Abstract
Xanthosoma sagittifolium Schott is a herb of the Araceae family, popularly known as taioba, which is consumed as food in some regions of Brazil, Africa, and Asia. This species has already been evaluated for the antifungal activities. However, based on its potential antitumor activity, the present study further aimed to examine the antitumor, as well as chelation, activity of X. sagittifolium leaf extract. Results showed that hydroethanolic extract of X. sagittifolium leaves (HEXs-L) exhibits cytotoxic effects against the immortalized line of human T-lymphocytic (Jurkat) and myelogenous (K562) leukemia cells, but not nontumor RAW 264.7 macrophages or NIH/3T3 fibroblasts. HEXs-L inhibited 50.3% of Jurkat cell proliferation, reducing by 20% cells in G2/M phase, but increasing cells in sub-G1 phase, thereby inducing apoptosis by 54%. In addition, HEXs-L inhibited NO production by 59%, as determined by Griess reaction, and chelated 93.8% of free Fe(II), as demonstrated by ferrozine assay. Phytochemical studies were carried out by ESI-MS, identifying apigenin di-C-glycosides as major compounds. Overall, this work revealed that leaf extract of Xanthosoma sagittifolium presented chelating activity and in vitro antitumor activity, arresting cell cycle and inducing apoptosis of leukemia cells, thus providing evidence that taioba leaves may have practical application in cancer therapy.
1. Introduction
Cancer is characterized by uncontrolled growth, invasion, and, sometimes, metastasis [1]. Its incidence grows annually worldwide, and, in Brazil, it is the second leading cause of death. According to the Brazilian National Cancer Institute (NCI), leukemia is a hematologic cancer characterized by abnormal and uncontrolled proliferation of white blood cells, grouped as lymphoid or myeloid cells, occurring in both children and adults. According to the National Cancer Institute [2], 9,370 new cases of leukemia would occur in Brazil, including 5,050 men and 4,320 women, with the potential to cause such pathophysiological changes as anemia, neutropenia, thrombocytopenia, fever, bleeding, osteoarticular pain, fatigue, and dyspnea [2–4].
Epidemiological evidence has pointed to a connection between chronic inflammation and predisposition for cancer development [5], suggesting that the inflammatory microenvironment can increase mutation rates and accompanying cell proliferation [6]. Such activated inflammatory cells serve as a source of high levels of reactive oxygen species (ROS), including superoxide anion, hydroxyl radicals, and hydroperoxide, as well as nitrogen reactive intermediates (NRS), such as nitric oxide (NO) or peroxynitrite. Together, ROS and NRS are capable of inducing genomic instability, cell damage, and/or death [7]. In addition, nitric oxide is an important mediator of chronic inflammation and regulates cell proliferation, survival, migration, angiogenesis, DNA repair, and tumorigenesis [8]. Accordingly, minimizing ROS and NRS production could protect normal cells from oxidative damage and tumorigenesis [9].
On the other hand, iron is essential for cell growth and proliferation, and perturbation in cellular iron uptake can arrest cell viability, both in vitro and in vivo [10–12]. Many studies have reported on the potential utility of iron deprivation for the treatment of multiple neoplasias [12–14]. Furthermore, bivalent transition metal ions play an important role as catalysts of oxidative processes, leading to the formation of hydroxyl radicals via Fenton reaction [15].
The identification of new compounds with potential anticancer activity derived from herbaceous foods or medicinal plant extracts has been widely explored in recent years [16], especially those that can serve as immunomodulators or antitumor and antioxidant agents. X. sagittifolium Schott, commonly known as taioba, tannia, or cocoyam, is an herbaceous plant of the Araceae family widely grown in many parts of Africa, America, and Asia [17]. The leaves of this species are an excellent source of calcium, phosphorus, iron, and some vitamins [18], and they are consumed as food in South America, especially in Brazil [19]. The rhizome is an important source of starch used as part of the diet in the Amazon region [18]. Despite its nutritional value, X. sagittifolium Schott is a poorly studied medicinal plant, being primarily used for treatment of bone diseases based on the results of free calcium (Ca2+) analyses [20]. In addition, antifungal activity has been demonstrated for the aqueous extract of X. sagittifolium [21].
Based on the antifungal activity of X. sagittifolium that antifungal extracts can also present antineoplastic activity [22–24] and considering that antitumor action has never been reported in this species this study aimed to determine the antileukemic potential of X. sagittifolium leaf extract, as well as its iron-chelating activity and inhibition of nitric oxide production.
2. Materials and Methods
2.1. Plant Material and Extract Preparation
Material of X. sagittifolium was collected at Nova Friburgo, Rio de Janeiro, Brazil, and identified by Dr. Cassia Sakuragui, Botanic Department of Rio de Janeiro Federal University. An exsiccate is deposited in the Herbarium of the Jardim Botânico do Rio de Janeiro, RJ, Brazil (RB 432051). Air-dried and powdered plant material was macerated in ethanol 70%. The rhizomes (HEXs-R) and leaves (HEXs-L) hydroethanolic extracts were obtained after filtration and solvent evaporation at 60°C (rotary evaporator, Pemen, with vacuum pump Cole Parmer, Instrument Company, Model 7049-50, and a bath, Fisatom, Brazil) and lyophilization (Labconco, Brazil). The extracts were stored at −20°C until use. For cell culture assays, the extracts were dissolved (50 mg/mL) in dimethyl sulfoxide (DMSO) and stored at −20°C. Each extract was diluted in RPMI-1640 medium supplemented with 10% fetal calf serum (RPMI supplemented medium) before experiments.
2.2. Cell Culture
Jurkat cells (T-lymphocytic leukemia cell line) were kindly donated by the Basic Research Center of Cancer National Institute of Rio de Janeiro, Rio de Janeiro, Brazil, while K562 cells (myelogenous leukemia cell line), NIH-3T3 (nontumor fibroblast cell line), and RAW-264.7 (macrophage cell line) cells were purchased from Rio de Janeiro Cell Bank, Brazil. The leukemia and NIH-3T3 cells were cultured in RPMI-1640 medium supplemented with 10% fetal calf serum (FCS). RAW 264.7 cells were maintained in DMEM supplemented with 10% FCS and 15 mM HEPES. The cells were expanded two-three times a week. Cultures were incubated at 37°C in humidified atmosphere with 5% CO2. Maximal DMSO concentration used in the experimental conditions was 0.05%. Cultures containing medium with DMSO did not present cytotoxic effects.
2.3. MTT Cytotoxicity Assay
Leukemia cells (1 × 105/mL) were cultured for 46 h (96-well plate) with different extract concentrations and the cytotoxicity was determined by the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] method [25]. RAW 264.7 (5 × 105 cells/mL) and NIH-3T3 cells (1 × 105 cells/mL) were seeded at 96-well plate and previously incubated 24 h for adhesion. Subsequently, NIH-3T3 cells were incubated for more 46 h with HEXs-L (10–30–50–70–100 μg/mL), and RAW 264.7 cells were incubated for more 22 h with HEXs-L (10–30–50–70–100 μg/mL), both in the presence of E. coli lipopolysaccharide (LPS) at 1 µg/mL. After cell incubation with plant samples, each well received 10 µL of MTT (5 mg/mL stock solution) and was incubated for 2 h at 37°C and 5% CO2. The formazan crystal was dissolved adding 100 μL/well of 10% sodium dodecyl sulfate solution with HCl 0.01 N. The absorbance was measured at 570 nm after 24 h incubation at 37°C (microplate reader μQuant, Bio-Tek Instruments, Inc.) and viability to each cell line was determined.
2.4. Cell Cycle Analysis
Jurkat cells (1 × 105/mL) were cultured in the absence or presence of HEXs-L 50 µg/mL or MTX 2 µg/mL for 48 h. After incubation, the viable cell number was determined (by Trypan blue dye exclusion) and the cell cycle evaluated [26]. After centrifugation, cells (1 × 106) of each experimental condition were suspended in 43 mM citrate buffer (500 µL), containing propidium iodide (PI) 50 μg/mL and Triton X-100 0.3%, homogenized, and remained at room temperature for 15 min in the dark. RNase (100 μg/mL in 43 mM citrate buffer) was added (500 µL/experimental condition) and incubation proceeded for 15 min at room temperature. PI fluorescence (FL-3 channel) was analyzed by flow cytometry on a Gallios Cytometer (Beckman Coulter). Results were analyzed using Summit 4.3v software.
2.5. Annexin V Apoptosis Assay
Jurkat cells (1 × 105/mL) were cultured in supplemented RPMI 1640 in the absence or presence of HEXs-L 50 µg/mL or MTX 2 µg/mL for 48 h at 37°C and 5% CO2 and humidified atmosphere. Apoptotic cells were labelled using the Annexin V-FITC Apoptosis Detection Kit (BioLegend). Cells (1 × 106) were washed with phosphate buffered saline, pH 7.4 (PBS), and suspended in 500 µL of “binding buffer.” Subsequently, 100 µL of this solution (1 × 105 cells) was transferred into another tube and Annexin V-FITC and PI (100 µg/mL stock solution) were added. The reaction was maintained for 15 min in ice. After this time, “binding buffer” was added (400 µL/tube), and the cells were analyzed by flow cytometry. The fluorescence was detected (5 × 104 events) on a Gallios Cytometer (Beckman Coulter). Data were analyzed using the Summit 4.3v software.
2.6. Iron (II)-Chelating Activity
This assay was measured by the decrease of iron(II)-ferrozine complex [27]. The EEXs-L (100 µL) was added to an aqueous solution (140 µL) of 2.0 mM FeCl2·4H2O. After 10 min, the reaction was initiated by addition of 10 µL of 5.0 mM ferrozine solution. The absorbance at 562 nm was recorded after 10 min of reaction. Quercetin was used as a positive control of chelating activity. The negative control tube contained all the reagents, except the HEXs-L or quercetin, and presented the highest absorbance at 562 nm. The blank tube contained reagents and HEXs-L or quercetin (except ferrozine). Chelating activity (%) was determined based on the formula
| (1) |
2.7. Nitric Oxide Production
Nitric oxide was indirectly determined by measuring nitrite content in the RAW 264.7 macrophage culture supernatant using Griess reagent [28]. Cells (5 × 105/mL) were previously cultured in a 96-well plate for 24 h for adhesion. They were then treated with LPS (1 μg/mL) and different HEXs-L concentrations (25–50–100 μg/mL) for more 24 h. The supernatant cell culture (50 μL) was taken from each well and incubated with 25 μL of sulfanilic acid for 10 min in the dark at room temperature. Then, 25 μL of N-(1-naphthyl)-ethylenediamine was added and the plate was incubated again for 10 min at room temperature in the dark. Finally, the absorbance was read at 550 nm (microplate reader μQuant, Bio-Tek Instruments, Inc.).
2.8. Phytochemical Analysis
The chemical characterization of the HEXs-L was achieved by electrospray ionization mass spectrometry (ESI-MS). LCQ equipment Fleet (Thermo Scientific) was used, equipped with electron-spray source operating in negative mode with the analyzer ion trap type. The samples were diluted in 200 µL methanol for 10 mg/mL concentration and injected by direct insertion. The source temperature was set at 180°C, the drying gas (nitrogen) flow rate was 4.0 L/min, and the nebulizer gas (nitrogen) pressure was 0.4 bar. In negative mode, the capillary voltage was 3.8 kV, the capillary exit voltage was −150 V, and the skimmers 1 and 2 voltages were 50 V and 15 V, respectively. Mass calibration was achieved by infusing ammonium formate in an isopropanol-water mixture (1 : 1, v/v) as an external standard. The Xcalibur software was used to obtain spectra in the range of m/z 100–1500. The detector was a mass spectrometer ion trap type equipped with an interface ESI and analyses were performed using a micrOTOF II mass spectrometer (Bruker Daltonics, Inc., Boston, MA, USA).
2.9. Statistical Analysis
The variance analysis was assessed by one-way ANOVA. Differences were considered statistically significant when p < 0.05, using Tukey's posttest for differences between three or more experimental groups and Student's t-test between two groups. The statistical comparisons were performed using the GraphPad Prism software, version 5.0 (GraphPad Software Inc., San Diego, USA).
3. Results
3.1. The In Vitro Cytotoxicity of X. sagittifolium Extracts
To investigate the effect of X. sagittifolium extracts on leukemia cells, the cytotoxicity of both leaf (HEXs-L) and rhizome (HEXs-R) extracts (50 µg/mL) was evaluated on lymphocytic (Jurkat) and myelocytic (K562) leukemia cells. The results, as shown in Figure 1, indicated that treatment for 48 h with HEXs-L partially inhibited mitochondrial reduction activity (MRA) of both leukemia cell lines, with Jurkat cells (Figure 1(a)) presenting higher inhibition index (33.6 ± 10.8%) than K562 cells (17.3 ± 7.5%) (Figure 1(b)). HEXs-R was not cytotoxic against leukemia cells. The effects of other HEXs-L concentrations (25–100 μg/mL) were evaluated as well, presenting IC50 of 95.9 μg/mL (Figure 2(a)). HEXs-L showed no cytotoxicity against NIH/3T3 fibroblasts (Figure 2(b)).
Figure 1.
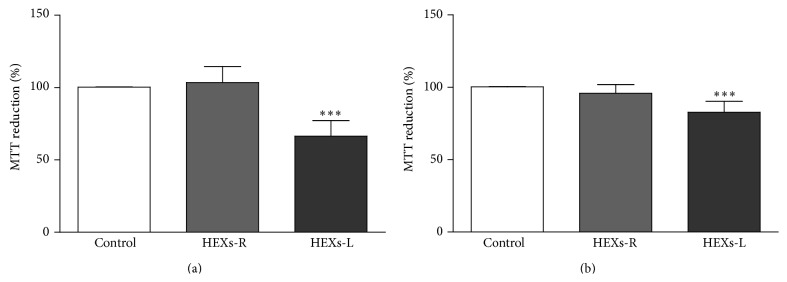
Cytotoxic effects of HEXs on leukemic cells. (a) Jurkat cells. (b) K562 cells. Cells (1 × 105/mL) were incubated in the absence (control) or presence of rhizome (HEXs-R) or leaf extracts (HEXs-L) at 50 µg/mL for 48 h. Cytotoxicity was determined by the MTT assay. The results express mean percentage of control ± S.D. of three experiments with triplicates. ∗∗∗ p < 0.001, related to control, by one-way ANOVA followed by Tukey's posttest.
Figure 2.
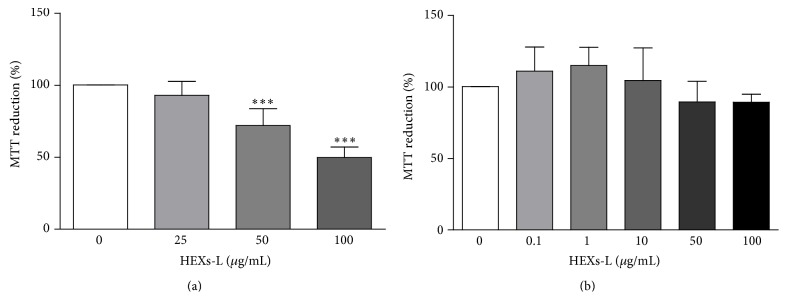
Cytotoxic effects of HEXs-L on leukemia and normal cells. (a) Jurkat cells. (b) Nontumor NIH fibroblasts. Cells were incubated in the absence (control) or presence of HEXs-L for 48 h. Cytotoxicity was determined by the MTT assay. The results express mean percentage of control ± S.D. of three experiments with triplicates. ∗∗∗ p < 0.001, related to control, by one-way ANOVA followed by Tukey's posttest.
3.2. Effects of HEXs-L on Jurkat Cell Proliferation and Apoptosis
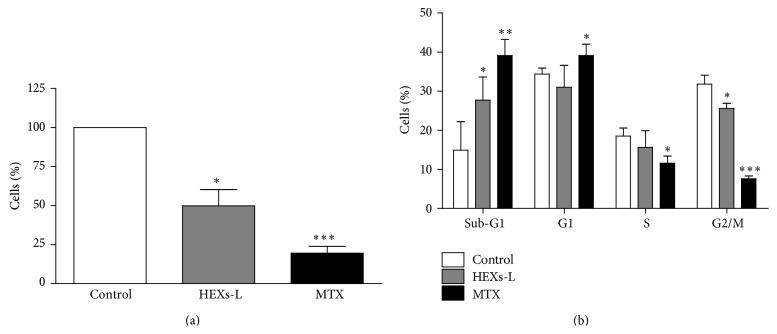
Treatment with HEXs-L for 48 h inhibited Jurkat cell proliferation, reducing significantly (50.7%) the viable cell number, compared to control (Figure 3(a)), as observed with methotrexate- (MTX) (80.5%) treated cultures. This HEXs-L effect was accompanied by an increase (85.9%) of cells in the sub-G1 phase and a reduction (20.0%) of cells in the G2/M phase (Figure 3(b)). These effects in the cell cycle were also observed with the MTX treatment, although at higher level than the plant extract (Figure 4(b)). In addition, MTX increased cells in the G1 phase (13%) and reduced them in the S phase (38%), compared to HEXs-L, 85.9% and 20%, respectively, as noted above.
Figure 3.
Effects of HEXs on Jurkat cell proliferation. (a) Viable cell number. (b) Cell cycle analysis. Cells (1 × 105/mL) were treated with HEXs-L (50 µg/mL) or MTX (2 µg/mL) or culture medium (control) for 48 h and then counted by contrast phase microscopy and processed for cell cycle analysis by flow cytometry, as described in Materials and Methods section. The results express mean ± S.D. of five independent experiments. ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001, related to control, by one-way ANOVA followed by Tukey's posttest.
Figure 4.
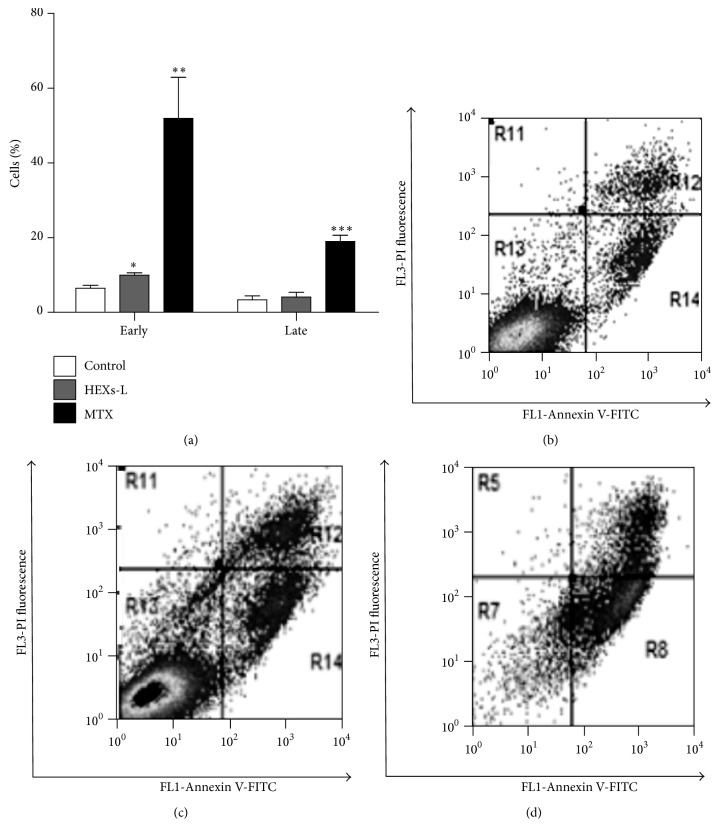
Effects of HEXs-L on Jurkat cell apoptosis by annexin-V-FITC labeling assay. (a) Early and late apoptotic cells (mean of three independent experiments). Cytograms of a representative experiment are shown in (b) control, (c) HEXs-L, and (d) MTX treated cultures. Cells (1 × 105/mL) were treated with HEXs-L (50 µg/mL) or MTX (2 µg/mL) or culture medium (control) for 48 h and then processed for flow cytometric analysis of apoptosis as described in Materials and Methods section. The results in (d) express mean ± S.D. ∗ p < 0.05, ∗∗ p < 0.01, and ∗∗∗ p < 0.001, related to control, by one-way ANOVA followed by Tukey's posttest.
Investigating the effects of HEXs-L (50 µg/mL) on cell death, the percentage of early apoptotic cells was increased (54.2%), compared to control culture, while MTX induced a higher level of early (7-fold) and late (4.6-fold) apoptosis, compared to control or HEXs-L treatment (Figure 4(a)). Cytograms of a representative apoptosis experiment are also shown in Figures 4(b)–4(d).
3.3. Iron Chelation Activity and NO Production
The inflammation microenvironment, rich in reactive oxygen (ROS) and NO/peroxynitrite, and cancer promotion have been linked. The antioxidant property of HEXs-L was then investigated by ferrous iron-chelating ability, since Fe2+ can lead to hydroxyl radical (OH∙) production via Fenton reaction. The results (Figure 5) indicated a high ferrous iron-chelating ability for HEXs-L that did not differ significantly from that of quercetin, the positive control of ferrous iron-chelating activity used in this assay. HEXs-L also reduced NO production by RAW 264.7 macrophages in a concentration-dependent manner (Figure 6(a)), showing IC50 of 86.3 µg/mL, without cytotoxic effects (Figure 6(b)).
Figure 5.
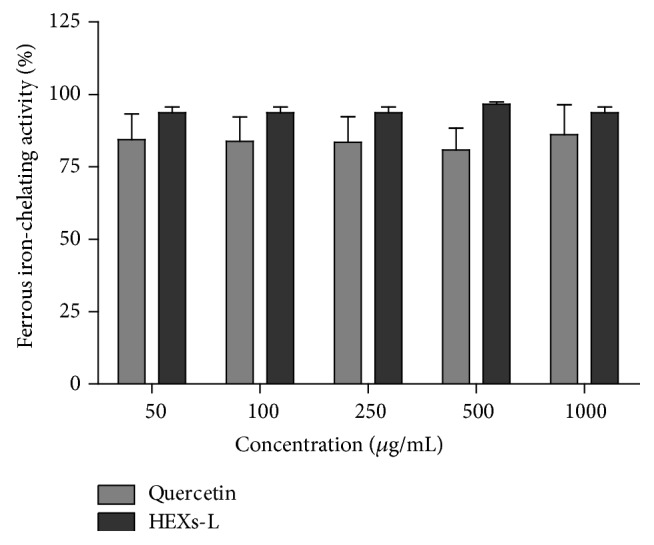
Effects of HEXs-L on iron(II)-chelating activity by the ferrozine assay. Quercetin was used as a chelating activity positive control. The results express mean ± S.D. of three experiments with triplicates. There were no significant differences between HEXs-L and quercetin, by Student's t-test.
Figure 6.
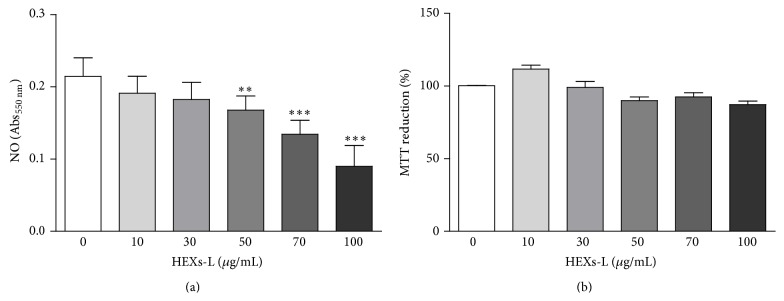
Effects of HEXs-L on RAW 264.7 macrophages. (a) NO production. (b) Cytotoxicity. Cells (5 × 105/mL) were treated or not with HEXs-L for 24 h and NO was then indirectly estimated in culture supernatant by nitrite determination (Griess reaction) and cell cytotoxicity was evaluated by MTT assay. The results express mean ± S.D. of three experiments with triplicates. ∗ p < 0.05 and ∗∗∗ p < 0.001, related to control, by one-way ANOVA followed by Tukey's posttest.
3.4. Phytochemical Analysis of the HEXs-L
The chemical analysis of HEXs-L showed two substances with molecular weight of 563.1422 and 593.1530 and molecular formulae of C26H27O14 and C27H29O15, respectively, as major compounds, proposed as apigenin di-C-glycosides, as well as sucrose, pyroglutamic acid (5-oxoproline), and some fatty acids (palmitic, stearic, and linolenic acids) by ESI-MS (Figure 7).
Figure 7.
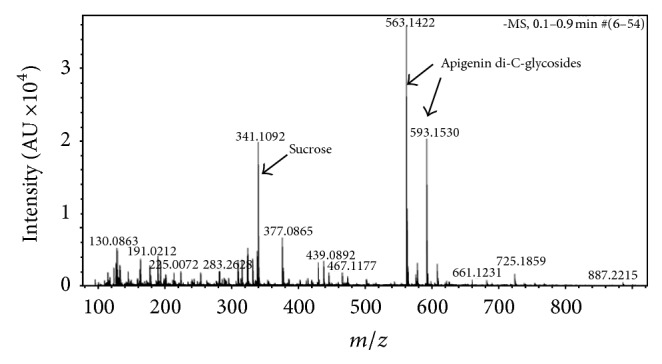
The electron-spray ionization (ESI) mass spectrum of 70% hydroalcoholic extract of Xanthosoma sagittifolium leaves. MS data were acquired in the negative ionization mode. The mass detector was an ion trap spectrometer equipped with an ESI interface. All data were analyzed using Bruker Daltonics ESI Compass Data Analysis version 4.0 SP1 (Bruker Daltonics Inc., MA, USA).
4. Discussion
The present study investigated the potential effects of X. sagittifolium Schott extract on the in vitro growth of leukemia cells, NO production, and iron-chelating activity. Mitochondria play a crucial role in cell survival and function by generating most of the cell's supply of adenosine triphosphate (ATP) [29]. The inhibition of mitochondrial reduction activity by the effect of HEXs-L on leukemia cells, as observed in this work, may be related to cytotoxic substances in the leaf, but not the rhizome (HEXs-R), since even higher concentrations of HEXs-R were not toxic. Leaves of plants of the Xanthosoma genus have previously been recognized as containing cytotoxic compounds, showing bactericidal activity, for example, X. robustum extract [30], and antifungal property, for example, X. sagittifolium extract [21]. In addition, aerial parts of plants have been used as popular medicines to treat a broad range of diseases, including cancer [31]. The toxicity of HEXs-L to leukemia cells does not result from dimethyl sulfoxide (DMSO), since control cultures with this solvent did not alter the responses (data not shown).
The lower sensitivity of myeloid K562 leukemia cells, compared to lymphocytic Jurkat cells, to the cytotoxic effects of HEXs-L, can be attributed to the lower expression of ABCG2 receptor in Jurkat cells, an ABC membrane transporter that induces drug resistance [32]. It is important to note that the treatment of nontumor NIH/3T3 fibroblasts with HEXs-L did not reduce their viability, which is an important finding since many antineoplastic drugs are extremely cytotoxic to nontumor cells [33]. The extract of other Araceae plants, such as Rhaphidophora korthalsii Scott [34] and Typhonium blumei Nicolson & Sivad [35], did not present cytotoxic effects to normal cells or affect the sensitivity of normal cells to a lesser extent than tumor cells, respectively. Or cytotoxicity to normal cells is less significant.
Since HEXs-L was demonstrated to reduce mitochondrial function in tumor cells, we evaluated if the extract could also inhibit Jurkat cell proliferation or only its activation. It was found that HEXs-L significantly reduced the number of Jurkat cells in the culture. This inhibitory effect on leukemia cell proliferation was corroborated by a decrease in the percentage of cells in the G2/M phase.
Iron is essential for neoplastic cell proliferation; it participates in DNA synthesis and macromolecule biosynthesis; accordingly neoplastic cells have a high iron requirement [36]. In the absence of iron, cells are unable to progress in the cell cycle. Recently, the role of iron chelation as antitumor agents has been investigated by targeting ribonucleotide reductase, the enzyme responsible for deoxyribonucleotide synthesis, which is the iron dependent enzyme involved in the rate limiting phase of DNA synthesis [37–39]. Therefore, we also studied the iron-chelating property of HEXs-L, which showed a high level of this activity. Meanwhile, other studies have shown that treatment with HEXs-L may result in a lower concentration of cellular deoxyribonucleotides in tumor cells, impairing their ability to complete the S phase of cell cycle. Consequently, cell growth and preparation for cell division would be curtailed in G2, leading to unsuccessful mitosis and cytokinesis in M phase, as described for the desferrioxamine, an iron-chelating agent that binds free iron in a stable complex, which is currently used to treat iron-overload disease and tumor cell growth [40–42]. Since several reports have also revealed the antiproliferative activity of desferrioxamine [43, 44], this hypothesis needs further investigation, which, however, is beyond the scope of the present report.
The increased number of leukemia cells in the sub-G1 phase after treatment with HEXs-L is an indication of DNA fragmentation in these cells. Since apoptosis is frequently associated with cell cycle arrest [45] and since apoptotic cells also show DNA fragmentation, we decided to investigate the effects of HEXs-L on the induction of apoptosis. Apoptosis is also characterized by other biochemical events, such as surface exposure of phosphatidylserine phospholipid (Ptd-L-Ser), mitochondrial membrane permeabilization, and activation of caspases [46]. Therefore, Jurkat cell apoptosis was evaluated by the surface exposure of Ptd-L-Ser. To accomplish this by performing flow cytometry and based on the increased number of Annexin V-positive cells, HEXs-L treatment was confirmed to induce apoptosis in Jurkat cells. This result is important because hyperproliferative conditions, such as autoimmune disease and cancer, are frequently associated with apoptosis impairment [46] while the induction of apoptosis is found as a mechanism of action for many traditional antineoplastic drugs [47].
Although low concentration of NO mediates important physiological processes [48] high levels play crucial role in antimicrobial and antiviral responses [49] and can trigger oxidative stress, chronic inflammation [50], and nitrosylation, in particular S-nitrosylation of mitochondrial caspase-8, caspase-9, and Bcl-2 results in the inhibition of apoptosis of tumor cells [51]. The reduction of the NO level in the culture supernatant of LPS-stimulated macrophages by treatment with HEXs-L, as described for other plants of the Araceae family [52], may represent an important finding since reduction of nitrosylation of caspases would, in turn, lead to the activation of caspases to cleave cellular organisms in the cell and cause apoptosis, thus contributing to the surveillance of tumor cell promotion/progression. Additionally, NO mediates inflammation and epidemiological studies have shown that chronic inflammation predisposes individuals to different types of cancer [53]. Many studies reporting on cancer development have focused on inflammation as a potential mechanism underlying the disease in its early stages [53]. Inhibition of NO production by HEXs-L can also represent a potential mechanism of inhibiting tumorigenesis.
Redox homeostasis is important for cell survival because it regulates the functions of transcription factors, signal transduction pathways, and mediators of cell proliferation and death [54]. High levels of ROS and NRS lead to oxidative stress and deleterious modifications to DNA, lipids, and proteins [55], inducing chronic inflammation and, potentially, cancer. Thus, the presence of free Fe2+, for example, can, through Fenton's reaction, lead to hydroxyl radical (OH∙) production, contributing to oxidative stress [56]. Furthermore, NO can react with superoxide, which results in the production of peroxynitrite, a more powerful oxidant. In this context, HEXs-L presents the property of chelating free Fe2+ which can promote antitumor activity by reducing ribonucleotide reductase activity and DNA synthesis, as well as reducing cell damage by oxidative stress, thus avoiding chronic inflammation and/or cancer development [56].
The use of plants as a source of phytochemical compounds in therapeutic medicine is increasingly being recognized [57]. In addition, flavonoids can affect the cell cycle regulation of cancer cells and apigenin glycoside has been found to be one of them [58]. The phytochemical analysis of HEXs-L by ESI-MS identified apigenin diglycosides as major compounds, corroborating reports [59, 60] that plants of this family are rich in apigenin and other flavonoids. Interestingly, apigenin and apigenin derivatives have previously been reported to inhibit the proliferation of leukemia cells [47, 61] chelating free Fe2+, induce leukemia apoptosis [62], and inhibit NO production [63]. Flavonoids are a group of natural compounds found in plants with variable phenolic structures. Their mechanisms of action can include scavenging superoxide, H2O2, nitric oxide, and hydroxyl radicals [64, 65]. Furthermore, flavonoid antioxidant capacity involves combined effect of radical scavenging activity, interaction with enzyme functions, or chelating elements involved in free radical generation [65]. In summary, Xanthosoma sagittifolium contains apigenin glycosides as major compounds and shows important biological activities, including Fe2+ chelation, inhibition of NO production, and inhibition of leukemia cell proliferation, mechanisms reported for traditional antitumor agents [66]. However, while antitumor activity may result from the presence of apigenin glycosides in the HEXs-L, it is also likely that other phytochemical compounds in the extract could be associated with this activity, as well as other biological effects noted above.
5. Conclusions
For the first time, the present work describes the antitumor activities of hydroethanolic extract of Xanthosoma sagittifolium Schott (taioba) leaves, including Fe2+ chelation, inhibition of NO production, and inhibition of leukemia cell proliferation. These properties may principally result from the presence of apigenin glycosides in HEXs-L. Therefore, by revealing the chelating activity of Xanthosoma sagittifolium leaf extract, as well as its antitumor activity, including cell cycle arrest and the induction of apoptosis, this work has provided evidence that taioba leaves may have practical application in cancer therapy.
Acknowledgments
The authors thank Fernanda Cristina Jordão Magalhães and the staff of LIA-BPPN for technical assistance. This work was supported by grants from FAPERJ (E26/101.478/2010, TCT-E26/102.211/2010), Rio de Janeiro, Brazil.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Kim H., Moon J. Y., Mosaddik A., Cho S. K. Induction of apoptosis in human cervical carcinoma HeLa cells by polymethoxylated flavone-rich Citrus grandis Osbeck (Dangyuja) leaf extract. Food and Chemical Toxicology. 2010;48(8-9):2435–2442. doi: 10.1016/j.fct.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 2.INCA. Instituto Nacional do Câncer, (Brazilian National Cancer Institute), 2014, http://www.inca.gov.br/estimativa/2014/sintese-de-resultados-comentarios.asp.
- 3.Brunton L. L., Lazo J. S., Parker K. L., editors. Goodman & Gilman's. The Pharmacological Basis of Therapeutics. 11th. New York, NY, USA: McGraw-Hill; 2006. [Google Scholar]
- 4.Cipolat S., Pereira B. B., Vargas F. Fisioterapia em Pacientes com Leucemia: Revisão Sistemática. Revista Brasileira de Cancerologia. 2011;2(57):229–236. [Google Scholar]
- 5.Ferreri A. J. M., Ernberg I., Copie-Bergman C. Infectious agents and lymphoma development: molecular and clinical aspects. Journal of Internal Medicine. 2009;265(4):421–438. doi: 10.1111/j.1365-2796.2009.02083.x. [DOI] [PubMed] [Google Scholar]
- 6.Chiba T., Marusawa H. A novel mechanism for inflammation-associated carcinogenesis; an important role of activation-induced cytidine deaminase (AID) in mutation induction. Journal of Molecular Medicine. 2009;87(10):1023–1027. doi: 10.1007/s00109-009-0527-3. [DOI] [PubMed] [Google Scholar]
- 7.Shukla S., Mehta A., John J., Singh S., Mehta P., Vyas S. P. Antioxidant activity and total phenolic content of ethanolic extract of Caesalpinia bonducella seeds. Food and Chemical Toxicology. 2009;47(8):1848–1851. doi: 10.1016/j.fct.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Farinati F., Piciocchi M., Lavezzo E., Bortolami M., Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Digestive Diseases. 2010;28(4-5):579–584. doi: 10.1159/000320052. [DOI] [PubMed] [Google Scholar]
- 9.Ebrahimzadeh M. A., Nabavi S. F., Nabavi S. M., Pourmorad F. Nitric oxide radical scavenging potential of some Elburz medicinal plants. African Journal of Biotechnology. 2010;9(32):5212–5217. [Google Scholar]
- 10.Yu Y., Wong J., Lovejoy D. B., Kalinowski D. S., Richardson D. R. Chelators at the cancer coalface: desferrioxamine to triapine and beyond. Clinical Cancer Research. 2006;12(23):6876–6883. doi: 10.1158/1078-0432.ccr-06-1954. [DOI] [PubMed] [Google Scholar]
- 11.Tam T. F., Leung-Toung R., Li W., Wang Y., Karimian K., Spino M. Iron chelator research: past, present, and future. Current Medicinal Chemistry. 2003;10(12):983–995. doi: 10.2174/0929867033457593. [DOI] [PubMed] [Google Scholar]
- 12.Gharagozloo M., Khoshdel Z., Amirghofran Z. The effect of an iron (III) chelator, silybin, on the proliferation and cell cycle of Jurkat cells: a comparison with desferrioxamine. European Journal of Pharmacology. 2008;589(1–3):1–7. doi: 10.1016/j.ejphar.2008.03.059. [DOI] [PubMed] [Google Scholar]
- 13.Richardson D. R. Iron chelators as therapeutic agents for the treatment of cancer. Critical Reviews in Oncology/Hematology. 2002;42(3):267–281. doi: 10.1016/s1040-8428(01)00218-9. [DOI] [PubMed] [Google Scholar]
- 14.Hileti D., Panayiotidis P., Hoffbrand A. V. Iron chelators induce apoptosis in proliferating cells. British Journal of Haematology. 1995;89(1):181–187. doi: 10.1111/j.1365-2141.1995.tb08927.x. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B. Antioxidants and human disease: a general introduction. Nutrition Reviews. 1997;55(1):S44–S49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 16.Rao P. S., Ramanadham M., Prasad M. N. V. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line—RPMI 8226. Food and Chemical Toxicology. 2009;47(2):283–288. doi: 10.1016/j.fct.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Jackix E. D. A., Monteiro E. B., Raposo H. F., Vanzela E. C., Amaya-Farfán J. Taioba (Xanthosoma sagittifolium) leaves: nutrient composition and physiological effects on healthy rats. Journal of Food Science. 2013;78(12):H1929–H1934. doi: 10.1111/1750-3841.12301. [DOI] [PubMed] [Google Scholar]
- 18.Carvalho E. F., Cordeiro J. A. D. Um método alternativo e eficiente de propagação vegetativa de inhame (Colocasia esculenta (L.) Schott e de taioba (Xanthosoma sagittifolium (L.) Schott. Acta Amazonica. 1990;20:11–18. [Google Scholar]
- 19.Pinto N. A. V. D., de Carvalho V. D., Corrêa A. D., de Oliveira Rios A. Avaliação de fatores antinutricionais das folhas da taioba (Xanthosoma sagittifolium Schoot) Ciência e Agrotecnologia. 2001;25:601–604. [Google Scholar]
- 20.de Oliveira G. L., Andrade L. H. C., de Oliveira A. F. M. Xanthosoma sagittifolium and Laportea aestuans: species used to prevent osteoporosis in Brazilian traditional medicine. Pharmaceutical Biology. 2012;50(7):930–932. doi: 10.3109/13880209.2011.637054. [DOI] [PubMed] [Google Scholar]
- 21.Schmourlo G., Mendonça-Filho R. R., Alviano C. S., Costa S. S. Screening of antifungal agents using ethanol precipitation and bioautography of medicinal and food plants. Journal of Ethnopharmacology. 2005;96(3):563–568. doi: 10.1016/j.jep.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Guo L., Wu J. Z., Han T., Cao T., Rahman K., Qin L.-P. Chemical composition, antifungal and antitumor properties of ether extracts of Scapania verrucosa Heeg. and its endophytic fungus Chaetomium fusiforme . Molecules. 2008;13(9):2114–2125. doi: 10.3390/molecules13092114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu L.-L., Han T., Wu J.-Z., et al. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine. 2009;16(6-7):609–616. doi: 10.1016/j.phymed.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 24.Ramírez J., Svetaz L., Quiroga J., et al. Synthesis of novel thiazole-based 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as potential antitumor and antifungal agents. European Journal of Medicinal Chemistry. 2015;92:866–875. doi: 10.1016/j.ejmech.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Pereira M. F., Martino T., Dalmau S. R., et al. Terpenic subfraction of Pterodon pubescens induces apoptosis of K562 leukemic cells by modulating gene expression. Oncology Reports. 2011;25(1):215–221. doi: 10.3892/or-00001063. [DOI] [PubMed] [Google Scholar]
- 27.Wang T., Jónsdóttir R., Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chemistry. 2009;116(1):240–248. doi: 10.1016/j.foodchem.2009.02.041. [DOI] [Google Scholar]
- 28.Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok J. S., Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-X. [DOI] [PubMed] [Google Scholar]
- 29.Fowler B. A., Kleinow K. M., Squibb K. S., Lucier G. W., Hayes W. Organelles as tools in toxicology. In: Hayes A. W., editor. Principles and Methods in Toxicology. 3rd. New York, NY, USA: Raven Press; 1994. pp. 1201–1230. [Google Scholar]
- 30.Kato T., Frei B., Heinrich M., Sticher O. Antibacterial hydroperoxysterols from Xanthosoma robustum . Phytochemistry. 1996;41(4):1191–1195. doi: 10.1016/0031-9422(95)00765-2. [DOI] [PubMed] [Google Scholar]
- 31.Kumar R. A., Sridevi K., Kumar N. V., Nanduri S., Rajagopal S. Anticancer and immunostimulatory compounds from Andrographis paniculata . Journal of Ethnopharmacology. 2004;92(2-3):291–295. doi: 10.1016/j.jep.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein B. E., Meissner A., Lander E. S. The mammalian epigenome. Cell. 2007;128(4):669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Nishanthini A., Mohan V. R. Antioxidant activites of Xanthosoma sagittifolium Schott using various in vitro assay models. Asian Pacific Journal of Tropical Biomedicine. 2012;2(3, supplement):S1701–S1706. doi: 10.1016/s2221-1691(12)60481-x. [DOI] [Google Scholar]
- 34.Yeap S. K., Alitheen N. B., Ali A. M., et al. Effect of Rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell (PBMC) proliferation and cytolytic activity toward HepG2. Journal of Ethnopharmacology. 2007;114(3):406–411. doi: 10.1016/j.jep.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 35.Hsu H. F., Huang K. H., Lu K. J., et al. Typhonium blumei extract inhibits proliferation of human lung adenocarcinoma A549 cells via induction of cell cycle arrest and apoptosis. Journal of Ethnopharmacology. 2011;135(2):492–500. doi: 10.1016/j.jep.2011.03.048. [DOI] [PubMed] [Google Scholar]
- 36.Heath J. L., Weiss J. M., Lavau C. P., Wechsler D. S. Effects of iron depletion on CALM-AF10 leukemias. Experimental Hematology. 2014;42(12):1022–1030. doi: 10.1016/j.exphem.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chenoufi N., Drénou B., Loréal O., Pigeon C., Brissot P., Lescoat G. Antiproliferative effect of deferiprone on the Hep G2 cell line. Biochemical Pharmacology. 1998;56(4):431–437. doi: 10.1016/s0006-2952(98)00071-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee D.-H., Jang P. S., Chung N. G., Cho B., Jeong D. C., Kim H. K. Deferasirox shows in vitro and in vivo antileukemic effects on murine leukemic cell lines regardless of iron status. Experimental Hematology. 2013;41(6):539–546. doi: 10.1016/j.exphem.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Sen S., Hassane D. C., Corbett C., Becker M. W., Jordan C. T., Guzman M. L. Novel mTOR inhibitory activity of ciclopirox enhances parthenolide antileukemia activity. Experimental Hematology. 2013;41(9):799–807. doi: 10.1016/j.exphem.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaston T. B., Lovejoy D. B., Watts R. N., Richardson D. R. Examination of the antiproliferative activity of iron chelators: multiple cellular targets and the different mechanism of action of triapine compared with desferrioxamine and the potent pyridoxal isonicotinoyl hydrazone analogue 311. Clinical Cancer Research. 2003;9(1):402–414. [PubMed] [Google Scholar]
- 41.Lucas J. J., Szepesi A., Domenico J., et al. Effects of iron-depletion on cell cycle progression in normal human T lymphocytes: selective inhibition of the appearance of the cyclin A-associated component of the p33cdk2 kinase. Blood. 1995;86(6):2268–2280. [PubMed] [Google Scholar]
- 42.Porreca E., Ucchino S., Di Febbo C., et al. Antiproliferative effect of desferrioxamine on vascular smooth muscle cells in vitro and in vivo . Arteriosclerosis and Thrombosis. 1994;14(2):299–304. doi: 10.1161/01.atv.14.2.299. [DOI] [PubMed] [Google Scholar]
- 43.Voest E. E., Vreugdenhil G., Marx J. J. M. Iron-chelating agents in non-iron overload conditions. Annals of Internal Medicine. 1994;120(6):490–499. doi: 10.7326/0003-4819-120-6-199403150-00008. [DOI] [PubMed] [Google Scholar]
- 44.Wang S. T., Jin K. L., Li T. H. The inhibitory effect of desferrioxamine on DNA synthesis in human lymphocytes. Chinese Medical Journal. 1989;102(12):902–905. [PubMed] [Google Scholar]
- 45.Piao W., Yoo J., Lee D. K., Hwang H. J., Kim J. H. Induction of G2/M phase arrest and apoptosis by a new synthetic anti-cancer agent, DW2282, in promyelocytic leukemia (HL-60) cells. Biochemical Pharmacology. 2001;62(11):1439–1447. doi: 10.1016/s0006-2952(01)00796-1. [DOI] [PubMed] [Google Scholar]
- 46.Kepp O., Galluzzi L., Lipinski M., Yuan J., Kroemer G. Cell death assays for drug discovery. Nature Reviews Drug Discovery. 2011;10(3):221–237. doi: 10.1038/nrd3373. [DOI] [PubMed] [Google Scholar]
- 47.Chen J., Li Z., Liu C., Yin L. The apoptotic effect of apigenin on human gastric carcinoma cells through mitochondrial signal pathway. Tumor Biology. 2014;35:7719–7726. doi: 10.1007/s13277-014-2014-x. [DOI] [PubMed] [Google Scholar]
- 48.de Palma C., Clementi E. Nitric oxide in myogenesis and therapeutic muscle repair. Molecular Neurobiology. 2012;46(3):682–692. doi: 10.1007/s12035-012-8311-8. [DOI] [PubMed] [Google Scholar]
- 49.Punturee K., Wild C. P., Vinitketkumneun U. Thai medicinal plants modulate nitric oxide and tumor necrosis factor-α in J774.2 mouse macrophages. Journal of Ethnopharmacology. 2004;95(2-3):183–189. doi: 10.1016/j.jep.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 50.Napolitano D. R., Mineo J. R., de Souza M. A., de Paula J. E., Espindola L. S., Espindola F. S. Down-modulation of nitric oxide production in murine macrophages treated with crude plant extracts from the Brazilian Cerrado. Journal of Ethnopharmacology. 2005;99(1):37–41. doi: 10.1016/j.jep.2005.01.059. [DOI] [PubMed] [Google Scholar]
- 51.Grimm E. A., Sikora A. G., Ekmekcioglu S. Molecular pathways: inflammation-associated nitric-oxide production as a cancer-supporting redox mechanism and a potential therapeutic target. Clinical Cancer Research. 2013;19(20):5557–5563. doi: 10.1158/1078-0432.ccr-12-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim O. K., Murakami A., Nakamura Y., Ohigashi H. Screening of edible Japanese plants for nitric oxide generation inhibitory activities in RAW 264.7 cells. Cancer Letters. 1998;125(1-2):199–207. doi: 10.1016/s0304-3835(97)00513-2. [DOI] [PubMed] [Google Scholar]
- 53.Mantovani A., Allavena P., Sica A., Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 54.Dajas F. Life or death: neuroprotective and anticancer effects of quercetin. Journal of Ethnopharmacology. 2012;143(2):383–396. doi: 10.1016/j.jep.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Fuchs-Tarlovsky V. Role of antioxidants in cancer therapy. Nutrition. 2013;29(1):15–21. doi: 10.1016/j.nut.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Liu S., Sun J., Yu L., et al. Antioxidant activity and phenolic compounds of Holotrichia parallela Motschulsky extracts. Food Chemistry. 2012;134(4):1885–1891. doi: 10.1016/j.foodchem.2012.03.091. [DOI] [PubMed] [Google Scholar]
- 57.Badmus J. A., Ekpo O. E., Hussein A. A., Meyer M., Hiss D. C. Antiproliferative and apoptosis induction potential of the methanolic leaf extract of Holarrhena floribunda (G. Don) Evidence-Based Complementary and Alternative Medicine. 2015;2015:11. doi: 10.1155/2015/756482.756482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meeran S. M., Katiyar S. K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Frontiers in Bioscience. 2008;13(6):2191–2202. doi: 10.2741/2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams C. A., Harborne J. B., Mayo S. J. Anthocyanin pigments and leaf flavonoids in the family araceae. Phytochemistry. 1981;20(2):217–234. doi: 10.1016/0031-9422(81)85096-0. [DOI] [Google Scholar]
- 60.Picerno P., Mencherini T., Lauro M. R., Barbato F., Aquino R. Phenolic constituents and antioxidant properties of Xanthosoma violaceum leaves. Journal of Agricultural and Food Chemistry. 2003;51(22):6423–6428. doi: 10.1021/jf030284h. [DOI] [PubMed] [Google Scholar]
- 61.Mahbub A. A., Maitre C. L. L., Haywood-Small S. L., McDougall G. J., Cross N. A., Jordan-Mahy N. Differential effects of polyphenols on proliferation and apoptosis in human myeloid and lymphoid leukemia cell lines. Anti-Cancer Agents in Medicinal Chemistry. 2013;13(10):1601–1613. doi: 10.2174/18715206113139990303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Solmaz S., Gokbulut A. A., Cincin B., et al. Therapeutic potential of apigenin, a plant flavonoid, for imatinib-sensitive and resistant chronic myeloid leukemia cells. Nutrition and Cancer. 2014;66(4):599–612. doi: 10.1080/01635581.2014.894099. [DOI] [PubMed] [Google Scholar]
- 63.Yang Z.-G., Jia L.-N., Shen Y., Ohmura A., Kitanaka S. Inhibitory effects of constituents from Euphorbia lunulata on differentiation of 3T3-L1 cells and nitric oxide production in RAW264.7 cells. Molecules. 2011;16(10):8305–8318. doi: 10.3390/molecules16108305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thomasset S. C., Berry D. P., Garcea G., Marczylo T., Steward W. P., Gescher A. J. Dietary polyphenolic phytochemicals—promising cancer chemopreventive agents in humans? A review of their clinical properties. International Journal of Cancer. 2007;120(3):451–458. doi: 10.1002/ijc.22419. [DOI] [PubMed] [Google Scholar]
- 65.Kumar S., Pandey A. K. Chemistry and biological activities of flavonoids: an overview. The Scientific World Journal. 2013;2013:16. doi: 10.1155/2013/162750.162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Papazisis K. T., Zambouli D., Kimoundri O. T., et al. Protein tyrosine kinase inhibitor, genistein, enhances apoptosis and cell cycle arrest in K562 cells treated with γ-irradiation. Cancer Letters. 2000;160(1):107–113. doi: 10.1016/s0304-3835(00)00569-3. [DOI] [PubMed] [Google Scholar]