Abstract
Background and study aims: It is difficult to perform endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) of small gastrointestinal (GI) subepithelial lesions (SELs) approximately 10 mm in diameter. This study was undertaken to evaluate the feasibility, safety, and diagnostic ability of EUS-FNA with a forward-viewing and curved linear-array echoendoscope (FVCLA-ES) that has a cap for small SELs.
Patients and methods: The study enrolled 8 patients who had small upper GI SELs approximately 10 mm in diameter. To fix the SELs during FNA, a cap device was attached to the scope tip.
Results: The mean (standard deviation [SD]) diameter of the SELs was 10.6 mm (2.94). Even small lesions were well targeted for FNA when the FVCLA-ES with a cap device was used. The mean (SD) number of passes was 4.6 (1.59). Adequate samples were obtained from 7 patients (87.5 %) – in 6 (75 %) for cytology and in 4 (50 %) for histologic examination with immunohistochemical (IHC) staining. No complication occurred. Gastrointestinal stromal tumor (GIST) in 2 patients and leiomyoma in 2 patients were definitively diagnosed with IHC staining.
Conclusions: EUS-FNA with an FVCLA-ES that has a cap device is feasible and safe. This technique is expected to contribute to histologic diagnosis, even in small SELs.
Introduction
Small subepithelial lesions (SELs) of the gastrointestinal (GI) tract are found incidentally during esophagogastroduodenoscopy (EGD). Until recently, their management consisted of observation without a definitive tissue diagnosis or surgical resection. SELs include a diverse array of benign, potentially malignant, and malignant lesions of still smaller size. Particularly, because every gastrointestinal stromal tumor (GIST) is regarded as potentially malignant, all GISTs may require resection or close observation, including even small intramural lesions of the GI tract 1. Nevertheless, an attempt at pathologic diagnosis should be made for additional management because not all intramural lesions of the GI tract are GISTs. Endoscopic ultrasonography (EUS), enabling intramural scanning of the GI tract, is extremely useful for SEL diagnosis. However, when a small SEL with a diameter of approximately 10 mm is found, EUS-guided fine-needle aspiration (EUS-FNA) biopsy is typically difficult to perform with a conventional oblique-viewing and curved linear-array echoendoscope because the target lesion is mobile during puncture.
Recently, the forward-viewing and curved linear-array echoendoscope (FVCLA-ES) has been developed. Several reports describe the usefulness of this new equipment for therapeutic interventional EUS 2 3. A salient benefit of the FVCLA-ES is that it enables the axial application of force during needle/device insertion under direct optical visualization. Furthermore, the endoscope's capability can be expanded by attaching a cap device to the tip 2. Therefore, the FVCLA-ES can probably scan and perform the interventional approach easily with fixation by the attached cap device, even if the target lesion moves continuously. This study was conducted to evaluate the feasibility, safety, and diagnostic capability of EUS-FNA with an FVCLA-ES that has a cap device attached on the scope tip for small SELs.
Patients and methods
From June to November 2013 at Fukushima Medical University, Aizu Medical Center Hospital, 8 consecutive patients (3 men, 5 women; mean age 59.8, range 25 – 84) who had small GI SELs approximately 10 mm in diameter and who underwent EUS-FNA with an FVCLA-ES (XGIF-UCT160 J-AL5; Olympus, Tokyo, Japan) were enrolled in this study. In all patients, small SELs were found incidentally during EGD for cancer screening. The existence of an SEL was confirmed by EUS with a mini-probe (20 MHz).
Five endosonographers performed EUS-FNA in patients who were moderately sedated with midazolam. To solve the difficulty of tumor scanning and fixation of the scope during EUS-FNA, a cap device was attached to the scope tip, and that fixed the SEL by suction (Fig. 1). For EUS-FNA, 22- or 25-gauge needles were used (Expect; Boston Scientific Japan, Tokyo, or EchoTip Ultra; Cook Japan, Tokyo). A target lesion was confirmed by using forward-viewing optics and a curved linear-array ultrasound transducer (Fig. 2). Then, the lesion was sucked into the attached cap device. With this technique, a small SEL was trapped and fixed for EUS-FNA (Fig. 3 a, b).
Fig. 1.

To solve the difficulty of tumor scanning and scope fixation during endoscopic ultrasound-guided fine-needle aspiration of a small gastrointestinal subepithelial lesion (SEL), a cap device is attached to the tip of a forward-viewing and curved linear-array echoendoscope scope tip; this device fixes the SEL.
Fig. 2.
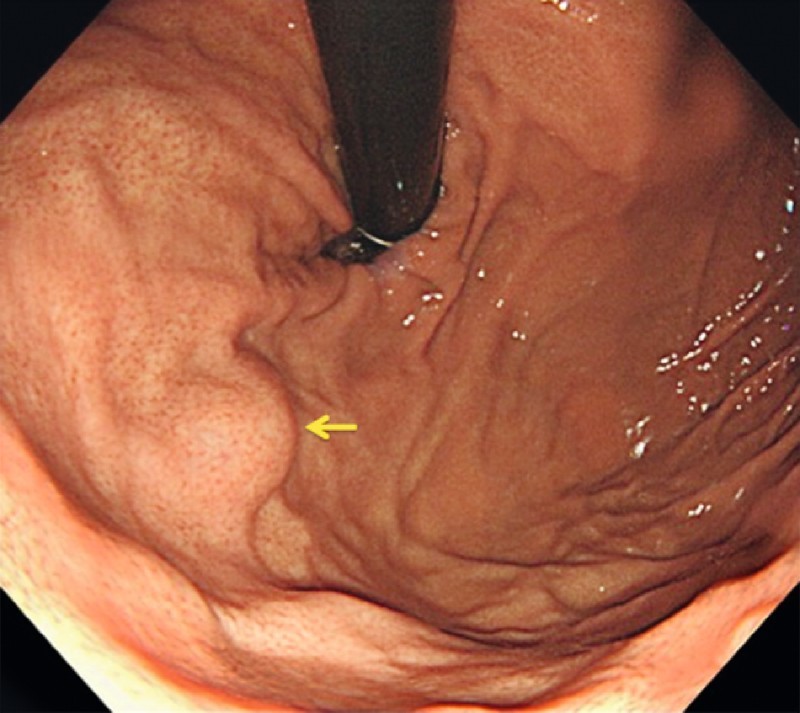
A small subepithelial lesion (arrow) is identified at the fundus of the stomach.
Fig. 3.
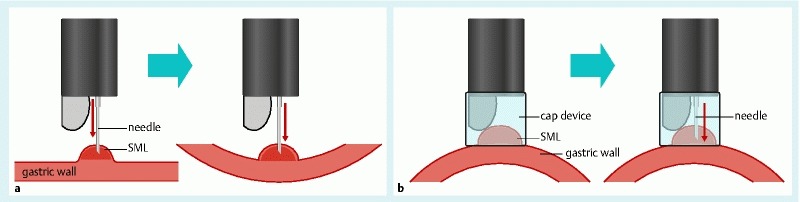
a A small subepithelial lesion (SEL) is difficult to puncture because the gastric wall bends and the SEL moves during the puncture. b An attached cap device can fix an SEL in the cap by absorption. Then, endoscopic ultrasound-guided fine-needle aspiration of a very small SEL can be performed easily without an object escaping. SML, submucosal lesion.
While suction of the lesion was maintained, EUS-FNA was performed. The needle was advanced under EUS guidance to the center of the lesion by using a quick, strong thrust of the handle (Fig. 4). Once the needle had been placed clearly into the target lesion, the stylet was withdrawn completely. Then, the needle was moved back and forth about 10 times within the lesion under EUS guidance while suction was applied with a 20-mL syringe and the lock device. Air with a syringe or a stylet was used to spray the aspirated materials onto glass slides, and these were processed for cytologic/histologic examination.
Fig. 4.
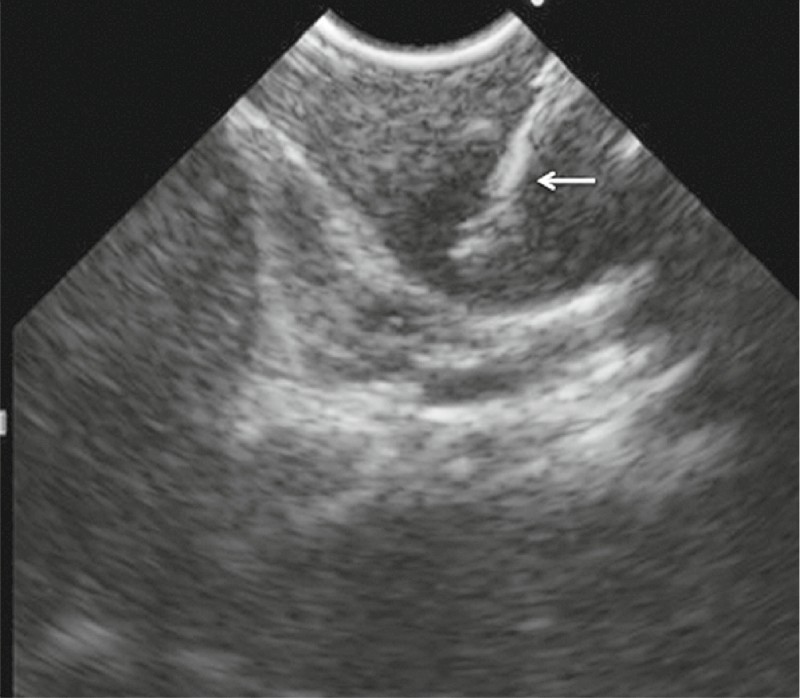
Endoscopic ultrasound-guided fine-needle aspiration is performed with absorption in a cap device. Good visualization of the aspiration needle is possible (arrow).
The specimens for histology were processed in the pathology laboratory for hematoxylin and eosin staining. Immunohistochemical (IHC) staining was subsequently performed on the histologically representative sections of each tumor. A tumor with a positive reaction to c-kit and/or CD34 was diagnosed as a GIST. A tumor with a negative reaction to c-kit, CD34, and S-100 and a positive reaction for muscle actin was diagnosed as a myogenic tumor (leiomyoma). A tumor with a negative reaction to c-kit, CD34, and muscle actin and a positive reaction for S-100 was diagnosed as a neurogenic tumor (neurinoma). When a histologic diagnosis was based on a surgically resected specimen or IHC staining, this was regarded as the definitive diagnosis. If only a cytologic examination was performed, the diagnosis or exclusion of malignancy was based on long-term clinical follow-up, including repeated EUS.
The study protocol was approved by the institutional review board for human research at the Fukushima Medical University. This study was conducted in accordance with the human and ethical principles of research set forth in the Declaration of Helsinki.
Results
Feasibility and safety
Features of the 8 GI SELs, including their location, size, layers of origin, and echo patterns, are presented in Table 1. The mean (standard deviation [SD]) diameter of the SELs was 10.6 mm (2.94); the range was 8 to 15 mm. The mean (SD) number of FNA passes was 4.6 (1.59); the range was 3 to 7. The FVCLA-ES with the attached cap device provided good visualization in all lesions. Good visualization of the FNA needle was obtained in all cases. Even very small lesions were well targeted for FNA with a cap (Table 1). The maneuverability of the scope and performance of FNA were rated as very satisfactory by the five endosonographers. No severe complication, such as bleeding, infection, or perforation, or any mild complication occurred during follow-up.
Table 1. Details and feasibility of endoscopic ultrasound (EUS)-guided fine-needle aspiration of small gastrointestinal subepithelial lesions with a forward-viewing and curved linear-array echoendoscope (n = 8).
| Location | Size, mm | EUS layer of origin | Echo pattern | Technical feasibility | Complication | Samples for | ||||
| Visualization | Absorption | Puncture | Histology | Cytology | ||||||
| 1 | Gastric fundus | 8.2 | Fourth | Homogeneoushypoechoic | Yes | Yes | Yes | No | No | Yes |
| 2 | Gastric body | 8.0 | Fourth | Heterogeneous | Yes | Yes | Yes | No | Yes | Yes |
| 3 | Gastric body | 8.1 | Fourth | Homogeneoushypoechoic | Yes | Yes | Yes | No | Yes | Yes |
| 4 | Gastric body | 10.7 | Fourth | Homogeneoushypoechoic | Yes | Yes | Yes | No | No | Yes |
| 5 | Gastric body | 15.0 | Fourth | Homogeneoushypoechoic | Yes | Yes | Yes | No | Yes | No |
| 6 | Gastric body | 10.8 | Fourth | Heterogeneous | Yes | Yes | Yes | No | No | No |
| 7 | Esophagus | 15.0 | Third | Homogeneoushypoechoic | Yes | Yes | Yes | No | No | Yes |
| 8 | Esophagus | 9.0 | Third | Heterogeneous | Yes | Yes | Yes | No | Yes | Yes |
Diagnostic ability
An EUS-guided needle puncture was done in all 8 cases. Adequate samples were obtained from 7 patients (87.5 %), in 6 (75 %) for cytology and in 4 (50 %) for histologic examination with IHC staining (Table 1). The sample of 1 patient (12.5 %) was judged as inadequate for both cytology and histology. In the 4 patients whose EUS-FNA specimens were adequate for histology, a definitive final diagnosis was achieved through IHC examination (GIST in 2, leiomyoma in 2). In 3 patients, the samples were adequate for cytologic examination but not for histology (GIST in 1, leiomyoma in 1, and glomus tumor in 1). In the patient in whom glomus tumor was suspected by cytology, leiomyoma was eventually diagnosed in the surgical specimen. In the 1 patient, in whom EUS-FNA failed to provide adequate samples, a final diagnosis was not obtained.
Discussion
The GI SELs include a diverse array of benign, potentially malignant, and malignant lesions: GIST, leiomyoma, schwannoma, glomus tumor, pancreatic rests, and others. When an endoscopist encounters such a lesion, important decisions related to its clinical significance must be made based on the probable diagnosis or diagnoses. Recently, every GIST has come to be regarded as potentially malignant. Some have suggested that all GISTs with or without metastasis must be resected 4. Miettinen et al. 1 reported that patients with gastric GISTs smaller than 20 mm in diameter and with no metastasis can achieve a 100 % cure rate after complete surgical resection. Therefore, early diagnosis for surgical resection while the tumor is still small is extremely important to improve the prognosis of this disease.
EUS is necessary for the diagnosis and preoperative staging of SELs because it can characterize a lesion by providing information related to its origin, size, borders, and echogenicity. Although EUS is extremely useful for an imaging diagnosis, its capability is limited for determining the final diagnosis in small subepithelial masses that are suspected to be GISTs. Particularly, it is difficult to distinguish a GIST from leiomyoma and schwannoma with only EUS imaging. Immunostaining is fundamentally necessary for a definitive diagnosis of GIST. Therefore, EUS-FNA is necessary for very small SELs if technically feasible.
EUS-FNA is widely recognized as an important modality for diagnosing SELs without surgery 5. Akahoshi et al. 6 demonstrated that the diagnostic rates for GISTs smaller than 20 mm, 20 to 40 mm, and 40 mm or larger were, respectively, 71 %, 86 %, and 100 %. In the present study, although EUS-FNA was conducted easily in large SELs, it was difficult to conduct in small SELs.
Part of the problem with small SELs in the stomach is that the lesions are mobile and, along with the lax gastric wall, difficult to puncture with a needle. Even after a successful puncture, it is difficult to move the needle back and forth within a small lesion for optimal sampling because frequently the lesion moves with the needle (i. e., the needle stays in a constant position within the lesion). Therefore, to diagnose small SELs definitively, we devised the technique described above, performing EUS-FNA for GI SELs approximately 10 mm in diameter by using an FVCLA-ES with a cap device. Regarding the FVCLA-ES, it is easy to transfer force to an object during EUS-FNA because the working channel in alignment with the endoscope shaft will facilitate the performance of EUS-FNA, in comparison with the oblique-viewing and curved linear-array echoendoscope 2.
Recently, Larghi et al. 7 reported the usefulness of the FVCLA-ES for the EUS-FNA of SELs. They performed EUS-FNA with a 19-gauge needle in lesions that had a mean (SD) size of 30.8 mm (18.1) and demonstrated that full histologic assessment, including IHC staining, could be completed in 93.4 % of cases. However, one 10-mm gastric SEL could not be placed in the target position after insertion of the biopsy needle into the working channel of the scope. Even with the FVCLA-ES, the puncture of small SELs is difficult because the gastric wall bends and the SEL moves during puncture (Fig. 3 a). Consequently, EUS-FNA in which an FVCLA-ES with an attached cap device is used to fix the SEL in a cap by absorption can be performed easily without an object escaping (Fig. 3 b).
In this study, we were able to scan and fix lesions, then perform EUS-FNA in all patients with small SELs by using an FVCLA-ES with an attached cap device. Consequently, we were able to obtain histopathologic samples from 7 patients (87.5 %). It is noteworthy that this method facilitated the puncture of small lesions located at the fornix/cardia, which are difficult to access with a conventional oblique-viewing and curved linear-array echoendoscope. Furthermore, although puncture in a longitudinal plane with the endoscope is regarded as contributing to the difficulty of using EUS-FNA for esophageal SELs, we were able to perform EUS-FNA with fixation by cap suction in these circumstances. A previous report 8 stated that the rates of successful EUS visualization and FNA of small GI SELs achieved by using an FVCLA-ES without a cap device were insufficient. Based on the previous discussion, the FVCLA-ES with a cap device is regarded as useful for EUS, including intervention in patients with small SELs.
In conclusion, EUS-FNA in which an FVCLA-ES with an attached cap device was used for small SELs approximately 10 mm in diameter was feasible and safe, with a high sampling rate. We believe that this technique will contribute to histologic diagnosis with immunostaining, even for small SELs. As a next step, the development of a new needle device (e. g., a biopsy cup) will be necessary to obtain samples for histologic testing.
Footnotes
Competing interests: None
References
- 1.Miettinen M, Sobin L H, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005;29:52–68. doi: 10.1097/01.pas.0000146010.92933.de. [DOI] [PubMed] [Google Scholar]
- 2.Irisawa A, Imaizumi H, Hikichi T. et al. Feasibility of interventional endoscopic ultrasound using forward-viewing and curved linear-array echoendoscope: a literature review. Dig Endosc. 2010;22:128–S131. doi: 10.1111/j.1443-1661.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 3.Iwashita T, Nakai Y, Lee J G. et al. Newly developed, forward-viewing echoendoscope: a comparative pilot study to the standard echoendoscope in the imaging of abdominal organs and feasibility of endoscopic ultrasound-guided interventions. J Gastroenterol Hepatol. 2012;27:362–367. doi: 10.1111/j.1440-1746.2011.06923.x. [DOI] [PubMed] [Google Scholar]
- 4.Casali P G, Jost L, Reichardt P. et al. Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20:64–67. doi: 10.1093/annonc/mdp131. [DOI] [PubMed] [Google Scholar]
- 5.Ando N, Goto H, Niwa Y. et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 6.Akahoshi K, Sumida Y, Matsui N. et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007;13:2077–2082. doi: 10.3748/wjg.v13.i14.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larghi A, Fuccio L, Chiarello G. et al. Fine-needle tissue acquisition from subepithelial lesions using a forward-viewing linear echoendoscope. Endoscopy. 2014;46:39–45. doi: 10.1055/s-0033-1344895. [DOI] [PubMed] [Google Scholar]
- 8.David D I, Amit J S, Viet-Nhan N H. et al. Use of a forward-viewing echoendoscope for evaluation of GI submucosal lesions. Gastrointest Endosc. 2012;75:428–431. doi: 10.1016/j.gie.2011.09.041. [DOI] [PubMed] [Google Scholar]


