Abstract
Aim
Patient setup errors were aimed to be reduced in radiotherapy (RT) of head-and-neck (H&N) cancer. Some remedies in patient setup procedure were proposed for this purpose.
Background
RT of H&N cancer has challenges due to patient rotation and flexible anatomy. Residual position errors occurring in treatment situation and required setup margins were estimated for relevant bony landmarks after the remedies made in setup process and compared with previous results.
Materials and methods
The formation process for thermoplastic masks was improved. Also image matching was harmonized to the vertebrae in the middle of the target and a 5 mm threshold was introduced for immediate correction of systematic errors of the landmarks. After the remedies, residual position errors of bony landmarks were retrospectively determined from 748 orthogonal X-ray images of 40 H&N cancer patients. The landmarks were the vertebrae C1–2, C5–7, the occiput bone and the mandible. The errors include contributions from patient rotation, flexible anatomy and inter-observer variation in image matching. Setup margins (3D) were calculated with the Van Herk formula.
Results
Systematic residual errors of the landmarks were reduced maximally by 49.8% (p ≤ 0.05) and the margins by 3.1 mm after the remedies. With daily image guidance the setup margins of the landmarks were within 4.4 mm, but larger margins of 6.4 mm were required for the mandible.
Conclusions
Remarkable decrease in the residual errors of the bony landmarks and setup margins were achieved through the remedies made in the setup process. The importance of quality assurance of the setup process was demonstrated.
Keywords: Radiotherapy, Head and neck cancer, Patient setup, Image guidance, Setup margins
1. Background
Image guided radiotherapy (IGRT) of head and neck (H&N) is a challenging task due to changes in patient anatomy caused by natural flexibility of the neck and potential patient weight loss. Position errors of the target and several risk organs should be determined to achieve correct patient positioning. 3D imaging is the best tool for this task1 but it is resource-intensive and routine patient verification is commonly based on 2D imaging. Therefore, routine IGRT is commonly based on bony landmarks visible in orthogonal 2D kV setup images.
Setup margins in RT of H&N cancer should be relatively small and margins from 3 to 5 mm have been recommended. This is because treatment volumes are typically close to several organs at risk (OAR), such as the parotid glands, the submandibular glands and the spinal cord.2–4 Adequate setup margins should cover total residual position errors caused by inter-fractional variation of treatment isocenter position, flexible patient anatomy (non-rigid target), patient rotation and inter-observer variation in isocenter selection.5–8 Unfortunately, the commonly accepted 3D margins of 5 mm have been reported inadequate or this can be calculated from the setup errors given.5–8 These results have been obtained with modern immobilization devices. Still the small margins are widely applied without any confirmation of patient setup accuracy or only inter-fractional isocenter variation has been taken into account.
Our group has previously determined contributions of all the sources of setup errors together for clinical data consisting of 80 H&N patients.8 It was found that the residual systematic displacement errors of the vertebrae C1–2, C5–7, the mandible and the occiput bone inside the target volume were approximately two times larger than inter-fractional couch corrections for a rigid target volume. With daily IGRT, the 3D margins of the C1–2, the mandible and the occiput bone, related to the most important OARs and the target volumes irradiated to the highest prescribed doses, were even 9 mm consistently with the existing literature.5–7 Further improvements in patient immobilization and image guidance practice were motivated by these results in order to reduce the setup errors and margins.8 Previously Outhwaite et al. have reported that setup reproducibility for H&N patients has improved after training and improved guidelines for patient immobilization.9
2. Aims of the study
A multi-disciplinary group was founded with an aim to reduce patient setup errors in RT of H&N cancer. The group consisted of radiation therapists, radiation oncologists and radiotherapy physicists. The main goals were to confirm that the technique used for the formation of the thermoplastic masks is appropriate, to develop the formation process, to establish the image matching guidelines in order to harmonize the choice of treatment isocenter and to decide how to deal with large residual errors. This study describes the practical improvements made in the patient setup process. The efficacy of the remedies was retrospectively investigated by determining residual position errors of the relevant bony landmarks and by determining the required setup margins. The results were compared to those obtained before the remedies. The residual position errors were determined in actual treatment situation and they included contributions from patient rotation, flexible anatomy and inter-observer variation in isocenter selection. To the best of our knowledge, data including contribution from all these factors together have earlier been reported for different landmarks only in one publication.8
3. Methods and materials
3.1. Patient group and RT procedure
748 image pairs of 40 consecutive H&N cancer patients were retrospectively analyzed. The patient material is described in Table 1. Patient fixation was carried out with 5-point C-frame Candor head and neck plate (Candor, Gislev, Denmark). 14 out of the 40 patients were treated with compression plate to immobilize the tongue while 26 patients were treated without it. CT imaging for treatment planning was done with either Toshiba Aquilion LB (Toshiba Medical System, Tokyo, Japan) or Philips Brilliance Big Bore (Philips Medical Systems, Eindhover, The Netherlands) with slice thickness of 3 mm and kVp of 120. The patients were treated with 6 MV photon beams of Clinac 2300 iX (Varian Medical Systems, Palo Alto, CA) and 7-field IMRT technique was used. The image guidance was performed bi-daily using orthogonal kV-images acquired with onboard imaging system (OBI). The anterior–posterior and lateral setup images were acquired at 100 kV and 70 kV, respectively. No tolerance was applied for translational couch corrections. If the couch correction was ≥3 mm, the image guidance was repeated on the next treatment fraction.
Table 1.
Patient and tumor characteristics.
| n (%) | ||
|---|---|---|
| Gender | ||
| Female | 13 (33) | |
| Male | 27 (67) | |
| Age (years) | ||
| Mean, median (range) | 63, 67 (22–87) | |
| Tumor site | ||
| Larynx | 3 (7.5) | |
| Oral cavity | 6 (15) | |
| Oropharynx | 18 (45) | |
| Hypopharynx | 5 (12.5) | |
| Nasopharynx | 3 (7.5) | |
| Parotid gland | 5 (12.5) | |
| TNM-stagea | ||
| T1 6 (15) | N0 13 (32.5) | I 3 (7.5) |
| T2 19 (47.5) | N1 11 (27.5) | II 8 (20) |
| T3 5 (12.5) | N2 10 (25) | III 10 (25) |
| T4 10 (25) | N3 6 (15) | IV 19 (47.5) |
| M1 8 (20) | ||
| Radiotherapy | ||
| Postoperative | 22 (55) | |
| Concurrent chemotherapy | 22 (55) | |
According to the American Joint Committee on Cancer (AJCC) 2010.
3.2. Basic verification of the patient immobilization procedure and the improvements made
In the first step a multi-disciplinary group was founded consisting of radiation therapists, radiation oncologists and radiotherapy physicists in our department. The aim was to optimize the patient immobilization process and to decide the remedies required. Especial aims were to reduce patient rotation and movement of the mandible because these were the largest obstacles in our previous data.8
It was first confirmed that the mask forming procedure is appropriate and the training was refreshed. It was checked that patient's mouth is closed during the mask formation and that the patient chin and neck are molded tightly but gently, while the mask is cooling on the patient's face. It was confirmed that there is no free space between the mask and the skin. The nose bone and the ear holes were carefully marked by pressing the mask gently with fingers.
First new action was to advice that the shoulders were kept relaxed and they were slightly pressed to caudal direction during the mask cooling. In addition, the mask was removed after it was cooled. Then the mask was further cooled to room temperature and re-worn before the treatment planning CT. Re-wearing of the mask cooled to room temperature and repositioning of the patient were considered to simulate the setup in treatment situation.
3.3. Image matching guidelines and actions to correct large position errors
Inter-observer variation in isocenter selection was attempted to be reduced by advising that the online match should be done to the vertebrae in the middle of the PTV (MID_PTV). The online match was performed by 25 experienced radiation therapists (one or two in time) in actual treatment situation. Compromise matching toward the mandible was discouraged. In 97.5% of the cases the MID_PTV was the vertebrae C3–4 enabling fair comparison with our previous data.
A threshold of 5 mm was applied for residual systematic position errors of the landmarks (1D). The relevant landmarks were the vertebrae C1–2, the C5–7, the mandible and the occiput bone shown in Fig. 1. If the average error in three consecutive fractions after the online match exceeded this threshold, patient positioning was attempted to be corrected first. If the problem was patient rotation, one or more plastic plates with thickness of 6 mm were inserted below the fixation base plate or head cushion to correct the rotation as presented in Fig. 2. When there was no improvement, immediate CBCT scan was acquired with the OBI system for the further evaluation of clinical importance of the large residual position error. A radiation oncologist (T.T.) decided further corrective actions and potential re-planning based on the CBCT scan (or scans). The use of an offline matching tool was recommended to optimally investigate, whether the position errors were really systematic. Inter-observer variation in image matching was estimated from the difference between the actual online match and consensus offline match performed by the multi-disciplinary group (M.K., M.L., T.T.) strictly based on the given guidelines. Finally, systematic residual 3D errors were retrospectively determined by the group to estimate the magnitude of residual position errors and their clinical importance.
Fig. 1.
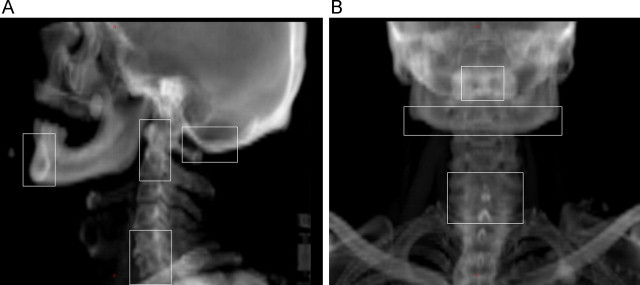
Selection of the bony landmarks relevant for the target volumes. The chosen landmarks in (a) lateral and (b) anterior reference image.
Fig. 2.

Patient immobilization system. A thermoplastic mask is attached to the base plate which is fixed to the treatment couch. Correction for patient rotation about lateral axis was carried out by placing plastic plates (lowest arrow) below the base plate. There are marks on the mask for the nose bone and deep holes for the ears (two upper arrows).
3.4. Data analysis
The position error of each landmark was measured by one observer (M.L.) from the online images collected during a treatment course for each individual patient. Thus the results present residual errors after daily IGRT and they actually occurred in treatment (residual errors were not estimated for fractions without image guidance). The position errors obtained implicitly include contributions from patient rotation, flexible anatomy and inter-observer variation in treatment isocenter selection. The position errors of the isocenter (rigid target) were determined also from the online match (M.L.) and these include the inter-observer variation. The variation of isocenter location was estimated for weekly IGRT. This was done by simulating the weekly IGRT by removing couch corrections from the data for one week intervals.
The systematic (Σ) and random (σ) components of the position errors both given as one standard deviation (SD) were determined for the patient group.10 The van Herk formula m = 2.5Σ + 0.7σ was used to calculate the adequate 3D setup margins.11 Anterior–posterior (vertical), superior–inferior (longitudinal) and lateral directions (denoted as AP, SI and LAT, respectively) were investigated. For the occiput bone, only the first two directions were investigated.
Current results were compared to our previously reported values8 to investigate whether the improvements made in patient setup process were statistically significant. The distributions of the systematic errors obtained were confirmed to be the Gaussian distributions with different standard deviations. Therefore, the two-tailed parametric F-test was chosen for the systematic errors (test for equality of variances). The distributions of the random errors were not Gaussians and they had quite equal variances. The non-parametric Wilcoxon rank sum test was chosen for the random errors (test for equality of means). A p-value ≤ 0.05 was considered statistically significant.
4. Results
Residual position errors of the landmarks, rigid target and inter-observer variation in the image matching are presented in Table 2. If the weekly IGRT protocol is used, the errors obtained for the rigid target should be summed in squares with the error of each landmark. With the daily IGRT, the 3D margins up to 4.4 mm were sufficient for all the other regions, except for the mandible. The larger margins up to 6.4 mm were required for the mandible. Systematic residual error of the mandible was approximately two times larger than that of the rigid target with weekly IGRT or inter-observer variation in image matching. Random errors were below 2 mm for all the investigated structures.
Table 2.
Residual errors (mm) of a rigid target and different bony landmarks relevant for the target after the online match. The van Herk margins (3D) are given in the parenthesis (n = 40).
| Structure | Systematic error Σ (1 SD) |
Random error σ (1 SD) |
||||
|---|---|---|---|---|---|---|
| AP/vertical | SI/longitudinal | LAT/lateral | AP/vertical | SI/longitudinal | LAT/lateral | |
| Rigid targeta | 0.7 (2.5) | 0.6 (2.4) | 0.8 (2.8) | 1.2 | 1.3 | 1.1 |
| Observer error | 0.6 (2.2) | 0.7 (2.6) | 0.8 (2.8) | 1.1 | 1.2 | 1.2 |
| C1–2b | 1.1 (3.7) | 0.5 (2.0) | 1.1 (3.6) | 1.2 | 1.0 | 1.2 |
| C5–7b | 0.9 (3.1) | 0.6 (2.3) | 1.3 (4.2) | 1.2 | 1.2 | 1.4 |
| Mandibleb | 2.0 (6.4) | 2.0 (6.3) | 1.5 (4.7) | 1.9 | 1.9 | 1.5 |
| Occiput boneb | 1.4 (4.4) | 1.2 (4.0) | – | 1.6 | 1.5 | – |
For weekly IGRT including inter-observer variation.
For daily IGRT including inter-observer variation.
Most of the residual errors and setup margins were significantly lower than in our previous study8 as can be seen in Table 3. Most importantly, the rotation errors of the vertebrae and the setup margins were significantly reduced. Also wide systematic errors were remarkably reduced. The inter-observer errors were significantly smaller in the vertical and lateral directions.
Table 3.
Differences in residual position errors of the landmarks after the described improvements made in the patient setup process (current − previous result, mm). Changes in the van Herk margins (3D) are given in the parenthesis.
| Structure | Systematic error Σ (1 SD) |
Random error σ (1 SD) |
||||
|---|---|---|---|---|---|---|
| AP/vertical | SI/longitudinal | LAT/lateral | AP/vertical | SI/longitudinal | LAT/lateral | |
| Rigid targeta | −0.1 (−0.7) | −0.3 (−0.8) | 0.1 (−0.5) | −0.5c | −0.3 | −0.5c |
| Observer error | −0.3 (−1.0)c | −0.1 (−0.5) | −0.1 (−0.5) | −0.3 | −0.3 | −0.4c |
| C1–2b | −0.9 (−2.6)c | −0.5 (−1.5)c | −0.5 (−1.5)c | −0.6c | −0.3c | −0.4c |
| C5–7b | −0.6 (−1.6)c | −0.4 (−1.5)c | 0.1 (0.0) | −0.2 | −0.6c | −0.3 |
| Mandibleb | −0.9 (−2.5)c | −0.7 (−2.2)c | −0.1 (−0.6) | −0.4 | −0.5c | −0.4c |
| Occiput boneb | −1.0 (−3.1)c | −0.3 (−1.0)c | – | −0.7c | −0.4c | – |
For weekly IGRT including inter-observer variation.
For daily IGRT including inter-observer variation.
The difference is statistically significant (p ≤ 0.05).
Immediate CBCT scan was performed for five out of the investigated 40 patients because the systematic 1D displacement for one or more of the landmarks (observed in online matching) was ≥5 mm in three successive treatment sessions. In these CBCTs, the 3D displacements were over 5 mm in four out of these five cases, but the displacements were not considered clinically important and none of the patients were re-planned. Two plastic plates had to be used only for one patient to reduce the vertebrae rotation to the acceptable level (Fig. 2). The plates were placed under the baseplate of the fixation system.
The retrospective analysis of the online images showed that the systematic 3D residual error of 5 mm was exceeded with eight patients in the mandible and with one patient in the occiput bone (2D). The corresponding maximal errors were 6.9 mm and 5.1 mm, respectively. The limit of 6 mm was exceeded with three patients. The limit of 4 mm, was exceeded with one patient in the C1-2, the C5-7 and the occiput bone, but even with 17 patients in the mandible. The corresponding maximal errors for the first two landmarks were 4.0 mm and 4.1 mm, respectively. The mean 3D systematic residual errors with and without the compression plate in the tongue were similar: 4.3 mm and 4.0 mm, respectively.
5. Discussion
The setup errors and margins were re-estimated for the most important bony landmarks in the RT of H&N cancer based on 2D IGRT. The patient setup process was re-checked and improved in order to reduce patient rotation, mandible movement and large position errors. The effort was motivated by our previous analysis of the clinical data.8 The 3D setup margins were considerably reduced even by 3.3 mm with the improvements made. Their efficacy of the remedies was demonstrated. To the best of our knowledge, this is the first study reporting that 3D margins equal to or less than 5 mm are sufficient for most of the landmarks when total position errors are accounted for. Such errors include contributions from inter-fractional variation of isocenter position, patient rotation, flexible patient anatomy and inter-observer variation in isocenter selection. Application of such small margins requires confirmation of patient setup accuracy. The obtained margins are suited for frequent 2D imaging used for routine patient setup. However, the 3D imaging should be done regularly (independently of the results of 2D IGRT) to confirm the relation between the soft tissues and the bony landmarks.2 The investigation of the frequency for the 3D imaging is beyond the scope of this study.
Our previous setup margins and setup errors of the landmarks8 were quite consistent with those reported in the existing literature5–7 Especially the margins of the C1–2 and the C5–7 indicating patient rotation were close to the values reported by van Kranen et al. obtained from CBCT data using offline position correction protocol and the same formula to calculate the margins.5 On the contrary, the new 3D margins presented in this study were from 0.2 to 3.1 mm (up to 57%) lower for the vertebrae C1–2, the C5–7 and the occiput bone than the previously reported values.5 In addition, the residual errors of the landmarks were on the average only half of those obtained with in-room CT by Zhang et al.7 Moreover, the 3D margins were up to 3.6 mm lower than the 1D margins reported by Prisciandaro et al.6 obtained using portal MV images. The comparison of the absolute results, however, has uncertainties because of different imaging modes (or modalities), image matching procedures and contribution of inter-observer variation. Moreover, different principles were used for treatment couch position corrections in the referred studies: van Kranen et al.5 have used a shrinking action level technique with offline couch corrections; Prisciandaro et al.6 matched the portal images after treatment delivery and Zhang et al.7 have reported their results for image co-registration based on radiopaque markers on the mask. Nevertheless, the importance of quality assurance was demonstrated in the present study. Also the efficacy of the remedies made in patient setup process was demonstrated to reduce the margins and it was shown how to achieve acceptably low 3D setup margins using daily 2D online IGRT. It should be noticed that daily CBCT imaging may give additional dose of even 3 Gy to radiosensitive organs12 while 2D imaging gives normally less than one-tenth of this dose.13
Some improvements have previously been proposed to increase accuracy of patient setup.9,14,15 Training of staff in mask formation has been shown to reduce overall 3D setup errors from 4.6 to 3.0 mm.9 In addition, making of tattoo marks on patient's chest has been demonstrated to reduce setup errors significantly.14 In this study, the weak points in the mask formation process were sought after and the efficacy of the remedies made was proven to reduce position errors of several bony landmarks. The thermoplastic masks were considered tighter after the described improvements and no new masks had to be made during a treatment course because of patient discomfort. Smaller residual random errors of the landmarks (all below 2 mm, mostly close to 1 mm) indicated improved reproducibility of patient setup. Especially rotation of the vertebrae and the head was remarkably reduced.
Image matching strategies have been investigated to minimize either the overall position error or the maximal error within the target.15 Image matching was advised to the middle of the target based on previous findings to obtain smallest overall errors.8,15 The target volumes receiving the highest prescribed doses and the regions of the spinal cord having the maximal dose are most usually close to the C3–4 region which was the average the MID_PTV level. The region near the C2 may be the most stable part of the head and neck.7 It was considered that if image matching is done more caudally than MID_PTV, the coverage of these target regions may be jeopardized and/or the planned dose to the spinal cord may be exceeded. For the target extending more caudally than C7, the use of additional caudal setup images may be useful because of limitations in the field sizes for the online images.
Our group has previously demonstrated that application of 3D margins of 5 mm is not possible unless a threshold of 4 mm is applied for systematic 3D position errors of the relevant landmarks.8 The previously obtained threshold was raised in this study because the margins of 5 mm were achieved through the presented improvements (except for the mandible). It was concluded that the verification and development of the mask making procedure together with image matching guidelines were the most important improvements while the application of the threshold only screened large position errors. Obviously accuracy can be further improved by using the stricter 3D threshold.
Patient weight changes and/or tumor regression may make it necessary to re-plan the patient even when the patient positioning process is of high quality. Re-planning, however, is not necessarily the best first choice to reduce systematic position errors because patient fixation system and practice may need improvements. Re-planning increases workload and re-imaging is always prone to random errors. The blind use of a threshold of 4–4.8 mm alone for systematic displacements for the bony structures has been estimated to result in a re-planning rate of even 22–38% for the H&N patients.5,8 In contrast, none of the investigated 40 patients were re-planned in this study and the presented improvements seem clinically attractive. The retrospective analysis of the online images showed that the presented actions have been successful because the 3D limit of 5 mm was clearly exceeded only for the mandible (eight out of 40 patients). On the other hand, potential target coverage in the region of the mandible was confirmed only for four out of these eight patients with immediate online CBCT. Fortunately, none of these position errors were considered clinically important. It was concluded, however, that the presented larger margins up to 6.4 mm or the tight threshold of 4 mm should be applied for systematic 3D errors of the mandible to consider early corrective actions when the target is very anterior. The treatment console should directly calculate also 3D translational shifts to facilitate the online evaluation of the 3D errors.
Large reduction of margins was achieved for the mandible, up to 2.5 mm, even though the image matching was done more strictly to the vertebrae than earlier. The large margins up to 6.4 mm have been determined for the most anterior part of the mandible having the largest movement. Most usually, however, the target does not extend far in the anterior direction in the mandible and lower margins of approximately 5 mm are sufficient but this has to be estimated individually. Smaller margins without a strict threshold for position errors would require further improvements in immobilization. Interestingly, the patients treated without compression plate for the tongue required smaller margins for the mandible in the SI direction than those treated with it, 5.5 mm vs. 7.5 mm (p = 0.17). The margin in the AP direction was also smaller 6.1 mm vs. 6.7 mm. It should be noticed that the 2D imaging may give maximal position errors for the mandible while average errors may be obtained in 3D studies.
6. Conclusions
To the best of our knowledge, this is the first report showing that the commonly accepted 3D setup margins of 5 mm can be achieved with daily online 2D image guidance in RT of head and neck cancer. These margins can compensate total residual position errors of the most relevant bony landmarks, such as the vertebrae C1–2, the C5–7 and the occiput bone. The use of these small margins, however, is not a straight-forward task requiring confirmation and potential improvements for the patient immobilization system and image guidance practice. Remedies were made in the setup process based on our previous clinical quality control data. The remedies were: confirmation of careful molding of the mask on the patient face, confirmation that patient mouth is closed, pressing the shoulders caudally, cooling and re-wearing of the mask before the planning CT. Also image matching was harmonized and advised to the vertebrae in the middle of the target volume. Moreover, a threshold of 5 mm was used for the residual systematic 1D position errors of all the landmarks relevant for the target. When this threshold was exceeded, an immediate 3D imaging was performed and an experienced oncologist evaluated the clinical importance of the displacement. After all these remedies, the 3D setup margins were reduced even by 3.1 mm and none of the investigated 40 patients had to be re-planned. In addition, inter-observer variation in the isocenter selection was reduced. The larger setup margin of 6.4 mm should be used for the mandible and further improvements are required to achieve smaller margins for very anterior targets in the mandible.
Conflict of interest
None declared.
Financial disclosure
This study was supported by the Pirkanmaa Hospital District, Elna Kaarina Savolainen Fund (Grant No. R12536).
Acknowledgments
Radiation therapists Helmi Luukkanen and Risto Manninen are greatly acknowledged for participating in the analysis of inter-observer variation.
Contributor Information
Mika Kapanen, Email: mika.kapanen@pshp.fi.
Marko Laaksomaa, Email: Marko.Laaksomaa@pshp.fi.
Tapio Tulijoki, Email: tapio.tulijoki@pshp.fi.
Pirkko-Liisa Kellokumpu-Lehtinen, Email: pirkko-liisa.kellokumpu-lehtinen@pshp.fi.
Simo Hyödynmaa, Email: simo.hyodynmaa@pshp.fi.
References
- 1.Schwartz D.L., Dong L. Adaptive radiation therapy for head and neck cancer – can an old goal evolve into a new standard? J Oncol. 2011;690595:e1–e13. doi: 10.1155/2011/690595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graff P., Hu W., Yom S.S., Pouliot J. Does IGRT ensure target dose coverage of head and neck IMRT patients? Radiother Oncol. 2012;104:83–90. doi: 10.1016/j.radonc.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Houghton F., Benson R.J., Tudor G.S.J. An assessment of action levels in imaging strategies in head and neck cancer using tomotherapy. Are our margins adequate in the absence of image guidance. Clin Oncol. 2009;21:720–727. doi: 10.1016/j.clon.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Han C., Chen Y.J., Liu A., Schultheiss T., Wong J. Actual dose variation of parotid glands and spinal cord for nasopharyngeal cancer patients during radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:1256–1262. doi: 10.1016/j.ijrobp.2007.10.067. [DOI] [PubMed] [Google Scholar]
- 5.Van Kranen S., van Beek S., Rasch C., van Herk M., Sonke J.J. Setup uncertainties of anatomical sub-regions in head-and-neck cancer patients after offline CBCT guidance. Int J Radiat Oncol Biol Phys. 2009;73:1566–1573. doi: 10.1016/j.ijrobp.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 6.Prisciandaro J., Frechette C., Herman M., Brown P., Garces Y., Foote R. A methodology to determine margins by EPID measurements of patient setup variation and motion as applied to immobilization devices. Med Phys. 2004;31:2978–2988. doi: 10.1118/1.1800712. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Garden A.S., Lo J. Multiple regions-of-interest analysis of setup uncertainties for head-and-neck cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:1559–1569. doi: 10.1016/j.ijrobp.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 8.Kapanen M., Laaksomaa M., Tulijoki T., Peltola S., Wigren T., Hyödynmaa S. Estimation of adequate setup margins and threshold for position errors requiring immediate attention in head and neck cancer radiotherapy based on 2D image guidance. Radiat Oncol. 2013;8:212. doi: 10.1186/1748-717X-8-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Outhwaite J.A., McDowall W.R., Marquart L., Rattray G., Fielding A., Hargrave C. Training programme impact on thermoplastic immobilization for head and neck radiation therapy. Radiography. 2013;19:28–34. [Google Scholar]
- 10.Greener T. Practical determination of systematic and random set-up errors, Σset-up and σset-up using portal imaging. In: Working Party of The British Institute of Radiology, editor. Geometric uncertainties in radiotherapy; defining the planning target volume. The British Institute of Radiology; London: 2003. pp. 36–43. [Google Scholar]
- 11.van Herk M., Remeijer P., Rasch C., Lebesque J. The probability of correct target dosage: dose–population histograms for deriving treatment margins in radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1121–1135. doi: 10.1016/s0360-3016(00)00518-6. [DOI] [PubMed] [Google Scholar]
- 12.Ding G.X., Coffey C.W. Radiation dose from kilovoltage cone beam computed tomography in an image-guided radiotherapy procedure. Int J Radiat Oncol Biol Phys. 2009;73:610–617. doi: 10.1016/j.ijrobp.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Hälg R.A., Besserer J., Schneider U. Systematic measurements of whole-body imaging dose distributions in image-guided radiation therapy. Med Phys. 2012;39:7650–7661. doi: 10.1118/1.4758065. [DOI] [PubMed] [Google Scholar]
- 14.Cronin B., McCarthy A., Claire K. Quality improvement investigation for head and neck stabilization in radiotherapy using setup tattoos. J Med Imag Radiat Sci. 2013;44:92–99. doi: 10.1016/j.jmir.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Van Kranen S., van Beek S., Mencarelli A., Rasch C., van Herk M., Sonke J.J. Correction strategies to manage deformations in head-and-neck radiotherapy. Radiother Oncol. 2010;94:199–205. doi: 10.1016/j.radonc.2009.12.016. [DOI] [PubMed] [Google Scholar]


