Abstract
Although wild chimpanzees and other African great apes live in regions endemic for African sleeping sickness, very little is known about their trypanosome infections, mainly due to major difficulties in obtaining their blood samples. In present work, we established a diagnostic ITS1-based PCR assay that allows detection of the DNA of all four Trypanosoma brucei subspecies (Trypanosoma bruceibrucei, Trypanosoma bruceirhodesiense, Trypanosoma bruceigambiense, and Trypanosoma bruceievansi) in feces of experimentally infected mice. Next, using this assay we revealed the presence of trypanosomes in the fecal samples of wild chimpanzees and this finding was further supported by results obtained using a set of primate tissue samples. Phylogenetic analysis of the ITS1 region showed that the majority of obtained sequences fell into the robust T. brucei group, providing strong evidence that these infections were caused by T. b. rhodesiense and/or T. b. gambiense. The optimized technique of trypanosome detection in feces will improve our knowledge about the epidemiology of trypanosomes in primates and possibly also other endangered mammals, from which blood and tissue samples cannot be obtained.
Finally, we demonstrated that the mandrill serum was able to efficiently lyse T. b. brucei and T. b. rhodesiense, and to some extent T. b. gambiense, while the chimpanzee serum failed to lyse any of these subspecies.
Keywords: Trypanosomes, Chimpanzee, Non-human primates, Transmission, Diagnostics
Graphical abstract

Highlights
-
•
ITS1-based PCR allows the detection of the Trypanosoma brucei in feces of non-human primates.
-
•
Wild chimpanzees are frequently infected with the T. brucei subspecies.
-
•
Mandrill serum efficiently lyses also Trypanosoma bruceigambiense.
1. Introduction
African trypanosomes of the Trypanosoma brucei group are the causative agents of sleeping sickness and nagana (Simarro et al., 2011). T. brucei has been found throughout sub-Saharan Africa and comprises three morphologically identical but genetically different subspecies, specifically Trypanosoma brucei brucei, Trypanosoma bruceirhodesiense, and Trypanosoma bruceigambiense (Tait et al., 2011), and Trypanosoma bruceievansi (Lai et al., 2008). Except the last one, these subspecies are transmitted by tsetse flies of the genus Glossina (Franco et al., 2014), with T. b. gambiense and T. b. rhodesiense, causing the West-African and East-African human sleeping sickness, respectively, both being lethal, yet having distinct clinical syndromes (Brun et al., 2010).
T. b. brucei is limited to animals and non-infective to humans due to trypanolytic factors found in human serum, whereas other two subspecies responsible for human trypanosomiasis, T. b. gambiense and T. b. rhodesiense, have developed mechanisms for escaping from lysis mediated by the trypanosome lytic factor (TLF) (Lugli et al., 2004; Wheeler, 2010), which is primarily composed of Apolipoprotein L1 (ApoL1) and a haptoglobin-related protein (Raper and Friedman, 2013). Both trypanosomes able to infect humans exhibit different ways of developing the TLF-mediated lysis. In the case of T. b. gambiense, the resistance is achieved by the interplay among a unique modification of the TLF receptor, expression of a specific TgSGP glycoprotein, and changes in lysosomal physiology (Uzureau et al., 2013). T. b. rhodesiense developed the serum-resistance-associated protein, which is alone sufficient to confer complete resistance to the human TLF (Xong et al., 1998; Vanhollebeke and Pays, 2010; Stephens et al., 2012). The sera of some non-human primates (baboons, sooty mangabeys, mandrills and gorillas) were shown to be capable of ApoL1-mediated killing of the flagellates, while the serum of chimpanzees showed no trypanolytic activity due to secondary loss of the ApoL1 gene (Lugli et al., 2004; Poelvoorde et al., 2004; Thomson et al., 2009, 2014). Moreover, early studies showed that experimental infections with T. b. rhodesiense and T. b. brucei mostly caused the death of untreated chimpanzees, while infections with T. b. gambiense were mild and did not result in apparent clinical symptoms (Baker, 1962, 1968; Baker and Taylor, 1971; Godfrey and Killick-Kendrick, 1967; Hoare, 1972).
Within recent years, with the advent of genetic characterization, molecular markers have been developed to study the genetic diversity of trypanosomes (Tait et al., 2011). These methods, such as determination of microsatellite markers for determination of allelic variations, demonstrated great diversity of the T. brucei group in humans due to frequent genetic exchange (Capewell et al., 2013a; Duffy et al., 2014). Recently, Echodu et al. (2015) showed the emergence of the human infective strains from the non-infective T. b. brucei strains of different genetic backgrounds and highlighted the importance of cattle as possible reservoir of sleeping sickness. Moreover, Capewell et al. (2013a) revealed the existence of hybrids of T. b. gambiense and T. b. brucei with novel resistance mechanism, which are able to infect humans. These facts showed the importance of research on the trypanosomatids in other mammalian host, especially free-ranging non-human primates.
Obtaining blood samples from wild African apes is complicated and in most cases ethically unacceptable (Leendertz et al., 2006). As a result, the blood and tissue samples have been collected mostly from chimpanzees and other primates that died of anthrax or respiratory diseases in the Taï National Park (NP), Ivory Coast (Leendertz et al., 2004; Kondgen et al., 2010) have been screened for the presence of Trypanosoma spp. Being aware of the breakthroughs in our understanding of the evolution of human Plasmodium species precipitated by their detection in feces of African apes (Kaiser et al., 2010), we designed an assay capable of amplifying trypanosome DNA from this material.
2. Methods
2.1. Ethics statement
General permission for sample collection from deceased wild primates was obtained from the authorities in charge of Côte d'Ivoire, Guinea, Uganda, and Democratic Republic of Congo. Deceased animals were found during the course of a long term project focused on the behavior and infectious diseases in wild primates (Leendertz et al., 2006). No animal was anaesthetized or handled for the sole purpose of sample collection. Fecal samples were collected without disturbing the animals within 2–3 min after defecation.
All samples from African sanctuary-living wild-born great apes were collected during routine health checks by the sanctuary on-site veterinarians. Since no animal was sampled specifically for this study, approval from the relevant institutional committee was not needed. All samples were collected according to the guidelines - Chimpanzee sanctuaries: guidelines and management workshop report (available at http://pages.ucsd.edu/∼jmoore/courses/methprimconsweb08/chimpsanct.pdf; date of access to website in 2004).
All sera from animals living in Czech zoological gardens were collected during preventive veterinary health checks. No animal was sampled specifically for this study. All samples were collected in accordance with legal requirements of the Czech Republic (Act no. 161/1992) and with the rules of the respective zoos. Samples collected during necropsies of primates that died from various causes in zoos and primate facilities were also included.
Imports of samples from free-ranging chimpanzees proceeded according to German veterinary regulations for import of organic materials. Tissue and blood samples were exported with the appropriate CITES permissions from the respective country and Germany. No permit was needed for transport between laboratories in Germany and Czech Republic as both are members of European Union. The experimental infection of mice was approved by the Ethical Committees of the Czech Ministry of Education and Biology Centre (no. 90/2013).
2.2. Detection of trypanosomes in feces of experimentally infected mice
Four laboratory mice were infected with T. b. brucei (STIB 920), T. b. rhodesiense (Etat 1.2 R variant), T. b. gambiense (LiTat 1.3) and T. b. evansi (STIB805) by intraperitoneal inoculation of 105 bloodstream stages (trypomastigotes), and the course of infection was controlled by searching for the flagellates in blood smears obtained as a drop from the tail. First search for the presence of parasites was performed 72 h post-infection and continued until trypomastigotes appeared in the blood every 8 h. At that time, fecal and blood samples were collected and used for isolation of total DNA using protocols described below.
Blood samples were placed on a filter paper for ethanol evaporation and total DNA was isolated by incubation in a 200 μL volume with Chelex 100 (Sigma–Aldrich, St. Louis, MO, USA) (final concentration 5%) at 56 °C for 1 h, followed by boiling for 10 min. Before use, samples were centrifuged for 1 min at 15,000 rpm, and 2.5 μL of supernatant was used for PCR. DNA from fecal samples was isolated by a commercial stool kit (Qiagen, Venlo, Limburg, Netherlands). PCR and sequencing were performed for each sample at least in triplicate and, to avoid contamination, in a laboratory that does not work with trypanosomes, always with positive and negative controls included.
A nested approach was used to amplify a region of the Internal Transcribed Spacer 1 (ITS-1) of Trypanosoma spp. (∼180-640 bp). Primers Tryp_3 (5′- TGCAATTATTGGTCGCGC -3′) and Tryp_4 (5′- CTTTGCTGCGTTCTT -3′) were used for the first round of PCR, while internal primers Tryp_1 (5′- AAGCCAAGTCATCCATCG -3′) and Tryp_2 (5′- TAGAGGAAGCAAAAG -3′) were used for the second round of PCR (Adams et al., 2006). PCR was performed with Taq-polymerase (TopBio, Prague, Czech Republic) using the following program: 1 min at 95 °C, 35 cycles of 1 min at 94 °C, 1 min at 54 °C, 30 s at 72 °C, and 5 min at 72 °C, and PCR products were resolved in ethidium-bromide stained agarose gels.
2.3. Collection of primate samples
Tissue samples were obtained from carcasses found mainly in Taï NP, Côte d'Ivoire. Furthermore, necropsy samples were collected from 93 wild primates from the Democratic Republic of Congo and Uganda. Additional 16 tissue samples were obtained from sanctuaries from Gambia, Guinea, Sierra Leone, Cameroon, Republic of Congo, Uganda, and two German zoos (Table 1). Finally, 13 fecal samples were collected without disturbing the animals within 2–3 min after defecation and fixed in 96% ethanol.
Table 1.
Summary of primate samples investigated for the presence of trypanosomes.
| Site (country) | Primate species | Origin | Examined/T-pos. Individuals (%) | Examined/T-pos. samples (%) | DNA source of T-pos. samples |
|---|---|---|---|---|---|
| Taï NP (Côte d'Ivoire) | Western chimpanzee | Wild | 16/8 | 28/11 (39) | Spleen (4×), liver (3×), lymph node (1×); feces (3×)a |
| Sooty mangabey | Wild | 10/1 | 10/1 (10) | Liver | |
| Western red colobus | Wild | 18/2 | 18/2 (11) | Blood | |
| Black-and-white colobus | Wild | 5/0 | 5/0 | – | |
| River Gambia NP (Gambia) | Chimpanzee | Semi-captive | 1/1 | 2/2 (100) | Spleen, livera |
| Chimpanzee Sanctuary (Guinea) | Western chimpanzee | Captive | 1/1 | 2/1 (50) | Liver |
| Budongo NP (Uganda) | Eastern chimpanzee | Wild | 9/1 | 9/1 (11) | Liver |
| Kasyoha-Kitomi FR (Uganda) | Eastern chimpanzee | Wild | 1/0 | 1/0 | – |
| Lui Kotale (DRC) | Western red colobus | Wild | 2/2 | 5/2 (40) | Spleen |
| ND | Pennant's red colobus | Wild | 3/0 | 3/0 | – |
| Limbe Wildlife Centre (Cameroon) | Western lowland gorilla | Semi-captive | 3/0 | 5/0 | – |
| ND (Kongo) | Western lowland gorilla | Captive | 1/0 | 1/0 | – |
| ND (Kongo) | Chimpanzee | Captive | 1/0 | 1/0 | – |
| Ngamba (Uganda) | Chimpanzee | Semi-captive | 27/0 | 27/0 | – |
| Tacugama (Sierra Leone) | Chimpanzee | Semi-captive | 6/0 | 8/0 | – |
| Limbe Wildlife Centre (Cameroon) | Chimpanzee | Captivity | 1/0 | 1/0 | – |
| Munster Zoo (Germany) | Colobus monkey | Captivity | 3/0 | 4/0 | – |
| Frankfurt Zoo (Germany) | Western lowland gorilla | Captivity | 1/0 | 2/0 | – |
| Suma | 109/16 (15%) | 132/20 (15%) | |||
T-pos. – Trypanosome-positive; NP – National Park; FR – Forest Reserve; % – prevalence; sanctuary ND – not determined (from sanctuary); DRC – Democratic Republic of Congo. Western chimpanzee – Pan troglodytes verus, Eastern chimpanzee – Pan t. schweinfurthii, Chimpanzee – Pan troglodytes, Western lowland gorilla – Gorilla gorilla gorilla, Western red colobus – Piliocolobus badius, Pennant's red colobus – P. pennantii, Black-and-white colobus – Colobus polykomos, Sooty mangabey – Cercocebus atys.
Several samples were collected from one individual.
DNA extractions and PCR were performed in physically isolated facilities. DNA from tissue samples and feces was extracted using the DNAeasy tissue kits (Qiagen, Venlo, Limburg, Netherlands) and the EURx Gene Matrix Stool Kit (Roboklon), respectively. All amplicons were gel-purified using the Gel Extraction Kit (Qiagen, Venlo, Limburg, Netherlands) and cloned in TOPO TA Cloning (Invitrogen, Carlsbad, CA, USA) as specified by the manufacturer.
Samples positive for ITS1 in nested PCR reactions were further analyzed for the presence of the ∼900 bp-long T. b. gambiense glycoprotein (TgSGP) gene, again via nested PCR, using outer primers 65-F (5′-GTGGCAATTACTAGCAATAGCG-3′) and 66-R (5′-GCCATCGTGCTTGCCGCTC-3′), and inner primers 61-F (5′-TCACGGCCATCAGACGGAGA-3′) and 62-R (5′-GGGCTCCTGCCTCAATTGCTGCA-3). Conditions were the same for both rounds: 2 min at 95 °C, 24 cycles of 1 min at 95 °C, 1 min at 55 °C, 2 min at 72 °C, and 2 min at 72 °C. As a positive control, DNA from blood of mouse experimentally infected with T. b. gambiense (LiTat 1.3) was used.
2.4. Phylogenetic analysis
Highly polymorphic ITS1 sequences obtained by nested PCR from primate fecal and tissue samples were aligned with Clustal-X (ver. 2.0; gap opening penalty 12; gap extension penalty 5) and neighbor joining clustering with K2P distances was performed on the unmodified alignment using PAUP (4.0, beta version). The final alignment included 452 characters. The accession numbers of sequences retrieved from the GenBank (Trypanosoma sp. ex Wildebeest JN673403, for Trypanosoma theileriJX178185, HQ664848, and HQ664849) and used in phylogenetic reconstructions and ITS1 alignment are available from the authors upon request.
2.5. Trypanosome lysis in primate sera
We used fresh sera from five non-human primate species (barbary macaque [Macaca sylvanus], mandrill [Mandrillus sphinx], spider monkey [Ateles geoffroyi], chimpanzee [Pan troglodytes], and Sumatran orangutan [Pongo abelii]) from the Plzeň, Liberec and Prague zoos, Czech Republic. At final concentration of 10% the sera were added to trypanosomatids cultivated in either HMI-9 or M199 medium with 10% fetal bovine serum (FBS), when concentration reached 5 × 105 cells/ml. The following isolates were used: T. b. brucei (STIB 920), T. b. rhodesiense (Etat 1.2 R variant), and T. b. gambiense (LiTat 1.3). Fresh normal human serum (NHS) obtained from a Central European individual and FBS were used as positive and negative controls, respectively. Trypanosomatids were cultivated in triplicate with the various primate sera for 24 h at 37 °C. After this period, resazurin (Sigma–Aldrich, St. Louis, MO, USA) was added to 1 ml of each culture (final concentration 58 pM) and cell viability was measured after 24 h (i.e. 48 hrs after the cells were mixed with primate sera) in triplicate using Tecan Infinite M200PRO under emission at 590 nm and excitation at 560 nm, and the results were analyzed using Anova. The relative survival was calculated as a ratio in a given serum compared to FBS.
3. Results
3.1. Detection of trypanosomes in feces of experimentally infected mice
Using the nested PCR protocol, the ITS1 region of T. b. brucei, T. b. gambiense, T. b. rhodesiense and T. b. evansi was successfully amplified from DNA isolated from fecal samples of infected mice, with relevant controls being negative (Fig. 1). Single abundant amplicons were of expected size (∼450 bp) and sequence (Fig. 2). This experiment proved that under our experimental conditions, the flagellate DNA can be reliably detected in feces of laboratory mice using a 35 cycle PCR protocol.
Fig. 1.
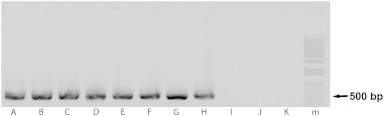
ITS1-based detection of trypanosomes in blood and feces of experimentally infected mice. (A–D, I) detection in blood; (E–H, J) detection in feces. (A, E) Trypanosoma b. brucei; (B, F) T. b. gambiense; (C, G) T. b. rhodesiense; (D, H) T. b. evansi; (I) blood from a non-infected mouse; (J) feces from a non-infected mouse; (K) negative control; (m) marker.
Fig. 2.
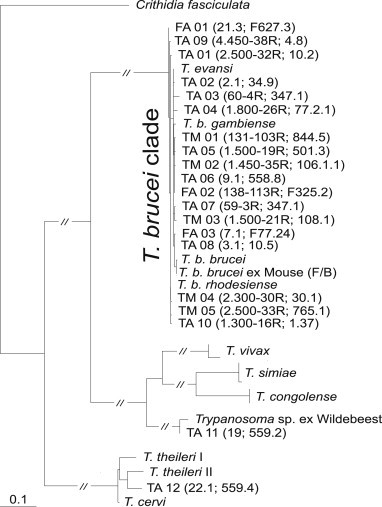
ITS1-based dendogram of trypanosomes from primate tissue and fecal samples. Sequences generated in this study are marked as follows: T – tissue samples of apes (TA) and monkeys (TM); F – fecal samples of apes (FA); sequences retrieved from GenBank are labeled with Latin names (Trypanosoma sp. ex Wildebeest JN673403, for T. theileriJX178185, HQ664848, and HQ664849).
3.2. Detection of trypanosomes in primates and phylogenetic analysis
Overall, 132 samples composed of 119 various tissues and 13 feces, collected from 109 individual primates belonging to six species were subjected to diagnostic PCR with trypanosome-specific primers (Tables 1 and 2). Nested PCR protocol established to amplify ITS1 from the fecal-derived DNA of laboratory-infected mice (Fig. 1) was applied to DNA isolated from the tissue and fecal samples. The sequences of amplicons obtained from 17 primate tissue samples were subjected to BLAST analysis, aligned and grouped in a dendrogram, which showed that our sequences belong to the T. brucei group (Fig. 2, see below).
Table 2.
List of primate samples positive for Trypanosoma spp. DNA.
| Primate species | DNA source | Country/origin | Label of ITS1 sequence | GenBank no. |
|---|---|---|---|---|
| Western chimpanzeea | Spleen | Côte d'Ivoire/wild | TA 01 (2.500-32R; 10.2) | KR092349 |
| Western chimpanzee | Spleen | Côte d'Ivoire/wild | TA 06 (9.1; 558.8) | KR092354 |
| Western chimpanzee | Spleen | Côte d'Ivoire/wild | TA 10 (1.300-16R; 1.37) | KR092358 |
| Western chimpanzee | Spleen | Côte d'Ivoire/wild | TA 12 (22.1; 559.2) | KR092365 |
| Western chimpanzee | Liver | Côte d'Ivoire/wild | TA 04 (1.800-26R; 77.2.1) | KR092352 |
| Western chimpanzeea | Liver | Côte d'Ivoire/wild | TA 08 (3.1; 10.5) | KR092356 |
| Western chimpanzee | Liver | Côte d'Ivoire/wild | TA 09 (4.450-38R; 4.7) | KR092357 |
| Western chimpanzee | Liver | Republic of Guinea/captive | TA 02 (2.1; 34.9) | KR092350 |
| Western chimpanzee | Lymph node | Côte d'Ivoire/wild | TA 11 (19; 559.4) | KR092364 |
| Western chimpanzee | Feces | Côte d'Ivoire/wild | FA 01 (21.3; F627.3) | KR092346 |
| Western chimpanzee | Feces | Côte d'Ivoire/wild | FA 02 (138-113R; F325.2) | KR092347 |
| Western chimpanzee | Feces | Côte d'Ivoire/wild | FA 03 (7.1; F77.24) | KR092348 |
| Eastern chimpanzee | Liver | Uganda (Budongo NP)/wild | TA 05 (1.500-19R; 501.3) | KR092353 |
| Chimpanzeea | Spleen | Gambia/semi-captive | TA 03 (60-4R; 347.2) | KR092351 |
| Chimpanzeea | Liver | Gambia/semi-captive | TA 07 (59-3R; 347.1) | KR092355 |
| Sooty mangabey | Liver | Côte d'Ivoire/wild | TM 01 (131-103R;844.5) | KR092359 |
| Red colobus | Spleen | DRC/wild | TM 02 (1.450-35R; 106.1.1) | KR092360 |
| Red colobus | Spleen | DRC/wild | TM 03 (1.500-21R; 108.1.) | KR092361 |
| Western red colobus | Blood | Côte d'Ivoire/wild | TM 04 (2.300-30R; 30.1) | KR092362 |
| Western red colobus | Blood | Côte d'Ivoire/wild | TM 05 (2.500-33R; 765.1) | KR092363 |
The same individual.
Since in the experimentally infected mice, the ITS1-specific primers were able to amplify the target region of trypanosomes from the fecal samples, we have performed the same PCR on DNA isolated from fecal samples of wild chimpanzees. Out of 13 freshly collected feces, three were positive for trypanosome DNA (Fig. 2), as confirmed by sequencing. DNA isolated from the same samples and analyzed independently in another laboratory by the same method lead to identical results.
Next, we have generated an alignment from all ITS1 sequences (for detail see Table 2) amplified from the tissue samples of chimpanzees (number of samples = 12), sooty mangabeys (n = 1) and Western red colobus monkeys (n = 4), and also from the feces of chimpanzees (n = 3) (Table 2). The dataset was complemented with relevant sequences available in GenBank, with Crithidia fasciculata used as outgroup. With two exceptions (TA11 and TA12, see Fig. 2), all newly obtained sequences fell into the T. brucei clade, as shown by a neighbor joining dendrogram (Fig. 2). The T. brucei clade contains sequences from all known subspecies and is characterized by extremely short branches, which is not surprising given the known minimal differences among individual subspecies. A total of 15 sequences obtained from the tissues of chimpanzees, Western red colobus monkeys and a sooty mangabey fell into this clade, as well as three sequences obtained from the feces of three chimpanzees, providing strong evidence for the infections by the T. brucei group parasites (Table 2; Fig. 2).
All our attempts to amplify the TgSGP gene specific for T. b. gambiense (Capewell et al., 2013b) from the tissues and feces of apes positive for Trypanosoma ITS1 PCR assay invariably failed, although this gene can be easily amplified from feces and blood of mice infected with this T. brucei subspecies (Supplementary data 1). Out of 18 positive samples belonging to the T. brucei clade, two samples, one from a spleen and second one from a lymph node of two post-mortem dissected wild Western chimpanzees originating from Taï NP, Côte d'Ivoire, fell outside of the T. brucei clade (Table 2; Fig. 2). While TA 11 is almost identical with an unnamed Trypanosoma sp. (Access. No. JN673403) from a wildebeest (Connochaetes) captured in Serengeti NP, Tanzania, TA 12 from an adult female chimpanzee from Taï NP is strongly affiliated with T. theileri (Fig. 2).
Due to the limited information residing in the obtained ITS1 region, multiple attempts to amplify (fragments of) the SSU rRNA gene of the above-described pathogens were performed seeking to establish more precisely their species status. Unfortunately, these attempts almost invariably failed. However, in the case of the TA 12 tissue sample, a fragment of the SSU rRNA gene was successfully amplified which allowed us to assign the respective parasite with high confidence to T. theileri.
3.3. Trypanolytic capacity of primate sera
Only mandrill and human sera (NHS) efficiently lysed T. b. brucei, with relative survival 0.101 ± 0.005 (mean ± SD; p < 0.001) and 0.068 ± 0.074 (p < 0.001), respectively (Supplementary data 2). When the cultured T. b. gambiense cells were subjected to the panel of different sera, the lytic factor allowing their limited killing was present only in the mandrill's serum (0.488 ± 0.087; p < 0.001). As anticipated, NHS failed to lyse T. b. gambiense, with a relative survival of 0.886 ± 0.170 (p > 0.05) (Supplementary data 2), while this pathogen also prospered in the other sera. The cultured T. b. rhodesiense cells were very efficiently lysed by the mandrill serum (0.138 ± 0.001; p < 0.001), with the sera of spider monkey and orangutan showing a moderate lysis (0.837 ± 0.054, p < 0.01 and 0.848 ± 0.067, p < 0.01 respectively). Under our conditions, NHS caused a very minor yet significant lysis as well (0.874 ± 0.075; p < 0.05).
4. Discussion
Within the last decade it has become abundantly clear that African great apes play a significant role in evolution, emergence, and transmission of a range of infectious diseases important for human populations. Simian immunodeficiency viruses and apicomplexan parasites of the genus Plasmodium are among the best known examples (Liu et al., 2010; Calvignac-Spencer et al., 2012; Liu et al., 2014). Although the wild chimpanzees and other African great apes live in regions endemic for African sleeping sickness, the research on their trypanosome infections has been neglected. This situation is mostly caused by the virtually insurmountable difficulties in obtaining blood samples from free-ranging animals and ethical unacceptability of experimental infections on captive animals.
Interestingly, African primates exhibit a widely different degree of resistance against parasites of the T. brucei group (Wheeler, 2010). In the literature, there are only very few notes on flagellates found in the blood of dead free-ranging chimpanzees, without any evidence that their death was a consequence of trypanosomiasis (Hoare, 1972). Early experimental infections of chimpanzees with different subspecies of T. brucei led to controversial results: while T. b. brucei and T. b. rhodesiense resulted in fatal parasitemia (Baker, 1962, 1968; Godfrey and Killick-Kendrick, 1967; Baker and Taylor, 1971), T. b. gambiense did not cause any obvious pathology despite the presence of trypomastigotes in the cerebro-spinal fluid (Godfrey and Killick-Kendrick, 1967; Hoare, 1972). This suggests that chimpanzees might serve as reservoir hosts for T. b. gambiense. Moreover, recent results demonstrated the existence of trypanosomatid hybrids among some subspecies of the T. brucei group, which have various animal reservoirs and are capable of infecting humans (Tait et al., 2011; Capewell et al., 2013a; Duffy et al., 2014; Echodu et al., 2015). Hence, addressing the question whether the free-ranging non-human primates may serve as a host or reservoir for some of these trypanosomes is relevant.
Inspired by recent advances in our understanding of mechanisms of resistance to trypanosomes mediated by the TLF (Raper and Friedman, 2013), we performed the experiments, in the frame of which three subspecies of the T. brucei group were incubated with sera from mandrill, barbary macaque, spider monkey, orangutan and chimpanzee. The obtained results on T. b. brucei and T. b. gambiense are in concordance with previous experiments of Lugli et al. (2004), though we also demonstrated that the serum from a mandrill is able to also lyse T. b. gambiense. Furthermore, the chimpanzee sera failed to lyse T. b. brucei, T. b. rhodesiense and also T. b. gambiense. However, results obtained with the latter subspecies are in contrast with the early infection experiments (Baker, 1962, 1968; Godfrey and Killick-Kendrick, 1967; Baker and Taylor, 1971), perhaps due to the fact that those have been conducted on animals originating from nature. This indicates that wild chimpanzees could have a (partial) resistance to T. b. gambiense, especially in the endemic regions. Our experiment confirmed the notion that chimpanzees are fully susceptible to T. b. brucei, against which humans and other primates developed efficient resistance (Xong et al., 1998; Raper and Friedman, 2013; Uzureau et al., 2013; Thomson et al., 2014). These results suggest that the transmission of any of the T. brucei group flagellates into the blood of chimpanzees shall result into an infection.
Inspired by successful detection of the Plasmodium DNA in primate feces (Liu et al., 2010; Jirků et al., 2012), we proved the ability of ITS1-based PCR assay to amplify the DNA of all four T. brucei subspecies in feces of experimentally infected mice. PCR-based screening of the available set of fecal samples from wild chimpanzees revealed the presence of trypanosomes, and this finding was further supported by positive results from a number of tissue samples taken from dead chimpanzees. By allowing the detection of trypanosomes in feces, this assay overcomes the inaccessibility of blood and tissue samples from animals that are endangered in the wild. This detection method provides new opportunities for studies of trypanosomatid parasites in the African great apes, and potentially in other rare or highly endangered mammals.
Our dendogram of the ITS1 region showed that absolute majority of the obtained sequences fell into the robust T. brucei clade, providing strong evidence that these infections were caused by one of its subspecies. However, since all attempts to amplify T. b. rhodesiense and T. b. gambiense-specific DNA regions failed, the subspecies status could not be established. In any case, the introduction of a PCR assay, capable of amplifying trypanosome DNA from the feces of great apes and potentially other infected mammals, will allow non-invasive large-scale screening for these important parasites. In summary, via feces- and tissue-based PCR assays, we have demonstrated that chimpanzees are frequently infected with trypanosomes from the T. brucei group and rarely also by other species. Future research might shed light on how chimpanzees control trypanosomiasis, and whether transmission occurs among them, other primates and humans.
Acknowledgments
We would like to thank Eva Černotíková-Stříbrná and Zdeněk Verner for technical assistance, Sebastian Calvignac-Spencer for the help in initial stages of the project, Lawrence Mugisha and veterinarians of the Taï chimpanzee project and sanctuary veterinarians for contributing some samples. We wish to acknowledge the Ivorian authorities for their long-term support, especially Ministry of the Environment and Forests, Ministry of Research, and the directorship of the Taï National Park. For sample collection we are grateful to S. Schenk, S. Metzger and the field assistants of the Tai Chimpanzee Project. We thank the Uganda Wildlife Authority and the Uganda National Council for Science and Technology for granting us permission to conduct this research. In Gabon, we acknowledge Agence Nationale des Parcs Nationaux and Centre National de la Recherche Scientifique et Technologique for permission to conduct research in Loango National Park, and the Société pour la Conservation et le Développement and Wildlife Conservation Society for financial and logistical support. We also thank the Centre International de Recherches Médicales de Franceville, especially E. Leroy and the Ministere des Eaux et Forets du Gabon. We appreciate the help of the CITES authorities of Cote d'Ivoire, Uganda, Republic of Congo and Germany in obtaining the necessary permission. This study was supported by Grant of the Czech Academy of SciencesM200961204, the Praemium Academiae Award to J.L., and Grant of USB 108/2013/P. Furthermore, we acknowledge the use of EU ‘Bioglobe grant’ (CZ.1.07/2.3.00/30.0032) and the EU 7th Framework Programme, grant agreement n° 316304.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
Supplementary Data 1.
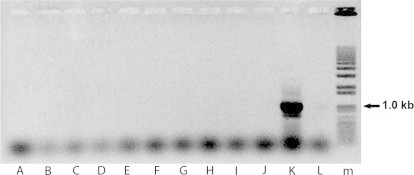
Unsuccessful detection of TgSGP gene in primate samples positive for trypanosomes based on the ITS1 region; (A–J) samples from infected primates, but negative for TgSGP; (K) positive control; (L) negative control; (M) marker.
Supplementary Data 2.
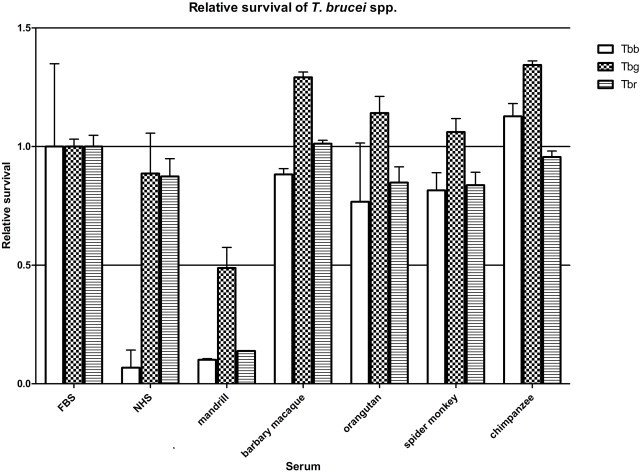
Survival of trypanosomes in sera. Trypanosomes were cultivated in media with human, mandrill, barbary macaque, orangutan, spider monkey and chimpanzee sera, and their relative survival was estimated using the Alamar Blue assay. The survival was standardized using FBS, with NHS used as a positive control. The experiment was performed in triplicate, bars indicate average ± SD values.2
References
- Adams E.R., Malele I.I., Msangi A.R., Gibson W.C. Trypanosome identification in wild tsetse populations in Tanzania using generic primers to amplify the ribosomal RNA ITS-1 region. Acta Trop. 2006;100:103–109. doi: 10.1016/j.actatropica.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Baker J.R. Infection of chimpanzee (Pan troglodytes verus) with Trypanosoma rhodesiense and T. brucei. Ann. Trop. Med. Parasitol. 1962;56:216–217. doi: 10.1080/00034983.1962.11686112. [DOI] [PubMed] [Google Scholar]
- Baker J.R. Experimental infections with Trypanosoma brucei and T. rhodesiense in chimpanzees. Trans. R. Soc. Trop. Med. Hyg. 1968;62:138. [Google Scholar]
- Baker J.R., Taylor A.E.R. Experimental infections of chimpanzee (Pan troglodytes) with Trypanosoma brucei brucei and Trypanosoma brucei rhodesiense. Ann. Trop. Med. Parasitol. 1971;65:471–485. doi: 10.1080/00034983.1971.11686780. [DOI] [PubMed] [Google Scholar]
- Brun R., Blum J., Chappuis F., Burri C. Human African trypanosomiasis. Lancet. 2010;375:148–159. doi: 10.1016/S0140-6736(09)60829-1. [DOI] [PubMed] [Google Scholar]
- Calvignac-Spencer S., Leendertz S.A.J., Gillespie T.R., Leendertz F.H. Wild great apes as sentinels and sources of infectious disease. Clin. Microbiol. Infect. 2012;18:521–527. doi: 10.1111/j.1469-0691.2012.03816.x. [DOI] [PubMed] [Google Scholar]
- Capewell P., Cooper A., Duffy C.W., Tait A., Turner M.R., Gibson W., Mehlitz D., MacLeod A. Human and animal trypanosomes in Côte d'Ivoire form a single breeding population. PLoS One. 2013;8:e67852. doi: 10.1371/journal.pone.0067852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capewell P., Clucas C., DeJesus E., Kieft R., Hajduk S., Veitch N., Steketee P.C., Cooper A., Weir W., MacLeod A. The TgsGP gene is essential for resistance to human serum in Trypanosoma brucei gambiense. PLoS Pathog. 2013;9:e1003686. doi: 10.1371/journal.ppat.1003686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy C.W., MacLean L., Sweeney L., Cooper A., Turner C.M.R., Tait A., Sternberg J., Morrison L.J., MacLeod A. Population genetics of Trypanosoma brucei rhodesiense: clonality and diversity within and between foci. PLoS Negl. Trop. Dis. 2014;7:e2526. doi: 10.1371/journal.pntd.0002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echodu R., Sistrom M., Bateta R., Murilla G., Okedi L., Aksoy S., Enyioha C., Enyaru J., Opiyo E., Gibson W., Caccone A. Genetic diversity and population structure of Trypanosoma brucei in Uganda: implications for the epidemiology of sleeping sickness and nagana. PLoS Negl. Trop. Dis. 2015;9:e3353. doi: 10.1371/journal.pntd.0003353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco J.R., Simarro P.P., Diarra A., Jannin J.G. Epidemiology of human African trypanosomiasis. Clin. Epidemiol. 2014;6:257–275. doi: 10.2147/CLEP.S39728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.G., Killick-Kendrick R. Cyclically transmitted infections of Trypanosoma brucei rhodesiense and T. gambiense in chimpanzees. Trans. R. Soc. Trop. Med. Hyg. 1967;61:781–791. doi: 10.1016/0035-9203(67)90035-1. [DOI] [PubMed] [Google Scholar]
- Hoare C.A. first ed. Blackwell Scientific Publications; Oxford: 1972. The Trypanosomes of Mammals. A Zoological Monograph. [Google Scholar]
- Jirků M., Pomajbíková K., Petrželková K.J., Hůzová z., Modrý D., Lukeš J. Detection of Plasmodium spp. in human feces. Emerg. Infect. Dis. 2012;18:634–636. doi: 10.3201/eid1804.110984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M., Lowa A., Ulrich M., Ellerbrok H., Goffe A.S., Blasse A., Zommers Z., Couacy-Hymann E., Babweteera F., Zuberbuhler K., Metzger S., Geidel S., Boesch C., Gillespie T.R., Leendertz F.H. Wild chimpanzees infected with 5 Plasmodium species. Emerg. Infect. Dis. 2010;16:1956–1959. doi: 10.3201/eid1612.100424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondgen S., Schenk S., Pauli G., Boesch C., Leendertz F.H. Noninvasive monitoring of respiratory viruses in wild chimpanzees. Ecohealth. 2010;7:332–341. doi: 10.1007/s10393-010-0340-z. [DOI] [PubMed] [Google Scholar]
- Lai D.H., Hashimi H., Lun Z.R., Ayala F.J., Lukeš J. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansii are petit mutans of T. brucei. Proc. Natl. Acad. Sci. U. S. A. 2008;105:1999–2004. doi: 10.1073/pnas.0711799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leendertz F.H., Ellerbrok H., Boesch C., Couacy-Hymann E., Matz-Rensing K., Hakenbeck R., Bergmann C., Abaza P., Junglen S., Moebius Y., Vigilant L., Formenty P., Pauli G. Anthrax kills wild chimpanzees in a tropical rainforest. Nature. 2004;430:451–452. doi: 10.1038/nature02722. [DOI] [PubMed] [Google Scholar]
- Leendertz F.H., Pauli G., Maetz-Rensing K., Boardman W., Nunn C., Ellerbrok H., Jensen S.A., Junglen S., Boesch C. Pathogens as drivers of population declines: the importance of systematic monitoring in great apes and other threatened mammals. Biol. Conserv. 2006;131:325–337. [Google Scholar]
- Liu W.M., Li Y.Y., Learn G.H., Rudicell R.S., Robertson J.D., Keele B.F., Ndjango J.B.N., Sanz C.M., Morgan D.B., Locatelli S., Gonder M.K., Kranzusch P.J., Walsh P.D., Delaporte E., Mpoudi-Ngole E., Georgiev A.V., Muller M.N., Shaw G.M., Peeters M., Sharp P.M., Rayner J.C., Hahn B.H. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–427. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.M., Li Y.Y., Shaw K.S., Learn G.H., Plenderleith L.J., Malenke J.A., Sundararaman S.A., Ramirez M.A., Crystal P.A., Smith A.G., Bibollet-Ruche F., Ayouba A., Locatelli S., Esteban A., Mouacha F., Guichet E., Butel C., Ahuka-Mundeke S., Inogwabini B.I., Ndjango J.B.N., Speede S., Sanz C.M., Morgan D.B., Gonder M.K., Kranzusch P.J., Walsh P.D., Georgiev A.V., Muller M.N., Piel A.K., Stewart F.A., Wilson M.L., Pusey A.E., Cui L.W., Wang Z.L., Farnert A., Sutherland C.J., Nolder D., Hart J.A., Hart T.B., Bertolani P., Gillis A., LeBreton M., Tafon B., Kiyang J., Djoko C.F., Schneider B.S., Wolfe N.D., Mpoudi-Ngole E., Delaporte E., Carter R., Culleton R.L., Shaw G.M., Rayner J.C., Peeters M., Hahn B.H., Sharp P.M. African origin of the malaria parasite Plasmodium vivax. Nat. Commun. 2014;5:3346. doi: 10.1038/ncomms4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugli E.B., Pouliot M., Portela M.D.M., Loomis M.R., Raper J. Characterization of primate trypanosome lytic factors. Mol. Biochem. Parasitol. 2004;138:9–20. doi: 10.1016/j.molbiopara.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Poelvoorde P., Vanhamme L., Van Den Abbeele J., Switzer W.M., Pays E. Distribution of apolipoprotein L-I and trypanosome lytic activity among primate sera. Mol. Biochem. Parasitol. 2004;134:155–157. doi: 10.1016/j.molbiopara.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Raper J., Friedman D.J. Parasitology: molecular one-upmanship. Nature. 2013;501:322–323. doi: 10.1038/501322a. [DOI] [PubMed] [Google Scholar]
- Simarro P.P., Diarra A., Postigo J.A.R., Franco J.R., Jannin J.G. The human African trypanosomiasis control and surveillance programme of the World Health Organization 2000–2009: the way forward. PLoS Negl. Trop. Dis. 2011;5:e1007. doi: 10.1371/journal.pntd.0001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens N.A., Kieft R., MacLeod A., Hajduk S.L. Trypanosome resistance to human innate immunity: targeting Achilles' heel. Trends Parasitol. 2012;28:539–545. doi: 10.1016/j.pt.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait A., Morrison L.J., Duffy C.W., Cooper A., Turner A.M.R., Macleod A. Trypanosome genetics: populations, phenotypes and diversity. Vet. Parasitol. 2011;181:61–68. doi: 10.1016/j.vetpar.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Thomson R., Samanovic M., Raper J. Activity of trypanosome lytic factor: a novel component of innate immunity. Future Microbiol. 2009;4:789–796. doi: 10.2217/FMB.09.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson R., Genovese G., Canon C., Kovacsics D., Higgins M.K., Carrington M., Winkler C.A., Kopp J., Rotimi C., Adayemo A., Doumatey A., Ayodo G., Alper S.L., Pollak M.R., Friedman D.J., Raper J. Evolution of the primate trypanolytic factor APOL1. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E2130–E2139. doi: 10.1073/pnas.1400699111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzureau P., Uzureau S., Lecordier L., Fontaine F., Tebabi P., Homble F., Grelard A., Zhendre V., Nolan D.P., Lins L., Crowet J.M., Pays A., Felu C., Poelvoorde P., Vanhollebeke B., Moestrup S.K., Lyngso J., Pedersen J.S., Mottram J.C., Dufourc E.J., Perez-Morga D., Pays E. Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature. 2013;501:430–434. doi: 10.1038/nature12516. [DOI] [PubMed] [Google Scholar]
- Vanhollebeke B., Pays E. The trypanolytic factor of human serum: many ways to enter the parasite, a single way to kill. Mol. Microbiol. 2010;76:806–814. doi: 10.1111/j.1365-2958.2010.07156.x. [DOI] [PubMed] [Google Scholar]
- Wheeler R.J. The trypanolytic factor-mechanism, impacts and applications. Trends Parasitol. 2010;26:457–464. doi: 10.1016/j.pt.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Xong H.V., Vanhamme L., Chamekh M., Chimfwembe C.E., Van Den Abbeele J., Pays A., Van Meirvenne N., Hamers R., De Baetselier P., Pays E. A VSG expression site-associated gene confers resistance to human serum in Trypanosoma rhodesiense. Cell. 1998;95:839–846. doi: 10.1016/s0092-8674(00)81706-7. [DOI] [PubMed] [Google Scholar]


