Abstract
Background
Patients with multiple brain metastases, especially those with more than 3 lesions, usually undergo to palliative whole brain (WB) radiotherapy (RT).
Methods
A breast cancer patient with 8 brain metastases was treated on the brain by a radical RT regimen. Prescription doses were according to the simultaneous integrated boost-intensity modulated radiation therapy (SIB-IMRT) technique with all lesions as well brain irradiated simultaneously in 20 daily fractions. Doses of 40.0 Gy (2.0 Gy/fraction) and 50.0 Gy (2.5 Gy/fraction) were prescribed to the whole brain and to eight individual metastases, respectively.
Results
Mean volume of the eight metastases was 8.1 cc (range: 3.8–10.1 cc). For all lesions, the volume receiving 95% of prescribed dose was 100% and dose homogeneity was within 3%. Moreover, maximum doses were less than 105% of prescribed dose, while average mean dose to lesions was 50.6 Gy (range: 49.7–51.5 Gy). Whole brain mean dose was 45.2 Gy. Maximum doses to brainstem and optic chiasma were limited to 44.5 Gy and 42.9 Gy, respectively, while maximum doses to eyes, lens and optic nerves were limited to 9.2 Gy, 4.9 Gy and 41.0 Gy, respectively. From a clinical point of view, subsequent MRI brain controls showed a complete clinical response. Forty months after treatment the patient is disease free and shows no late brain and skin toxicities.
Conclusion
This case demonstrates the technical feasibility of a SIB-IMRT treatment in patients with more than 3 brain metastases.
Keywords: Brain metastases, SIB-IMRT, Simultaneous integrated boost-intensity modulated radiation therapy
1. Introduction
Development of brain metastases is a common problem in oncology. Given the large number of patients and the important consequences for individual patients and health care systems, intense research activity is directed towards prevention, therapy and quality of life monitoring.1–4 Whole brain (WB) radiotherapy (RT) is the treatment of choice, especially when more than 3 brain lesions are present. In patients with 3 or less brain metastases, a stereotactic radiotherapy boost is indicated after WB-RT, based on the results of the 9508 RTOG study.5 In this paper we report the case of a young patient bearing 8 brain recurrent lesions from breast carcinoma, who was successfully treated with simultaneous integrated boost intensity modulated radiation therapy (SIB-IMRT) technique.
2. Case report
2.1. Clinical history
In October 2008, a 37 years old patient, with idiopathic thrombocytopenia (storage pool disease), noticed a lump in right breast. Mammogram and ultrasound (US) examinations of the breast and ipsilateral axillary region were highly suggestive of a malignant nodule, and US-guided needle core biopsy revealed the presence of an infiltrating ductal carcinoma. Immunohistochemical characterization of breast cancer tissue documented the presence of oestrogen and progesterone receptors in 30% and 20% of neoplastic elements, respectively, while c-erb-B2 immunoreaction was 3+. Positive staining for Ki67 was found in 20% of tumour cells. At the staging work up (chest X-rays, US liver examination, bone scan, CT scan of the brain) the patient was staged as cT3N1M0, so that underwent 6 cycles of neo-adjuvant chemotherapy with docetaxel and trastuzumab. At the end of systemic treatment, the patient underwent radical mastectomy and axillary lymphadenectomy. Histological examination showed negative axillaries lymph nodes and microscopic residual disease in the breast. After surgery the patient was triaged to adjuvant hormone therapy with tamoxifen and LH–RH analogue. Subsequent controls showed no recurrence of disease until May 2010 when an MRI, performed due to moderate headache, documented the presence of 8 sub-centimetric lesions in the brain (Fig. 1). Given the young age and excellent performance status (ECOG: 0) a potentially curative treatment was recommended, based on step and shoot IMRT planning. Patient underwent computed tomography (CT) simulation (2 mm slice spacing) and T1-weighted magnetic resonance (MR) scan with contrast. Patient immobilization was achieved by using a thermoplastic mask. Image fusion was performed based on anatomy surface-matching approach. All clinical structures were delineated individually on the CT/MR axial slices by both a radiologist and a radiation oncologist after the fusion was confirmed. Clinical Target Volume 2 (CTV2) was defined as the whole brain; to account for set-up uncertainties, a Planning Target Volume 2 (PTV2) was generated from CTV2 by a geometrical expansion of 5 mm. Eight Clinical Target Volumes 1 (CTV1s, defined as the GTV) were contoured and a 5 mm margin was added to each metastatic lesion obtaining eight PTV1s. Critical organs at risk (OAR) including brainstem, chiasm, optical nerves and eyes were all contoured. Dose was prescribed according to the SIB-IMRT technique with all PTVs irradiated simultaneously in 20 daily fractions. Doses of 40.0 Gy (2.0 Gy/fraction) and 50.0 Gy (2.5 Gy/fraction) were prescribed to the PTV2 and to eight PTV1s, respectively. The dose normalization was chosen in such a way that 95% of the prescribed dose covers 100% of PTVs volumes. Treatment plan was generated by Oncentra Masterplan treatment planning system and delivered by an Elekta Precise linear accelerator, equipped with a 40-leaf dynamic multileaf collimator. A beams arrangement based on nine fields (seven coplanar, two non-coplanar) was adopted and 6-MV photon beam energy was used. All beams shared a single isocenter, located near to the centre of the brain, not within any particular lesion, so that no patient repositioning was needed during beams delivery. To evaluate plan optimization dose-volume histogram for PTVs and OARs was analyzed. In particular, PTVs mean, maximum and minimum doses were recorded to estimate dose homogeneity (Dmax/Dmin), while for OARs exclusively maximum doses were considered. Mean volume of the eight PTV1 was 8.1 cc (range: 3.8–10.1 cc). For all PTV1s, the volume receiving 95% of prescribed dose was 100% and dose homogeneity was within 3%. In all PTV1s, maximum doses were less than 105% of prescribed dose, while average mean dose to PTV1s was 50.6 Gy (range: 49.7–51.5 Gy). Concerning whole brain mean dose (PTV2), it was 45.2 Gy. Maximum doses to brainstem and optic chiasma were limited to 44.5 Gy and 42.9 Gy, respectively, while maximum doses to eyes, lens and optic nerves were limited to 9.2 Gy, 4.9 Gy and 41.0 Gy, respectively. Fig. 2 illustrates the dose distributions in representative transverse, coronal and sagittal planes obtained with SIB-IMRT based treatment planning, highlighting a highly shaped dose distribution and sharp peripheral falloff.
Fig. 1.
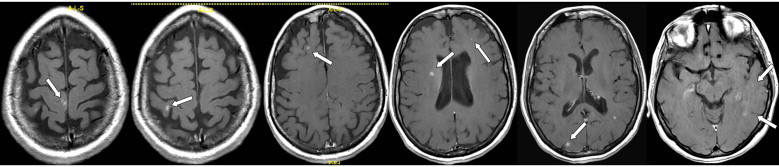
Pre-treatment MRI (white arrows indicate brain metastases).
Fig. 2.
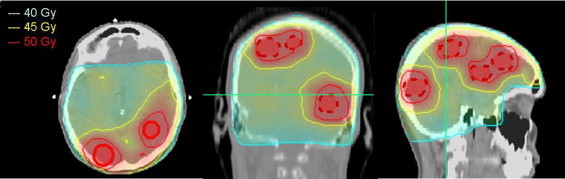
Treatment planning: dose distribution on axial, coronal and sagittal planes for the SIB-IMRT technique; the dose conformity is shown at the level of 40 Gy, 45 Gy and 50 Gy dose levels. Metastases are highlighted.
2.2. Treatment
The single fraction treatment time lasted about 18 min with 364 monitor units per fraction. Concurrent temozolomide at a dosage of 75 mg/mq/day, 5 days/week, was administered.
2.3. Quality assurance
Daily portal images in the first phase of irradiation (5–10 MU) were acquired.6 Deviations larger than 3 mm in the isocenter position were immediately corrected. For quality assurance through treatment planning and delivery, two independent checks were performed by medical and physics staff, as previously described.7 During radiotherapy course the patient reported mild headache and dizziness.
2.4. Follow up
After 3 months MRI imaging showed the complete response of most of the lesions with only two contrast enhancement areas (diameter: 1 mm) visible (Fig. 3). Subsequent MRIs performed at 3 month-intervals for 2 years showed only the presence of two millimetric areas, at the site of larger metastases. Last MRI examination performed 40 months after treatment evidenced no brain disease any more. On the other hand, regular checks by i.v. enhanced CT scans, at 6 month-intervals, showed the absence of extracranial disease. To date, the patient's neurological examination is negative and alopecia is not present, moreover the patient still denies visual disturbances, headache or drowsiness, and reports normal family and work activities.
Fig. 3.
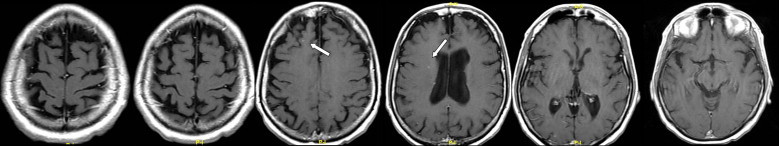
Post-treatment MRI (white arrows indicate two small residual lesions) performed 3 months after SIB-IMRT.
3. Discussion
A class solution in a patient with multiple (8) brain metastases treated by IMRT-SIB has been reported. More than 3 years after treatment, the patient is radiological free from disease with good general condition and absence of neurological symptoms. The IMRT use has been previously proposed for primitive brain tumours8 and in the stereotactic treatment of malignant gliomas9 with the aim of reducing the irradiation of healthy brain hence allowing dose-escalation. IMRT has been also proposed for brain metastases treatment. In particular, IMRT has been evaluated in several planning studies, with the aim to spare neural stem cells during WB irradiation,10–12 to treat by stereotactic technique multiple metastases13, to simultaneously boost the dose to 2–3 brain metastases during WB14 and for radiosurgery of multiple brain metastases or large irregular lesions.15 Furthermore, IMRT has been used in some clinical studies on brain metastases. In fact, IMRT was retrospectively evaluated in the treatment of patients with oligometastatic brain disease (1–4 metastases) with or without WB,16 and used as adjuvant stereotactic radiosurgery or stereotactic radiotherapy in patients with resected brain metastases.17 Finally IMRT has been used to boost up to 40 Gy the gross tumour volume of single bulky metastatic disease during a standard 30 Gy WB schedule. No acute or subacute morbidity was recorded and encouraging early control data were reported.18 More generally, the results of all these previous analyses confirmed the advantages of IMRT-SIB, represented by: (1) reduction in overall treatment, due to the simultaneous administered boost; (2) greater biological anticancer boost effectiveness, due to the accelerated treatment at gross tumour volume; (3) improved sparing of healthy brain tissue, due to the integration of the boost within the WB treatment. However, to the best of our knowledge this is the first case where such a large number of brain metastases received concomitant boost radiotherapy and was clinical followed for so long time (40 months).
4. Conclusions
Our case demonstrated for the first time the clinical as well the technical feasibility of a boost administered over a large number of metastatic lesions. The positive result recorded in our patient in terms of response and absence of neurological symptoms or other toxicities, justifies the design of prospective studies (such as the ongoing EORTC trial #22111-26111) to assess the role of the SIB-IMRT in the treatment of multiple (>3) brain metastases in patients without extracranial disease.
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Nieder C., Spanne O., Mehta M.P., Grosu A.L., Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117:2505–2512. doi: 10.1002/cncr.25707. [DOI] [PubMed] [Google Scholar]
- 2.Fabi A., Felici A., Metro G. Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res. 2011;30:10. doi: 10.1186/1756-9966-30-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caravatta L., Deodato F., Ferro M. A phase I study of short course accelerated whole brain radiation therapy (SHARON) for multiple brain metastases. Int J Radiat Oncol Biol Phys. 2012;84(4):e464. doi: 10.1016/j.ijrobp.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez G., Pocinho R., Travancinha C. Quality of life and radiotherapy in brain metastasis patients. Rep Pract Oncol Radiother. 2012;17:281–287. doi: 10.1016/j.rpor.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews D.W., Scott C.B., Sperduto P.W. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 6.Deodato F., Cilla S., Massaccesi M. Daily on-line set-up correction in 3D-conformal radiotherapy: is it feasible? Tumori. 2012;98:441–444. doi: 10.1177/030089161209800407. [DOI] [PubMed] [Google Scholar]
- 7.Morganti A.G., Deodato F., Zizzari S. Complexity index (COMIX) and not type of treatment predicts undetected errors in radiotherapy planning and delivery. Radiother Oncol. 2009;89:320–329. doi: 10.1016/j.radonc.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Morganti A.G., Balducci M., Salvati M. A phase I dose-escalation study (ISIDE-BT-1) of accelerated IMRT with temozolomide in patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2010;77:92–97. doi: 10.1016/j.ijrobp.2009.04.064. [DOI] [PubMed] [Google Scholar]
- 9.Voynov G., Kaufman S., Hong T., Pinkerton A., Simon R., Dowsett R: Treatment of recurrent malignant gliomas with stereotactic intensity modulated radiation therapy. Am J Clin Oncol. 2002;25:606–611. doi: 10.1097/00000421-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Kirby N., Chuang C., Pouliot J., Hwang A., Barani I.J. Physics strategies for sparing neural stem cells during whole-brain radiation treatments. Med Phys. 2011;38:5338–5344. doi: 10.1118/1.3633946. [DOI] [PubMed] [Google Scholar]
- 11.Tarnawski R., Michalecki L., Blamek S. Feasibility of reducing the irradiation dose in regions of active neurogenesis for prophylactic cranial irradiation in patients with small-cell lung cancer. Neoplasma. 2011;58:507–515. doi: 10.4149/neo_2011_06_507. [DOI] [PubMed] [Google Scholar]
- 12.Gondi V., Tolakanahalli R., Mehta M.P. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1244–1252. doi: 10.1016/j.ijrobp.2010.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma Y., Li M., Yin Y. Hypofractionated stereotactic radiotherapy for brain metastases: a dosimetric and treatment efficiency comparison between volumetric modulated arc therapy and intensity modulated radiotherapy. Technol Cancer Res Treat. 2010;9:499–507. doi: 10.1177/153303461000900508. [DOI] [PubMed] [Google Scholar]
- 14.Wolff D., Abo-Madyan Y., Dobler B. Serial tomotherapy vs. MLC-IMRT (multileaf collimator intensity modulated radiotherapy) for simultaneous boost treatment large intracerebral lesions. Z Med Phys. 2009;19:58–66. doi: 10.1016/j.zemedi.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Lawson J.D., Wang J.Z., Nath S.K. Intracranial application of IMRT based radiosurgery to treat multiple or large irregular lesions and verification of infra-red frameless localization system. J Neurooncol. 2010;97:59–66. doi: 10.1007/s11060-009-9987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S.J., Choi M., Fuller C.D., Salter B.J., Fuss M. Intensity-modulated radiosurgery for patients with brain metastases: a mature outcomes analysis. Technol Cancer Res Treat. 2007;6:161–168. doi: 10.1177/153303460700600302. [DOI] [PubMed] [Google Scholar]
- 17.Do L., Pezner R., Radany E., Liu A., Staud C., Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys. 2009;73:486–491. doi: 10.1016/j.ijrobp.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 18.Edwards A.A., Keggin E., Plowman P.N. The developing role for intensity-modulated radiation therapy (IMRT) in the non-surgical treatment of brain metastases. Br J Radiol. 2010;83:133–136. doi: 10.1259/bjr/28596848. [DOI] [PMC free article] [PubMed] [Google Scholar]


