Abstract
Biomedical imaging techniques can provide a vast amount of anatomical information, enabling diagnosis and the monitoring of disease and treatment profile. MRI uniquely offers convenient, non-invasive, high resolution tomographic imaging. A considerable amount of effort has been invested, across several decades, in the design of non toxic paramagnetic contrast agents capable of enhancing positive MRI signal contrast. Recently, focus has shifted towards the development of agents capable of specifically reporting on their local biochemical environment, where a switch in image contrast is triggered by a specific stimulus/biochemical variable. Such an ability would not only strengthen diagnosis but also provide unique disease-specific biochemical insight. This feature article focuses on recent progress in the development of MRI contrast switching with molecular, macromolecular and nanoparticle-based agents.
1. Introduction
There exist a wide variety of spatially resolved clinical imaging modalities, including positron emission tomography (PET), computed tomography (CT), ultrasound, optical imaging and magnetic resonance imaging (MRI).1 Of these, MRI stands out through its combination of convenient non-invasive application, high spatial resolution, and tomographic capability. This modality can provide images of the anatomy and physiology of living subjects by rapidly mapping out the spatial distribution of the proton (1H) signal intensity. Originally termed nuclear magnetic resonance imaging upon its discovery in the 1940s (renamed MRI in the 1970s due to deemed negative connotations associated with the term ‘nuclear’), this imaging technique works by exploiting the phenomenon of nuclear magnetic resonance (NMR) and, specifically, the reaction to a strong external magnetic field (B0) of magnetic atomic nuclei, which absorb and re-emit electromagnetic waves at a characteristic radio frequency (RF). In a static magnetic field, nuclei process at a (Larmor) frequency (ω0), which is linearly dependent on B0 and the gyromagnetic ratio of the nucleus γ, according to eqn (1).
| (1) |
The application of a RF pulse causes the net magnetisation vector associated with these processing and thermally equilibrated nuclei to flip from a position parallel to the external field to one transverse. The process of their relaxation back to the equilibrium state can occur by two different mechanisms, namely those which are longitudinal (or spin–lattice, T1) or transverse (or spin–spin, T2) in nature. An MR imaging system exploits the current generated by the motion of these relaxing magnetic moments by constructing a time domain NMR signal and using a Fourier transform to generate a frequency domain spectrum from which relaxation times may be derived. Images can be generated from these signals in different ways, most commonly by monitoring nuclear relaxation after a series of spaced RF pulses, which can then be spatially resolved electronically. Subsequently acquired image contrast in anatomical models is generated, in the first instance, from variance in water content across different body tissues. The inherently low sensitivity of MRI (arising primarily from the small energetic differential associated with nuclear Zeeman splitting) generally requires that this contrast be boosted through the use of added contrast agents, if it is to be diagnostically useful. Contrast agents work to enhance MR contrast by locally reducing T1 and T2 relaxation times.2-5 Those that predominantly reduce T1 are referred to as “positive” contrast agents and result in increases in signal intensity (bright contrast), whereas those which primarily affect T2 are commonly known as “negative”, providing reductions in signal intensity (dark contrast). Clinically, T1 agents offer higher spatial resolution and are not associated with false signal reading due to the existence of other signal draining sources in tissues that can plague T2 modalities. The most common T1 contrast agents currently used are paramagnetic gadolinium ion complexes (Gd3+), due to their seven unpaired electrons, large magnetic moment and long electronic relaxation time (9–10 s), which contribute to enhanced relaxation according to the parameters set out in the Solomon, Bloembergen and Morgan (SBM) theory (vide infra).6,7 Free Gd3+, however, is toxic, disrupting physiological Ca2+ signalling;8,9 kinetically robust chelation of Gd3+ with ligands such as tetraazacyclododecane-tetraacetic acid (DOTA) is, thus, commonly employed.10,11 Such agents make up the majority of those in current clinical use, including gadopentetate dimeglumine (Magnevist), gadoterate meglumine (Dotarem), gadoteridol (ProHance) and gadodiamide (Omniscan).12,13 This article will focus on progress made in further engineering such Gd binding scaffolds so as to engender high image contrast with additional responsiveness to chemical or biological stimuli of physiological relevance.
2. Molecular contrast agents (CAs)
In order to enhance image contrast obtained from molecular T1 agents (such as those listed above), optimisation of the parameters that govern relaxivity have been investigated in detail for several decades, most commonly with concurrent reference to the SBM theory.3,14,15 Indeed, relaxivity (r1), defined by eqn (2), describing the change in relaxation rate (Δ(1/T1) = ΔR1) of water protons in the presence of a specified concentration of contrast agent ([CA]), is dependent on external field, temperature, the electronic properties of the paramagnetic centre, water residence time (τm), rotational correlation time (τR), first and second coordination sphere hydration (q), and the ion to water proton distance.3 In general, enhanced relaxivity can be achieved by increasing the q value, shortening τm and increasing τR.
| (2) |
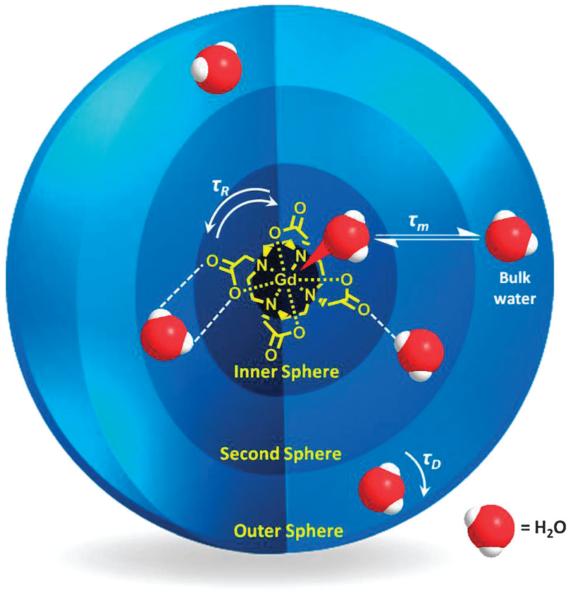
T1 relaxation originates, in part, from dipolar interactions between the imaged water protons and local paramagnetic species. The former may be inner-sphere (IS, those directly coordinated to the Gd3+ centre), second-sphere (SS, those hydrating the complex) or outer-sphere (OS, those diffusing near the chelate, governed by translational diffusion, τD), as depicted in Fig. 1, with their relaxation rates contributing to the overall relaxation rate according to eqn (3).
Fig. 1.
Schematic representation of inner, second and outer sphere water interaction with a typical T1 contrast agent, a Gd–DOTA chelate.
Inner-sphere contributions are thought to be the most important in the relaxation of molecular paramagnetic species (eqn (4), where T1m is the longitudinal water proton relaxation time)16 and have hence dominated investigations where chelating ligand structure has been tuned to facilitate relaxation enhancement,3,6 or, more recently, report on immediate microenvironment.3,17,18
| (3) |
| (4) |
2.1 Responsive molecular contrast agents
In living organisms, variations in tissue and cellular microenvironment can provide vital information about the status of healthy or diseased tissues, organs and tumours. A specific sensitivity of MR image contrast to a physiological or biochemical reaction in tissue is the main focus of the emerging discipline of functional MRI.19 The most well-known example of this is blood-oxygen-level-dependent (BOLD) contrast, which depicts differences in blood oxygenation related to neural activity.20 This technique provides MR contrast change through the imaging of haemoglobin (Hb) and the extreme sensitivity of this to oxygenation (an accompanying transformation of Hb from paramagnetic to diamagnetic).
The most common method of facilitating an MRI contrast response to environment with lanthanide macrocycles is through variations in hydration state (q value), often facilitated by conformation change.16,21 The second-sphere water molecule dynamics of a chelate can also be manipulated by an environmental trigger, providing variation in MRI signal contrast.22-25 Responsive conformation changes can alternatively result in a change in contrast agent molecular volume, affecting τR and hence T1m.26 Increases in the molecular weight of a contrast agent, through cross-linking or polymerisation, can also be prompted by an environmental stimulus, resulting in image contrast enhancement through the reduction of molecular tumbling rates (increasing τR). It is worth noting here that, in the design of agents for conventional MRI, permanent enhancement of signal contrast is the desired goal; in functional MRI, specific change in response to a physiological trigger (and the degree and specificity of relaxivity change) is more important than the absolute magnitude.
2.2 Bio-responsive molecular contrast agents
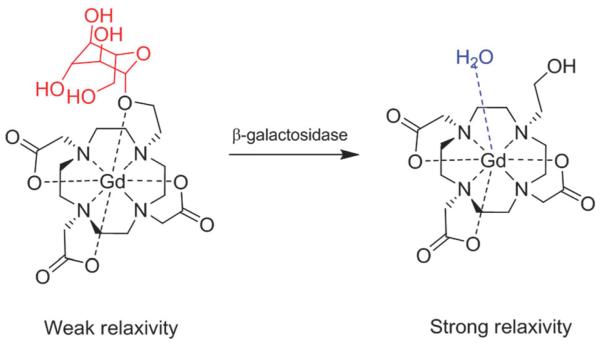
Potentially the most important developing class of responsive MRI is that based on agents that are acted on by pathologically relevant enzymes such as those associated with disease states including stroke, cerebral ischemia, cardiovascular or neurodegenerative inflammatory processes.27 Pioneering work by Meade and co-workers in the late 1990s, for example, paved the way towards the development of responsive or ‘smart’ MRI contrast agents specifically designed to respond to an enzymatic cleavage.28 Their work describes a family of 4,7,10-tri(acetic acid)-1-(2-β-galactopyranosylethoxy)-1,4,7,10-tetraazacyclo-dodecane gadolinium (EGad) contrast agents, whose galactopyranose groups are removed by β-galactosidase (an important reporter marker for monitoring gene expression), resulting in an irreversible transition from a weak to a strong relaxivity state. A 20% change in relaxation rate is observed through the removal of a H2O blocking group, increasing q and, thus, improving inner sphere T1 relaxation (Fig. 2). Since then, several similar approaches have been published describing the modulation of MRI contrast through the control of water access to a chelated paramagnetic centre.29-31 These have achieved 3-fold increases in relaxation rate upon enzymatic cleavage of the hydration-hindering group.29 An alternative approach has been offered by Giardiello et al., who prepared a neutral complex that binds with high affinity to H2O-blocking HCO3− anions and displays low relaxivity. The action of a specific enzyme (porcine liver esterase) on the modified side arms of this DOTA derivative introduces new anionic charge, repels the chelating HCO3− ions increases metal hydration, and triggers a ~90% increase in signal contrast.32 Reliance on inner sphere hydration as the mechanism of MRI contrast modulation, however, is not ideal. Anion interactions with the cleaved (more solvated/accessible) paramagnetic species, has, in particular, been identified to be a considerable interfering factor, particularly in vivo, where water-competing anions are abundant.30 Such interactions can have a significant detrimental effect on resulting relaxivity and any assumptions therein; alternative mechanisms to achieve MRI activation have, therefore, been investigated.
Fig. 2.
Schematic representation of Egad MRI contrast agent; galactopyranose groups (red) are removed via β-galactosidase cleavage, resulting in an irreversible transition from a weak to a strong relaxivity state, reproduced with permission from ref. 28. Copyright 1997 Wiley-VCH Verlag GmbH & Co. KGaA.
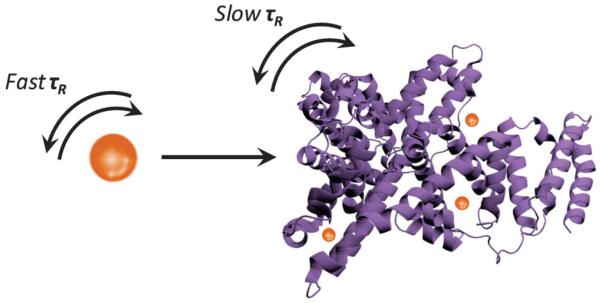
One such mechanism is the so-called receptor-induced magnetisation effect (RIME), which utilises binding of a target moiety, such as a protein, to a paramagnetic chelate, resulting in the formation of a bulky, slow-tumbling macromolecular contrast agent with increased τR (and thus relaxation/contrast relative to background; Fig. 3).33 In this vein, Breckwoldt et al. have employed a bis-5-hydroxytryptamide-diethylenetriamine-pentatacetate gadolinium species, which, in the presence of the enzyme myeloperoxidase (MPO, a key enzyme secreted in the inflammatory response to tissue injury), oxidises and radicalises, leading to cross-linking, polymerisation and subsequent protein binding.27 Successful triggering of relaxation enhancement was non-invasively monitored in vivo, allowing tracking of MPO activity in stroke-affected mice models. A Gd3+-diethylenetriaminepentaacetic acid (DTPA) type construct with a phosphonate side-chain, termed MS-325 by Caravan et al., which targets human serum albumin (HSA), again employs the RIME strategy to provide selective vascular MRI enhancement. In this case, HSA binding limits extravasation of the free chelate from the blood pool into the non-vascular space, slowing renal excretion and contributing to an extended blood half-life and also thus providing vascular-specific relaxation rate enhancement. A 9-fold increase in relaxivity (at 20 MHz) was observed upon non-covalent HSA binding in ex situ studies, due to slowing molecular rotation (τR) of the HSA-bound MS-325 entity.33 A similar strategy employs a Gd3+-chelate functionalised with a trilysine masking group with associated poor native HSA affinity (and hence low relaxivity, r1 = 9.8 mM−1 s−1 at 20 MHz, 37 °C). Upon cleavage of the lysine residues by human carboxy peptidase B, a thrombin-activatable fibrinolysis inhibitor (TAFI, an important enzyme in thrombotic disease), the chelate–HSA affinity increases substantially due to the exposure of aryl groups with high HSA binding affinity, a transition with an associated 170% relaxivity enhancement (to 26.5 mM−1 s−1 at 20 MHz, 37 °C).34 Sherry and co-workers have designed a Gd–DO3A-peptide-based CA that is τR activated upon binding to a specific target protein in a similar vein.35 The relaxivity of this CA (r1 = 8.3 ± 0.2 mM−1 s−1 at 20 MHz) increases substantially upon addition of the target protein and phantom T1-weighted imaging demonstrates a 10-fold improvement in image intensity for the chelate in the presence of the binding protein. More recently, Gd3+–DO3A ligands bearing a pendant diphenylphosphinamide arm have been shown to possess a high affinity for HSA, boosting r1 by 54–119% due to increases in τR.36 The degree of relaxivity switching, however, was adversely affected by the displacement of inner sphere water molecules by carboxylate residues of the protein, an inherent problem often observed in the presence of oxygen-based chelates.
Fig. 3.
Illustration of the generic association between a Gd3+ chelate (brown sphere) and a protein target (purple) resulting in lengthened τR and improved MRI contrast.
The vast array of diagnostically potent functional enzymes and proteins present physiologically makes this class of bioresponsive CAs arguably the most important in detecting and monitoring disease pathology. The examples of bio-responsive switchable CAs referred to herein represent some of the most effective (in terms of the degree of switching) of this class so far available and exploit robust change in q or τR. It should be highlighted, however, that the majority of these cases present irreversible changes in contrast, with a ‘one-off’ trigger facilitating the contrast enhancement or reduction. Further work will be required to generate derived CAs capable of long-term use or disease treatment profiling.
2.3 Cation responsive molecular contrast agents
Another important class of biologically activatable CAs are those which can be triggered by the presence of metal ions. Metal ions are vital in a variety of physiological pathways. Ca2+ ions for example, play an important role in neural signalling and changes in brain activity can lead to variations in its concentration. Similarly, increased Zn2+ ion concentrations have been implicated in environments commonly associated with Alzheimer’s disease.37 Cation responsive contrast would therefore undoubtedly be diagnostically potent.38 In the design of such, signal changes are once again most commonly effected through variance in hydration number of paramagnetic CA complexes. There have been several examples exploiting this in recent literature, most of which employ conformation changes and associated perturbations in inner-sphere water access and q. The first reports of a Zn2+-specific CA reporter were by Hanaoka et al., who described DTPA–bisamide chelators which respond sensitively and selectively to Zn2+ through the displacement of inner sphere water arising from a Zn2+ binding induced geometrical reconfiguration.39,40 Such systems observed a ~33% decrease in relaxivity due to the inhibition of water access to the Gd3+-chelator upon cation binding. Gadolinium complexes with bis-15-crown-5 ether or β-diketone recognition sites have also been prepared to enable the detection of K+, Mg2+ or Ca2+ ions, with MRI signal contrast again decreasing due to a geometrical rearrangement in the presence of the metal ion, and associated change in the second sphere of paramagnet hydration.41 Within this study, the most efficient MRI response was observed for β-diketone tethered Gd–DTPA species, whose r1 relaxivity (4.98 mM−1 s−1 at 20 MHz) decreased 20.7% (to 3.95 mM−1 s−1) in the presence of Mg2+ ions.
These modulations, based on decreases or ‘turning-off’ of image contrast, are less desirable than systems which result in image brightening through specifically triggered relaxivity increase. Groups such as that of De Leon-Rodriguez and co-workers have prepared Gd3+–DOTA chelates appended with N,N-bis-(2-pyridylmethyl) ethylene diamine (bisBPEN) diamide functionalities which are capable of binding Zn2+. Such species successfully exhibit a modest ion-specific increase in r1 relaxivity of 20% (from 5 to 6 mM−1 s−1 at 23 MHz) with the introduction of 2 equivalents of Zn2+ ions (with similar changes observed in the presence of Cu2+ ions).42 These changes were attributed to an increase in water exchange rate upon ion binding, or alternatively, the creation of a more organised second sphere of water molecules (bound to the Zn2+ or Cu2+ ions) in close proximity to the single Gd3+-bound water molecules of the complex. This group has recently improved upon this by using a Gd–DOTA derivative containing two bis-(3-pyrazolyl) units, yielding a 64% relaxivity enhancement with introduction of Zn2+ ions, also demonstrating successful in vivo application.43 Interestingly, these groups also describe significant further r1 relaxation enhancement of the Zn2+-coordinated complex upon binding to human serum albumin (HSA), providing a 165% increase (from 6.6 to 17.4 mM−1 s−1), a rotational correlation time (τR) effect only occurring in the presence of the metal ions.42 Major et al. have demonstrated that the design of the chelate species can play a vitally important role in relaxivity modulation by specific cations.44,45 Their asymmetric chelates display an acetate pendant arm capable of switching its coordination to either the paramagnetic centre of the contrast agent or a cation, such as Zn2+. Coordination to the former centre generates a coordinatively saturated chelate, with q = 0 and hence low MRI contrast. Coordination to a Zn2+ ion causes a change in the molecular geometry and the Gd3+ coordination sphere, leading to an increase in hydration number to q = 1 (Fig. 4), and 121% increase in r1 relaxivity (from r1 = 2.3 to 5.1 mM−1 s−1 at 60 MHz). Subsequent in vitro studies demonstrated a qualitative increase in T1-weighted image contrast of the agent in the presence of physiologically relevant concentrations of Zn2+.44
Fig. 4.
A proposed mechanism of MRI relaxivity modulation based on hydration (q) alterations due to pendant acetate coordination in the presence of Zn2+ ions, adapted with permission from ref. 45. Copyright 2008 American Chemical Society.
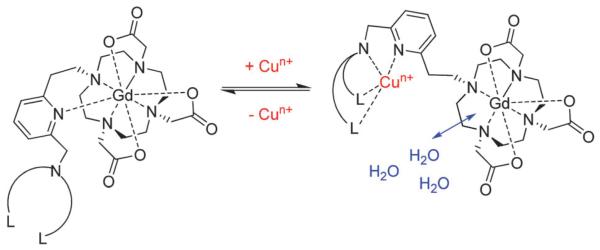
Specificity amongst metal ions can, of course, be an important factor for consideration in the design of metal ion-modulating MRI contrast. Gd-chelates have been designed to recognise various different metal ions, including Cu2+, Ca2+, Mg2+ and K+ with selectivities generally utilising Irving-Williams governed trends in affinity as well as coordination environment preferences.46 Ethylene glycol tetraacetic acid (EGTA, a highly selective Ca2+ chelator) has, for example, been bound to two macrocyclic Gd3+-containing moieties, with Ca2+ ion binding causing increases in the inner-sphere hydration number of the chelate and concomitant modest associated protic longitudinal relaxivity increases (32% from r1 = 5.4 to 7.1 mM−1 s−1 at 500 MHz).46 Similar constructs exploiting q to effect a contrast change have shown 83% increases in relaxivity in the presence of Ca2+ (from r1 = 3.4 to 6.3 mM−1 s−1 at 500 MHz); at physiological concentrations, such as those in the relevant range for Ca2+ modulation in the brain (0.8–1.2 mM), relaxivity changes of ~10% are observed.47 Similarly, DOPTA–Gd complexes structurally modulate inner-sphere water access to the chelated Gd3+ ion using iminoacetate arms, which shield the paramagnetic centres from water in the absence of Ca2+ ions (q = 0).48 Upon binding of Ca2+, the complex undergoes a reorganisation leading to an increase in q and relaxivity by 80% to 5.8 mM−1 s−1 (at 500 MHz) over Ca2+ concentrations ranging 0.1–10 μM. Cu+/Cu2+ ion selectivity has been achieved by Que et al. through the design of Gd–DO3A chelates coupled to acetate or thioether-rich receptor ligands, which rely upon modest q modulation through Cu2+ binding (eliciting a 40% change in relaxivity).49,50 Similarly, Pope and co-workers use a bis-macrocylic ligand which recognises Hg2+ to generate a 24% increase in relaxivity.51 The highest increase in longitudinal MRI relaxation exploiting a hydration change mechanism has been observed for thioether-tethered DO3A chelates, demonstrating a 360% increase in r1 upon binding 1 equivalent of Cu2+ (from 1.5 to 6.9 mM−1 s−1 at 20 MHz).50 The initially low r1 observed in the absence of metal ions in this work is suggestive of a q = 0, with the acetate or pyridine moieties present on the linker initially capping inner-sphere water access (a cap that is removed on the association of Cu2+ ions; Fig. 5). A polyarginine modified version of this chelate has demonstrated some promise in initial intracellular work.52 Recently, several others have reported a variety of Gd–DOTA-based chelates with pendant arms capable of cation binding, providing relaxivity responses due to q modulation.53-55
Fig. 5.
Representation of MRI contrast agent species with Cu+/2+ selectivity, where L is a ligand, such as a thioether-based donor, n = 1, 2; adapted with permission from ref. 50. Copyright 2009 American Chemical Society.
Modulation of MRI contrast based on metal ion recognition by a CA can also be achieved through exploitation of rotational correlation time. Peters and co-workers have investigated bisphosphonate appended coordination oligomers of a DOTA-like chelator as a means of generating cation triggered changes in rotational correlation time.56 This resulted in 200–500% increases in r1 (depending on Zn2+ concentration), although it was noted that selectivity would be poor under physiological conditions. A 250% relaxivity enhancement of Gd–DOTA–diBPEN in response to Zn2+ ions has recently been translated reasonably well to both ex vivo and in vivo studies, an effect which relies upon binding of the Zn2+-bound-chelate species to HSA and resulting changes in τR.57 Several groups have investigated heterometallic complexes featuring Gd-chelates which self-assemble to form bulky macromolecules upon coordination to iron ions.58-61 Comblin et al., for example, have designed a Gd(phen)HDO3A chelate (r1 = 3.7 mM−1 s−1 at 20 MHz) which uses its phenanthroline-like unit to complex Fe2+ ions, forming a tris-complex with very high molecular weight, with an associated relaxivity increase to 12.2 mM−1 s−1.11 Toth and co-workers have developed a metallostar structure comprising six densely packed Gd3+–diethylenetriaminetetraacetic acid (DTTA) chelates around an Fe2+ ion, resulting in an almost 2-fold increase in relaxivity (from 12.4 to 20.2 mM−1 s−1 at 20 MHz).59 The formation of slowly rotating macromolecular species with increased τR is responsible for the observed enhancements throughout all these works. An alternative method for metal ion detection employing relaxivity responses which has been explored by Muller et al. describes the transmetallation of a DTPA-derived chelate (MS-325) which releases Gd3+ into solution in the presence of Zn2+ ions, resulting in insoluble Gd–phosphate complexes which precipitate and hence no longer contribute to the observed 1H r1 relaxivity.62 This process leads to a 25% reduction in r1 after 5000 min. The timescale and associated toxicity risk of this method, however, is unlikely to make it practically relevant.
There have been, then, a number of reported approaches to tune r1 relaxation through association with physiologically relevant levels of cations. In nearly all cases this has been through the modulation of water access or rotational correlation time and accompanied by varying degrees of relaxivity switching (20–360%). Bar some obvious exceptions, performance is both modest and detrimentally affected in attempts to extrapolate to physiological in vivo conditions. Though much work clearly remains to be done, particularly in safeguarding specificity, these prior reports show promise and are likely to underpin future developments.
2.4 pH-responsive molecular contrast agents
MRI contrast agents capable of detecting variations in environmental pH can be of particular use for the non-invasive detection of disease or metabolic disorder.63 Ischemia, for example, is often defined by low pH (caused by amide exchange due to regional neural ischemia, for instance) and can characterise heart disease. Similarly, regions of acidity can indicate the presence of hypoxia, tumour growth and metastases (malignant tumours present with pHs ranging from 6.8–7.2).64 pH reporters can hence provide important information which can impact directly upon selected treatment (e.g. hypoxic tumour cells are resistant to radiation and to many anticancer drugs65) and a monitoring of their efficacy. It is unsurprising then that a range of paramagnetic agents have been developed in which hydration state, leading to either increases or decreases in signal, is highly pH dependent.
Pagliarin and co-workers have, for example, described a series of Ln3+ macrocyclic complexes in which the presence of a β-arylsulfonamide group on the chelate species supports pH dependent relaxivity (as well as luminescence).16 These systems are based on protonation of the sulphonamide nitrogen and the associated increase from q = 0 to q = 1 (with a 48% increase in r1 relaxivity values over the pH range 7.4–6.8 and concomitant decrease in photoluminescent emissivity due to water based quenching). Hall et al. have synthesised terpyridine-based Gd3+-chelates which demonstrate decreasing relaxivities with increasing pH (from r1 = 12.8 mM−1 s−1 at pH 6 to r1 = 2 mM−1 s−1 at pH 11, at 20 MHz), attributed to an overall decrease in q from 3 to 0, due to the successive deprotonation of water molecules and subsequent formation of dimeric complexes possessing no bound water molecules.66
An alternative approach to pH modulation of MRI is to exploit proton exchange between inner sphere coordinated water molecules and bulk water due to highly acidic/basic environments. This approach has been employed by Aime et al., who have described a range of C4-symmetric Gd3+–DOTA-type chelates with different pendent arms demonstrating largely invariant relaxivity between pH 2–8 (where r1 ≈ 2.5 mM−1 s−1 at 20 MHz, due to only outer-sphere contributions), but marked increases in r1 at very high (>10; r1 ≈ 5.7 mM−1 s−1 at 20 MHz) and very low (<2; r1 ≈ 5 mM−1 s−1 at 20 MHz) pH environments.22,23 The observed increases in relaxation behaviour at these two extremes are attributed to the formation of a well-defined second hydration sphere, where water molecules are in rapid exchange. In strongly basic media this is ascribed to deprotonation of bound water molecules or proximate ligand amide NH protons. In acidic media these beneficial changes are ascribed to protic-catalysed dissociation of the ion-paired water–Gd-complexes, promoting water exchange. Similar work by Sherry and co-workers has described Gd3+–DOTA complexes with phosphonate pendent arms with strong pH-dependent r1 behaviour, demonstrating two relaxivity troughs, the first having a 40% variation between pH 2–6 and 70% between pH 6–12, with an r1 maximum at pH 6, observations again ascribed to protic exchange between bound water protons and bulk solvent protons contributing to a second hydration sphere possessing rapidly exchanging water molecules.24,25
A major limitation inherent in the field of responsive contrast lies in the lack of ability to quantify the concentration of contrast agent present at the site of interest (meaning, in turn, that relaxivities resulting from any trigger can only be qualitative). There is, then, much interest in the development of ratiometric responsive CAs and most development thus far has been associated with those which respond to local pH. Sherry and co-workers have, for example, investigated such a system (employing a mixture of pH-insensitive GdDOTP5− and pH sensitive GdDOTA-4AmP5− chelates with necessary assumptions about identical biodistribution) and demonstrated its efficacy both in vitro and in vivo.17,67 An alternative approach is to measure the ratio between the transverse and longitudinal paramagnetic relaxation rates of water protons in response to a single contrast agent (R2/R1), as described by Terreno and co-workers.68 This approach, based on a Gd3+-complex having τm or rotational mobility dependent on pH, allows an assessment which is independent of contrast agent concentration. Bimodal agents employing a second imaging technique using a dual-functional probe can also provide quantification of local contrast agent concentration and hence relaxivity,69 an approach adopted in work by Frullano et al., which saw the production of a dual MRI-PET agent composed of the pH-dependent GdDOTA-4AMP chelate appended with a18/19F functionality.70 A linear relationship between the PET and MRI signals allowed determination of the concentration of the agent through comparison with a pH calibration curve, providing a quantitatively accurate non-invasive probe of great promise for in vivo application.
To summarise thus far, the most common and controllable approach to effect relaxation change by pH modulation is through exploitation of the hydration state (q) of molecular contrast agents. In this way, enhancement or reduction of signal contrast can be attained, with modest (up to 70%) changes over relevant physiological pH ranges (pH 6–8).24 The use of protic exchange and second hydration sphere dynamics can provide an alternative pH responsive route to MRI contrast modulation, although the most significant responses occur at very high (>10) or very low (<2) pH regions, making such responses less physiologically relevant. To allow unambiguous assessment of MRI contrast modulation, the contrast agent concentration must be accurately known, a particularly difficult prospect in site-targeting and in vivo applications. Responsive ratiometric contrast agents offer great potential in this area and some initial investigations have demonstrated promise.
2.5 Redox responsive molecular contrast agents
Redox reactions are widespread in biochemical systems, with organisms integrating them directly within fundamental methods of energy generation. A change in oxidising/reducing condition can result from variations in blood flow, oxygenation and other variables and can have a profound effect on physiological function, such as those in the brain accompanying stroke, Parkinson’s and Alzheimer’s diseases.71 Very often such physiological stress is associated with increased reactive oxygen species (ROS) and decreased antioxidant levels.72,73 One can seek to directly acquire tomographic mapping of such conditions through the use of paramagnetic agents which are, in some capacity, redox active.
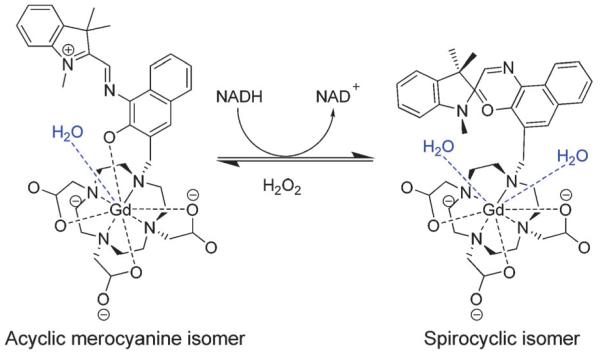
One non-metallic class of such are the nitroxides, which undergo reduction to diamagnetic hydroxylamines, resulting in a marked and reversible contrast change.71,73 These aside, T1-weighted contrast agents demonstrating redox triggered contrast switching are rare.74-76 Louie and co-workers have described the only Gd3+-based T1-contrast agents capable of redox sensing using a Gd3+–DOTA system featuring a spiropyran/merocyanine motif, demonstrating 26% relaxivity decreases upon redox activation (from 2.5 to 1.9 mM−1 s−1 at 60 MHz), caused by decreasing hydration number (from q = 1.16 to 0.44) as a result of a conformation change.74,75 An 8-coordinate chelate complex tethered with acyclic merocyanine converts to its spirocyclic isomer, a 7-coordinate chelate, following reduction using nicotinamide adenine dinucleotide (NADH). This structural change increases the hydration number of the complex (from q = 1 to q = 2), resulting in a 54% increase in relaxivity; a change which is reversible upon treatment with hydrogen peroxide (Fig. 6).74
Fig. 6.
Redox-sensitive structural isomerisation of spiropyran–merocyaninetethered GdDOTA, adapted with permission from ref. 74. Copyright 2009 Wiley-VCH Verlag GmbH & Co. KGaA.
A slightly different mechanistic approach has also been explored towards the development of redox switchable CAs sensitive to environmental O2 partial pressure (pO2, which is relevant in various pathological diseases, including stroke and tumours), exploiting variations in the chelate metal centre oxidation state, similar to the BOLD method of activated detection earlier described.77 In this vein, Terreno and co-workers have investigated manganese (Mn2+/3+) porphyrin complexes which were encapsulated into cyclodextrin (CD) hosts.78 Oxidation of Mn2+ complexes to Mn3+ by O2 was associated with ~50% decreases in MR signal intensity (r1 = 5 mM−1 s−1 to 2.5 mM−1 s−1 at 20 MHz); changes attributed to a combination of electron spin density delocalisation and changes in the number of labile protons at the metal centre.78-80 This system allows quantification of pO2 (0–40 Torr) in the region of interest. Some Eu2+ analogues have also been investigated as potential redox-responsive probes, due to their isoelectronic relationship with Gd3+. Burai et al., for example, have investigated chelates based on cryptates, which, although not experimentally verified, have potential for strong redox triggered MRI contrast switching capabilities upon oxidation of Eu2+ to Eu3+.77,81
Though there exist, then, a number of elegant examples of redox responsive MR contrast which have been applied in vitro, it is clear that much more work needs to be done before the realisation of reliable and marked switching of signal contrast in vivo.
2.6 Light responsive molecular contrast agents
Bioluminescence imaging is a useful non-invasive modality providing potentially excellent signal-to-noise, spatial refinement and high throughput. Although poor tissue penetration makes it a less tractable means of switching contrast than other methods noted thus far, light emitting gene markers, such as those based on lucerase–luciferin could potentially provide an appropriate application for photoactivatable agents.21,82 To this end, a small number of articles have hence described the development of light-sensitive MRI contrast agents based on spirobenzopyrans, which undergo an isomeric conformation change upon light irradiation, affecting water coordination (q) at the paramagnetic metal centre.21,75 This transformation (which is reversible) prevents hydration at the metal centre, resulting in a reduction in relaxivity of about 21% (from r1 = 3.7 mM−1 s−1 to 2.9 mM−1 s−1 at 60 MHz).75 Though modest, this work may find application if, for example, larger relaxivity changes can be married with NiR wavelength triggering.
3. Macromolecular and nanoparticle contrast agents
Small molecule CAs have paved the way for enhanced medical imaging (including that which is targeted and/or multimodal), and facilitated most of what we understand in terms of tuning CA behaviour in vitro and in vivo. However, their limitations, in particular rapid elimination, and the quest for signal amplification, have resulted in the increased utilisation of higher molecular weight species offering inherently striking and, in many cases, profoundly advantageous characteristics.13
Relaxivity can be improved by the incorporation of paramagnetic gadolinium centres directly into a nanosized matrix to obtain, for example, GdF3:CeF3 nanoaggregates coated with poly(acrylic acid) chains,83 doped zeolite GdNaY,84 gadofullerenes,85 gadonanotubes,86 GdF3:citrate,87 or PEG–phosphate coated NaGdF4 nanoparticles.88 Though a number of these derivations are associated with high MR contrast, they very often exhibit low kinetic stability and hence potentially high levels of toxicity. A preferred method of increasing signal contrast is by embedding or conjugating numerous clinically approved Gd3+-chelates to macromolecules or nanoparticulates based on polymers,89 dendrimers,90 liposomes,91 micelles,92 proteins,93 virus capsids,94 gold glyconanoparticles,95 or silica.96 These approaches primarily exploit the inherently large particle surface area and size to improve MRI signal. Before looking at some of the characteristics associated with the paramagnetic modification of such species, we briefly review the mechanisms associated with the dipole–dipole longitudinal relaxation mechanism. As outlined earlier (eqn (3) and (4)), inner-sphere contributions generally dominate relaxation enhancement. The longitudinal relaxation rate of the bound water (1/T1m) is given by eqn (5). These expressions confirm that the relaxation behaviour of a complex depends on a number of parameters, including the distance between the unpaired electron spin of Gd3+ and protons of the coordinated H2O (rGdh), the angular proton Larmor frequency (ωI) and the global and local correlation times (τCG and τCL, respectively, defined by eqn (6) and (7)).
| (5) |
| (6) |
| (7) |
where μ0 is the permeability of vacuum (μ0 = 1.257 × 10−6 N A−2), γI is the gyromagnetic constant for protons (γI = 2.675 × 108 T−1 s−1), g is the electronic g-factor (g = 2), μB is the Bohr magneton (μB = 9.274 × 10−24 J T−1), S is the total electron spin of the material ion (S = 7/2 for Gd3+), F2 denotes the order parameter (vide infra) and T1e is the electron spin longitudinal relaxation time.97
As a result of the natively increased steric bulk of nanoparticles or macromolecules, tumbling of the appended molecular paramagnetic probe is, relatively, slowed down; the associated increase in overall rotational correlation time (τR) providing concurrent improvement of relaxivity.98 The length of τR for small molecular weight Gd3+-chelates is in the picosecond range (50–200 ps),99 1–3 orders smaller than τm and T1e, and so τC is dominated by τR.100 On the other hand, τR of their higher molecular weight counterparts is in the nanosecond range (0.5–50 ns), a timescale which is comparable or even longer than τm and T1e. Since eqn (4) predicts a higher relaxivity for a shorter τm value (τm << T1m) and T1m can already be more than one order of magnitude shorter in such systems (eqn (5) and (6)), the relaxivity of nanoparticulate contrast agents is characteristically limited by rather long τm and thus slow kex (kex = 1/τm).97
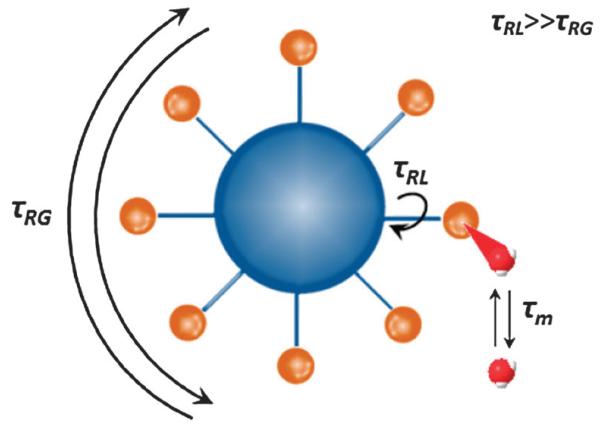
An important consideration in the design of macromolecular or nanoparticulate CAs with high relaxivity is the structure and flexibility of the linking moiety (it is not necessarily the case that the particle structural rigidity and rotational time are directly translated to appended complexes). Fast rotation around the Gd3+-complex linker compared to the motion of the nanoparticle can be a limiting factor in the relaxivity enhancement; if Gd3+-complexes are covalently attached to the surface of the particle through a flexible linker, their local rotational motion (τRL) around the axis of the linker in solution is much faster than the global rotation of the nanoparticles (τRG) (Fig. 7). Using the Lipari–Szabo approach85,86 incorporated into the SBM equations, the relaxation parameters of nanoparticulate MRI probes can be obtained through fitting of experimental nuclear magnetic resonance dispersion (NMRD) profiles.101 The degree of spatial restriction of the motion, i.e., the extent to which τRL and τRG contribute to τR is described by the order parameter F2 (0 ≤ F2 ≥ 1). If τRL is negligible, as in a perfectly rigid system, F2 equals 1. In more conformationally flexible environments, F2 approaches 0, and internal motions are completely independent of global motion.96 These nanoscale effects are summarised in eqn (5), which is valid at B0 > 1.5 T if the global molecular reorientation is isotropic.3,97
Fig. 7.
The relaxivity of a nanoparticulate MRI probe (blue sphere) with conjugated Gd3+-complexes (brown spheres) is determined by the local rotational time of the complex around the linker (τRL), the global rotational motion (τRG), and the coordinated water exchange rate (kex = 1/τm). Adapted with permission from ref. 97. Copyright 2012 Wiley-VCH Verlag GmbH & Co. KGaA.
This theoretical framework enables evaluation of the rigidity and the internal motions of the nanosized system, the influence of the length and flexibility of the contrast agent linker on the relaxation parameters, and the contributions emerging from both the appended molecular MRI probe and from the nanoparticle to the overall relaxation of the composite.102 The number of nanoparticulate-based MRI contrast agents has rapidly increased in recent years, due not only to their capacity to deliver a large number of paramagnetic ions per nanostructure, allowing improvements in relaxivity per unit dose compared to individual Gd3+-chelates, but especially, as noted, due to their ability to boost relaxivity by retarding the chelate tumbling rate.98
The conjugation of Gd3+-chelates to block, graft, or micellar macromolecular carriers generates a class of CA whose relaxivity is dependent on the polymer rigidity.90,103 This, in turn, is chemically tuneable through the overall molecular weight,104 by the incorporation of monomers with high glass transition temperatures,105 by grafting of conjugated polymers possessing a more rigid backbone,106 enhancing the degree of cross-linking,107 lengthening and making side chains hydrophobic,102 or by increasing the number of internal hydrogen bonds (and hence reducing internal motion).90 Improved H2O exchange can be achieved by the incorporation of a hydrophilic shell layer that serves as a reservoir for water molecules.108 On the other hand, grafting polyethyleneglycol (PEG) chains onto hyperbranched polymers and dendrimers is known to slightly decrease relaxivity because of hindered water exchange.103,109 In dendrimers, relaxivity can be maximised with each increase in branching generation, through τR effects. This trend, however, is not exhaustive and has been found to decrease once the G9 generation is reached.13 Macromolecular formulations with an MRI sensitivity that is environmentally responsive in a simple manner can be achieved by selecting monomers with responsive functional groups,110 by gating the water accessibility,111 by conformational conversion between globular and extended form,107 through the incorporation of cleavable linkers,103 and by switching between hydrophobic interaction and electrostatic repulsion,112 amongst others.
Cone-like or truncated cone-like amphiphiles that self-aggregate into micelles or liposomes under specific conditions have also been utilised to improve CA relaxivity.13,97 Memsomes (Fig. 8) are a class of liposomes, where a paramagnetic complex can be attached to the hydrophilic heads of the lipid molecules in the bilayer, allowing easy access to solvent.13 In this case, the attachment of the paramagnetic complex to the membrane is not rigid (τRL << τRG), and so the size of the memsome itself has no effect on relaxivity.113 Instead, the latter is observed to be dependent on the rotational diffusion of the Gd3+-complex on the surface of the liposome,114 as well as on the length of the linker, tuning water accessibility to the lanthanide CA centre.115 On increasing the alkyl chain length from C10 to C18, τRG can increase from 500 to 2800 ps. However, the advantage of high τRG is reduced in all liposomal systems due to the high internal mobility of the Gd3+-complexes (short τRL and low F2), and slow kex.102 The latter is highly dependent on the phospholipid membrane’s water permeability in enosomes, which comprise a group of liposomes that encapsulate water-soluble Gd3+-chelates.97 Enhancement of the permeability concomitant with a shortened τio (i.e. water exchange time between internal, i, and external, o, parts of the liposome)116 can be achieved by increasing the temperature towards the phase transition temperature (TM) that causes phospholipids to undergo a gradual increase in fluidity. In this system, quenched r1 can only be recovered when the liposome composition allows for rapid water exchange between the interior and exterior of the enosomes (τio << T1i). Subsequently, relaxivity of the paramagnetic agent encapsulated within the liposome becomes similar to that in the solution. Higher concentrations of metal chelate encapsulated within the liposome and higher internal viscosity, further lengthens the rotational and diffusional correlation times, contributes to a slightly higher relaxivity compared to non-encapsulated Gd3+-complex.91,116
Fig. 8.
Schematic representation of local and global mobility processes relevant to Gd3+-chelates (brown spheres) in (a) cross-linked polymeric nanoparticles, (b) dendrites, (c) enosomes, and (d) memsomes with τRG and τRL representing global and local rotational correlation times, respectively. Paramagnetic chelates are covalently bound in (a) and (b); for the paramagnetic chelates encapsulated (in (c) and (d)) in the membrane or aqueous phase of a liposome, τio denotes water exchange rate between the interior and exterior and τRi the rotational correlation time of the internalised complex.
Another important class of highly tuneable nanoparticulate CAs consists of inorganic biocompatible Gd3+-chelate decorated mesoporous silica nanoparticles (MSNs) with uniform mesopore compartments and large silanol surface areas.94,117,118 The geometric confinement of the former increases τR of the paramagnetic complex and τD of water molecules (increasing the H2O–lanthanide interaction time) and, through this, relaxivity.85,119 The tuneable surface chemistry can be further exploited to improve the accessibility of water and increase the water-exchange rate, kex. For example, a threefold faster kex was reported after acetylation of surface amine groups otherwise used for conjugation to Gd3+-chelates.120
It is clear, then, that nanostructured and macromolecular materials can provide improved MRI signal contrast due to their behaviour in suspension. Such materials can be further engineered to offer stimuli responsive contrast in a predictable and useful manner. Their characteristics can be tuned through design and synthesis in terms of q,121 τRL,122 τRG,89, τio123 or τm,114 and have the capability to additionally provide specificity to a site of interest, high in vivo mobility, and multimodality as well as multifunctionality. As such, they represent an increasingly important class of MRI contrast agent.
3.1 pH-responsive particulate contrast agents
Nanoparticulate MRI probes can be designed to exhibit significant contrast response to physiological or pathological pH change in the region of interest.124 Most typically, this is through the incorporation of pH-responsive Gd3+-chelates,125 acid labile linkers such as ketals126 or pH-responsive groups into a nanoparticle scaffold,127 whose pH-responsiveness triggers a global response across the particle, such as a change in hydration state (leading to swelling/collapse), propensity to degrade/dissolve, hydrophilic/hydrophobic change, hydrodynamic diameter, conformational change (globular/linear), micellisation, or change in water permeability. These changes can result in marked changes in F2,112 q,128 or τm,68 and thus T1 contrast.
Such structural changes are, of course, markedly more useful if the responsive functional groups possess pKa values around physiological pHs (frequently employed groups which affect the hydration state q as a result of a response to a stimuli, include arylsulfonamide,16 imidazole,124 nitrophenol,129 and phosphonate125). Aime and co-workers have, for example, reported an adamantane derivative of a sulfonamide based Gd3+-chelate that was non-covalently hosted by a macromolecular carrier consisting of 8–10 poly-β-cyclodextrin units. With a pKa of 6.7, the sulfonamide nitrogen deprotonates at basic pH and ligates to the metal centre, generating an “MRI silent” q = 0 state (a reversible change to q = 2 at acidic pH, ‘turning on’ relaxivity contrast). An additional integration into the same cyclodextrin scaffold of an adamantane functionalised 19F-containing reporter can additionally engender ratiometric 1H/19F mapping of pH.128
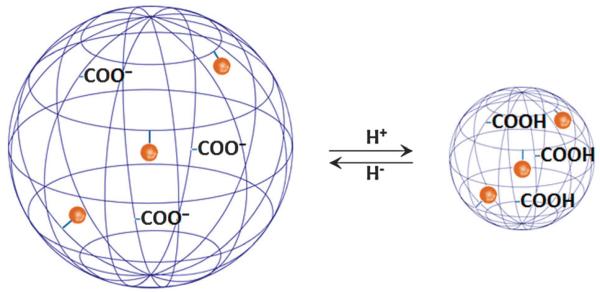
On the other hand, anionic, pH-sensitive polymers, such as those based on polymethacrylic acid (PMMA), can accept or release protons (Fig. 9), resulting in swelling/contraction that modifies τR.112 At pH 7, a Gd3+–DO3A loaded spherical copolymer consisting of PMMA cross-linked by N,N′-methylenebisacrylamide becomes swollen due to electrostatic repulsion between the carboxy groups. At pH 4 carboxy group protonation induces the formation of a compact globule conformation of restricted molecular motion and r1 increases from 13.6 mM−1 s−1 to 28.0 mM−1 s−1.107
Fig. 9.
The carboxylic groups of anionic polymeric nanoparticulates are progressively deionised when exposed to an acidic environment resulting in polymer shrinking, restricted side chain mobility, and significantly increasing relaxivity.107 The blue net represents the polymer and brown spheres the Gd3+-chelate groups covalently conjugated to polymer side chains. Picture adapted with permission from ref. 130.
The remarkably higher τR of a generation-5 poly(amidoamine) (G5-PAMAM) dendrimer loaded with Gd3+–EPTPA in an acidic environment was studied in detail by Merbach and co-workers using the Lipari–Szabo approach to fit NMRD profiles. Under acidic conditions, repulsions between positively charged atoms associated with protonation of tertiary amines were increased, leading to an extended dendritic structure of slower global rotation (longer τR due to increased τRG = 4.04 ns) and increased hydrogen bond governed rigidity (F2 = 0.43). The increases in τRG and F2 above those noted at basic pH (τRG = 2.95 ns, F2 = 0.36), generated relaxivity increases from 13.7 to 23.9 mM−1 s−1 at low pH.90
The opposite effect of pH on τR was reported for a macromolecular probe comprising 114 ornithine residues, 30 of which were linked to aminoethyl-functionalised Gd3+–DO3A through a squaric acid moiety. At acidic pH, cationic terminal amine side chains were highly hydrated and repelling, generating a highly flexible structure of low τR and r1. At basic pH, deprotonation of the amine groups led to formation of a rigid α-helix structure, with an associated increase in r1 to 32 mM−1 s−1.131 This behaviour was consistent with increasing τR with pH as determined by NMRD. Above pH 7, the R2/R1 ratio was independent of the local concentration of the paramagnetic agent allowing ratiometric imaging.132
Both τm and τR contributed to responsiveness when 96 Gd3+-chelate moieties, each with four pH-sensitive phosphonate pendant arms that form hydrogen-bonds with bulk water (phosphonate triggered protic exchange in the second-hydration sphere133 is described in Section 2.3), were covalently coupled to a G5-PAMAM dendrimer by the group of Sherry. At pH 9, r1 (10.8 mM−1 s−1) was reportedly limited by slow water exchange. As the pH fell below 8.5, the phosphonates were consecutively protonated, and their effectiveness at catalysing proton exchange at Gd3+-coordinated H2O with bulk H2O increased. The combined effects of faster inner-sphere water exchange, longer τm of the second sphere due to either a larger or more ordered second coordination shell (via protonated phosphonates), and longer τR as a result of protonation of amines within the dendrimer, (turning on hydrogen bond mediated rigidification),90 increase r1 to 24.0 mM−1 s−1 at pH 6. However, the ultimate relaxivity here was limited by relatively slow water exchange in the inner-sphere (in the microsecond range) and by relatively fast protic exchange in the second-sphere (in the nanosecond range).134
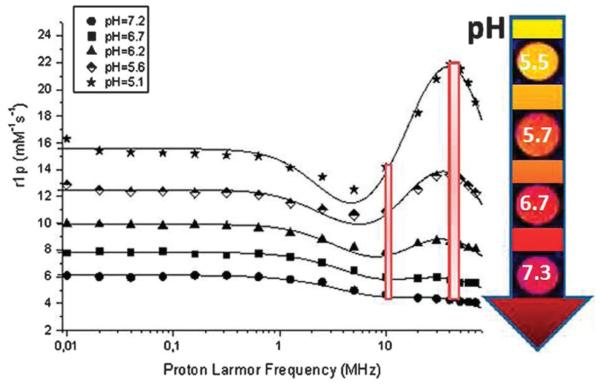
τm and τR have also been pH tuned through the loading of an amphiphilic Gd3+–DO3A derivative bearing a sulfonamide moiety into large unilamellar vesicles (LUV). Subsequent relaxivity decreases at high pH were ascribed not only to changes in inner-sphere hydration of the Gd3+-chelate (from q = 2 to q = 0), but also to its changed intraliposomal distribution and water exchange across the vesicle’s membrane. In an acidic environment, protonation of the sulfonamide moiety with concomitant removal of the arm from the coordination cage of the Gd3+-ion caused its incorporation deeper into the liposomal membrane. As a result, the increased number of Gd3+-complexes intercalated into the membrane led to a higher τR with respect to the free complex, an elongated τm, an increased membrane permeability to water and consequently an enhanced relaxation rate R1. In a rather unusual step, the authors finally report that, in mapping the dependence of the ratio of the 1H R1 relaxation rates at two different magnetic fields (40 MHz and 8.5 MHz) on pH (Fig. 10) ratiometric pH MR imaging can be achieved (image contrast independent of CA concentration).135
Fig. 10.
NMRD profile showing the pH tuned1H longitudinal relaxivity of LUV loaded with a Gd3+–DO3A derivative (1 mM [Gd]) as a function of applied magnetic field. The colour scale bar in the arrow denotes the signal intensities of T1-weighted images, and the two red bars represent 1H relaxivities at two different magnetic fields (8.5 MHz and 40 MHz). Adapted with permission from ref. 135. Copyright 2012 American Chemical Society.
There are, then, several different approaches which can be employed in the generation of high r1 CAs with pH sensitive contrast. The most common approaches utilise either pH dependent swelling, causing variations in τR, or pH dependent metal hydration/water exchange rate. In most cases, relatively modest (~2-fold) changes in relaxivity have been achieved to date. The potential within this field, however, is vast, with a range of disease states triggering notable variation in local pH profile. This is likely to be an area of active publishing in the coming years.
3.2 Redox-responsive particulate contrast agents
In comparison to pH, there are relatively few examples of particulate-based T1 MRI probes for which contrast is tuned through a redox event. To date, examples are primarily based on changes in rotational correlation time. For example, the redox-responsive relaxivity change of a nanoparticulate MRI probe based on a thiol/disulfide redox couple is caused by variation in molecular dynamics (τR) as a result of degradation. Nanocapsules based on several β-CD units linked by disulfide bridges incorporated Gd3+-chelates that were able to interact with β-CD via hydrophobic pendant aromatic residues. The incorporated Gd3+-chelates exhibited high relaxivity (15.2 mM−1 s−1 at 70 MHz) due to restricted mobility and good water permeability through the β-CD shell. The addition of a reducing agent caused the nanocapsules to disassemble, releasing the β-CD monomers and Gd3+-complexes. As a result, τR was shortened and relaxivity decreased to 8.2 mM−1 s−1.136
In comparable work but with converse observations, Liang and others obtained an enhanced relaxivity through elongated τR as a consequence of self-assembly of gadolinium doped nanoparticles triggered by enzymatic and reductive cleavage.137 After entry into cancer cells, the disulfide bond of an MRI contrast agent (r1 = 6.0 mM−1 s−1) consisting of Gd3+–DOTA, 2-cyanobenzothiazole and a peptide sequence containing an S–S bond, was reduced by glutathione and the peptide sequence cleaved by furin, a trans-Golgi protease overexpressed in tumours. The so-generated intermediate Gd3+-complex condensed into an amphiphilic dimer with increased relaxivity (13.2 mM−1 s−1), self-assembling via π–π stacking into gadolinium containing nanoparticles, with τR once again being responsible for contrast modulation.138
These examples demonstrate that effective relaxation responses can be observed using a redox stimulus, although reductions in relaxivity resulting from dissolution are less useful from a potential toxicity perspective, increases due to lengthened τR can provide >100% contrast signal enhancement.
3.3 Thermo-responsive particulate contrast agents
Temperature sensitive nanoparticulate MRI probes can potentially be used for both measuring temperature distribution in the human body, and thermometry, the latter largely based on local hyperthermia for either chemodosimetry or controllably killing cancer cells.123,139,140
In terms of CA relaxivity, work to date has focused on either temperature induced release of Gd3+-chelates from a liposomal core140 or by a change in water access to particle internalised Gd3+-complexes.141,142 In both cases all prior work has been based on the transition of the liposomal phospholipid bilayer from a highly ordered gel-like phase that serves as a diffusion barrier to a fluid-like disordered state with increased membrane permeability (at T > TM).123
Grüll and others have, for example, investigated liposomes consisting of several different lipids including a thermoresponsive 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC, TM = 41.5 °C140), and a lysolipid 1-palmitoyl-sn-glycero-3-phosphocholine (MPPC). T1 measurements at T < TM imply that encapsulated Gd3+-complexes remain inside the aqueous lumen of the liposome so that relaxivity is limited by the water diffusion across the bilayer. This increases with increasing temperature, such that there is a sharp reduction in T1 (from 1828 ms to 394 ms) above the TM, upon incubation of the liposomes at 45 °C for 30 min.143
In vivo studies were performed by Li et al., who developed a DPPC-based thermo-sensitive liposomal formulation with encapsulated Gd3+–DTPA and doxorubicin. Chelate release was triggered by local hyperthermia and quantified by MRI. T1 relaxation of a tumour in a BALB/c mouse treated by such liposomes was 2878 ms. After hyperthermic treatment in a hot bath at 43 °C, T1 was accelerated to 1509 ms in the rim (i.e. 1–2 mm band around tumour periphery) and to 2482 ms in the core of the tumour due to Gd3+–DTPA release.144
A two-point thermometry system with an “off–on” and “on–off” transition using lipid nanoparticles containing P(NIPAM)-co-P(AM) crosslinked with a Gd3+-chelate bi-linker was developed by Wu and others.145 Poly(N-isopropylacrylamide) P(NIPAM) exhibits a unique temperature sensitivity, undergoing a reversible phase transition at 32 °C, expelling water and forming a contracted hydrophobic globule of low associated relaxivity.146 The critical temperature of this transition could be raised with increasing amounts of copolymerised temperature insensitive monomer acrylamide (AM). At temperatures above the critical point the hydrophilic hydrogel containing the Gd3+-chelate crosslinker was exposed to water, increasing T1 signal.145
These investigations into the use of temperature activatable contrast agents exploiting tuneable interactions between bulk water and paramagnetic chelate have been successfully translated to in vivo studies and shown great promise,144 particularly considering the demonstrable biocompatibility of the materials involved. Further analyses will undoubtedly present systems of greater control, tuneability and temperature definition.
3.4 Bio-responsive particulate contrast agents
Smart MRI nanoparticulate probes endeavour to sense molecular events, such as enzymatic activities and gene expression patterns at the level of the entire organism.147 A number of attempts have been made to generate macromolecular contrast agents that respond significantly to the presence of a biomolecule of interest based on the so-called receptor-induced magnetisation-enhancement (RIME) effect.148 In many cases this has been achieved through a modulation of τR. Biotinylated DNA aptamers 3′ conjugated to Gd3+–DOTA have, for example, been observed to switch the terminal r1 relaxivity by ~40% in the presence/absence of adenosine. This sensing occurs through binding induced changes in DNA hybridisation with its associated change in steric bulk.149 Similarly, T1 contrast of a G5-PAMAM dendrimer conjugated to folic acid and Gd3+-complex can be enhanced upon binding to a folate receptor due to resulting slower tumbling rates.150 Similarly, the relaxivity of a gadolinium metallopeptide consisting of a DNA-binding transcription factor and a Ca2+-binding moiety increased r1 by 100% to 42.4 mM−1 s−1 at 60 MHz upon binding to DNA, due to an increased τR effect.151 A τR dependent relaxometric procedure was also developed by Aime and others, who exploited a ratiometric couple based on Gd3+–DOTA covalently linked to a peptide sequence and a lipophilic tail that was incorporated into a liposome (large R2/R1). As the matrix metalloproteinase-2 cleaved the sequence between serine and leucine, the gadolinium complex was released from the liposomal membrane, and, due to increased τR, R2/R1 was reduced by ~46% at 300 MHz independent of the total concentration of gadolinium.152
In addition, a Gd3+ complex itself can be responsive to direct ligation to a biomolecule, whereby the number of inner sphere bound water molecules q changes upon binding. This conceptually and chemically simple mechanism has, for example, been demonstrated with an octahedral nanocage consisting of four rigid tridentate ligands coordinating six gadolinium atoms that showed a r1 of 388.5 mM−1 s−1, at 400 MHz. Addition of glucosamine triggered a decrease in r1 to 62.1 mM−1 s−1 due to substitution of coordinated water molecules and decreased q (selectivity over glucose was notably observed).110
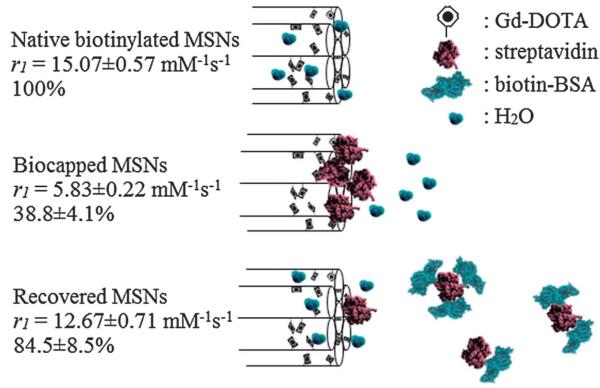
Davis and co-workers have explored the first inorganic nanoparticle-based contrast system triggered by protein–protein recognition based on tuned water access and τm (Fig. 11).111 They specifically reported the reversible contrast switching of Gd3+-chelate doped MSNs (r1 = 15.1 ± 0.57 mM−1 s−1 at 300 MHz) by tuning the water access to internally biased paramagnetic centres (and thus kex) using a surface immobilised protein of sufficient steric bulk (in the protein gated state r1 = 5.8 ± 0.22 mM−1 s−1). In the presence of a partner biomolecule (in this case a biotinylated protein), this steric cap was observed to be competed off, and both water access and high relaxivity were subsequently restored. This proof of principal serves to illustrate the significant potential of nanoparticle-based functional MR imaging in reporting on specific biorecognition processes.
Fig. 11.
Schematic summary of the protein gating of MSNs. Externally biotinylated Gd–DOTA MSNs enjoy good water accessibility and a high relaxivity that can be reversibly capped by the steric bulk of bound streptavidin. In the presence of low μM of biotinylated BSA, the gating protein is competed off the particle surface and relaxivity recovers. Adapted from ref. 111 with permission from The Royal Society of Chemistry.
The wide variety of potential bio-responsive triggers available which are associated with various disease and tumour states should stimulate further investigations into nanoparticle scaffolds, wherein both inherently high signal contrast and potentially dramatic contrast switching are possible.
4. Conclusions and future outlooks
There exist a plethora of MRI CAs which are capable of displaying strong positive or negative signals in vitro and in vivo. The prospect of CAs which can detect and respond to specific stimuli is an exciting one, with huge potential for non-invasive disease diagnosis and, more generally, the (potentially whole body) “mapping” of local biochemistry. This article has focused on T1 paramagnetic centre-based contrast agents, and described some of the mechanisms which can be exploited to facilitate change in MRI contrast signal in response to a specific environmental trigger (see Table 1). Molecular, macromolecular and particulate-based contrast agents can, specifically, be engineered such that variations in hydration number (q), rotational correlation (τR) or water exchange (τm) follow specific chemical or physical interactions with their environment.
Table 1.
Summary of relaxivity responses to various environmental stimuli of molecular, macromolecular, polymeric and particulate MRI CAs
| Responsive MRI probe | Stimulus | Mechanistic change | r1 switch [mM−1 s−1] | Percentage change | Ref. |
|---|---|---|---|---|---|
| Molecular MRI CAs | |||||
| 4,7,10-Tri(acetic acid)-1-(2-β-galactopyranosylethoxy)- 1,4,7,10-tetraazacyclododecane gadolinium (EGad) |
β-Galactosidase | q | Not reported | 20% decrease | 28 |
| (l-(2-(β-Glalactopyranosyloxy)propyl) -4,5,10-tris(carboxymethyl-1,4,7,10-tetra- azacyclododecane))gadolinium (EGadMe) |
β-Galactosidase | q | 0.90–2.72 s−1 a at 500 MHz | 200% increase | 29 |
| Gd–DO3A bearing a pendant β-glucuronic acid moiety connected by a self-immolative linker |
β-Glucuronidase | q | 4.75–3.90 at 60 MHz | 20% decrease | 30 |
| Gd–DO3A appended with acetoxymethyl esters | Porcine liver esterase |
q | 5.7–10.8 at 20 MHz | 89% increase | 32 |
| Bis-5-hydroxytryptamide-diethylenetriamine-pentatacetate gadolinium (MPO–Gd) |
Myeloperoxidase (MPO) |
τ R | Not reported | Not reported | 27 |
| Gd–DTPA with phosphonate side chain (MS-325) | Human serum albumin (HSA) |
τ R | 5.6–50.8 at 20 MHz | 800% increase at 20 MHz | 33 |
| 5.0–25.0 at 64 MHz | 400% increase at 64 MHz | ||||
| Gd–DTPA appended with lysine residues | Thrombin-activa- table fibrinolysis inhibitor (TAFI) |
τ R | 12.5–25.2 at 20 MHz | 100% increase | 34 |
| Gd–DOTA with pendant diphenylphosphinamide groups | Human serum albumin (HSA) |
τ R | 7.3–16.0 at 20 MHz | 119% increase | 36 |
| Gd–DO3A–glutamate | Glutamic acid decarboxylase (GAD) |
q, τR | 8.0–11.5 at 20 MHz | 44% increase | 31 |
| Peptide-labelled GdDO3A (Gd3+–G80BP) | Ga180 protein | τ R | 8.3–44.8 at 20 MHz | 440% increase | 35 |
| Gd–DTPA bisamide | Zn2+ | q | 6.06–3.98 at 300 MHz | 34% decrease | 39 |
| Gd–DTPA bisamide | Zn2+ | q | 4.8–3.5 at 300 MHz | 27% decrease | 40 |
| Gd–DTPA-appended β-diketone (KMR–Mg) | Mg2+ (also Ca2+) | q | 4.98–3.95 at 20 MHz | 21% decrease | 41 |
| Gd–DOTA appended with N,N-bis(2-pyridyl-methyl) ethylene diamine (bisBPEN) diamide | Zn2+, Cu2+ | k ex | 5.0–6.0 at 23 MHz | 20% increase | 42 |
| Gd–DOTA appended with two bis-(3-pyrazolyl) units | Zn2+, Cu2+ | k ex | 4.2–6.9 at 23 MHz | 64% increase | 43 |
| Gd–diaminoacetate with 3 methylenes (Gd–daa3) | Zn2+ | q | 2.3–5.1 at 60 MHz | 121% increase | 44, 45 |
| Gd[l,2-bis[{[({l-[l,4,7-tris(carboxymethyl)-1,4,7,10-tetra- azacyclododecane-10-yl]eth-2-yl}amino)carbonyl]methyl}- (carboxymethyl)amino]ethane] |
Ca2+ | q | 5.4–7.1 at 500 MHz | 32% increase | 46 |
| Ethylene glycol tetraacetic acid (EGTA) linked to 2 Gd–DO3A chelates |
Ca2+ | q | 3.44–6.29 at 500 MHz | 83% increase | 47 |
| DOPTA–Gd | Ca2+ | q | 3.26–5.76 at 500 MHz | 80% increase | 48 |
| Gd–DO3A chelate with pyro-EGTA moiety | Ca2+, Zn2+ | q | 3.8–6.6 at 60 MHz | 73% increase | 55 |
| Gd–DO3A with a pendant iminodiacetate | Cu2+ | q | 3.76–5.29 at 400 MHz | 41% increase | 49 |
| Gd–DO3A with tethered thioether groups | Cu2+ | q | 1.5–6.9 at 20 MHz | 360% increase | 50 |
| Octaarginine-conjugated Gd–DO3A with tethered thioether groups | Cu+ | q | 3.9–12.5 at 60 MHz | 220% increase | 52 |
| Gd–DO3A with appended 8-amidequinoline (Gd-QDOTAMA) | Cu2+ | q | 4.27–7.29 at 400 MHz | 71% increase | 53 |
| Tryptophan-appended Gd–TTDA [Gd(Try-TTDA)(H2O)]2− | Cu2+ | q | 4.22–7.42 at 20 MHz | 76% increase | 54 |
| Di-metallic-DO3A with piperazine bridge | Hg2+ | q | 8.3–10.3 at 30 MHz | 24% increase | 51 |
| GdDOTA–diBPEN | Zn2+ | τ R | 5.0–17.5 at 23 MHz | 250% increase | 57 |
| Gd(phen)HDO3A chelate | Fe2+ | τ R | 3.7–12.2 at 20 MHz | 230% increase | 11 |
| [Gd2bpy(DTTA)2(H2O)4]2− | Fe2+ | τ R | 12.44–20.17 at 20 MHz | 62% increase | 59 |
| Terpyridine–Gd3+ chelate | pH | q | 12.8–2.0 at 20 MHz | 84% | 66 |
| [Gd(DOTA tetrakis(methylamide))]3+ | pH | τ m | 2.5–5.7 at 20 MHz | 128% | 22, 23 |
| Dinitrospiropyran–GdDO3A | NADH | q | 2.51–1.86 at 60 MHz | 26% decrease | 74, 75 |
| Spiropyran–GdDO3A | NADH | q | 5.58–8.60 at 60 MHz | 54% increase | 74 |
| Spiropyran–GdDO3A | Light | q | 3.72–2.93 at 60 MHz | 21% decrease | 75 |
| Macromolecular, polymeric and particulate MRI CAs | |||||
| G5-PAMAM dendrimer with Gd-complexes bearing phosphonate pendant arms |
pH | τR, τm | 10.8–24.0 at 20 MHz | 122% | 134 |
| Large unilamellar vesicles (POPC/DPPC) loaded with the an amphiphilic GdDO3A derivative |
pH | τR, τm | 4.5–13.5 at 43 MHz | 200% | 135 |
| Sulfonamide arm containing Gd-chelate hosted by poly-β-cyclodextrin |
pH | q | 8–16 at 43 MHz | 100% | 128 |
| Diacylphosphatidylethanolamine/ dipalmitoylglycerosuccinate based enosome loaded with a GdDTPA-derivative |
pH | τ io | 2.3–0.7 at 20 MHz | 228% | 159 |
| Poly-l-ornithine | pH | τR, τm | 23.0–32.0 at 20 MHz | 39% | 131 |
| Methacrylic acid based polymer cross-linked by N,N′-methylenebisacrylamide |
pH | Swelling (τR) | Non-cross-linked polymer: 6.7–12.1 Cross-linked polymer: 13.6–28.0 at 20 MHz | 106% | 107 |
|
n-Octylamine modified poly(SM-EVE) polymer loaded with aminoethyl-modified GdDO3A |
pH | Electrostatic repulsion (high pH), hydrophobic interaction (low pH) (τR) |
8.0–9.0 at 20 MHz | 12% | 112 |
| Avidin conjugated to GdDOTA derivative with a phosphonate pendant arm |
pH | τR, q | 10.4–12.6 at 128 MHz | 21% | 160 |
| Ketal-based polymer with a GdDTPA derivative | pH | Polymer degradation (τR) |
8.2–3.8 at 64 MHz | 115% | 126 |
| Gadonanotubes | pH | Gd3+ loss | 40.0–133.0 at 64 MHz | 233% | 86 |
| Gadofullerenes | pH | Aggregation (τR) | 10.4–38.5 at 30–60 MHz | 270% | 161 |
| Manganese oxide nanoparticles | pH | Release of Mn2+ ions |
8.8–27.7 at 64 MHz | 215% | 162 |
| Manganese oxide functionalised mesoporous silica nanoparticle |
pH | Dissolution | 0.8–8.8 at 128 MHz | 1015% | 163 |
| GdDOTA doped mesoporous silica nanoparticles capped by streptavidin | Biotinylated BSA | τ m | 5.8–12.7 at 300 MHz | 219% increase | 111 |
| Hexanuclear gadolinium organic octahedron | Glucosamine | kex, q | 388.5–62.1 at 400 MHz | 526% decrease | 110 |
| Manganese loaded apoferritin | Melanin | Reduction, dissolution (τR) |
0.3–6.0 at 300 MHz | 1900% increase | 164 |
| DNA aptamer conjugated to streptavidin and GdDOTA |
Adenosine | Release of Gd-DNA strand (τR) |
12.2–9.2 at 60 MHz | 33% decrease | 149 |
| Liposome with incorporated amphiphilic GdDOTA covalently linked to a peptide sequence |
Metalloproteinase-2 | τ R | 8.9–7.5 at 60 MHz | 16% decrease | 152 |
| GdDOTA covalently coupled to a chemically modified peptide sequence bearing a cyano and 1,2-aminothiol group |
Furin | Reduction, self-assembly into nanoparticles (τR) |
6.0–13.2 at 64 MHz | 220% increase | 137 |
| Perthiolated β-cyclodextrin-based nanocapsules | Reductive/hypoxic environment |
Reduction, degradation (τR) |
15.2–8.2 at 70 MHz | 46% decrease | 136 |
| P(NIPAM)-co-P(AM) hydrogel nanoparticles cross-linked by a GdDTPA-derivative and loaded into solid lipid nanoparticles |
Temperature | kex, τio, τR | 12.4–8.6 at 300 MHz | 44% decrease | 145 |
| POEGMA-b-P(NIPAM-co-NBA-co-Gd) diblock copolymer | Temperature | k ex | 6.5–12.1 at 300 MHz | 86% increase | 165 |
| Liposomes loaded with Gd-complexes | Temperature | τ io | 1828–394 msb at 300 MHz | 364% decrease | 143 |
Relaxation rate provided in s−1.
Relaxation time provided in ms.
In general, macromolecular and particle-based CAs provide higher relaxivity values than molecular agents alone, due in part to significant lengthening of the paramagnet rotational correlation times through its association with a rigid, bulky, species. The degree of relaxivity modulation observed, however, is, thus far, similar for both molecular and nanoparticulate agents (see Table 1). As protocols for the synthesis of homogeneous and well-characterised particles become widely used, this area is likely to develop markedly over the next few years. Tactics to facilitate response are likely to involve designed switching of τR and τm, as well as gating water access to encapsulated paramagnetic centres. The possibility of utilising additional (native or added) multimodal characteristics (nuclear or otherwise), high local concentrations and targeting would make these agents exceedingly powerful from a diagnostic perspective.
To date, it is clear that the chemical complexities of an in vivo environment often prove detrimental in keeping image contrast switches both substantial and selective. The development of improved particle characterisation methodologies together with chemistries that introduce other magnetic nuclei, chemically exchangeable groups, or hyperpolarized centres, will, however, almost certainly underpin beneficial developments.153-158
Few areas of research have exploded quite as dramatically as the development of both molecular and nanoparticle based image contrast and therapeutic delivery systems during the past decade. Applying the chemists’ toolkit and additionally integrating a non-invasive means of diagnosing and monitoring response to a treatment regime would, quite simply, herald a new age in clinical healthcare.
Acknowledgements
The authors acknowledge financial support from the Wellcome Trust (WT094114MA).
Biographies

Gemma-Louise Davies
Gemma-Louise Davies received her degree (2006) and PhD degree (2011) in Chemistry from Trinity College Dublin, Ireland. Her PhD involved the development of new silica and magnetic-luminescent silica nanostructured materials. She is currently working in the Department of Chemistry at the University of Oxford, UK with Dr Jason Davis. Her research interests encompass the preparation and characterisation of multifunctional nanomaterials as diagnostic and therapeutic tools in healthcare.

Iris Kramberger
Iris Kramberger received her Bachelor degree (2011) from University of Ljubljana, Slovenia and her Masters degree (2012) in Nanomaterials from Imperial College London, UK. She is currently studying towards her DPhil in Inorganic Chemistry from the University of Oxford, UK and is interested in the design and fabrication of ratiometric and stimuli-responsive inorganic and hydrogel based nanoparticle MRI agents.

Jason J. Davis
Jason Davis obtained a PhD in Chemistry from Oxford in 1998. After a Royal Society University Research Fellowship, he took up a lectureship at the University of Oxford before becoming a Reader in Chemistry. His research interests are broad and primarily focused on the use and development of state-of-the-art molecular, theranostic and medical imaging technologies as well as the design, analysis and manipulation of functional molecular interfaces.
Notes and references
- 1.Huang W-Y, Davis JJ. Dalton Trans. 2011;40:6087. doi: 10.1039/c0dt01656j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pankhurst QA, Connolly J, Jones SK, Dobson J. J. Phys. D: Appl. Phys. 2003:R167. [Google Scholar]
- 3.Caravan P. Chem. Soc. Rev. 2006;35:512. doi: 10.1039/b510982p. [DOI] [PubMed] [Google Scholar]
- 4.Corr SA, Byrne SJ, Tekoriute R, Meledandri CJ, Brougham DF, Lynch M, Kerskens C, O’Dwyer L, Gun’ko YK. J. Am. Chem. Soc. 2008;130:4214. doi: 10.1021/ja710172z. [DOI] [PubMed] [Google Scholar]
- 5.Davies G-L, Corr SA, Meledandri CJ, Briode L, Brougham DF, Gun’ko YK. ChemPhysChem. 2011;12:772. doi: 10.1002/cphc.201000853. [DOI] [PubMed] [Google Scholar]
- 6.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Chem. Rev. 1999;99:2293. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 7.Werner EJ, Datta A, Jocher CJ, Raymond KN. Angew. Chem., Int. Ed. 2008;47:8568. doi: 10.1002/anie.200800212. [DOI] [PubMed] [Google Scholar]
- 8.Lin C, Kadono T, Yoshizuka K, Furuichi T, Kawano T. Z. Naturforsch., C: J. Biosci. 2006;61:74. doi: 10.1515/znc-2006-1-214. [DOI] [PubMed] [Google Scholar]
- 9.Oksendal AN, Hals P-A. J. Magn. Reson. Imaging. 1993;3:157. doi: 10.1002/jmri.1880030128. [DOI] [PubMed] [Google Scholar]
- 10.Stasiuk GJ, Long NJ. Chem. Commun. 2013;49:2732. doi: 10.1039/c3cc38507h. [DOI] [PubMed] [Google Scholar]
- 11.Comblin V, Gilsoul D, Hermann M, Humblet V, Jacques V, Mesbahi M, Sauvage C, Desreux JF. Coord. Chem. Rev. 1999;185:451. [Google Scholar]
- 12.Aime S, Caravan P. J. Magn. Reson. Imaging. 2009;30:1259. doi: 10.1002/jmri.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villaraza AJL, Bumb A, Brechbiel MW. Chem. Rev. 2010;110:2921. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon I. Phys. Rev. 1955;99:559–565. [Google Scholar]
- 15.Bloembergen N, Purcell EM, Pound RV. Phys. Rev. 1948;73:679. [Google Scholar]
- 16.Lowe MP, Parker D, Reany O, Aime S, Botta M, Castellano G, Gianolio E, Pagliarin R. J. Am. Chem. Soc. 2001;123:7601. doi: 10.1021/ja0103647. [DOI] [PubMed] [Google Scholar]
- 17.De Leon-Rodriguez LM, Lubag AJM, Malloy CR, Martinez GV, Gillies RJ, Sherry AD. Acc. Chem. Res. 2009;42:948. doi: 10.1021/ar800237f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu C, Osborne EA, Louie AY. Ann. Biomed. Eng. 2011;39:1335. doi: 10.1007/s10439-011-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Major JL, Meade TJ. Acc. Chem. Res. 2009;42:893. doi: 10.1021/ar800245h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Logothetis NK. Nature. 2008;453:869. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 21.Tu C, Louie AY. Chem. Commun. 2007:1331. doi: 10.1039/b616991k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aime S, Barge A, Botta M, Parker D, De Sousa AS. J. Am. Chem. Soc. 1997;119:4767. [Google Scholar]
- 23.Aime S, Barge A, Bruce JI, Botta M, Howard JAK, Moloney JM, Parker D, de Sousa AS, Woods M. J. Am. Chem. Soc. 1999;121:5762. [Google Scholar]
- 24.Zhang S, Wu K, Sherry AD. Angew. Chem., Int. Ed. 1999;38:3192. [PubMed] [Google Scholar]
- 25.Kálmán FK, Woods M, Caravan P, Jurek P, Spiller M, Tircsó G, Király R, Brücher E, Sherry AD. Inorg. Chem. 2007;46:5260. doi: 10.1021/ic0702926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aime S, Crich SG, Botta M, Giovenzana G, Palmisano G, Sisti M. Chem. Commun. 1999:1577. [Google Scholar]
- 27.Breckwoldt MO, Chen JW, Stangenberg L, Aikawa E, Rodriguez E, Qiu S, Moskowitz MA, Weissleder R. Proc. Natl. Acad. Sci. U. S. A. 2008;105:18584. doi: 10.1073/pnas.0803945105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moats RA, Fraser SE, Meade TJ. Angew. Chem., Int. Ed. Engl. 1997;36:726. [Google Scholar]
- 29.Louie AY, Huber MM, Ahrens ET, Rothbacher U, Moats R, Jacobs RE, Fraser SE, Meade TJ. Nat. Biotechnol. 2000;18:321. doi: 10.1038/73780. [DOI] [PubMed] [Google Scholar]
- 30.Duimstra JA, Femia FJ, Meade TJ. J. Am. Chem. Soc. 2005;127:12847. doi: 10.1021/ja042162r. [DOI] [PubMed] [Google Scholar]
- 31.Napolitano R, Pariani G, Fedeli F, Baranyai Z, Aswendt M, Aime S, Gianolio E. J. Med. Chem. 2013;56:2466. doi: 10.1021/jm301831f. [DOI] [PubMed] [Google Scholar]
- 32.Giardiello M, Lowe MP, Botta M. Chem. Commun. 2007:4044. doi: 10.1039/b711989e. [DOI] [PubMed] [Google Scholar]
- 33.Caravan P, Cloutier NJ, Greenfield MT, McDermid SA, Dunham SU, Bulte JWM, Amedio JC, Looby RJ, Supkowski RM, Horrocks WD, McMurry TJ, Lauffer RB. J. Am. Chem. Soc. 2002;124:3152. doi: 10.1021/ja017168k. [DOI] [PubMed] [Google Scholar]
- 34.Nivorozhkin AL, Kolodziej AF, Caravan P, Greenfield MT, Lauffer RB, McMurry TJ. Angew. Chem., Int. Ed. 2001;40:2903. [PubMed] [Google Scholar]
- 35.De León-Rodríguez LM, Ortiz A, Weiner AL, Zhang S, Kovacs Z, Kodadek T, Sherry AD. J. Am. Chem. Soc. 2002;124:3514. doi: 10.1021/ja025511v. [DOI] [PubMed] [Google Scholar]
- 36.Giardiello M, Botta M, Lowe M. J. Inclusion Phenom. Macrocyclic Chem. 2011;71:435. [Google Scholar]
- 37.Noy D, Solomonov I, Sinkevich O, Arad T, Kjaer K, Sagi I. J. Am. Chem. Soc. 2008;130:1376. doi: 10.1021/ja076282l. [DOI] [PubMed] [Google Scholar]
- 38.Frederickson CJ, Koh J-Y, Bush AI. Nat. Rev. Neurosci. 2005;6:449. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- 39.Hanaoka K, Kikuchi K, Urano Y, Nagano T. J. Chem. Soc., Perkin Trans. 2. 2001:1840. [Google Scholar]
- 40.Hanaoka K, Kikuchi K, Urano Y, Narazaki M, Yokawa T, Sakamoto S, Yamaguchi K, Nagano T. Chem. Biol. 2002;9:1027. doi: 10.1016/s1074-5521(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 41.Hifumi H, Tanimoto A, Citterio D, Komatsu H, Suzuki K. Analyst. 2007;132:1153. doi: 10.1039/b707225b. [DOI] [PubMed] [Google Scholar]
- 42.Esqueda AC, López JA, Andreu-de-Riquer G, Alvarado-Monzón JC, Ratnakar J, Lubag AJM, Sherry AD, De León-Rodríguez LM. J. Am. Chem. Soc. 2009;131:11387. doi: 10.1021/ja901875v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Leon-Rodriguez LM, Lubag AJM, Lopez JA, Andreu-de-Riquer G, Alvarado-Monzon JC, Sherry AD. MedChemComm. 2012;3:480. doi: 10.1039/C2MD00301E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Major JL, Parigi G, Luchinat C, Meade TJ. Proc. Natl. Acad. Sci. U. S. A. 2007;104:13881. doi: 10.1073/pnas.0706247104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Major JL, Boiteau RM, Meade TJ. Inorg. Chem. 2008;47:10788. doi: 10.1021/ic801458u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishra A, Fousková P, Angelovski G, Balogh E, Mishra AK, Logothetis NK, Tóth É. Inorg. Chem. 2008;47:1370. doi: 10.1021/ic7017456. [DOI] [PubMed] [Google Scholar]
- 47.Angelovski G, Fouskova P, Mamedov I, Canals S, Toth E, Logothetis NK. ChemBioChem. 2008;9:1729. doi: 10.1002/cbic.200800165. [DOI] [PubMed] [Google Scholar]
- 48.Li W-H, Fraser SE, Meade TJ. J. Am. Chem. Soc. 1999;121:1413. [Google Scholar]
- 49.Que EL, Chang CJ. J. Am. Chem. Soc. 2006;128:15942. doi: 10.1021/ja065264l. [DOI] [PubMed] [Google Scholar]
- 50.Que EL, Gianolio E, Baker SL, Wong AP, Aime S, Chang CJ. J. Am. Chem. Soc. 2009;131:8527. doi: 10.1021/ja900884j. [DOI] [PubMed] [Google Scholar]
- 51.Andrews M, Amoroso AJ, Harding LP, Pope SJA. Dalton Trans. 2010;39:3407. doi: 10.1039/b923988j. [DOI] [PubMed] [Google Scholar]
- 52.Que EL, New EJ, Chang CJ. Chem. Sci. 2012;3:1829. doi: 10.1039/C2SC20273E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W-S, Luo J, Chen Z-N. Dalton Trans. 2011;40:484. doi: 10.1039/c0dt01141j. [DOI] [PubMed] [Google Scholar]
- 54.Kasala D, Lin T-S, Chen C-Y, Liu G-C, Kao C-L, Cheng T-L, Wang Y-M. Dalton Trans. 2011;40:5018. doi: 10.1039/c1dt10033e. [DOI] [PubMed] [Google Scholar]
- 55.Mishra A, Logothetis NK, Parker D. Chem.-Eur. J. 2011;17:1529. doi: 10.1002/chem.201001548. [DOI] [PubMed] [Google Scholar]
- 56.Kubíček V, Vitha T, Kotek J, Hermann P, Vander Elst L, Muller RN, Lukeš I, Peters JA. Contrast Media Mol. Imaging. 2010;5:294. doi: 10.1002/cmmi.386. [DOI] [PubMed] [Google Scholar]
- 57.Lubag AJM, De Leon-Rodriguez LM, Burgess SC, Sherry AD. Proc. Natl. Acad. Sci. U. S. A. 2011;108:18400. doi: 10.1073/pnas.1109649108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aime S, Botta M, Fasano M, Terreno E. Spectrochim. Acta, Part A. 1993;49:1315. [Google Scholar]
- 59.Livramento JB, Sour A, Borel A, Merbach AE, Tóth É. Chem.-Eur. J. 2006;12:989. doi: 10.1002/chem.200500969. [DOI] [PubMed] [Google Scholar]
- 60.Parac-Vogt TN, Vander Elst L, Kimpe K, Laurent S, Burtéa C, Chen F, Van Deun R, Ni Y, Muller RN, Binnemans K. Contrast Media Mol. Imaging. 2006;1:267. doi: 10.1002/cmmi.114. [DOI] [PubMed] [Google Scholar]
- 61.Paris J, Gameiro C, Humblet V, Mohapatra PK, Jacques V, Desreux JF. Inorg. Chem. 2006;45:5092. doi: 10.1021/ic0603050. [DOI] [PubMed] [Google Scholar]
- 62.Muller RN, Radüchel B, Laurent S, Platzek J, Piérart C, Mareski P, Vander Elst L. Eur. J. Inorg. Chem. 1999:1949. [Google Scholar]
- 63.Gillies RJ, Raghunand N, Garcia-Martin ML, Gatenby RA. IEEE Eng. Med. Biol. 2004;23:57. doi: 10.1109/memb.2004.1360409. [DOI] [PubMed] [Google Scholar]
- 64.Wike-Hooley JL, Haveman J, Reinhold HS. Radiother. Oncol. 1984;2:343. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 65.Rockwell S, Dobrucki IT, Kim EY, Marrison ST, Vu V. T. h. u. c. Curr. Mol. Med. 2009;9:442. doi: 10.2174/156652409788167087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall J, Haner R, Aime S, Botta M, Faulkner S, Parker D, de Sousa AS. New J. Chem. 1998;22:627. [Google Scholar]
- 67.Garcia-Martin ML, Martinez GV, Raghunand N, Sherry AD, Zhang S, Gillies RJ. Magn. Res. Med. 2006;55:309. doi: 10.1002/mrm.20773. [DOI] [PubMed] [Google Scholar]
- 68.Aime S, Fedeli F, Sanino A, Terreno E. J. Am. Chem. Soc. 2006;128:11326. doi: 10.1021/ja062387x. [DOI] [PubMed] [Google Scholar]
- 69.Gianolio E, Maciocco L, Imperio D, Giovenzana GB, Simonelli F, Abbas K, Bisi G, Aime S. Chem. Commun. 2011;47:1539. doi: 10.1039/c0cc03554h. [DOI] [PubMed] [Google Scholar]
- 70.Frullano L, Catana C, Benner T, Sherry AD, Caravan P. Angew. Chem., Int. Ed. 2010;49:2382. doi: 10.1002/anie.201000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hyodo F, Chuang K-H, Goloshevsky AG, Sulima A, Griffiths GLL, Mitchell JBB, Koretsky AP, Krishna MC. J. Cereb. Blood Flow Metab. 2008;28:1165. doi: 10.1038/jcbfm.2008.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Klaidman LK, Leung AC, Adams JD. Anal. Biochem. 1995;228:312. doi: 10.1006/abio.1995.1356. [DOI] [PubMed] [Google Scholar]
- 73.Hyodo F, Soule BP, Matsumoto K-I, Matusmoto S, Cook JA, Hyodo E, Sowers AL, Krishna MC, Mitchell JB. J. Pharm. Pharmacol. 2008;60:1049. doi: 10.1211/jpp.60.8.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tu C, Nagao R, Louie AY. Angew. Chem., Int. Ed. 2009;48:6547. doi: 10.1002/anie.200900984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tu C, Osborne EA, Louie AY. Tetrahedron. 2009;65:1241. doi: 10.1016/j.tet.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Raghunand N, Jagadish B, Trouard TP, Galons J-P, Gillies RJ, Mash EA. Magn. Res. Med. 2006;55:1272. doi: 10.1002/mrm.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Burai L, Scopelliti R, Toth E. Chem. Commun. 2002:2366. doi: 10.1039/b206709a. [DOI] [PubMed] [Google Scholar]
- 78.Aime S, Botta M, Gianolio E, Terreno E. Angew. Chem., Int. Ed. 2000;39:747. doi: 10.1002/(sici)1521-3773(20000218)39:4<747::aid-anie747>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 79.Hernandez G, Bryant RG. Bioconjugate Chem. 1991;2:394. doi: 10.1021/bc00012a002. [DOI] [PubMed] [Google Scholar]
- 80.Bryant LH, Hodges MW, Bryant RG. Inorg. Chem. 1999;38:1002. doi: 10.1021/ic981197n. [DOI] [PubMed] [Google Scholar]
- 81.Burai L, Tóth É, Moreau G, Sour A, Scopelliti R, Merbach AE. Chem.-Eur. J. 2003;9:1394. doi: 10.1002/chem.200390159. [DOI] [PubMed] [Google Scholar]
- 82.de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Mol. Cell. Biol. 1987;7:725. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheung ENM, Alvares RDA, Oakden W, Chaudhary R, Hill ML, Pichaandi J, Mo GCH, Yip C, Macdonald PM, Stanisz GJ, van Veggel FCJM, Prosser RS. Chem. Mater. 2010;22:4728. [Google Scholar]
- 84.Platas-Iglesias C, Vander Elst L, Zhou WZ, Muller RN, Geraldes C, Maschmeyer T, Peters JA. Chem.-Eur. J. 2002;8:5121. doi: 10.1002/1521-3765(20021115)8:22<5121::AID-CHEM5121>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 85.Ananta JS, Godin B, Sethi R, Moriggi L, Liu X, Serda RE, Krishnamurthy R, Muthupillai R, Bolskar RD, Helm L, Ferrari M, Wilson LJ, Decuzzi P. Nat. Nanotechnol. 2010;5:815. doi: 10.1038/nnano.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartman KB, Laus S, Bolskar RD, Muthupillai R, Helm L, Toth E, Merbach AE, Wilson LJ. Nano Lett. 2008;8:415. doi: 10.1021/nl0720408. [DOI] [PubMed] [Google Scholar]
- 87.Evanics F, Diamente PR, van Veggel F, Stanisz GJ, Prosser RS. Chem. Mater. 2006;18:2499. [Google Scholar]
- 88.Hou Y, Qiao R, Fang F, Wang X, Dong C, Liu K, Liu C, Liu Z, Lei H, Wang F, Gao M. ACS Nano. 2013;7:330. doi: 10.1021/nn304837c. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Beija M, Laurent S, vander Elst L, Muller RN, Duong HTT, Lowe AB, Davis TP, Boyer C. Macromolecules. 2012;45:4196. [Google Scholar]
- 90.Laus S, Sour A, Ruloff R, Toth E, Merbach AE. Chem.-Eur. J. 2005;11:3064. doi: 10.1002/chem.200401326. [DOI] [PubMed] [Google Scholar]
- 91.Fossheim SL, Fahlvik AK, Klaveness J, Muller RN. Magn. Reson. Imaging. 1999;17:83. doi: 10.1016/s0730-725x(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 92.Mi P, Cabral H, Kokuryo D, Rafi M, Terada Y, Aoki I, Saga T, Takehiko I, Nishiyama N, Kataoka K. Biomaterials. 2013;34:492. doi: 10.1016/j.biomaterials.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 93.Caravan P, Parigi G, Chasse JM, Cloutier NJ, Ellison JJ, Lauffer RB, Luchinat C, McDermid SA, Spiller M, McMurry TJ. Inorg. Chem. 2007;46:6632. doi: 10.1021/ic700686k. [DOI] [PubMed] [Google Scholar]
- 94.Carniato F, Tei L, Dastru W, Marchese L, Botta M. Chem. Commun. 2009:1246. doi: 10.1039/b820591d. [DOI] [PubMed] [Google Scholar]
- 95.Marradi M, Alcantara D, de la Fuente JM, Garcia-Martin ML, Cerdan S, Penades S. Chem. Commun. 2009:3922. doi: 10.1039/b900957d. [DOI] [PubMed] [Google Scholar]
- 96.Peters JA, Djanashvili K. Eur. J. Inorg. Chem. 2012:1961. doi: 10.1021/ic201393n. [DOI] [PubMed] [Google Scholar]
- 97.Botta M, Tei L. Eur. J. Inorg. Chem. 2012:1945. [Google Scholar]
- 98.Bottrill M, Kwok L, Long NJ. Chem. Soc. Rev. 2006;35:557. doi: 10.1039/b516376p. [DOI] [PubMed] [Google Scholar]
- 99.Lowe MP. Aust. J. Chem. 2002;55:551–556. [Google Scholar]
- 100.Lauffer RB, Brady TJ. Magn. Reson. Imaging. 1985;3:11. doi: 10.1016/0730-725x(85)90004-9. [DOI] [PubMed] [Google Scholar]
- 101.Kielar F, Tei L, Terreno E, Botta M. J. Am. Chem. Soc. 2010;132:7836. doi: 10.1021/ja101518v. [DOI] [PubMed] [Google Scholar]
- 102.Nicolle GM, Toth E, Eisenwiener KP, Macke HR, Merbach AE. J. Biol. Inorg. Chem. 2002;7:757. doi: 10.1007/s00775-002-0353-3. [DOI] [PubMed] [Google Scholar]
- 103.Tang J, Sheng Y, Hu H, Shen Y. Prog. Polym. Sci. 2013;38:462. [Google Scholar]
- 104.Zong Y, Guo J, Ke T, Mohs AM, Parker DL, Lu Z-R. J. Controlled Release. 2006;112:350. doi: 10.1016/j.jconrel.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 105.Peng H, Blakey I, Dargaville B, Rasoul F, Rose S, Whittaker AK. Biomacromolecules. 2009;10:374. doi: 10.1021/bm801136m. [DOI] [PubMed] [Google Scholar]
- 106.Xu Q, Zhu L, Yu M, Feng F, An L, Xing C, Wang S. Polymer. 2010;51:1336. [Google Scholar]
- 107.Okada S, Mizukami S, Kikuchi K. Bioorg. Med. Chem. 2012;20:769. doi: 10.1016/j.bmc.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 108.Turner JL, Pan D, Plummer R, Chen Z, Whittaker AK, Wooley KL. Adv. Funct. Mater. 2005;15:1248. [Google Scholar]
- 109.Kojima C, Turkbey B, Ogawa M, Bernardo M, Regino CAS, Bryant LH, Jr, Choyke PL, Kono K, Kobayashi H. Nanomed.: Nanotechnol., Biol. Med. 2011;7:1001. doi: 10.1016/j.nano.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.He C, Wu X, Kong J, liu T, Zhang X, Duan C. Chem. Commun. 2012;48:9290. doi: 10.1039/c2cc33177b. [DOI] [PubMed] [Google Scholar]
- 111.Huang W-Y, Davies G-L, Davis JJ. Chem. Commun. 2013;49:60. doi: 10.1039/c2cc37545a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Okada S, Mizukami S, Kikuchi K. ChemBioChem. 2010;11:785. doi: 10.1002/cbic.200900649. [DOI] [PubMed] [Google Scholar]
- 113.Tilcock C, Ahkong QF, Koenig SH, Brown RD, Davis M, Kabalka G. Magn. Res. Med. 1992;27:44. doi: 10.1002/mrm.1910270106. [DOI] [PubMed] [Google Scholar]
- 114.Alhaique F, Bertini I, Fragai M, Carafa M, Luchinat C, Parigi G. Inorg. Chim. Acta. 2002;331:151. [Google Scholar]
- 115.Storrs RW, Tropper FD, Li HY, Song CK, Kuniyoshi JK, Sipkins DA, Li KCP, Bednarski MD. J. Am. Chem. Soc. 1995;117:7301. doi: 10.1002/jmri.1880050617. [DOI] [PubMed] [Google Scholar]
- 116.Pütz B, Barsky D, Schulten K. J. Liposome Res. 1994;4:771. [Google Scholar]
- 117.Davis JJ, Huang W-Y, Davies G-L. J. Mater. Chem. 2012;22:22848. doi: 10.1039/C2JM35116A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor KML, Kim JS, Rieter WJ, An H, Lin W, Lin W. J. Am. Chem. Soc. 2008;130:2154. doi: 10.1021/ja710193c. [DOI] [PubMed] [Google Scholar]
- 119.Sethi R, Ananta JS, Karmonik C, Zhong M, Fung SH, Liu X, Li K, Ferrari M, Wilson LJ, Decuzzi P. Contrast Media Mol. Imaging. 2012;7:501. doi: 10.1002/cmmi.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Carniato F, Tei L, Cossi M, Marchese L, Botta M. Chem.-Eur. J. 2010;16:10727. doi: 10.1002/chem.201000499. [DOI] [PubMed] [Google Scholar]
- 121.Dhingra Verma K, Mishra A, Engelmann J, Beyerlein M, Maier ME, Logothetis NK. ChemPlusChem. 2012;77:758. [Google Scholar]
- 122.Datta A, Hooker JM, Botta M, Francis MB, Aime S, Raymond KN. J. Am. Chem. Soc. 2008;130:2546. doi: 10.1021/ja0765363. [DOI] [PubMed] [Google Scholar]
- 123.Hossann M, Wang T, Syunyaeva Z, Wiggenhorn M, Zengerle A, Issels RD, Reiser M, Lindner LH, Peller M. J. Controlled Release. 2013;166:22. doi: 10.1016/j.jconrel.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 124.Lin C-W, Tseng SJ, Kempson IM, Yang S-C, Hong T-M, Yang P-C. Biomaterials. 2013;34:4387. doi: 10.1016/j.biomaterials.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 125.Vibhute SM, Engelmann J, Verbic T, Maier ME, Logothetis NK, Angelovski G. Org. Biomol. Chem. 2013;11:1294. doi: 10.1039/c2ob26555a. [DOI] [PubMed] [Google Scholar]
- 126.Schopf E, Sankaranarayanan J, Chan M, Mattrey R, Almutairi A. Mol. Pharmaceutics. 2012;9:1911. doi: 10.1021/mp2005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Schmaljohann D. Adv. Drug Delivery Rev. 2006;58:1655. doi: 10.1016/j.addr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 128.Gianolio E, Napolitano R, Fedeli F, Arena F, Aime S. Chem. Commun. 2009:6044. doi: 10.1039/b914540k. [DOI] [PubMed] [Google Scholar]
- 129.Woods M, Kiefer GE, Bott S, Castillo-Muzquiz A, Eshelbrenner C, Michaudet L, McMillan K, Mudigunda SDK, Ogrin D, Tircsó G, Zhang S, Zhao P, Sherry AD. J. Am. Chem. Soc. 2004;126:9248. doi: 10.1021/ja048299z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Almeida H, Amaral MH, Lobão P. J. Appl. Pharm. Sci. 2012;2:1. doi: 10.18433/j3hw2b. [DOI] [PubMed] [Google Scholar]
- 131.Aime S, Botta M, Crich SG, Giovenzana G, Palmisano G, Sisti M. Chem. Commun. 1999:1577. [Google Scholar]
- 132.Aime S, Fedeli F, Sanino A, Terreno E. J. Am. Chem. Soc. 2006;128:11326. doi: 10.1021/ja062387x. [DOI] [PubMed] [Google Scholar]
- 133.Borel A, Helm L, Merbach AE. Chem.-Eur. J. 2001;7:600. doi: 10.1002/1521-3765(20010202)7:3<600::aid-chem600>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 134.Ali MM, Woods M, Caravan P, Opina ACL, Spiller M, Fettinger JC, Sherry AD. Chem.-Eur. J. 2008;14:7250. doi: 10.1002/chem.200800402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gianolio E, Porto S, Napolitano R, Baroni S, Giovenzana GB, Aime S. Inorg. Chem. 2012;51:7210. doi: 10.1021/ic300447n. [DOI] [PubMed] [Google Scholar]
- 136.Martinelli J, Fekete M, Tei L, Botta M. Chem. Commun. 2011;47:3144. doi: 10.1039/c0cc05428c. [DOI] [PubMed] [Google Scholar]
- 137.Liang G, Ronald J, Chen Y, Ye D, Pandit P, Ma ML, Rutt B, Rao J. Angew. Chem., Int. Ed. 2011;50:6283. doi: 10.1002/anie.201007018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao C-Y, Shen Y-Y, Wang J-D, Li L, Liang G-L. Sci. Rep. 2013;3:1. doi: 10.1038/srep01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Galiana G, Branca RT, Jenista ER, Warren SW. Science. 2008;322:421. doi: 10.1126/science.1163242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grüll H, Langereis S. J. Controlled Release. 2012;161:317. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 141.Terreno E, Sanino A, Carrera C, Castelli DD, Giovenzana GB, Lombardi A, Mazzon R, Milone L, Visigalli M, Aime S. J. Inorg. Biochem. 2008;102:1112. doi: 10.1016/j.jinorgbio.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 142.Strijkers GJ, Mulder WJM, van Heeswijk RB, Frederik PM, Bomans P, Magusin PCMM, Nicolay K. Magn. Reson. Mater. Phys., Biol. Med. 2005;18:186. doi: 10.1007/s10334-005-0111-y. [DOI] [PubMed] [Google Scholar]
- 143.de Smet M, Langereis S, van den Bosch S, Grüll H. J. Controlled Release. 2010;143:120. doi: 10.1016/j.jconrel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 144.Tagami T, Foltz WD, Ernsting MJ, Lee CM, Tannock IF, May JP, Li S-D. Biomaterials. 2011;32:6570. doi: 10.1016/j.biomaterials.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 145.Shuhendler AJ, Staruch R, Oakden W, Gordijo CR, Rauth AM, Stanisz GJ, Chopra R, Wu XY. J. Controlled Release. 2012;157:478. doi: 10.1016/j.jconrel.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 146.Lehner R, Wang X, Wolf M, Hunziker P. J. Controlled Release. 2012;161:307. doi: 10.1016/j.jconrel.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 147.Vandsburger MH, Radoul M, Cohen B, Neeman M. NMR Biomed. 2012:1. doi: 10.1002/nbm.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang Z, Greenfield MT, Spiller M, McMurry TJ, Lauffer RB, Caravan P. Angew. Chem., Int. Ed. 2005;44:6766. doi: 10.1002/anie.200502245. [DOI] [PubMed] [Google Scholar]
- 149.Xu W, Lu Y. Chem. Commun. 2011;47:4998. doi: 10.1039/c1cc10161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swanson SD, Kukowska-Latallo JF, Patri AK, Chen C, Ge S, Cao Z, Kotlyar A, East AT, Baker JR. Int. J. Nanomed. 2008;3:201. [PMC free article] [PubMed] [Google Scholar]
- 151.Caravan P, Greenwood JM, Welch JT, Franklin SJ. Chem. Commun. 2003:2574. doi: 10.1039/b307817e. [DOI] [PubMed] [Google Scholar]
- 152.Catanzaro V, Gringeri CV, Menchise V, Padovan S, Boffa C, Dastrù W, Chaabane L, Digilio G, Aime S. Angew. Chem., Int. Ed. 2013;52:3926. doi: 10.1002/anie.201209286. [DOI] [PubMed] [Google Scholar]
- 153.Villaraza AJL, Bumb A, Brechbiel MW. Chem. Rev. 2010;110:2921. doi: 10.1021/cr900232t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Viswanathan S, Kovacs Z, Green KN, Ratnakar SJ, Sherry AD. Chem. Rev. 2010;110:2960. doi: 10.1021/cr900284a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ward KM, Aletras AH, Balaban RS. J. Magn. Reson. 2000;143:79. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 156.Wolff SD, Balaban RS. Magn. Res. Med. 1989;10:135. doi: 10.1002/mrm.1910100113. [DOI] [PubMed] [Google Scholar]
- 157.Ward KM, Balaban RS. Magn. Res. Med. 2000;44:799. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 158.Glunde K, Artemov D, Penet M-F, Jacobs MA, Bhujwalla ZM. Chem. Rev. 2010;110:3043. doi: 10.1021/cr9004007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Løkling K-E, Skurtveit R, Dyrstad K, Klaveness J, Fossheim SL. Int. J. Pharm. 2004;274:75. doi: 10.1016/j.ijpharm.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 160.Vibhute SM, Engelmann J, Verbic T, Maier ME, Logothetis NK, Angelovski G. Org. Biomol. Chem. 2013;11:1294. doi: 10.1039/c2ob26555a. [DOI] [PubMed] [Google Scholar]
- 161.Toth E, Bolskar RD, Borel A, Gonzalez G, Helm L, Merbach AE, Sitharaman B, Wilson LJ. J. Am. Chem. Soc. 2005;127:799. doi: 10.1021/ja044688h. [DOI] [PubMed] [Google Scholar]
- 162.Kim T, Cho E-J, Chae Y, Kim M, Oh A, Jin J, Lee E-S, Baik H, Haam S, Suh J-S, Huh Y-M, Lee K. Angew. Chem., Int. Ed. 2011;50:10589. doi: 10.1002/anie.201103108. [DOI] [PubMed] [Google Scholar]
- 163.Chen Y, Yin Q, Ji X, Zhang S, Chen H, Zheng Y, Sun Y, Qu H, Wang Z, Li Y, Wang X, Zhang K, Zhang L, Shi J. Biomaterials. 2012;33:7126. doi: 10.1016/j.biomaterials.2012.06.059. [DOI] [PubMed] [Google Scholar]
- 164.Szabo I, Crich SG, Alberti D, Kalman FK, Aime S. Chem. Commun. 2012;48:2436. doi: 10.1039/c2cc17801j. [DOI] [PubMed] [Google Scholar]
- 165.Li Y, Qian Y, Liu T, Zhang G, Liu S. Biomacromolecules. 2012;13:3877. doi: 10.1021/bm301425j. [DOI] [PubMed] [Google Scholar]