Abstract
Childhood trauma exposure is a potent risk factor for psychopathology. Emerging research suggests that aberrant saliency processing underlies the link between early trauma exposure and later cognitive and socioemotional deficits that are hallmark of several psychiatric disorders. Here, we examine brain and behavioral responses during a face categorization conflict task, and relate these to intrinsic connectivity of the salience network (SN). The results demonstrate a unique pattern of SN dysfunction in youth exposed to trauma (n = 14) relative to comparison youth (n = 19) matched on age, sex, IQ, and sociodemographic risk. We find that trauma-exposed youth are more susceptible to conflict interference and this correlates with higher fronto-insular responses during conflict. Resting-state functional connectivity data collected in the same participants reveal increased connectivity of the insula to SN seed regions that is associated with diminished reward sensitivity, a critical risk/resilience trait following stress. In addition to altered intrinsic connectivity of the SN, we observed altered connectivity between the SN and default mode network (DMN) in trauma-exposed youth. These data uncover network-level disruptions in brain organization following one of the strongest predictors of illness, early life trauma, and demonstrate the relevance of observed neural effects for behavior and specific symptom dimensions. SN dysfunction may serve as a diathesis that contributes to illness and negative outcomes following childhood trauma.
Keywords: Salience network, Child, Conflict, Adolescent, Resting-state
Abbreviations: SN, salience network; RS, reward sensitivity; rFIC, right fronto-insular cortex; dACC, dorsal anterior cingulate cortex; DMN, default mode network
Graphical abstract
Highlights
-
•
Youth exposed to trauma are more susceptible to interference during conflict.
-
•
Higher conflict interference is related to increased right fronto-insular response.
-
•
Trauma-exposed youth show higher salience network (SN) connectivity within the insula.
-
•
SN dysfunction may contribute to cognitive and affective deficits following trauma.
1. Introduction
Information about the world is funneled into our brain through sensory organs. Various inputs compete for our attention, and we prioritize and weigh these inputs in favor of those most relevant to our goals. The process by which we select among competing stimuli is rapid and predominately automatic. Current neurobiological models hold that aberrant filtering, detection, and mapping of salient external stimuli or internal mental events play a significant role in psychopathology (Menon, 2011). According to these models, increased bottom-up detection of salient events impairs the ability to recruit higher-order brain systems mediating attention and cognitive control. Elevated interference by inappropriately assigned salient information may underlie cognitive dysfunction and emotion regulatory deficits hallmark of several psychiatric disorders.
Childhood trauma exposure is a critical and significant risk factor, associated with ~50% of childhood psychiatric disorders and ~30% of later-onset clinical disorders (Green et al., 2010). A growing body of evidence indicates that disruptions in cognitive processes and their associated neural underpinnings may contribute to the elevated risk in these individuals. These studies show altered perceptual sensitivity and attention control (review by Pollak, 2008), and potentiated neural responses to salient stimuli in individuals who have experienced early adversity or trauma (Dannlowski et al., 2012; Herringa et al., 2013; McCrory et al., 2011). Hyperactivity of brain regions that detect and enhance biologically-relevant information, such as the amygdala, fronto-insular cortex (comprising the anterior insula and ventrolateral prefrontal cortex), and dorsal anterior cingulate cortex (dACC), are consistently reported findings. Activity in these regions correlates with emotional arousal (Taylor et al., 2003), autonomic activity (Critchley, 2005), and anticipation of aversive events (Kalisch et al., 2005). Notably, these regions are key nodes of the salience network (SN), an intrinsic connectivity network involved in detecting, integrating, and filtering relevant interoceptive, autonomic, and emotional information (Seeley et al., 2007; Taylor et al., 2009). Prior studies also demonstrate that activity and connectivity within the SN is elevated in adults with major depressive disorder (Hamilton et al., 2012; Manoliu et al., 2013), posttraumatic stress disorder (PTSD; Sripada et al., 2012), and anxiety disorders (Etkin and Wager, 2007). It has been postulated that aberrant function and interaction of the SN may contribute to negative biases in attention and thought inherent in these disorders (Etkin and Wager, 2007; Hamilton et al., 2012). It is possible that functional changes in the SN are a consequence of childhood trauma that also serve to increase psychiatric risk.
Neurobiological investigations have yet to provide clarity regarding functional brain changes that link the experience of childhood trauma with the development of psychopathology. First, the majority of neuroimaging studies are conducted in adults with histories of childhood trauma. Effects observed in adults may reflect secondary compensatory mechanisms rather than those primarily associated with trauma. Evaluation of childhood and adolescence, periods proximal to the time of the traumatic experience, may inform understanding of early emergence of neurological traits that are precursors to mental illness. Recent pediatric research has begun to address this gap, revealing heightened sensitivity of SN regions to emotional stimuli in youth who have experienced early adversity or trauma (Maheu et al., 2010; McCrory et al., 2011; Tottenham et al., 2011; White et al., 2012). To our knowledge, SN engagement during interference processing has yet to be examined in a high-risk developmental trauma framework. Second, because most either lack behavioral measures or find no differences in behavior, changes in brain activation may reflect core deficits, compensation, or both. In addition, few studies link neural changes to psychiatric symptoms, and even fewer examine specific symptom dimensions (e.g., reward sensitivity) which may show greater correspondence with neurobiological variation (Morris and Cuthbert, 2012). Third, we are aware of no pediatric studies that examine the impact of childhood trauma on resting-state functional connectivity of the SN.
Based on prior findings of altered perceptual sensitivity and attention control and heightened neural responses to salient stimuli in adults that experience early trauma, we test the hypothesis that salience processing and functional organization of the SN becomes disrupted in youth — proximal to the traumatic experience. An overactive SN alerting system may contribute to cognitive and socioemotional deficits observed in individuals who have experienced trauma in early life. Tasks that create conflict (i.e., tension between two competing stimuli) provide a reliable behavioral metric of the ability to detect and filter extraneous stimuli, and the degree to which conflicting information interferes with ongoing cognitive processing. Evaluation of neurocognitive function during conflict allows us to measure engagement of SN regions and the relevance of neural changes for behavior. A prior study in women with PTSD (related to interpersonal trauma) found higher responses to interference in the insula, supporting the notion of increased salience detection in the SN in those who have experienced trauma and/or secondary disease symptomology (Bruce et al., 2012). While the amygdala is traditionally thought of in the context of emotional conflict, research shows that core SN regions, including the dACC and fronto-insular cortex, track conflict more broadly (Aupperle et al., 2015; Egner et al., 2008).
The fronto-insular cortex (FIC) is considered an integral hub of the brain, regulating information flow across other large-scale brain networks involved in attentional processing and cognitive control (Sridharan et al., 2008). Research shows that the right FIC (rFIC), in particular, mediates switching between self-directed (e.g., default mode network) and executive control networks (Sridharan et al., 2008), and that this switch has relevance for mental health (Hamilton et al., 2011). The FIC contains a specialized class of neurons with large axons that facilitate rapid relay of control signals to other cortical regions (Cauda et al., 2014), and is thus well suited to initiate network switching. Developmental research shows that the FIC is one of the earliest developing structures in the prenatal period (Afif et al., 2007), and its role as an integral hub of the brain is established within the first years of life (Fransson et al., 2011; Gao et al., 2011). Although the critical role of this structure is evident in early life, there is evidence that large-scale functional brain connectivity continues to undergo significant restructuring throughout childhood (Menon, 2013). The FIC, in particular, shows weak within- and between-network functional connectivity in childhood (Uddin et al., 2011), highlighting its potential source of vulnerability for developmental psychopathology.
The FIC is also an important site of convergence for salient proprioceptive, interoceptive, emotional, cognitive, homeostatic and environmental inputs. Information originating from sensory perceptive regions is received by the amygdala and FIC, extending into mid-posterior insular regions (Cauda et al., 2014). The amygdala and mid-posterior insula are considered part of the extended SN, given evidence from both human neuroimaging (Seeley et al., 2007; Taylor et al., 2009) and primate tract tracing (Mesulam and Mufson, 1982) literatures. Although salience filtering likely occurs at multiple levels of processing, current theory holds that the SN (and the rFIC in particular), triggers a cascade of cognitive control signals, impacting how stimuli are subsequently processed (Menon and Uddin, 2010). This unique role underscores the potential for profound disruptions in cognitive and affective functioning should insular function or connectivity be altered.
Here, we describe research that examined the impact of childhood trauma on connectivity within the SN during rest and neurocognitive function during a face-categorization conflict task. We hypothesized that youth exposed to trauma would show greater behavioral decrements to conflicting stimuli (i.e., higher conflict interference), increased response to conflict in SN brain regions, and increased connectivity within the SN. While we focus on function and connectivity within the SN, we also tested for altered connectivity between the SN and the default mode network (DMN), given the SN's critical role in initiating network switching and prior research showing changes in SN–DMN connectivity in adults with MDD (Manoliu et al., 2013) and PTSD (Sripada et al., 2012). Finally, we evaluated the correspondence between observed variation in the developing connectome and individual variation in positive and negative valence systems. These dispositions are relevant for psychopathology (as outlined by the Research Domain Criteria initiative; Morris and Cuthbert, 2012) and offer insight into the manner of differences observed between groups, aiding interpretation of neural effects as risk or adaptation factors. A self-report measure of trait reward sensitivity (RS) was used to assess the positive valence systems, while validated anxiety and depressive symptom measures were used to assess negative valence systems.
2. Materials and methods
2.1. Participants
A total of 51 youth, recruited locally through advertisements or child psychiatry clinics (Detroit, Michigan), participated in this functional magnetic resonance imaging (fMRI) study. Exclusionary criteria included: English as a second language, lower than a 2nd grade reading level, history of brain injury, neurological or movement disorders, or presence of MRI contraindications. Parental informed written consent and child/adolescent assent were obtained prior to participation. The Human Investigation Committee of Wayne State University approved the study protocol.
Both trauma and comparison participants were recruited based on high sociodemographic risk. Prior research shows that trauma frequency is extreme among African Americans living in impoverished areas (nearly 90%; Gillespie et al., 2009). Moreover, minority, urban residents are nearly two times more likely to develop emotional psychopathology following trauma exposure (Gillespie et al., 2009; Goldmann et al., 2011; Kessler et al., 1995). Despite this population's apparent increased susceptibility to mental illness following trauma, little research has examined trauma and its neural correlates in high-risk, urban residents.
IQ was evaluated using the Kaufman Brief Intelligence Test (KBIT v.2; Kaufman and Kaufman, 2004). Pubertal maturation was assessed using Tanner staging (Marshall and Tanner, 1968). Following prior work (Forbes et al., 2009), participants were categorized as pre/early (Tanner stages 1–2) or mid/late pubertal (stages 3–5). Resting-state data from 22 participants have been reported previously (Thomason et al., 2013). Face categorization conflict task data presented here have never been previously reported, however data from an analogous emotion-categorization stroop task in 29 participants included here have previously been described (Marusak et al., 2015; tasks counterbalanced for order of presentation). Although we did not conduct diagnostic testing or exclude individuals with attention-deficit/hyperactivity disorder (ADHD), data from initial study screening noted 3 trauma and 1 comparison participants for potential ADHD-like behavior. Two participants were on psychotropic medications: one trauma participant was taking strattera and sertraline, and one comparison participant was taking trazadone, intuniv, metadate CD, zoloft, and colonidine. Follow-up analyses excluding the two participants on medications yielded no changes to observed effects.
2.2. Trauma and clinical measures
Utilizing parent and youth reports, youth participants who experienced at least one trauma indicated on the Children's Trauma Assessment Center Screen Checklist (source: Michigan Trauma Assessment Center) were categorized as ‘trauma’. Number and type of endorsed traumas are provided in Table 1. Participants with movement exceeding 4 mm or 3 rotational degrees (n = 12; 3 trauma, 9 comparison), conflict task accuracy <50% (n = 4; 1 trauma, 3 comparison), or errors in behavioral data collection (n = 2; 1 trauma, 1 comparison) were excluded from analyses. Therefore, all data are reported for 14 trauma-exposed and 19 age-, sex-, and IQ-matched comparison youth.
Table 1.
Participant demographics by group.
| Trauma (n = 14) | Comparison (n = 19) | |
|---|---|---|
| Age, m (SD) | 12.61 (2.11) | 12.06 (2.66) |
| Sex (female), n (%) | 10 (71.4) | 15 (78.9) |
| Pubertal development, n (%) | ||
| Pre/early pubertal (Tanner stages 1–2) | 5 (35.71) | 8 (42.1) |
| Mid/late pubertal (Tanner stages 3–5) | 9 (64.29) | 11 (57.9) |
| IQ, m (SD) | 100.14 (13.17) | 104.29 (14.34) |
| Race/ethnicity, n (%) | ||
| African American | 5 (35.71) | 9 (47.37) |
| Caucasian | 3 (21.43) | 8 (42.11) |
| Hispanic | 2 (14.29) | 0 |
| Biracial | 1 (7.14) | 1 (5.26) |
| Not reported | 3 (21.43) | 1 (5.26) |
| Household annual income, n (%) | ||
| Less than $40,000 | 10 (71.43) | 9 (47.37) |
| $40–60,000 | 2 (14.29) | 4 (21.05) |
| $60–80,000 | 1 (7.14) | 3 (15.79) |
| Over $80,000 | 0 | 3 (15.79) |
| Not reported | 1 (7.14) | 0 |
| Type of trauma endorsed, n (%) | ||
| Physical abuse | 2 (14)a | 0a |
| Neglectful home environment | 2 (14)a | 0a |
| Exposure to domestic violence | 7 (50)a | 0a |
| Exposure to any other violence not already identified | 7 (50)a | 0a |
| Multiple separations from parent or caregiver | 2 (14)a | 0a |
| Sexual abuse or exposure | 3 (21)a | 0a |
| Anxiety symptomology (SCR), m (SD) | 19.38 (13.97) | 13.38 (8.94) |
| Depressive symptomology (CDI), m (SD) | 2 (2.56) | 2.26 (3.01) |
| Reward sensitivity (BAS, z-scores), m (SD) | −0.2 (0.59) | 0.07 (0.94) |
| Reward responsivity | 17.21 (1.58) | 18 (2.09) |
| Fun seeking | 12.07 (1.9) | 12.44 (2.67) |
| Drive | 10.43 (2.47) | 11 (2.56) |
| Motion during cognitive conflict taskb, m (SD) | ||
| Translational mean movement | 0.05 (0.03) | 0.05 (0.03) |
| Rotational mean movement | 3.44 (4.01) | 4.01 (5.16) |
| Translational RMS | 0.05 (0.03) | 0.05 (0.03) |
| Rotational RMS | 0.06 (0.03) | 0.06 (0.04) |
| Translational max excursion | 0.47 (0.5) | 0.44 (0.26) |
| Rotational max excursion | 0.45 (0.36) | 0.46 (0.29) |
| Motion during resting-state scanb, m (SD) | ||
| Translational mean movement | 0.15 (0.12) | 0.18 (0.13) |
| Rotational mean movement | 0.12 (0.12) | 0.14 (0.1) |
| Translational RMS | 0.1 (0.06) | 0.11 (0.05) |
| Rotational RMS | 0.001 (0.001) | 0.001 (0.001) |
| Translational max excursion | 0.66 (0.43) | 0.85 (0.39) |
| Rotational max excursion | 0.47 (0.31) | 0.57 (0.28) |
Chi-square tests were used for sex, race/ethnicity, puberty, and trauma-type comparisons; two-sample t-tests for age, symptomology, and motion comparisons; Mann–Whitney U for income.
Abbreviations: standard deviation, SD; mean, m; Intelligence Quotient, IQ; Screen for Child Anxiety Related Emotional Disorders, SCR; Children's Depression Inventory, CDI; Behavioral Activation Subscale of the BIS/BAS, BAS; root-mean-square (head position change), RMS.
Indicates group comparison is significant at p ≤ 0.05.
Translational (x, y, z) movement is reported in mm; rotational, in degrees. Parenthetical values given by totals or means represent percentages and SDs, respectively.
Individual variation in positive and negative valence systems was assessed, as outlined by the Research Domain Criteria initiative (Morris and Cuthbert, 2012). Variation in negative valence systems were measured using two validated self-report measures of anxiety and depressive symptoms: the 41-item Screen for Child Anxiety-Related Emotional Disorders (SCR; Birmaher et al., 1997) and the 10-item Children's Depression Inventory (CDI; Saylor et al., 1984). The 20-item Behavioral Inhibition and Activation Scales (BIS/BAS; Carver and White, 1994) was used to measure variation in positive valence systems. Trait RS was conceptualized as the Behavioral Activation (BAS) component of the BIS/BAS, following prior work (Garner et al., 2012; Marusak et al., 2015). Scores for each of the three BAS subscales (representing different aspects of reward function: reward responsiveness, fun seeking, drive) were converted to z-scores and averaged to form RS, an overall index of reward function. RS data were not available for one comparison participant. A visual analog scale (VAS) was used to obtain an average rating of fear/anxiety during the MRI visit (repeat measures at 30-minute intervals) as previously described (Thomason et al., 2013).
2.3. Experimental paradigms and procedures
See Supplemental Material for MRI acquisition parameters and summary of approach taken for mitigation of possible motion related confounds.
2.3.1. Conflict task
During fMRI, participants underwent a face categorization conflict task adapted from Egner et al. (2008). The task consisted of 163 presentations of happy or fearful facial expression photographs, overlaid with the words “FEMALE” or “MALE” to create categorically congruent and incongruent stimuli (see Fig. 1A). Participants were instructed to identify the gender of the face stimuli with a button press response, while trying to ignore the task-irrelevant gender word stimuli. Stimuli were presented for 1000 ms, with a varying interstimulus interval of 2000–4000 ms (mean = 3000 ms), in a pseudorandom order, counterbalanced across trial types for expression, word, response button, and gender. The original task (Egner et al., 2008) utilized adult face stimuli. Here, we adapted the task for children by utilizing an established set of child and adolescent face stimuli (Egger et al., 2011), minimizing the complex relations inherent in adult face stimuli (Marusak et al., 2013). Stimuli were presented with EPrime Software v.2.0 (Psychology Software Tools, Inc., Pittsburgh, PA) during fMRI scanning and displayed on a back-projection screen viewed by participants via a mirror attached to the head coil. Task duration was 12:46. Participants with poor task performance (<50% accuracy) or errors in behavioral data collection were not included in the study sample. For the remaining participants, task accuracy was fair (mean = 86.4%, SD = 9.92%). Reaction time (RT) was unavailable for one participant due to errors in data collection, and this participant was therefore not included in RT analyses but retained in all other analyses for completeness.
Fig. 1.
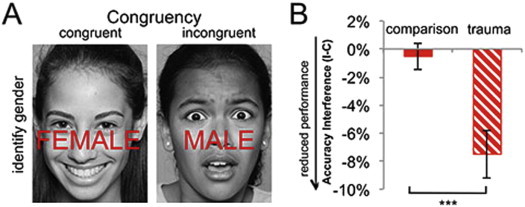
(A) Face categorization conflict task and (B) group differences in conflict interference. Participants were instructed to identify the underlying face gender (male or female) while ignoring an overlying gender word (‘MALE’ or ‘FEMALE’). Trials varied such that distracter words either matched (“congruent”) or conflicted (“incongruent”) with the underlying face. Trauma-exposed youth show a greater loss of accuracy for incongruent relative to congruent trials (I − C). Negative values indicate a loss in performance. ***p < 0.001, two-sample t-test. Error bars represent standard error.
2.3.2. Resting-state paradigm
Following the conflict task, the participants underwent a 6-minute resting-state paradigm. The participants were asked to lie quietly in the scanner with their eyes closed for the duration of the scan.
2.4. Imaging Data analysis
2.4.1. Preprocessing
BOLD fMRI data were processed using SPM8 software (Statistical Parametric Mapping; http://www.fil.ion.ucl.ac.uk/spm/) implemented in MATLAB (MathWorks, Inc., Natick, MA). The first four image volumes were excluded to allow for signal equilibration effects. Preprocessing steps included: (i) slice-time correction, (ii) image realignment, (iii) spatial transformation to the Montreal Neurological Institute (MNI) template using the participant-specific transformation parameters created by fitting mean functional images to the single reference EPI standard template (in SPM). Data were not resampled during normalization, thus retained the native resolution (3.44 × 3.44 × 4 mm) for subsequent analysis. (iv) Images were then spatially smoothed with a Gaussian kernel (6 mm full width at half maximum [FWHM] for task data; 8 mm FWHM for resting-state data).
2.4.2. Conflict task
A 128-s temporal high-pass filter was applied to the data, and temporal autocorrelation was estimated using a first-order autoregressive model. Two independent participant-level models were created in the context of a general linear model to examine effects of (1) conflict and (2) conflict regulation. In the first model, separate regressors for the stimulus events (convolved with a canonical hemodynamic response function) were created for incongruent (I) and congruent (C) trials. For the second model, trial types were broken down based on the preceding trial type: regressors were created for postcongruent incongruent trials (cI), postincongruent incongruent trials (iI), postcongruent congruent trials (cC), and postincongruent congruent trials (iC). All participant-level models included regressors of no interest corresponding to the six motion parameters, and modeled error and post-error trials separately. Participant-level contrasts isolated (1) conflict-related neural activity by subtracting congruent from incongruent trials (I − C), and (2) conflict regulation by subtracting post-congruent incongruent trials from post-incongruent incongruent trials (iI − cI). The contrast iI − cI isolates activity during conflict trials for which behavior differs by virtue only of priming induced by conflict demands of the previous trial type (i.e., previous trial is either congruent or incongruent). Engagement of the conflict system on the preceding trial should ready the system for reengagement. Group-level random-effects two-sample t-tests were used to test for group differences in neural activity during (1) cognitive conflict (I − C), and (2) conflict regulation (iI − cI).
2.4.3. Resting-state
Connectivity analyses were performed using the CONN fMRI functional connectivity toolbox (version 12.1; http://www.nitrc.org/projects/conn). Resting-state fMRI volumes were submitted to seed-based connectivity analyses to assess connectivity within the SN, and between the DMN and SN. First, seed time series data were extracted from SN and DMN masks comprising 6 mm radii spheres centered at Montreal Neurological Institute (MNI) coordinates of peak-valued loci. These coordinates were determined by group averaged independent components analysis of intrinsic functional networks identified in an independent N = 65 pediatric sample (Thomason et al., 2011): (41, 21, −5), (−2, 23, 33), and (−46, 15, −5) for the SN, and (5, −53, 13), (−2, 57, −18), and (52, −63, 26) for the DMN, see Fig. S1. Correlation estimates controlled for estimated translational and rotational motion as well as a white matter and cerebral spinal fluid nuisance time course. A band-pass filter was applied to investigate low-frequency correlations (between 0.01 and 0.1 Hz; Van Dijk et al., 2010). Pearson bivariate correlation coefficients were calculated between average time courses in the SN seed region mask and all other voxels of the brain. Group-level random-effects two-sample t-tests were used to test for group differences in intrinsic connectivity of the SN, and connectivity between the DMN and key SN regions.
2.4.4. Regions of interest
To examine the relevance of intrinsic SN connectivity for neural engagement during a cognitive task, all between-group effects were considered within SN regions known to be recruited in the conflict task: (i) bilateral amygdala (left, x = −30, y = −6, z = −14; right, x = 32, y = 0, z = −12), (ii) dACC (x = 2, y = 32, z = 31) and (iii) rFIC (x = 40, y = 30, z = −7) using coordinates derived from our prior work (Egner et al., 2008; Etkin et al., 2006). (iv) We also examined a mid-posterior insula region (x = −38, y = −13, z = −8) that showed higher responses in adults with PTSD during a similar task (Bruce et al., 2012). Regional masks (10 mm radii spheres) were created around each peak and then intersected with a gray matter mask. Group differences were examined within each region separately using a threshold of p < 0.05, small-volume family-wise error (FWE) corrected. All coordinates provided in this report are given in MNI convention.
2.4.5. Exploratory whole-brain results
Between-group whole-brain effects of I − C are also reported at a threshold of p < 0.005, cluster minimum = 10 voxels. This threshold was derived from suggested standards for whole-brain analyses (Lieberman and Cunningham, 2009).
2.5. Relations among measures
2.5.1. Correlations among measures
Average signal change/connectivity strength was extracted from peaks of group difference (4 mm radii spheres) and plotted for visualization, and/or submitted to 4 planned Pearson correlation analyses in IBM SPSS v.22 to evaluate correspondence among brain activation, connectivity, task performance, and symptom severity. Specifically, we tested for relations between insula connectivity and (i) insula reactivity to conflict, (ii) behavioral response to conflict, and (iii) RS, and (iv) between insular and behavioral responses to conflict.
2.5.2. Mediation analysis
PROCESS Software v.2.11 (Hayes, 2013) implemented in SPSS was used to test for the mediating effects of insula–SN connectivity in the association between trauma exposure and RS. We focused on RS due to prior research documenting relations between neural effects related to trauma exposure and this symptom dimension (Bogdan et al., 2013; Marusak et al., 2015). Relations between neural connectivity and symptoms related to negative valence (anxiety, depression) were also examined. The mediation model assumes significant relations between trauma exposure and connectivity, and between connectivity and RS. Connectivity values were extracted from the insula region showing increased SN connectivity in trauma-exposed youth. We then evaluated the correspondence between connectivity strength and RS. This approach uses bootstrapping, and indirect effects are considered significant when confidence intervals do not overlap zero (Hayes, 2013).
3. Results
Trauma and comparison groups were matched on age, sex, pubertal maturation, IQ, race, annual household income, and movement during both fMRI experiments (Table 1). Two trauma participants were left-handed. Groups did not differ on RS, anxiety, or depressive symptoms (Table 1). Participants also did not differ on ratings of state anxiety obtained via visual analog scale (VAS), t(31) = 0.08, p = 0.94. Lack of differences between groups in state and trait mood symptoms suggests that observed effects are not influenced by group differences in fear and anxiety.
3.1. Greater behavioral decrements to conflict in youth exposed to trauma
Consistent with the conflict effect, incongruent trials (relative to congruent trials) caused significant accuracy decreases and slowing in reaction time (RT) across the sample, accuracy: t(32) = 3.26, p = 0.003; RT: t(31) = 3.8, p = 0.001. Relative to comparison youth, trauma participants showed a greater impairment in accuracy for incongruent vs. congruent trials (I − C), t(31) = 3.8, p < 0.001 (Fig. 1B). Specifically, conflict trials caused ~8% loss in accuracy in trauma-exposed youth, while performance was relatively consistent across incongruent and congruent trials in comparison youth. This is in line with the notion that trauma-exposed youth show greater behavioral interference by task-irrelevant distracters. Breakdown by trial type (I, C; see Fig. S2) showed that this group difference was driven by lower accuracy in trauma participants during incongruent rather than congruent trials (group × trial-type interaction, F(1,31) = 14.42, p = 0.001). No group differences in RT interference or conflict regulation were observed, p's > 0.17 (Table S1). Groups did not differ on overall task accuracy or RT (p's > 0.29; see Table S1 for full summary).
3.2. Trauma-exposed youth show elevated insula reactivity to conflict
We observed greater rFIC response to conflict (I − C) in trauma-exposed relative to comparison youth, x = 32, y = 34, z = −10, Z = 2.98, pFWE = 0.031, Fig. 2A. Across the sample, higher rFIC reactivity was associated with greater performance decrements to conflict (I − C), r(33) = −0.375, p = 0.032. A similar pattern was observed in left mid-posterior insula; trauma-exposed youth showed higher response to conflict (x = −34, y = −10, z = 10, Z = 3.89, pFWE = 0.002) which was associated with greater performance decrements, r(33) = −0.433, p = 0.012, Fig. 2B. Amygdala and dACC SN regions did not show group differences in responses to conflict, and neural activity did not differ between groups during conflict regulation (iI − cI). Exploratory whole-brain effects of I − C are provided in Table S2. Briefly, trauma-exposed youth showed higher response to conflict (I − C) in the FIC, putamen, inferior parietal lobe, and sensorimotor areas; comparison youth showed higher response to conflict in the inferior parietal lobe.
Fig. 2.

Trauma-exposed youth show greater (A) right fronto-insular cortex (rFIC) and (B) left mid-posterior insula response to conflict, that correlates with greater performance decrements. Fronto-insular response to conflict is exaggerated in trauma-exposed youth, especially those that demonstrate large interference values (incongruent − congruent trials [I − C]). Clusters are significant at pFWE < 0.04, small-volume corrected. X, Y, Z coordinates are given in MNI convention. Error bars represent standard error.
3.3. Aberrant salience network (SN) connectivity in trauma-exposed youth
Relative to comparison participants, trauma-exposed youth demonstrated increased SN connectivity within the left amygdala (x = −28, y = −6, z = −18, Z = 3.13, pFWE = 0.046; Fig. 3A) and left middle insula (x = −28, y = 8, z = −16, Z = 3.98, pFWE = 0.011; Fig. 3C), and reduced SN connectivity in the right dACC (x = 4, y = 36, z = 34, Z = 3.14, pFWE = 0.044; Fig. 3B). Increased SN connectivity in the left middle insula was associated with higher rFIC response to conflict (I − C; Fig. 3D), r(33) = 0.516, p = 0.002, and greater performance decrements to incongruent trials, although the latter effect was a non-significant trend, r(33) = −0.335, p = 0.057. SN connectivity across the sample is presented in Fig. S3.
Fig. 3.
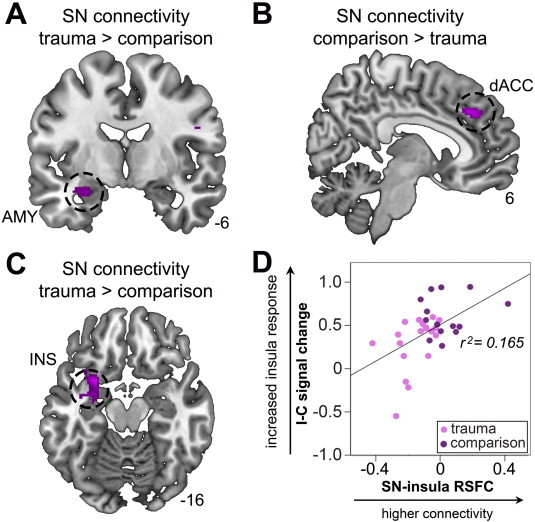
Group differences in salience network (SN) connectivity. Trauma-exposed youth show increased amygdala (AMY), A, decreased dorsal anterior cingulate cortex (dACC), B, and increased middle insula (INS), C, to SN signal covariance. Increased SN connectivity within the left insula (x = −28, y = 8, z = −16) was associated with greater right fronto-insular (x = 32, y = 34, z = −10) response to conflict, D. Results are significant at pFWE < 0.05, small-volume corrected. X, Y, Z coordinates are given in MNI convention. Error bars represent standard error.
3.4. Altered connectivity within the salience network (SN) is associated with variation in trait reward sensitivity (RS)
Driven by prior work showing relationships between depressive symptoms and altered SN connectivity in the insula (Manoliu et al., 2013), and by our recent work showing associations between trait RS and altered function of emotional conflict neural systems in trauma-exposed youth (Marusak et al., 2015), we tested associations between SN-insula connectivity and RS. The strength of SN connectivity was extracted from the peak of the insula region that showed higher connectivity in trauma-exposed youth. We then tested for associations between connectivity in this region and RS across the sample. We observed that higher SN to left insula connectivity was associated with diminished RS, r(32) = −0.373, p = 0.036. Mediation analyses showed that the association between trauma exposure and RS was mediated by SN-insula connectivity (β = −0.4, standard error (SE) = 0.21, lower limit confidence interval = −0.89, upper limit confidence interval = −0.007; see Fig. 4). Neither anxiety nor depressive symptoms were related to SN connectivity in the insula (p's > 0.27).
Fig. 4.
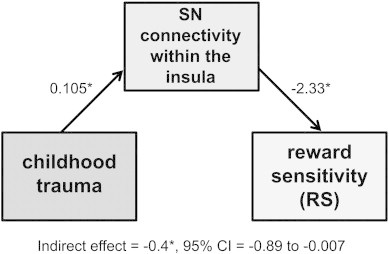
Salience network (SN) connectivity within the insula mediates the relationship between trauma exposure and reward sensitivity (RS). Unstandardized regression coefficients and bias-corrected 95% confidence interval (CI) for the indirect effect from a bootstrap-mediation analysis. Specifically, trauma exposure led to diminished RS through increased SN connectivity within the insula. *p < 0.05.
3.5. Aberrant connectivity between the salience network (SN) and default mode network (DMN) in trauma-exposed youth
Next, we evaluated connectivity between the SN and the DMN. As shown in Fig. 5, trauma-exposed youth showed reduced DMN to SN connectivity, particularly in the dACC (x = 2, y = 26, z = 40, Z = 4.05, pFWE = 0.011). DMN connectivity across the sample is presented in Fig. S3.
Fig. 5.

Altered connectivity between the salience network (SN) and the default mode network (DMN) in trauma-exposed youth. Trauma-exposed youth show lower DMN connectivity with the right dorsal anterior cingulate cortex (dACC), a key SN node. Resting-state functional connectivity is depicted as Fisher-transformed r values. Results are significant at pFWE = 0.011, small-volume corrected. Coordinates are given in MNI convention. Error bars represent SEM.
4. Discussion
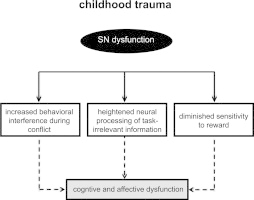
Contemporary neurobiological models suggest that inappropriate assignment of saliency to external stimuli or internal mental events leads to aberrant interactions within and between large-scale neurocognitive networks, and plays a significant role in several psychiatric disorders (Menon, 2011). Recent research in adults supports this conceptualization (e.g., Manoliu et al., 2013; Sripada et al., 2012). However, it is unknown if changes are evident in the brain prior to the emergence of clinically significant symptoms; these may underlie vulnerability. The present study is the first to link early life trauma exposure — a major predisposing factor for the development of psychopathology — to dysfunctional architecture of large-scale neurocognitive networks in a sample of high-risk urban youth. We demonstrate increased SN connectivity within the insula in trauma-exposed youth that has cognitive repercussions: increased SN connectivity corresponds with suboptimal brain and behavioral responses during a conflict task. Further, we demonstrate that altered SN connectivity is associated with individual variation in positive valence systems. That is, higher SN connectivity within the insula was associated with lower RS. These results suggest that enhanced salience detection, diminished sensitivity to reward, and connectome-level brain changes may contribute to later cognitive and affective deficits observed in individuals who have experienced trauma. A schematic representation of the overarching framework is provided in Fig. 6.
Fig. 6.
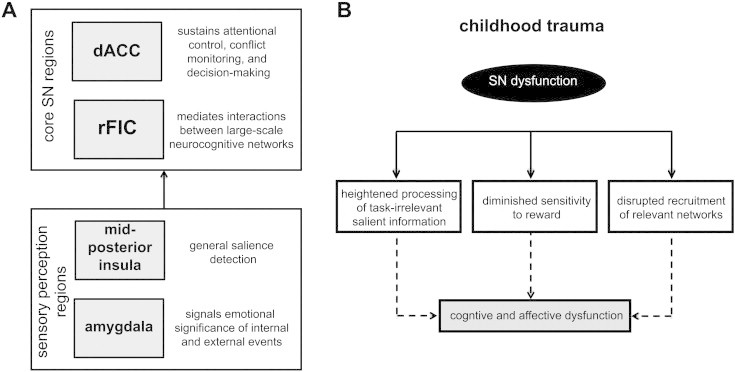
Schematic representation of (A) salience network (SN) organization and (B) model of SN dysfunction in youth exposed to trauma. (A) The normal function of the SN is to detect relevant internal and external cues among myriad inputs. Ventral sensory pathways, including the amygdala and mid-posterior insular regions, feed forward stimulus information to core SN regions (i.e., right fronto-insular cortex (rFIC) and dorsal anterior cingulate cortex (dACC)). Engaged rFIC may influence dominance in other neurocognitive systems that are relevant for goal-directed cognitive processes (e.g., conflict regulation). This is consistent with suggestions that the FIC is an integrative hub that filters how sensory inputs are further processed (Menon and Uddin, 2010). (B) The present results demonstrate that childhood trauma, a major predisposing factor for psychopathology, is associated with altered function and connectivity of the SN. Fitting with conceptual models of posttraumatic stress disorder (Patel et al., 2012) and major depressive disorder (Hamilton et al., 2012), changes within the SN may lead to a cascade of events that increase risk for cognitive and affective dysfunction hallmark of these disorders. Our results suggest that altered salience processing may underlie the link between early life trauma and development of psychopathology. Specifically, we observed greater performance decrements to conflict trials, which corresponded with increased fronto-insular responses in trauma-exposed youth. We also observed altered connectivity within the SN, and between the SN and the default mode network (DMN). Network-level disruptions may underlie the observed sub-optimal brain and behavioral responses during the conflict task in trauma-exposed youth. Finally, strength of SN connectivity within the insula was associated with reduced reward sensitivity, an affective trait emerging as an important risk/resilience factor in the aftermath of early trauma exposure.
Trauma-exposed youth show elevated rFIC and left mid-posterior insula response to conflict relative to comparison youth. The latter is striking given that a similar left mid-posterior insula region (x = −37, y = −21, z = 12) was shown to be hyperreactive in women with PTSD during an emotional interference task analogous to the paradigm employed in the current study (Bruce et al., 2012). In that study, participants were instructed to indicate whether two non-emotional house stimuli in the horizontal axis were the same or different, while ignoring distracting emotion–face pairs in the vertical axis. Posterior insular regions are thought to interact with anterior fronto-insular regions to modulate autonomic reactivity to salient stimuli. Once a salient stimulus is detected, the rFIC initiates attentional control signals and facilitates network switching. In the current study, higher reactivity of both rFIC and mid-posterior insula to conflict was associated with greater performance decrements. This suggests that increased salience detection in fronto-insular regions may interfere with the ability to recruit higher-order cognitive systems necessary for task execution, or cause inappropriate engagement of higher-order systems to task-irrelevant, conflicting stimuli.
We observed greater contributions of the insula and amygdala to the SN in trauma-exposed youth. This finding is consistent with prior research in adults with PTSD (Sripada et al., 2012). Our results thus extend earlier observations and suggest that strengthening of connectivity within a network that detects salient external and internal events begins proximally to the traumatic experience, during youth. Moreover, we observed that patterns detected at rest corresponded with those observed during a neurocognitive task. Specifically, SN connectivity was higher in the insula, which was more responsive to conflict in trauma-exposed youth. These converging results support the notion that elevated SN activity is a pervasive phenomenon that may affect these individuals across a variety of contexts. These data are also fitting with prior behavioral reports of sustained attention to extraneous stimuli in youth who have endured early life trauma (Pechtel and Pizzagalli, 2011). Inappropriate assignment of salience to mundane events could interfere with ongoing cognitive or affective processes by biasing attentional resources. For instance, elevated salience detection could diminish a child's ability to focus on the task at hand, or to regulate emotional responses.
Trauma and comparison groups did not differ on demographic factors measured, allowing us to compare the effects of trauma exposure in groups that were similar in sociodemographic risk. Given also that the trauma group did not present with marked clinical symptomology relative to the comparison group, observed neural changes may represent either risk or adaptation to adverse early environments. Our results support the former. Specifically, we observed that youth showing the most aberrant pattern of insula–SN covariance (e.g., elevated connectivity) showed diminished levels of RS. RS is an affective trait that may contribute to the emergence of stress-related psychopathology during adolescence (Bogdan et al., 2013) and as such, dysfunctional SN connectivity within the insula may represent a neural substrate of increased risk. Longitudinal follow-up planned in this sample may allow for further evaluation of this potential mechanism.
Our analyses indicate that SN connectivity is lower in the dACC in trauma-exposed youth. This is in contrast to the observed strengthening of SN connectivity in the amygdala and middle insula. This result suggests reduced reliance on a core SN region (i.e., dACC), with a concomitant increase in regions thought to carry salient interoceptive, homeostatic and emotional information to core SN nodes (i.e., amygdala, middle insula). In line with a recent conceptual framework (Menon, 2011), we demonstrate a link between aberrant connectivity of the rFIC within the SN and aberrant connectivity between the SN and DMN, a network involved in self-referential processing (Buckner et al., 2008). Prior research shows that altered connectivity of large-scale neural networks has relevance for mental health. For instance, depressed adults show altered connectivity within the SN's rFIC and strength of connectivity relates to symptom severity (Manoliu et al., 2013). The network-level brain changes observed in the present study therefore hold implications for psychiatric risk. Altered network connectivity may also provide an avenue for intervention. For example, a recent study showed that transcranial magnetic stimulation (TMS) was capable of attenuating aberrant network connectivity in depressed individuals by modulating interactions between networks (Liston et al., 2014), an encouraging result.
Study limitations warrant mention. First, sample size was limited, and replication in larger samples is warranted to improve generalizability of the observed effects. Thus, results are presented as preliminary, but highlight important neural and behavioral differences in trauma-exposed youth in an understudied population of urban-dwelling, minority youth with a high stress burden. Next, we lack information about the onset (age) and duration of trauma experienced by these study participants. While retrospective analyses show that trauma onset and type relate to distinct emotional outcomes (English et al., 2005), prior studies also document nonspecific effects of these variables on outcomes (Arata et al., 2007; Collishaw et al., 2007) and some suggest that disentangling unique effects may result in overly narrow interpretations (Green et al., 2010). Future studies should also consider obtaining trauma documentation beyond self- and parent-report measures (e.g., positive forensic investigation by Child Protective Services). Next, it is unclear why groups did not differ in levels of internalizing symptomology (anxiety, depression, RS). This may be because both groups were drawn from the same sociodemographic risk community, and did not differ on IQ, race, or income. Comparing groups with similar backgrounds provides a unique opportunity to isolate effects of trauma in the context of high-risk youth. However, future research might consider comparing trauma groups with and without diagnoses or presence of elevated symptom levels. Additionally, since this research is cross-sectional, we cannot examine which, if any, of these participants will go on to develop stress-related clinical disorders. While this work provides the first characterization of SN integrity and conflict systems in trauma-exposed youth at high risk for clinical disorders, future longitudinal work is needed to evaluate disease trajectories. Finally, while we observed a significant relationship between trauma and SN function, other factors are likely to influence and be influenced by SN function, such as age and gender. Effects of additional factors and their potential interactions with trauma is important for future work that may seek to develop personalized interventions to promote optimal outcomes in children who have experienced trauma.
4.1. Conclusions
The present results demonstrate a compelling pattern of SN dysfunction, particularly in the insula, in youth exposed to trauma. We show that trauma-exposed youth are more susceptible to interference during conflict and this correlates with higher fronto-insular responses to conflict, and increased tethering of the insula to the SN. We therefore provide evidence of trauma-related changes across multiple domains of neural function, and show that the observed effects have relevance for behavior. Our data also suggest a direct link between connectome-level brain organization and specific symptom dimensions associated with psychiatric risk. In particular, increased insula engagement in the SN was associated with diminished RS, an affective trait that is emerging as a critical risk/resilience factor in the aftermath of stress. Overall, these results support the notion that childhood trauma exposure is associated with disrupted saliency processing at the level of large-scale neural networks. Our findings are preliminary, but may aid the formulation of hypotheses about neural processing differences that result from significant traumatic life events. Further research will be needed to advance discovery in this area to development of behavioral interventions, a much-needed direction for follow-up work. For instance, mindfulness, cognitive training, and neural stimulation have potential to quell overactive SN nodes and alter the functional connectome (Liston et al., 2014; Lutz et al., 2013).
Conflicts of interest
Ms. Marusak, Dr. Etkin, and Dr. Thomason declare no biomedical financial interests or potential conflicts of interest.
Acknowledgements
The authors thank Zahid Latif and Yashwanth Katkuri of Wayne State University (WSU) for their assistance in neuroimaging data acquisition, Kayla Martin, Gregory H. Baldwin, Melissa Youmans, Mallory Gardner, Amy Katherine Swartz, Timothy Lozon, Berta Rihan, Stephen Shen, Amy Anderson, and Ali Daher of WSU for assistance in participant recruitment and data collection, and thank the children and their families who generously shared their time.
Research reported in this publication was supported, in part, by the Merrill Palmer Skillman Institute and the Department of Pediatrics, Wayne State University School of Medicine, and by a NARSAD Young Investigator Award to MET. AE was supported by the Sierra-Pacific Mental Illness Research, Education and Clinical Center (MIRECC) at the VA Palo Alto Health Care System.
Appendix A. Supplementary data
Supplementary material. Supplemental data and Supplemental experimental procedures.
References
- Afif A., Bouvier R., Buenerd A., Trouillas J., Mertens P. Development of the human fetal insular cortex: study of the gyration from 13 to 28 gestational weeks. Brain Struct. Funct. 2007;212(3–4):335–346. doi: 10.1007/s00429-007-0161-1. 17962979 [DOI] [PubMed] [Google Scholar]
- Arata C.M., Langhinrichsen-Rohling J., Bowers D., O'Brien N. Differential correlates of multi-type maltreatment among urban youth. Child Abus. Negl. 2007;31(4):393–415. doi: 10.1016/j.chiabu.2006.09.006. 17412420 [DOI] [PubMed] [Google Scholar]
- Aupperle R.L., Melrose A.J., Francisco A., Paulus M.P., Stein M.B. Neural substrates of approach-avoidance conflict decision-making. Hum. Brain Mapp. 2015;36(2):449–462. doi: 10.1002/hbm.22639. 25224633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B., Khetarpal S., Brent D., Cully M., Balach L., Kaufman J., Neer S.M. The screen for child anxiety related emotional disorders (SCARED): scale construction and psychometric characteristics. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. 9100430 [DOI] [PubMed] [Google Scholar]
- Bogdan R., Nikolova Y.S., Pizzagalli D.A. Neurogenetics of depression: a focus on reward processing and stress sensitivity. Neurobiol. Dis. 2013;52:12–23. doi: 10.1016/j.nbd.2012.05.007. 22659304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce S.E., Buchholz K.R., Brown W.J., Yan L., Durbin A., Sheline Y.I. Altered emotional interference processing in the amygdala and insula in women with post-traumatic stress disorder. Neuroimage Clin. 2012;2:43–49. doi: 10.1016/j.nicl.2012.11.003. 24179757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Andrews-Hanna J.R., Schacter D.L. The brain's default network: anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. 18400922 [DOI] [PubMed] [Google Scholar]
- Carver C.S., White T.L. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS Scales. J. Personal. Soc. Psychol. 1994;67(2):319–333. [Google Scholar]
- Cauda F., Geminiani G.C., Vercelli A. Evolutionary appearance of von Economo's neurons in the mammalian cerebral cortex. Front. Hum. Neurosci. 2014;8:104. doi: 10.3389/fnhum.2014.00104. 24672457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collishaw S., Pickles A., Messer J., Rutter M., Shearer C., Maughan B. Resilience to adult psychopathology following childhood maltreatment: evidence from a community sample. Child Abus. Negl. 2007;31(3):211–229. doi: 10.1016/j.chiabu.2007.02.004. 17399786 [DOI] [PubMed] [Google Scholar]
- Critchley H.D. Neural mechanisms of autonomic, affective, and cognitive integration. J. Comp. Neurol. 2005;493(1):154–166. doi: 10.1002/cne.20749. 16254997 [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. 22112927 [DOI] [PubMed] [Google Scholar]
- Egger H.L., Pine D.S., Nelson E., Leibenluft E., Ernst M., Towbin K.E., Angold A. The NIMH child emotional faces picture set (NIMH-ChEFS): a new set of children's facial emotion stimuli. Int. J. Methods Psychiatr. Res. 2011;20(3):145–156. doi: 10.1002/mpr.343. 22547297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner T., Etkin A., Gale S., Hirsch J. Dissociable neural systems resolve conflict from emotional versus nonemotional distracters. Cereb. Cortex. 2008;18(6):1475–1484. doi: 10.1093/cercor/bhm179. 17940084 [DOI] [PubMed] [Google Scholar]
- English D.J., Upadhyaya M.P., Litrownik A.J., Marshall J.M., Runyan D.K., Graham J.C., Dubowitz H. Maltreatment's wake: the relationship of maltreatment dimensions to child outcomes. Child Abus. Negl. 2005;29(5):597–619. doi: 10.1016/j.chiabu.2004.12.008. 15970327 [DOI] [PubMed] [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. 16982430 [DOI] [PubMed] [Google Scholar]
- Etkin A., Wager T.D. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am. J. Psychiatry. 2007;164(10):1476–1488. doi: 10.1176/appi.ajp.2007.07030504. 17898336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes E.E., Hariri A.R., Martin S.L., Silk J.S., Moyles D.L., Fisher P.M., Brown S.M., Ryan N.D., Birmaher B., Axelson D.A., Dahl R.E. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am. J. Psychiatry. 2009;166(1):64–73. doi: 10.1176/appi.ajp.2008.07081336. 19047324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P., Aden U., Blennow M., Lagercrantz H. The functional architecture of the infant brain as revealed by resting-state fMRI. Cereb. Cortex. 2011;21(1):145–154. doi: 10.1093/cercor/bhq071. 20421249 [DOI] [PubMed] [Google Scholar]
- Gao W., Gilmore J.H., Giovanello K.S., Smith J.K., Shen D., Zhu H., Lin W. Temporal and spatial evolution of brain network topology during the first two years of life. PLOS One. 2011;6(9):e25278. doi: 10.1371/journal.pone.0025278. 21966479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner K.G., Dux P.E., Wagner J., Cummins T.D., Chambers C.D., Bellgrove M.A. Attentional asymmetries in a visual orienting task are related to temperament. Cogn. Emot. 2012;26(8):1508–1515. doi: 10.1080/02699931.2012.666205. 22650182 [DOI] [PubMed] [Google Scholar]
- Gillespie C.F., Bradley B., Mercer K., Smith A.K., Conneely K., Gapen M., Weiss T., Schwartz A.C., Cubells J.F., Ressler K.J. Trauma exposure and stress-related disorders in inner city primary care patients. Gen. Hosp. Psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. 19892208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldmann E., Aiello A., Uddin M., Delva J., Koenen K., Gant L.M., Galea S. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American urban community: the Detroit Neighborhood Health Study. J. Trauma. Stress. 2011;24(6):747–751. doi: 10.1002/jts.20705. 22144187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J.G., McLaughlin K.A., Berglund P.A., Gruber M.J., Sampson N.A., Zaslavsky A.M., Kessler R.C. Childhood adversities and adult psychiatric disorders in the national comorbidity survey replication I: associations with first onset of DSM-IV disorders. Arch. Gen. Psychiatry. 2010;67(2):113–123. doi: 10.1001/archgenpsychiatry.2009.186. 20124111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Chen G., Thomason M.E., Schwartz M.E., Gotlib I.H. Investigating neural primacy in Major depressive disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol. Psychiatry. 2011;16(7):763–772. doi: 10.1038/mp.2010.46. 20479758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J.P., Etkin A., Furman D.J., Lemus M.G., Johnson R.F., Gotlib I.H. Functional neuroimaging of major depressive disorder: a meta-analysis and new integration of baseline activation and neural response data. Am. J. Psychiatry. 2012;169(7):693–703. doi: 10.1176/appi.ajp.2012.11071105. 22535198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-based Approach. Guilford Publications; 2013. [Google Scholar]
- Herringa R.J., Phillips M.L., Fournier J.C., Kronhaus D.M., Germain A. Childhood and adult trauma both correlate with dorsal anterior cingulate activation to threat in combat veterans. Psychol. Med. 2013;43(7):1533–1542. doi: 10.1017/S0033291712002310. 23171514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R., Wiech K., Critchley H.D., Seymour B., O'Doherty J.P., Oakley D.A., Allen P., Dolan R.J. Anxiety reduction through detachment: subjective, physiological, and neural effects. J. Cogn. Neurosci. 2005;17(6):874–883. doi: 10.1162/0898929054021184. 15969906 [DOI] [PubMed] [Google Scholar]
- Kaufman A.S., Kaufman N.L. Kaufman Brief Intelligence Test—Second Edition (KBIT-2) American Guidance Service; Circle Pines, MN: 2004. [Google Scholar]
- Kessler R.C., Sonnega A., Bromet E., Hughes M., Nelson C.B. Posttraumatic stress disorder in the National Comorbidity Survey. Arch. Gen. Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. 7492257 [DOI] [PubMed] [Google Scholar]
- Lieberman M.D., Cunningham W.A. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. 20035017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C., Chen A.C., Zebley B.D., Drysdale A.T., Gordon R., Leuchter B., Voss H.U., Casey B.J., Etkin A., Dubin M.J. Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol. Psychiatry. 2014;76(7):517–526. doi: 10.1016/j.biopsych.2014.01.023. 24629537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A., McFarlin D.R., Perlman D.M., Salomons T.V., Davidson R.J. Altered anterior insula activation during anticipation and experience of painful stimuli in expert meditators. Neuroimage. 2013;64:538–546. doi: 10.1016/j.neuroimage.2012.09.030. 23000783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu F.S., Dozier M., Guyer A.E., Mandell D., Peloso E., Poeth K., Jenness J., Lau J.Y., Ackerman J.P., Pine D.S., Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 2010;10(1):34–49. doi: 10.3758/CABN.10.1.34. 20233954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A., Meng C., Brandl F., Doll A., Tahmasian M., Scherr M., Schwerthöffer D., Zimmer C., Förstl H., Bäuml J., Riedl V., Wohlschläger A.M., Sorg C. Insular dysfunction within the salience network is associated with severity of symptoms and aberrant inter-network connectivity in major depressive disorder. Front. Hum. Neurosci. 2013;7:930. doi: 10.3389/fnhum.2013.00930. 24478665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall W.A., Tanner J.M. Growth and physiological development during adolescence. Annu. Rev. Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. 4297619 [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Carré J.M., Thomason M.E. The stimuli drive the response: an fMRI study of youth processing adult or child emotional face stimuli. Neuroimage. 2013;83:679–689. doi: 10.1016/j.neuroimage.2013.07.002. 23851324 [DOI] [PubMed] [Google Scholar]
- Marusak H.A., Martin K.R., Etkin A., Thomason M.E. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. 25413183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E.J., De Brito S.A., Sebastian C.L., Mechelli A., Bird G., Kelly P.A., Viding E. Heightened neural reactivity to threat in child victims of family violence. Curr. Biol. 2011;21(23):R947–R948. doi: 10.1016/j.cub.2011.10.015. 22153160 [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 2011;15(10):483–506. doi: 10.1016/j.tics.2011.08.003. 21908230 [DOI] [PubMed] [Google Scholar]
- Menon V. Developmental pathways to functional brain networks: emerging principles. Trends Cogn. Sci. 2013;17(12):627–640. doi: 10.1016/j.tics.2013.09.015. 24183779 [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. 20512370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M.M., Mufson E.J. Insula of the old world monkey. III: efferent cortical output and comments on function. J. Comp. Neurol. 1982;212(1):38–52. doi: 10.1002/cne.902120104. 7174907 [DOI] [PubMed] [Google Scholar]
- Morris S.E., Cuthbert B.N. Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues Clin. Neurosci. 2012;14(1):29–37. doi: 10.31887/DCNS.2012.14.1/smorris. 22577302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Spreng R.N., Shin L.M., Girard T.A. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2012;36(9):2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. 22766141 [DOI] [PubMed] [Google Scholar]
- Pechtel P., Pizzagalli D.A. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2011;214(1):55–70. doi: 10.1007/s00213-010-2009-2. 20865251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak S.D. Mechanisms linking early experience and the emergence of emotions: illustrations from the Study of Maltreated Children. Curr. Dir. Psychol. Sci. 2008;17(6):370–375. doi: 10.1111/j.1467-8721.2008.00608.x. 21701602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saylor C.F., Finch A.J., Jr., Spirito A., Bennett B. The children's depression inventory: a systematic evaluation of psychometric properties. J. Consult. Clin. Psychol. 1984;52(6):955–967. doi: 10.1037//0022-006x.52.6.955. 6520288 [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. 17329432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. 18723676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada R.K., King A.P., Welsh R.C., Garfinkel S.N., Wang X., Sripada C.S., Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom. Med. 2012;74(9):904–911. doi: 10.1097/PSY.0b013e318273bf33. 23115342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor K.S., Seminowicz D.A., Davis K.D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009;30(9):2731–2745. doi: 10.1002/hbm.20705. 19072897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S.F., Phan K.L., Decker L.R., Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. 12667842 [DOI] [PubMed] [Google Scholar]
- Thomason M.E., Dennis E.L., Joshi A.A., Joshi S.H., Dinov I.D., Chang C., Henry M.L., Johnson R.F., Thompson P.M., Toga A.W., Glover G.H., Van Horn J.D., Gotlib I.H. Resting-state fMRI can reliably map neural networks in children. Neuroimage. 2011;55(1):165–175. doi: 10.1016/j.neuroimage.2010.11.080. 21134471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M.E., Tocco M.A., Quednau K.A., Bedway A.R., Carré J.M. Idle behaviors of the hippocampus reflect endogenous cortisol levels in youth. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52(6):642–652. doi: 10.1016/j.jaac.2013.04.004. 23702453 [DOI] [PubMed] [Google Scholar]
- Tottenham N., Hare T.A., Millner A., Gilhooly T., Zevin J.D., Casey B.J. Elevated amygdala response to faces following early deprivation. Dev. Sci. 2011;14(2):190–204. doi: 10.1111/j.1467-7687.2010.00971.x. 21399712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Supekar K.S., Ryali S., Menon V. Dynamic reconfiguration of structural and functional connectivity across core neurocognitive brain networks with development. J. Neurosci. 2011;31(50):18578–18589. doi: 10.1523/JNEUROSCI.4465-11.2011. 22171056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Hedden T., Venkataraman A., Evans K.C., Lazar S.W., Buckner R.L. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. 19889849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White M.G., Bogdan R., Fisher P.M., Muñoz K.E., Williamson D.E., Hariri A.R. FKBP5 and emotional neglect interact to predict individual differences in amygdala reactivity. Genes Brain Behav. 2012;11(7):869–878. doi: 10.1111/j.1601-183X.2012.00837.x. 22979952 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material. Supplemental data and Supplemental experimental procedures.


