barriers for cross-species transmission of viruses, they may impede the use of animal models to study HIV. Thus, understanding the antiviral mechanisms of host restriction factors will improve the study of HIV infection in animal models. Even after the many recent advances discussed above, much research is still required to fully understand the mechanisms of action of the anti-HIV factors currently known, and more host restriction factors that are likely to be discovered in the future. Structural biology is expected to continue to provide major insights into the mechanisms of these complex host-viral interactions.
Highlights.
Trim5α senses HIV capsid patterns and achieves strong binding by avidity
MxB binds to the HIV capsid assembly and may interfere with HIV nuclear import
HIV-1 Vif hijacks a host E3 ubiquitin ligase to antagonize APOBEC3 proteins
Allosteric GTP and dNTPs together activate the tetrameric SAMHD1 dNTPase
HIV-1 Vpu hijacks clathrin AP1 for the mistrafficking of BST2/tetherin
Acknowledgements
We would like to thank Jennifer Fribourgh, Henry Nguyen, Xiaoyun Ji, Chenxiang Tang, Brady Summers, Olga Buzovetsky, and Wei Wang for stimulating discussions and editorial contributions. This work was supported by the NIH grants (AI102778 and AI097064).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Sayah DM, Sokolskaja E, Berthoux L, Luban J. Cyclophilin a retrotransposition into TRIM5 explains owl monkey resistance to HIV-1. Nature. 2004;430(6999):569–573. doi: 10.1038/nature02777. [DOI] [PubMed] [Google Scholar]
- 2.Stremlau M, Owens CM, Perron MJ, Kiessling M, Autissier P, Sodroski J. The cytoplasmic body component TRIM5alpha restricts HIV-1 infection in old world monkeys. Nature. 2004;427(6977):848–853. doi: 10.1038/nature02343. [DOI] [PubMed] [Google Scholar]
- 3.Stremlau M, Perron M, Lee M, Li Y, Song B, Javanbakht H, Diaz-Griffero F, Anderson DJ, Sundquist WI, Sodroski J. Specific recognition and accelerated uncoating of retroviral capsids by the TRIM5alpha restriction factor. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(14):5514–5519. doi: 10.1073/pnas.0509996103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao G, Ke D, Vu T, Ahn J, Shah VB, Yang R, Aiken C, Charlton LM, Gronenborn AM, Zhang P. Rhesus TRIM5alpha disrupts the HIV-1 capsid at the inter-hexamer interfaces. PLoS pathogens. 2011;7(3):e1002009. doi: 10.1371/journal.ppat.1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5•.Biris N, Yang Y, Taylor AB, Tomashevski A, Guo M, Hart PJ, Diaz-Griffero F, Ivanov DN. Structure of the rhesus monkey TRIM5alpha pryspry domain, the HIV capsid recognition module. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(33):13278–13283. doi: 10.1073/pnas.1203536109. [The crystal structure of the rhesus TRIM5α PRY/SPRY domain was determined using a construct where the v1 loop was replaced by a two-amino acid linker. The conformation of the v1 loop was characterized using NMR.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6•.Yang H, Ji X, Zhao G, Ning J, Zhao Q, Aiken C, Gronenborn AM, Zhang P, Xiong Y. Structural insight into HIV-1 capsid recognition by rhesus TRIM5alpha. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(45):18372–18377. doi: 10.1073/pnas.1210903109. [The crystal structure of the rhesus TRIM5α PRY/SPRY domain was determined as a fusion chimera with the maltose-binding protein. Detailed models of how the PRY/SPRY may recognize HIV capsid with varying curvature were proposed.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Li X, Stremlau M, Lee M, Sodroski J. Removal of arginine 332 allows human TRIM5alpha to bind human immunodeficiency virus capsids and to restrict infection. Journal of virology. 2006;80(14):6738–6744. doi: 10.1128/JVI.00270-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stremlau M, Perron M, Welikala S, Sodroski J. Species-specific variation in the b30.2(spry) domain of TRIM5alpha determines the potency of human immunodeficiency virus restriction. Journal of virology. 2005;79(5):3139–3145. doi: 10.1128/JVI.79.5.3139-3145.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yap MW, Nisole S, Stoye JP. A single amino acid change in the spry domain of human TRIM5alpha leads to HIV-1 restriction. Current biology : CB. 2005;15(1):73–78. doi: 10.1016/j.cub.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 10.Ganser-Pornillos BK, Chandrasekaran V, Pornillos O, Sodroski JG, Sundquist WI, Yeager M. Hexagonal assembly of a restricting TRIM5alpha protein. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(2):534–539. doi: 10.1073/pnas.1013426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11•.Goldstone DC, Walker PA, Calder LJ, Coombs PJ, Kirkpatrick J, Ball NJ, Hilditch L, Yap MW, Rosenthal PB, Stoye JP, Taylor IA. Structural studies of postentry restriction factors reveal antiparallel dimers that enable avid binding to the HIV-1 capsid lattice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(26):9609–9614. doi: 10.1073/pnas.1402448111. [The crystal structure of the B-box and coiled coil domains of rhesus TRIMCyp was determined (with the CypA domain replaced by T4 lysozyme), presenting a more complete picture of the TRIM5 protein structures.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12•.Sanchez JG, Okreglicka K, Chandrasekaran V, Welker JM, Sundquist WI, Pornillos O. The tripartite motif coiled-coil is an elongated antiparallel hairpin dimer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(7):2494–2499. doi: 10.1073/pnas.1318962111. [The crystal structure of the human TRIM25 coiled-coil domain together with a portion of the L2 linker was determined, demonstrating the antiparallel arrangement of the coiled-coil domain and providing an updated model of the hexagon lattice formed by TRIM5α on HIV capsid.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goujon C, Moncorge O, Bauby H, Doyle T, Ward CC, Schaller T, Hue S, Barclay WS, Schulz R, Malim MH. Human Mx2 is an interferon-induced post-entry inhibitor of HIV-1 infection. Nature. 2013;502(7472):559–562. doi: 10.1038/nature12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kane M, Yadav SS, Bitzegeio J, Kutluay SB, Zang T, Wilson SJ, Schoggins JW, Rice CM, Yamashita M, Hatziioannou T, Bieniasz PD. Mx2 is an interferon-induced inhibitor of HIV-1 infection. Nature. 2013;502(7472):563–566. doi: 10.1038/nature12653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Pan Q, Ding S, Qian J, Xu F, Zhou J, Cen S, Guo F, Liang C. The interferon-inducible MxB protein inhibits HIV-1 infection. Cell host & microbe. 2013;14(4):398–410. doi: 10.1016/j.chom.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Busnadiego I, Kane M, Rihn SJ, Preugschas HF, Hughes J, Blanco-Melo D, Strouvelle VP, Zang TM, Willett BJ, Boutell C, Bieniasz PD, et al. Host and viral determinants of Mx2 antiretroviral activity. Journal of virology. 2014;88(14):7738–7752. doi: 10.1128/JVI.00214-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goujon C, Moncorge O, Bauby H, Doyle T, Barclay WS, Malim MH. Transfer of the amino-terminal nuclear envelope targeting domain of human Mx2 converts Mx1 into an HIV-1 resistance factor. Journal of virology. 2014;88(16):9017–9026. doi: 10.1128/JVI.01269-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Fribourgh JL, Nguyen HC, Matreyek KA, Alvarez FJ, Summers BJ, Dewdney TG, Aiken C, Zhang P, Engelman A, Xiong Y. Structural insight into HIV-1 restriction by MxB. Cell host & microbe. 2014 doi: 10.1016/j.chom.2014.09.021. [The first structural report for MxB, which reveals separate determinants for the antiviral functions of MxA and MxB. A direct interaction between MxB and the assembled HIV capsid, but not individual capsid proteins, was demonstrated.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418(6898):646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 20.Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, Watt IN, Neuberger MS, Malim MH. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113(6):803–809. doi: 10.1016/s0092-8674(03)00423-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Yang B, Pomerantz RJ, Zhang C, Arunachalam SC, Gao L. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature. 2003;424(6944):94–98. doi: 10.1038/nature01707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS pathogens. 2008;4(12):e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwatani Y, Chan DS, Wang F, Maynard KS, Sugiura W, Gronenborn AM, Rouzina I, Williams MC, Musier-Forsyth K, Levin JG. Deaminase-independent inhibition of HIV-1 reverse transcription by APOBEC3G. Nucleic acids research. 2007;35(21):7096–7108. doi: 10.1093/nar/gkm750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302(5647):1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 25•.Aydin H, Taylor MW, Lee JE. Structure-guided analysis of the human APOBEC3-HIV restrictome. Structure. 2014 doi: 10.1016/j.str.2014.02.011. [A comprehensive analysis summarizing the recent advances in the structural studies of the A3 proteins and their antagonization by Vif.] [DOI] [PubMed] [Google Scholar]
- 26••.Guo Y, Dong L, Qiu X, Wang Y, Zhang B, Liu H, Yu Y, Zang Y, Yang M, Huang Z. Structural basis for hijacking CBF-beta and Cul5 E3 ligase complex by HIV-1 Vif. Nature. 2014;505(7482):229–233. doi: 10.1038/nature12884. [The crystal structure of HIV-1 Vif in complex with CBFβ and components of the hijacked E3 ligase complex (EloB, EloC, and Cullin 5 N-terminus) was determined, providing the structural basis of the engagement of the E3 ligase complex by HIV-1 Vif for antagonizing APOBEC3 proteins.] [DOI] [PubMed] [Google Scholar]
- 27.Fribourgh JL, Nguyen HC, Wolfe LS, Dewitt DC, Zhang W, Yu XF, Rhoades E, Xiong Y. Core binding factor beta plays a critical role by facilitating the assembly of the Vif-cullin 5 E3 ubiquitin ligase. Journal of virology. 2014;88(6):3309–3319. doi: 10.1128/JVI.03824-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hrecka K, Hao C, Gierszewska M, Swanson SK, Kesik-Brodacka M, Srivastava S, Florens L, Washburn MP, Skowronski J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature. 2011;474(7353):658–661. doi: 10.1038/nature10195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berger A, Sommer AF, Zwarg J, Hamdorf M, Welzel K, Esly N, Panitz S, Reuter A, Ramos I, Jatiani A, Mulder LC, et al. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutieres syndrome are highly susceptible to HIV-1 infection. PLoS pathogens. 2011;7(12):e1002425. doi: 10.1371/journal.ppat.1002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstone DC, Ennis-Adeniran V, Hedden JJ, Groom HC, Rice GI, Christodoulou E, Walker PA, Kelly G, Haire LF, Yap MW, de Carvalho LP, et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature. 2011;480(7377):379–382. doi: 10.1038/nature10623. [DOI] [PubMed] [Google Scholar]
- 31.Franzolin E, Pontarin G, Rampazzo C, Miazzi C, Ferraro P, Palumbo E, Reichard P, Bianchi V. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proceedings of the National Academy of Sciences of the United States of America. 2013 doi: 10.1073/pnas.1312033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White Tommy E, Brandariz-Nuñez A, Valle-Casuso Jose C, Amie S, Nguyen Laura A, Kim B, Tuzova M, Diaz-Griffero F. The retroviral restriction ability of SAMHD1, but not its deoxynucleotide triphosphohydrolase activity, is regulated by phosphorylation. Cell host & microbe. 2013;13(4):441–451. doi: 10.1016/j.chom.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kretschmer S, Wolf C, König N, Staroske W, Guck J, Häusler M, Luksch H, Nguyen LA, Kim B, Alexopoulou D, Dahl A, et al. SAMHD1 prevents autoimmunity by maintaining genome stability. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford R, Louis T, Robbe P, Ackroyd S, Burns A, Timbs AT, Wright Colopy G, Dreau H, Sigaux F, Judde JG, Rotger M, et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood. 2014;123(7):1021–1031. doi: 10.1182/blood-2013-04-490847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Ji X, Wu Y, Yan J, Mehrens J, Yang H, DeLucia M, Hao C, Gronenborn AM, Skowronski J, Ahn J, Xiong Y. Mechanism of allosteric activation of SAMHD1 by dGTP. Nature structural & molecular biology. 2013;20(11):1304–1309. doi: 10.1038/nsmb.2692. [The crystal structure of the catalytic core of SAMHD1 in the active, tetrameric conformation, explained the mechanism of dGTP-induced tetramerization and activation of the dNTPase activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Zhu C, Gao W, Zhao K, Qin X, Zhang Y, Peng X, Zhang L, Dong Y, Zhang W, Li P, Wei W, et al. Structural insight into dGTP-dependent activation of tetrameric SAMHD1 deoxynucleoside triphosphate triphosphohydrolase. Nature communications. 2013;4:2722. doi: 10.1038/ncomms3722. [The work also presents the crystal structure of the catalytic core of SAMHD1 in the tetramer form, showing dNTPase activation through binding of allosteric dGTPs.] [DOI] [PubMed] [Google Scholar]
- 37••.Ji X, Tang C, Zhao Q, Wang W, Xiong Y. Structural basis of cellular dNTP regulation by SAMHD1. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(41):E4305–4314. doi: 10.1073/pnas.1412289111. [Using a comprehensive approach combining crystallography (26 crystal structures) and biochemistry, this paper describes a complete spectrum of nucleotide binding to SAMHD1, demonstrating a robust SAMHD1 activation mechanism by a collaborative action of GTP and dNTPs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Koharudin LM, Wu Y, DeLucia M, Mehrens J, Gronenborn AM, Ahn J. Structural basis of allosteric activation of sterile alpha motif and histidine-aspartate domain-containing protein 1 (SAMHD1) by nucleoside triphosphates. The Journal of biological chemistry. 2014;289(47):32617–32627. doi: 10.1074/jbc.M114.591958. [Five crystal structures of SAMHD1-nucleotide complexes were determined, providing insight into SAMHD1 activation by GTP and dNTPs.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn J, Hao C, Yan J, DeLucia M, Mehrens J, Wang C, Gronenborn AM, Skowronski J. Hiv/simian immunodeficiency virus (SIV) accessory virulence factor vpx loads the host cell restriction factor SAMHD1 onto the E3 ubiquitin ligase complex CRL4DCAF1. The Journal of biological chemistry. 2012;287(15):12550–12558. doi: 10.1074/jbc.M112.340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lim ES, Fregoso OI, McCoy CO, Matsen FA, Malik HS, Emerman M. The ability of primate lentiviruses to degrade the monocyte restriction factor SAMHD1 preceded the birth of the viral accessory protein Vpx. Cell host & microbe. 2012;11(2):194–204. doi: 10.1016/j.chom.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fregoso OI, Ahn J, Wang C, Mehrens J, Skowronski J, Emerman M. Evolutionary toggling of Vpx/Vpr specificity results in divergent recognition of the restriction factor SAMHD1. PLoS pathogens. 2013;9(7):e1003496. doi: 10.1371/journal.ppat.1003496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42••.Schwefel D, Groom HC, Boucherit VC, Christodoulou E, Walker PA, Stoye JP, Bishop KN, Taylor IA. Structural basis of lentiviral subversion of a cellular protein degradation pathway. Nature. 2014;505(7482):234–238. doi: 10.1038/nature12815. [The mechanism by which Vpx targets SAMHD1 C-terminal tail was illustrated by a crystal structure of SIVsmm Vpx in complex with the C-terminal domain of the E3 ligase substrate adaptor DCAF1 and the C-terminal region of human SAMHD1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Schwefel D, Boucherit VC, Christodoulou E, Walker PA, Stoye JP, Bishop KN, Taylor IA. Molecular determinants for recognition of divergent SAMHD1 proteins by the lentiviral accessory protein Vpx. Cell host & microbe. 2015 doi: 10.1016/j.chom.2015.03.004. (In press). [The mechanism by which Vpx targets SAMHD1 N-terminal region was illustrated by a crystal structure of SIVmnd-2 Vpx in complex with the C-terminal domain of the E3 ligase substrate adaptor DCAF1 and the N-terminal region of mandrill SAMHD1.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beloglazova N, Flick R, Tchigvintsev A, Brown G, Popovic A, Nocek B, Yakunin AF. Nuclease activity of the human SAMHD1 protein implicated in the Aicardi-Goutieres syndrome and HIV-1 restriction. The Journal of biological chemistry. 2013 doi: 10.1074/jbc.M112.431148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goncalves A, Karayel E, Rice GI, Bennett KL, Crow YJ, Superti-Furga G, Burckstummer T. SAMHD1 is a nucleic-acid binding protein that is mislocalized due to Aicardi-Goutieres syndrome-associated mutations. Human mutation. 2012;33(7):1116–1122. doi: 10.1002/humu.22087. [DOI] [PubMed] [Google Scholar]
- 46.White TE, Brandariz-Nunez A, Carlos Valle-Casuso J, Amie S, Nguyen L, Kim B, Brojatsch J, Diaz-Griffero F. Contribution of SAM and HD domains to retroviral restriction mediated by human SAMHD1. Virology. 2012 doi: 10.1016/j.virol.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryoo J, Choi J, Oh C, Kim S, Seo M, Kim SY, Seo D, Kim J, White TE, Brandariz-Nunez A, Diaz-Griffero F, et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nature medicine. 2014;20(8):936–941. doi: 10.1038/nm.3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451(7177):425–430. doi: 10.1038/nature06553. [DOI] [PubMed] [Google Scholar]
- 49.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson MC, Stephens EB, Guatelli J. The interferon-induced protein BST-2 restricts HIV-1 release and is downregulated from the cell surface by the viral Vpu protein. Cell host & microbe. 2008;3(4):245–252. doi: 10.1016/j.chom.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galao RP, Le Tortorec A, Pickering S, Kueck T, Neil SJ. Innate sensing of HIV-1 assembly by tetherin induces NFkappaB-dependent proinflammatory responses. Cell host & microbe. 2012;12(5):633–644. doi: 10.1016/j.chom.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. Stimulation of NF-kappaB activity by the HIV restriction factor BST2. Journal of virology. 2013;87(4):2046–2057. doi: 10.1128/JVI.02272-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hinz A, Miguet N, Natrajan G, Usami Y, Yamanaka H, Renesto P, Hartlieb B, McCarthy AA, Simorre JP, Gottlinger H, Weissenhorn W. Structural basis of HIV-1 tethering to membranes by the BST-2/tetherin ectodomain. Cell host & microbe. 2010;7(4):314–323. doi: 10.1016/j.chom.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schubert HL, Zhai Q, Sandrin V, Eckert DM, Garcia-Maya M, Saul L, Sundquist WI, Steiner RA, Hill CP. Structural and functional studies on the extracellular domain of BST2/tetherin in reduced and oxidized conformations. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(42):17951–17956. doi: 10.1073/pnas.1008206107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swiecki M, Scheaffer SM, Allaire M, Fremont DH, Colonna M, Brett TJ. Structural and biophysical analysis of BST-2/tetherin ectodomains reveals an evolutionary conserved design to inhibit virus release. The Journal of biological chemistry. 2011;286(4):2987–2997. doi: 10.1074/jbc.M110.190538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang H, Wang J, Jia X, McNatt MW, Zang T, Pan B, Meng W, Wang HW, Bieniasz PD, Xiong Y. Structural insight into the mechanisms of enveloped virus tethering by tetherin. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(43):18428–18432. doi: 10.1073/pnas.1011485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56••.Venkatesh S, Bieniasz PD. Mechanism of HIV-1 virion entrapment by tetherin. PLoS pathogens. 2013;9(7):e1003483. doi: 10.1371/journal.ppat.1003483. [The membrane-tethering topology of BST2/tetherin was thoroughly probed by using rationally designed epitopes and protease cleavage sites in BST2.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauter D. Counteraction of the multifunctional restriction factor tetherin. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bartee E, McCormack A, Fruh K. Quantitative membrane proteomics reveals new cellular targets of viral immune modulators. PLoS pathogens. 2006;2(10):975–988. doi: 10.1371/journal.ppat.0020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Jia X, Weber E, Tokarev A, Lewinski M, Rizk M, Suarez M, Guatelli J, Xiong Y. Structural basis of HIV-1 Vpu-mediated BST2 antagonism via hijacking of the clathrin adaptor protein complex 1. eLife. 2014;3:e02362. doi: 10.7554/eLife.02362. [The structural basis of Vpu-mediated BST2 mistrafficking was established by the crystal structure of the cytoplasmic domains of BST2 and HIV-1 Vpu in complex with the activated clathrin AP1.] [DOI] [PMC free article] [PubMed] [Google Scholar]