Abstract
Objective
Given reports of high pain thresholds and reduced auditory response in individuals with Agenesis of the Corpus Callosum (AgCC), this study investigated whether affected participants report atypical experiences and behaviors on a well-established sensory processing measure.
Methods
Fourteen participants with AgCC (ages 11-59) completed the Adolescent/Adult Sensory Profile (Brown & Dunn, 2001). Sensory profile scales were classified as “Atypical” if they were more than one standard deviation from the mean.
Results
Fifty-seven percent of participants with AgCC reported reduced sensory registration as compared to an expected 16% of the normative sample. Similarly, 50% of the AgCC participants reported atypically increased auditory processing difficulties.
Conclusions
Using a well-established sensory processing questionnaire, participants with AgCC reported measurable differences in multiple aspects of sensory processing. The most notable difference was in the quadrant of low sensory registration, suggesting that individuals with AgCC may require sensory information to be presented more slowly or at a higher intensity for adequate processing. The sensory modality that was most affected was the auditory system, which is consistent with increased rates of language disorders and Autism Spectrum Disorders in this population. Understanding sensory processing in individuals with AgCC can both elucidate the role of inter-hemispheric transfer in the development of intact sensory processing as well as contribute to our knowledge of the role of the corpus callosum in a range of disorders in which sensory processes are impacted.
Keywords: sensory processing, agenesis of the corpus callosum, Sensory Profile
Introduction
The corpus callosum in humans is comprised of approximately 190 million axons connecting the left and right cerebral hemispheres which can facilitate the long-distance processing of information between these hemispheres (Paul, 2011). One out of every 4000 individuals is born without all (complete agenesis) or part (partial agenesis) of the corpus callosum (Glass, Shaw, Ma, & Sherr, 2008; Wang, Huang, & Yeh, 2004). Furthermore, 3-5% of individuals assessed for neurodevelopmental disabilities are estimated to have agenesis of the corpus callosum (AgCC; Bodensteiner, Schaefer, Breeding, & Cowan, 1994). We have reported that over 30% of individuals with AgCC exhibit traits of autism, including deficits in language and social skills (Lau et al., 2013; Paul, 2011). These deficits are likely secondary, in part, to cognitive processing inefficiency, as we previously have shown that reduction in processing speed for word reading and color naming account for measured differences in executive function tasks of cognitive inhibition and flexibility (Marco et al., 2012). Disrupted inter-hemispheric function likely affects not only the movement of information from one hemisphere to the other but also contralateral inhibition of homologous cortex during sensory processing tasks which are highly lateralized, such as auditory processing leading to speech. The ability to rapidly perceive, integrate, organize, and respond to incoming sensory information is critical to adaptive functioning. As we better understand the neural connectivity differences in individuals with atypical sensory processing, we will be able to recognize the contributions of domain specific white matter pathways and networks, particularly the role of inter-hemispheric communication (Owen et al., 2013), in the development of dysfunctional sensory processing. Individuals with AgCC provide a unique opportunity for investigating the role of the corpus callosum in sensory perception, integration, and response.
There is, however, limited information regarding sensory processing behaviors for individuals with AgCC. Anecdotal reports and parent surveys have suggested that individuals with AgCC can have diminished pain perception (Moes, Schilmoeller, & Schilmoeller, 2009). Further, studies have shown that affected individuals have both diminished pain and increased touch sensitivity relative to their unaffected siblings (Doherty, Tu, Schilmoeller, & Schilmoeller, 2006), suggesting that diminished pain sensitivity is not secondary to reduced processing of tactile sensory information. Given the varied comorbidities associated with AgCC, some researchers have attempted to discern whether these processing difficulties are related to callosal agenesis or other conditions. In a study examining differences between patients with AgCC plus complicating conditions (e.g., autism, IQ below 80, hydrocephalus, spina bifida, etc.) and “simple AgCC”, results indicated that both groups had increased frequency of hearing problems, diminished pain perception, and atypical sensitivity to cold when compared to their typically developing siblings (Moes et al., 2009). These data suggest that sensory differences in individuals with AgCC are not exclusively secondary to cormorbid conditions.
AgCC may impact these sensory processes indirectly as well. For example, in a study examining microelectrode recording in the anterior cingulate cortex of patients undergoing awake cingulotomy for treatment of psychiatric disorder, Hutchison et al. (1999) found that while most neurons responded only to painful and not innocuous stimuli, the painful stimulation did not elicit unpleasant sensation for the patients. To account for this discordance between brain activity and reported experience, the authors suggested three possible explanations: (1) the type of pain stimulation used in the experimental paradigm did not sufficiently reproduce a normal pain response in the brain, (2) pain-induced activation of the anterior cingulate is related to pain modulation rather than perception, or (3) pain perception requires bilateral cingulate activation or simultaneous activation of other cortical regions. The latter of these hypotheses is interesting to consider in the context of reduced pain perception in individuals with AgCC when taking into account the role of the cingulate cortex in early callosal development (Koester & O'Leary, 1994; Piper et al., 2009).
Taken together, research on sensory processing in individuals with AgCC suggests that these individuals may experience reduced pain perception as well as some emerging evidence of temperature sensitivity. However, to date, comprehensive examination and characterization of sensory processing behavior across multiple sensory modalities (i.e. auditory, tactile, taste, smell, and visual) has not been systematically addressed. The Adolescent/Adult Sensory Profile is a standardized self-report questionnaire that classifies sensory experiences and behaviors (Brown & Dunn, 2001). Based on the extant literature, we hypothesize that individuals with AgCC will report a pattern of sensory processing characterized by a high neurological threshold for sensory input or (“low registration”), and differences in tactile and auditory processing compared to the normative sample.
Methods
Participants
The Adolescent/Adult Sensory Profile was sent to individuals ages 11 and older diagnosed with AgCC (n=20) in the Brain Development Research Program database at the University of California, San Francisco. Participants were recruited if records indicated that their full scale intelligence quotient was greater than 70 and if they were able to complete the self-report form independently or with minimal parent assistance. Fourteen out of 20 (70%) were returned and fully completed. In our final sample, the AgCC cohort consisted of ten males and four females between the ages of 11 and 59 years. Nine participants identified as Caucasian and the remaining five declined to report their group identification. There were nine participants with complete AgCC and five with partial AgCC. Four individuals were left handed and two were ambidextrous. Participant characteristics are presented in Table 1.
Table 1.
Individual and Group Participant Characteristics with Sensory Profile Quadrant Scores
| Subject/Group | Age | FSIQ | AgCC Type | Low Registration | Sensation Seeking | Sensory Sensitivity | Sensation Avoiding |
|---|---|---|---|---|---|---|---|
| 1 | 17 | 81 | Complete | 59 | 47 | 41 | 25 |
| 2 | 26 | 94 | Complete | 47 | 55 | 39 | 40 |
| 3 | 59 | 78 | Complete | 50 | 33 | 57 | 43 |
| 4 | 11 | 85 | Partial | 31 | 57 | 32 | 49 |
| 5 | 49 | 129 | Partial | 17 | 61 | 25 | 27 |
| 6 | 32 | 84 | Complete | 25 | 50 | 23 | 28 |
| 7 | 13 | 94 | Complete | 41 | 31 | 51 | 47 |
| 8 | 24 | 91 | Complete | 46 | 38 | 38 | 54 |
| 9 | 13 | 100 | Complete | 35 | 50 | 27 | 28 |
| 10 | 16 | 90 | Partial | 40 | 42 | 29 | 34 |
| 11 | 21 | 88 | Complete | 26 | 47 | 28 | 26 |
| 12 | 38 | 100 | Partial | 38 | 50 | 32 | 39 |
| 13 | 20 | 111 | Complete | 51 | 48 | 32 | 47 |
| 14 | 14 | 83 | Partial | 59 | 47 | 41 | 25 |
| Complete M(SD) | 25.00 (14.14) | 91.22 (10.16) | 42.22 (11.56) | 44.33 (8.31) | 37.33 (11.28) | 39.13 (10.56) | |
| Partial M(SD) | 25.60 (16.89) | 97.40 (18.85) | 37.00 (15.25) | 51.40 (7.64) | 31.80 (5.89) | 34.80 (9.71) | |
| All AgCC M(SD) | 25.21 (14.52) | 93.43 (13.50) | 40.36 (12.67) | 46.86 (8.53) | 35.36 (9.83) | 37.46 (10.06) |
AgCC participants were assessed in accordance with IRB approval and gave consent or assented to participation with guardian consent. Individuals with Aicardi syndrome (N=82) or other primary brain malformations (N=27) were excluded. A diagnosis of AgCC was confirmed by review of their brain MRI at UCSF. MR images were analyzed by one of two pediatric neuroradiologists and one pediatric neurologist (EHS). Images were evaluated for the presence and size of the corpus callosum, the anterior commissure, the hippocampal commissure, cortical malformations (e.g. polymicrogyria, periventricular and subcortical heterotopia), Probst bundles, white matter abnormalities, and dysgenesis of the posterior fossa. For more information on the analysis of MRI data, see Hetts et al. (2006). Data were compared to that of the normative sample from the published Sensory Profile validation studies (Brown & Dunn, 2001), which consisted of 950 adolescents and adults without known disabilities or psychiatric conditions as indicated by self-report. This sample was primarily derived from the mid-western region of the United States and was 92% Caucasian.
Measures
The Adolescent/Adult Sensory Profile is a 60-item self-report questionnaire which characterizes sensory experiences and behavior and evaluates their impact on functional abilities and daily life (Brown & Dunn, 2001). The respondent is asked to rate each item as applying to their experience or behavior: (1) almost never, (2) seldom, (3) occasionally, (4) frequently, or (5) almost always. The ratings range from one to five with three representing typical experience or behavior. Ratings of one or two suggest reduced frequency of behavior or experience and ratings of four or five indicate increased frequency. The Sensory Profile categorizes sensory processing into six domains: auditory, tactile, visual, taste/smell, movement, and activity level. Items from each domain are incorporated into four distinct quadrants to characterize experience and behavior: low registration, sensation seeking, sensory sensitivity and sensation avoiding.
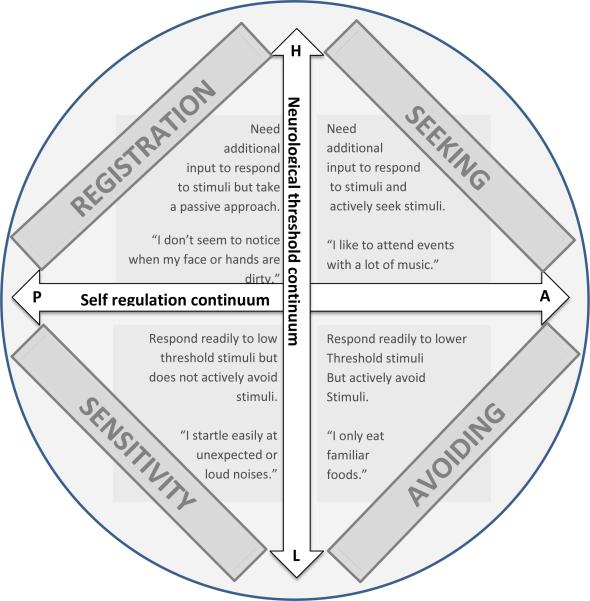
The low registration quadrant refers to the tendency to show a delayed or absent response to sensory stimuli across sensory domains. Individuals with low registration may have difficulty responding to low intensity or rapidly presented stimuli. Examples of items in the low registration quadrant reference not smelling things that others smell, taking longer than others to wake up in the morning,, not noticing others entering a room or calling the participant's name.
Individuals high in the sensation seeking quadrant may create or seek out sensory stimulation to meet their high thresholds for sensory input. They generally find sensory experiences pleasurable and may have difficulty tolerating environments with low stimulation. This scale includes items which reference enjoyment of colorful or brightly lit settings, making noises such as humming and whistling, and smelling flowers.
In contrast, individuals with high sensory sensitivity quadrant scores have low thresholds for sensory input (high registration) and are highly responsive to stimuli. This sensitivity can be associated with superior environmental awareness and attention to detail but often at the cost of discomfort and distractibility. Items on this scale reference dislikes for strong tastes, car rides, and certain types of clothing fabrics.
Finally, individuals with high sensation avoiding quadrant scores are overwhelmed or bothered by certain sensory experiences and actively avoid exposing themselves to disturbing stimuli. Examples of sensory avoiding items reference preference for familiar foods, avoidance of elevators or escalators due to their type of movement, and use of strategies to minimize sounds like closing doors, use of earplug, covering ears, etc. These quadrants are not necessarily mutually exclusive. The differences between quadrants may relate to either the response to sensation or the experience of it. For example, respondents with elevated scores on low registration or sensation seeking both need additional sensory information in order to process it adequately. This is thought to represent a high neurologic perception threshold; however, these quadrants differ in their approach. For the low registration quadrant respondents take on a passive approach. For example, respondents may not be aware of dirt on their hands or face. In contrast, for the sensory seeking quadrant respondents actively seek additional information from their environment, such as attendance of music events. The sensory sensitivity and sensation avoiding quadrants are comprised of items assessing whether the respondents are easily bothered by stimuli. Figure 1 illustrates the relationships between the different quadrants of sensory experience and behavior.
Figure 1.
Quadrants of the Sensory Profile
Application of Dunn's Sensory Processing Framework
Quadrant and domain totals are summed for each participant and are compared to a bell curve distribution. Scores were then categorized as “Typical” if they were within one standard deviation of the mean and “Atypical” if they were more than one standard deviations from the mean in either direction. The “Atypical” categories combine what is referred to as probable difference and definite difference in the Sensory Profile Manual. Deviations from the mean were analyzed separately for each direction on the curve. Atypically High designates scores that are more than one standard deviation above the mean and Atypically Low indicates scores that are more than one standard deviation below the mean. The expected prevalence for scores within each of these categories is 16% based on the normative distribution. We additionally categorized scores that were two or more standard deviations from the mean as Definitely High or Definitely Low. This corresponds to the definite difference categories in the Sensory Profile Manual (Dunn, 1999). The expected prevalence for scores within each of these categories is 2% based on the normative distribution. Construct validity of these self-reported sensory experiences has been demonstrated through evidence of a relationship between quadrant scores and physiologic measures of skin conductance (Brown, Tollefson, Dunn, Cromwell, & Filion, 2001).
Results
Statistical Analysis
To explore our primary hypothesis that the percentage of individuals with AgCC who display atypical sensory behavior will differ from the normative sample on the four Sensory Profile quadrants (low registration, sensory seeking, sensory sensitivity, and sensory avoiding), we calculated the percentage of individuals in the AgCC cohort who scored in the Atypically High category and Atypically Low category. We then conducted one-sample z-tests of proportion comparing the percentages of participants with AgCC in each of these categories against a test value of 16%. In a second level analyses, we compared percentages of AgCC participants in the Definitely High and Definitely Low categories against a test value of 2%. We adjusted for multiple comparisons (eight in total for high and low difference categories across four quadrants) using a Bonferroni correction of p <0.00625. In a post-hoc comparison, we investigated six sensory domains: auditory, tactile, visual, taste/smell, movement, and activity level using the same analysis method as outlined for the primary hypothesis. A Bonferroni correction of p<0.00417 was used to address multiple comparison (12 in total for high and low difference categories across six domains).
Sensory Profile Quadrant Comparisons
57.14% of individuals with AgCC were categorized as Atypically High in the low registration quadrant as compared to 16% of the normative sample (z=4.20, p<0.0001). In contrast, only one individual with AgCC (7.14%) was categorized as Atypically Low in the low registration quadrant (z=0.90, p=0.37), suggesting that low registration for sensory information is more common for individuals with AgCC than for the general population. Comparisons for the Atypically High and Atypically Low categories were not statistically significant for the remaining quadrants after correction for multiple comparisons. In a second level analysis, 42.86% of participants with AgCC were categorized as Definitely High (>2SD difference) in the low registration quadrant compared to the expected proportion of 2% (z=10.92, p<.0001). For the sensory sensitivity and sensory avoiding quadrants, 14.29% of participants with AgCC were categorized a Definitely High versus an expected 2% prevalence (z=3.29, p=.001).
Sensory Profile Domain Comparisons
Given the group differences identified in our primary quadrant analysis, we conducted domain specific analyses to examine rates of atypical sensory experience in auditory, tactile, visual, taste/smell, movement, and activity level domains. Fifty percent of participants with AgCC scored in the Atypically High category compared to the expected proportion of 16% for the auditory processing and taste/smell domains (z=3.47, p=0.0005). The proportions of participants scoring in the Atypically High category for the remaining sensory domains were not significantly different from expected proportions after correction for multiple comparisons. In second level analyses, 14.29% of AgCC participants scored in the Definitely High category compared to the expected proportion of 2% on the taste/smell domain (z=3.29, p=.001). For the activity level and visual domains, 21.43% of AgCC participants were categorized as Definitely High (z=5.19, p<.0001). Proportions of participants scoring in the Definitely High and Definitely Low categories for the remaining sensory domains were not significantly different from the expected proportion of 2%. These percentages are presented in Table 2 for both the combined sample as well as separately for individuals with complete versus partial AgCC.
Discussion
This study examined self-reported sensory experience and behavior on the Sensory Profile (Brown & Dunn, 2001) in high functioning adolescents and adults with AgCC. As hypothesized, proportionately more participants with AgCC than the normative sample endorsed low registration of sensory experience in both levels of analysis—Atypically High (> 1 SD) and Definitely High (> 2 SD). These results are consistent with previous research in which individuals with AgCC were reported to have high pain tolerance (Doherty et al., 2006; Moes et al., 2009), or “low registration” for pain sensation. Current results suggest that this high tolerance for pain may be related to a higher threshold for detection of pain or slower response to painful input. Interestingly, significant differences were not detected between the normative and AgCC samples for tactile sensory processing despite reports of high pain tolerance. In fact, previous studies have indicated that affected individuals can have both diminished pain and increased touch sensitivity (Doherty et al., 2006). These findings, in combination with the results of the current study, suggest that diminished pain sensitivity in AgCC is not secondary to reduced processing of tactile sensory information. Rather, response to pain-inducing stimulation and sensitivity of tactile sensory processing appear to be differentially impacted via separate processes in AgCC.
Consistent with this profile of low registration, results of domain specific analyses indicated that both Atypically Low and Definitely High levels of taste/smell experiences were more prevalent in this sample, suggesting that altered taste and smell sensation is common for individuals with AgCC in both directions of difference. Also seemingly contradictory to a low registration profile, 14.29% of participants were categorized as Definitely High in sensory sensitivity and sensation avoiding behavior. Although not statistically significant, it is noteworthy that this percentage rises to 35.71% for sensation avoiding and 29% for sensory sensitivity when more moderate (Atypically High) categorizations are considered. This suggests that some individuals with AgCC are more likely to experiences sensitivity and avoid exposure to certain sensory experiences. Previous research has identified sensory hypersensitivity in individuals with AgCC, particularly in the tactile sensory domain (Doherty et al., 2006). In the context of the data suggesting that these individuals experience low registration of sensory input, it is possible that high ratings on the sensory avoiding domain relate to difficulty keeping up with processing demands in certain situations. For example, individuals with low registration tendencies find it difficult to react to rapidly presented information (Brown & Dunn, 2001). These difficulties may shape their behavior to avoid situations with rapid processing demands. This is consistent with previous work in which slow processing speed was reported with individuals with AgCC (Marco et al., 2012).
These difficulties with rapid processing may be related to the domain specific findings in the current study. Specifically, sensory processing in the auditory modality was more frequently impacted in the AgCC group than for those in the normative sample. Moreover, the highest scoring auditory domain items were related to both low registration for auditory sensory information as well as language processing, which requires rapid integration of auditory information (i.e., difficulty understanding fast speech, need for repetition, etc.). Difficulties in language processing and understanding the social aspects of language have been documented in individuals with AgCC. For example, individuals with AgCC have impairments in language pragmatics, including understanding of idioms, proverbs, vocal prosody, non-literal interpretation, and humor (Brown, Paul, Symington, & Dietrich, 2005; Brown, Symingtion, VanLancker-Sidtis, Dietrich, & Paul, 2005; Huber-Okrainec, Blaser, & Dennis, 2005; Paul, Van Lancker-Sidtis, Schieffer, Dietrich, & Brown, 2003). Further, studies of functional connectivity in individuals with AgCC have identified correlations between slower verbal processing speed and reduced resting state connectivity of the left medial and superior temporal lobe (Hinkley et al., 2012). Language skills require rapid integration of basic sensory information with higher order cognitive functions. Given the report of low registration for sensory information in many individuals with AgCC, as well as specific differences affecting auditory processing behaviors, the rapid processing demands of language comprehension and social cognitive language skills may be overwhelming to affected individuals. Taken together, results suggest that for individuals with AgCC, lower sensory registration does not motivate sensory seeking behavior. In fact, results suggest that low registration may instead motivate avoidance behavior for situations in which the individual is at a sensory processing disadvantage due to information processing inefficiency (i.e., situations in which rapid processing and integration of multiple sources of information is required). Given prior evidence of reductions in posterior regions of the corpus callosum in individuals with Attention-Deficit/Hyperactivity Disorder (Seidman, Valera, & Makris, 2005; Valera, Faraone, Murray, & Seidman, 2007) and the current findings of increased activity level (50%) in the absence of evidence for increased sensation seeking (7.14%), it is possible that increased activity may reflect this motivation to avoid such situations as well.
The findings from the current study may suggest that reduced neuroanatomical capacity for information transfer has a negative impact on sensory processing and, subsequently, the cognitive processes that rely on rapid integration of sensory information. This reduced capacity for inter-hemispheric transfer has been implicated in other clinical populations in which sensory dysfunction and callosal abnormalities are prevalent. Clinically, there is a growing recognition that individuals with neurodevelopmental disabilities and brain injury, such as autism, premature delivery, and traumatic brain injury, will also show pronounced dysregulation in the processing of sensory information and that this dysregulation, in turn, affects participation in activities of daily living (Adamson, Hare, & Graham, 2006; Galvin, Froude, & Imms, 2009; Rogers & Ozonoff, 2005; Wickremasinghe et al., 2013). In these populations, exploration of the underlying mechanisms of sensory dysfunction have begun but remains difficult to reconcile, in part due to the etiologic heterogeneity of the individuals studied within autism cohorts (Marco, Hinkley, Hill, & Nagarajan, 2011) as well as the variation of injury patterns and ages for traumatic brain injury. However, individuals with autism as well as those with TBI often have diminished corpus callosum volumes and differences in white matter connectivity (Hardan et al., 2009; Schmidt et al., 2013).
Conversely, an estimated 40% of individuals with AgCC will meet screening criteria for Autism Spectrum Disorder (Lau et al., 2013). Based on pediatric data from the Sensory Profile (Dunn, 1999), individuals with ASD experience a more diffuse pattern of sensory dysfunction compared to the AgCC participants in the current study. For example, sensation seeking behaviors are often very conspicuous in individuals with ASD (e.g., making unusual sounds, hand flapping, visual inspection of objects, sniffing things that aren't typically associated with a strong smell, enjoying running water on hands, etc.). In contrast, results of the current study suggest that individuals with AgCC do not engage in more sensation seeking behavior despite low registration of sensory information. This discrepancy highlights the need for studies focused on understanding symptom dimensions that relate to differences in neurobiology. Because AgCC is a congenital condition, early intervention efforts targeting sensory processing may translate to better outcomes in other domains of functioning that rely on sensory processing for learning and development. Likewise, adaptations to facilitate adequate sensory processing in impacted domains, such as reducing the rate or providing repetition for auditory information, may have potential to improve functional outcomes for affected individuals.
Limitations and Future Research
There were several limitations of the current study. First, the sample size was small, which limited both statistical power and study design. Specifically, individuals with partial and complete AgCC were grouped together to increase power; however, this may have obscured finding specific to differences in neuroanatomy that could be explored with a larger sample. Future studies with more participants with both partial and complete AgCC should assess relationships between abnormalities in specific callosal subdivisions and characterization of sensory processing across domains to further our understanding of the impact of callosal agenesis on sensory dysfunction. Second, our AgCC sample was also heterogeneous with respect to etiology. A sample of individuals with AgCC resulting from a specific genetic cause, for example a single gene or genetic loci, might have resulted in a more distinct or homogenous sensory profile. Our assessment of sensory processing also was limited to self-report and prior evidence suggests that individuals with AgCC may under-report symptoms compared to caregiver ratings (Lau et al., 2012). Future research should incorporate objective methods for direct measurement of sensory processing in combination with self and informant report in order to make inferences about relationships between brain activity and experience or behavior. Further, studies concurrently examining performance on cognitive tasks and sensory processing may provide insights about the impact of sensory dysfunction on cognitive development in this population. Finally, researchers need to study interventions informed by sensory patterns to identify effective treatment for individuals with AgCC. Longitudinal intervention studies can inform our understanding of the role of intact sensory processing on neurodevelopment and the impact of its disruption.
Supplementary Material
Figure 2.
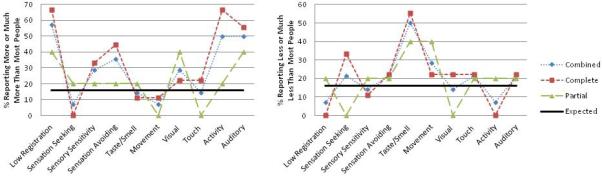
Percentages of Participants in the Partial, Complete, or Combined AgCC Groups Scoring in the Atypical and Definite Difference Categories Across Quadrants and Domains
References
- Adamson A, Hare AO, Graham C. Impairments in Sensory Modulation in Children with Autistic Spectrum Disorder. British Journal of Occupational Therapy. 2006;69(8):357–364. [Google Scholar]
- Bodensteiner J, Schaefer GB, Breeding L, Cowan L. Hypoplasia of the Corpus Callosum: A Study of 445 Consecutive MRI Scans. Journal of Child Neurology. 1994;9(1):47–49. doi: 10.1177/088307389400900111. doi:doi:10.1177/088307389400900111. [DOI] [PubMed] [Google Scholar]
- Brown C, Dunn W. Adolescent/Adult Sensory Profile: User’s Manual. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Brown C, Tollefson N, Dunn W, Cromwell R, Filion D. The Adult Sensory Profile: measuring patterns of sensory processing. The American Journal of Occupational Therapy: Official Publication of the American Occupational Therapy Association. 2001;55(1):75–82. doi: 10.5014/ajot.55.1.75. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11216370. [DOI] [PubMed] [Google Scholar]
- Brown WS, Paul LK, Symington M, Dietrich R. Comprehension of humor in primary agenesis of the corpus callosum. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2004.09.008. Retrieved from http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=med4&NEWS=N&AN=15716161. [DOI] [PubMed]
- Brown WS, Symingtion M, VanLancker-Sidtis D, Dietrich R, Paul LK. Paralinguistic processing in children with callosal agenesis: emergence of neurolinguistic deficits. Brain and Language. 2005;93:135–139. doi: 10.1016/j.bandl.2004.09.003. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15781301. [DOI] [PubMed] [Google Scholar]
- Doherty D, Tu S, Schilmoeller K, Schilmoeller G. Health-related issues in individuals with agenesis of the corpus callosum. Child: Care, Health and Development. 2006;32(3):333–42. doi: 10.1111/j.1365-2214.2006.00602.x. doi:doi:10.1111/j.1365-2214.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- Dunn W. Sensory Profile User’s Manual. Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Galvin J, Froude EH, Imms C. Sensory processing abilities of children who have sustained traumatic brain injuries. American Journal of Occupational Therapy. 2009;63:701–709. doi: 10.5014/ajot.63.6.701. [DOI] [PubMed] [Google Scholar]
- Glass HC, Shaw GM, Ma C, Sherr EH. Agenesis of the corpus callosum in California 1983-2003: a population-based study. American Journal of Medical Genetics. Part A. 2008;146A(19):2495–500. doi: 10.1002/ajmg.a.32418. doi:doi:10.1002/ajmg.a.32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan A, Pabalan M, Gupta N, Bansal R, Melhem N, Fedorov S, Minshew N. Corpus callosum volume in children with autism. Psychiatry Research. 2009;174(1):57–61. doi: 10.1016/j.pscychresns.2009.03.005. doi:doi:10.1016/j.pscychresns.2009.03.005.Corpus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetts SW, Sherr EH, Chao S, Gobuty S, Barkovich a J. Anomalies of the corpus callosum: an MR analysis of the phenotypic spectrum of associated malformations. AJR. American Journal of Roentgenology. 2006;187(5):1343–8. doi: 10.2214/AJR.05.0146. doi:doi:10.2214/AJR.05.0146. [DOI] [PubMed] [Google Scholar]
- Hinkley LBN, Marco EJ, Findlay AM, Honma S, Jeremy RJ, Strominger Z, Sherr EH. The role of corpus callosum development in functional connectivity and cognitive processing. PloS One. 2012;7(8):e39804. doi: 10.1371/journal.pone.0039804. doi:doi:10.1371/journal.pone.0039804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber-Okrainec J, Blaser SE, Dennis M. Idiom comprehension deficits in relation to corpus callosum agenesis and hypoplasia in children with spina bifida meningomyelocele. Brain and Language. 2005;93(3):349–68. doi: 10.1016/j.bandl.2004.11.002. doi:doi:10.1016/j.bandl.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Hutchison WD, Davis KD, Lozano AM. Pain-related neurons in the human cingulate. Nature Neuroscience. 1999;2(5):403–405. doi: 10.1038/8065. [DOI] [PubMed] [Google Scholar]
- Koester S, O’Leary D. Axons of Early Generated Corpus Callosum Neurons in Cingulate Cortex Pioneer the. The Journal of Neuroscience. 1994;14(November):6608–6620. doi: 10.1523/JNEUROSCI.14-11-06608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YC, Hinkley LBN, Bukshpun P, Strominger Z. a, Wakahiro MLJ, Baron-Cohen S, Marco EJ. Autism traits in individuals with agenesis of the corpus callosum. Journal of Autism and Developmental Disorders. 2012;43(5):1106–1118. doi: 10.1007/s10803-012-1653-2. doi:doi:10.1007/s10803-012-1653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau YC, Hinkley LBN, Bukshpun P, Strominger Z. a, Wakahiro MLJ, Baron-Cohen S, Marco EJ. Autism traits in individuals with agenesis of the corpus callosum. Journal of Autism and Developmental Disorders. 2013;43(5):1106–18. doi: 10.1007/s10803-012-1653-2. doi:doi:10.1007/s10803-012-1653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Harrell KM, Brown WS, Hill SS, Jeremy RJ, Kramer JH, Paul LK. Processing speed delays contribute to executive function deficits in individuals with agenesis of the corpus callosum. Journal of the International Neuropsychological Society: JINS. 2012;18 J(3):521–9. doi: 10.1017/S1355617712000045. doi:doi:10.1017/S1355617712000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EJ, Hinkley LBN, Hill SS, Nagarajan SS. Sensory processing in autism: a review of neurophysiologic findings. Pediatric Research. 2011;69(5 Pt 2):48R–54R. doi: 10.1203/PDR.0b013e3182130c54. doi:doi:10.1203/PDR.0b013e3182130c54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moes P, Schilmoeller K, Schilmoeller G. Physical, motor, sensory and developmental features associated with agenesis of the corpus callosum. Child: Care, Health and Development. 2009;35(5):656–72. doi: 10.1111/j.1365-2214.2009.00942.x. doi:doi:10.1111/j.1365-2214.2009.00942.x. [DOI] [PubMed] [Google Scholar]
- Owen JP, Marco EJ, Desai S, Fourie E, Harris J, Hill SS, Mukherjee P. Abnormal white matter microstructure in children with sensory processing disorders. NeuroImage. Clinical. 2013;2:844–53. doi: 10.1016/j.nicl.2013.06.009. doi:doi:10.1016/j.nicl.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK. Developmental malformation of the corpus callosum: a review of typical callosal development and examples of developmental disorders with callosal involvement. Journal of Neurodevelopmental Disorders. 2011;3(1):3–27. doi: 10.1007/s11689-010-9059-y. doi:doi:10.1007/s11689-010-9059-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul LK, Van Lancker-Sidtis D, Schieffer B, Dietrich R, Brown WS. Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain and Language. 2003;85:313–324. doi: 10.1016/s0093-934x(03)00062-2. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0093934X03000622. [DOI] [PubMed] [Google Scholar]
- Piper M, Plachez C, Zalucki O, Fothergill T, Goudreau G, Erzurumlu R, Richards LJ. Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cerebral Cortex (New York, N.Y.: 1991) 2009;19(Suppl 1)(July):i11–21. doi: 10.1093/cercor/bhp027. doi:doi:10.1093/cercor/bhp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Ozonoff S. Annotation: what do we know about sensory dysfunction in autism? A critical review of the empirical evidence. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2005;46(12):1255–68. doi: 10.1111/j.1469-7610.2005.01431.x. doi:doi:10.1111/j.1469-7610.2005.01431.x. [DOI] [PubMed] [Google Scholar]
- Schmidt AT, Hanten G, Li X, Wilde E. a, Ibarra AP, Chu ZD, Levin HS. Emotional prosody and diffusion tensor imaging in children after traumatic brain injury. Brain Injury: [BI] 2013;27(13-14):1528–35. doi: 10.3109/02699052.2013.828851. doi:doi:10.3109/02699052.2013.828851. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57(11):1263–72. doi: 10.1016/j.biopsych.2004.11.019. doi:doi:10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Valera EM, Faraone SV, Murray KE, Seidman LJ. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61(12):1361–9. doi: 10.1016/j.biopsych.2006.06.011. doi:doi:10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Wang LW, Huang CC, Yeh TF. Major brain lesions detected on sonographic screening of apparently normal term neonates. Neuroradiology. 2004;46(5):368–73. doi: 10.1007/s00234-003-1160-4. doi:doi:10.1007/s00234-003-1160-4. [DOI] [PubMed] [Google Scholar]
- Wickremasinghe a C., Rogers EE, Johnson BC, Shen a, Barkovich a J, Marco EJ. Children born prematurely have atypical sensory profiles. Journal of Perinatology: Official Journal of the California Perinatal Association. 2013;33(8):631–5. doi: 10.1038/jp.2013.12. doi:doi:10.1038/jp.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.