Abstract
Management of patients who experience biochemical failure after radical radiotherapy with or without hormonal therapy is highly challenging. The clinician must not only choose the type of treatment, but also the timing and optimal sequence of treatment administration. When biochemical failure occurs, numerous treatment scenarios are possible, thus making it more difficult to select the optimal approach. Moreover, rapid and ongoing advances in treatment options require that physicians make decisions that could impact both survival and quality of life.
The aim of the present consensus statement, developed by the Urological Tumour Working Group (URONCOR) of the Spanish Society of Radiation Oncology (SEOR), is to provide cancer specialists with the latest, evidence-based information needed to make the best decisions for the patient under all possible treatment scenarios.
The structure of this consensus statement follows the typical development of disease progression after biochemical failure, with the most appropriate treatment recommendations given for each stage. The consensus statement is organized into three separate chapters, as follows: biochemical failure with or without local recurrence and/or metastasis; progression after salvage therapy; and treatment of castration-resistant patients.
Keywords: Prostate cancer, Radiotherapy, Hormone therapy, PSA, Biochemical failure, Castration-resistant prostate cancer
1. Introduction, objectives and methodology
In most studies, biochemical relapse-free survival is the main outcome measure used to evaluate the efficacy of local treatment of prostate cancer (PCa). However, an important aspect of PCa that has received less attention is the patient's clinical course following biochemical recurrence (BCR). The long natural history of PCa, including failure following initial local treatment, raises the question of which salvage treatment should be administered and in what sequence. Salvage therapy will depend on the primary treatment, the patient's clinical characteristics, and the aggressiveness of both the primary and relapsing tumours.
The definition of biochemical recurrence after radiotherapy (RT) is clear and universally accepted. As a result, the same diagnostic criteria and objectives for salvage treatment can be used in all clinical studies. However, level IA evidence for salvage treatment and follow up in these patients is scant. In contrast to surgical patients, local relapse after RT must be confirmed by biopsy before local salvage therapy can be considered. Moreover, no comparative studies have been conducted to evaluate the various salvage treatments and no consensus has yet been reached regarding the most appropriate indication for each salvage therapy. Similarly, due to insufficient experience, it is not yet possible to precisely define the imaging criteria (whether by MRI or choline PET-CT) required to confirm radiological relapse in an irradiated prostate.
Given this context, androgen deprivation therapy (ADT) is sometimes initiated early – at times indiscriminately – in patients with BCR who do not present signs of clinical or radiological progression. ADT is often administered in such cases even though evidence for such an indication is limited. Moreover, due to lack of data, we do not know if early initiation of ADT – implemented when patients fulfil specific criteria (as yet undetermined) – could delay metastatic progression and therefore – more importantly – improve overall survival.
Only 10% of low-risk patients who experience BCR will go on to develop metastasis or die due to prostate cancer. However, in intermediate and high risk patients, 35% of those with BCR will develop clinical progression and/or die.1,2 These figures reflect a reality seen in everyday clinical practice: biochemical relapse does not necessarily imply worse overall survival. Given this background, it is essential to understand how salvage treatment impacts the subsequent course of disease in patients with BCR. The main objective of any study of BCR should be to evaluate the sequence and timing of salvage treatments, the causes of death in this patient group, and overall survival. A better understanding of the role of these variables will help physicians to select those patients that will truly benefit from salvage therapy.
In the present document, the Urological Tumour Working Group (URONCOR) of the Spanish Society of Radiation Oncology (SEOR) has evaluated and compiled all the published evidence regarding biochemical recurrence after radiotherapy. The present consensus statement is, therefore, based on data from the most relevant studies available in the literature on this subject to date. The objective of the present report is to harmonize the criteria that Radiation Oncologists use to approach the diagnosis, treatment and follow up of patients with BCR. The URONCOR will periodically review this consensus guideline to incorporate new advances made in this field. Likewise, URONCOR is committed to disseminating and promoting the use of the contents of this document among professionals that specialize in treating PCa. An important aim of URONCOR is to establish the foundations necessary to promote a multidisciplinary patient care model, close coordination among professionals, and optimization of available resources.
2. Chapter I. Patient management from biochemical relapse until first-line hormonal therapy
-
1.
Definition of biochemical relapse. Prognostic factors.
-
2.
Work-up in patients with biochemical relapse.
-
3.
Options and indications for local treatment.
-
4.
Treatment of M0 patients with biochemical relapse who are not candidates for local salvage.
-
5.
Treatment options for biochemical relapse in M1 patients.
-
6.
Continuous or intermittent hormonotherapy.
-
7.
Follow up.
2.1. Definition of biochemical relapse. Prognostic factors
The therapeutic strategy for localized PCa is based on stratification by risk group.3 Depending on the risk group, treatment could involve radical prostatectomy (RP), brachytherapy (BT), or external beam radiotherapy (EBRT) with or without hormonotherapy (HT). However, a third of patients who undergo RP,4 and approximately 20–30% of patients treated with EBRT and HT,5 develop BCR accompanied or not by subsequent local failure or metastasis. Salvage treatment is administered in only 16–35% of cases. Because only those patients whose cancer metastasizes are at risk of death due to PCa, and, given that most patients with BCR die from causes other than the cancer, it is essential that we appropriately select only those patients who can benefit from treatment after BCR.
Various prognostic factors and nomograms are available to guide physicians in determining the likelihood that a patient will develop local or distant relapse after BCR. The nomograms can also help to select the optimal treatment approach in each case. Nowadays, PSA kinetics – specifically the PSA doubling time (PSADT) – is unanimously accepted as one of the most important prognostic factors for the development of BCR: a PSADT >10–12 months correlates with a greater likelihood of local relapse, while a PSADT ≤10–12 months is associated with a higher risk of developing distant disease. Other factors that confer a poor prognosis are: Gleason score at diagnosis (≥8); clinical stage (T3,N1); PSA at diagnosis (>20 ng/ml); the time interval between initial treatment and the emergence of biochemical failure (≤2 years); and absence of biochemical control after local treatment.3–5
2.2. Work-up in patients with biochemical relapse
To properly evaluate tumour dissemination in a patient with BCR, we must first attempt to determine whether the recurrence is a local failure or metastatic disease; if local failure is suspected, we must then decide if the patient is a candidate for salvage therapy.
Although evidence for the specific case of patients who develop BCR following radiotherapy is scant, we can extrapolate from published recommendations about patients who develop BCR after RP. Based on these data, a reasonable approach would be to recommend bone scans and computed tomography (CT) in those patients who present PSA values >20 ng/ml (after BCR diagnosis) and/or PSADT <10–12 months and/or with high risk factors at diagnosis.6
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) (or, alternatively, MRI with spectroscopy) is recommended in cases of biochemical failure following EBRT or BT due to its ability to detect local recurrence and a greater sensitivity of this modality compared to ultrasound. In addition, DCE-MRI provides important information about the presence of extracapsular extension or seminal vesicle invasion, with a sensitivity of 86% and specificity of 96%.7–9
In terms of the value of choline positron-emission tomography (PET)-CT, a recent meta-analysis10 concluded that this modality has a sensitivity of 85%, specificity of 88%, and a DOR (diagnostic odds ratio) of 41%. On the basis of this evidence, the authors of that meta-analysis suggest that choline PET-CT is superior to CT, MRI, and bone scintigraphy in detecting the presence of disease in lymph nodes, bones, and the prostate gland. Those authors, consequently, recommend choline PET-CT for re-staging patients with BCR before any therapeutic decisions are made. Choline PET-CT appears to be capable of detecting recurrences in 80% of patients with PSA progression after RT. However, at present, it is not known whether a specific PSA value is predictive of a positive choline PET-CT scan in patients with post-RT biochemical failure. In contrast, the PSADT (> or <6 months) can help predict the likelihood of detecting local or distant clinical relapse: the probability of developing distant lesions is 65% with a PSADT <6 months vs. 19% with PSADT >6 months.11
Choline PET-CT can be used to detect lymph node recurrence12 (sensitivity close to 100%); however, the decreased specificity (82%) of this test could result in unnecessary surgical treatments. For this reason, the value of a single imaging scan (choline PET-CT) that can be used to detect all disease sites after BCR should not be underestimated, particularly when bone metastases are suspected. The strongest predictors of PET-CT positivity for relapse in PCa patients are a PSA value >1 ng/ml, PSA velocity >1 ng/ml/year, and a PSADT <3 months. On-going hormonal therapy does not limit diagnostic accuracy. However, choline PET-CT does not appear to be indicated for detecting local recurrence (sensitivity 75%, specificity 82%) because of two main limitations in detecting local recurrence: (1) the presence of microscopic disease spread is not detectable (due to limits in resolution power), and (2) there may be inflammatory uptake at the prostatic site. Loco-regional recurrence in patients with BCR can be detected more accurately with combined proton magnetic resonance spectroscopic imaging (MRSI) and DCE-MRI at 3 T magnet than with choline PET-CT (89% vs. 60%).13
Detection rates for 11C-Choline PET in patients who develop BCR after RP or EBRT are <50% when PSA is <2 ng/ml and/or PSA velocity <1 ng/ml/year.14 According to Greco et al.,15 the patient population with a rising post-treatment PSA without evidence of disease on standard imaging tests currently represents the second largest group of PCa patients. At present, little information is available regarding the specificity and sensitivity of PET tracers in the assessment of early BCR. Ideally, PET imaging would allow physicians to accurately discriminate between local, nodal, and distant relapse, thus enabling the appropriate selection of patients for local salvage therapy; however, a vast majority of studies show a relatively poor yield of positive scans when the PSA is <4 ng/ml. To date, no tracer has proven capable of detecting local recurrence within the clinically useful 1 ng/ml PSA threshold, a clear limitation for the use of PET imaging in the post-surgical setting. Preliminary evidence, however, suggests that 11C-Choline PET may be useful in selecting patients with early BCR (PSA < 2 ng/ml) who have pelvic lymph node oligometastases that are potentially amenable to local treatment. Greco and colleagues conclude that the role of PET imaging in PCa is gradually evolving but still remains within the experimental realm. Well-conducted studies comparing the merits of different tracers are needed.
In patients who develop BCR following RT that are considered potential candidates for local salvage therapy, a prostate biopsy should be performed (once metastatic disease has been reasonably ruled out) to confirm the presence of local recurrence. To assure evaluability, the biopsy should be performed at least 24 months after completion of EBRT or BT. In patients with biochemical failure without disease spread (including a negative prostate biopsy), the recommended approach is observation and follow up,3,4,6 as we discuss in Section 4.
2.3. Options and indications for local salvage therapy
Ideal candidates for local salvage treatment after RT should meet all of the following characteristics: age <70 years; life expectancy >10 years16; diagnosis of low or intermediate risk PCa before initial treatment; PSADT ≥10–12 months; progression or relapse-free interval ≥2 years; an absolute PSA value at salvage ≤10 ng/ml; and absence of significant intestinal or urinary co-morbidities that would contraindicate treatment. In scenarios other than those described above, salvage therapy should be considered on a case-by-case basis after mutual consensus with the patient.
The treatment options for local salvage after BCR depend on the primary treatment and on patient comorbidities, and include the following interventions: RP, BT, cryosurgery, EBRT and high-intensity focused ultrasound (HIFU).17–21 Additional research and randomized clinical trials are required to determine which salvage modality is superior in terms of oncologic efficacy and reduced morbidity. Table 1 summarizes the outcomes of these various techniques. Treatmentalgorithmdetails are illustrated in figure 1.
Table 1.
Summary of results of local salvage therapy after radical radiotherapy in prostate cancer.
| Salvage treatment | Primary treatment | Results | Complications | Observations |
|---|---|---|---|---|
| Radical prostatectomy | EBRT/BT | BRFS/5 years: 50% (47–82%) BRFS/10 years: 28% CSS/10 years: 70% OS/10 years: 54–89% |
Incontinence: 50% (44–77%) Rectal fistula: 2.5% (2–10%) Stenosis: 25% (22–41%) |
The treatment with the most extensive clinical experience, largest series, and longest published follow up. |
| Cryotherapy | EBRT/BT | BRFS/5years: 45% (30–50)% OS/5 years: 73%-85% DFS/5 years: 30–60% |
Incontinence: 17% (10–73%) Fistula: 2% (1–10%) Stenosis: 7% (10–45%) |
Patients not candidates for RP. Short follow up. |
| HIFU | EBRT | BRFS/5 years: 40% (30–50%) OS/5 years: 84% |
Incontinence: 37% (6–50%) Fistulas: 4% (2–7%) Stenosis: 7% (4–35%) |
Very limited experience and short follow up. |
| Brachytherapy | EBRT and/or BT | BRFS/5 years: 55% (35–70%) | Incontinence: 6% (5–30%) Fistula: 3% (0–6%) Stenosis: 7–8% Rectal ulcers: 2–4% GI Tox gr 4: 2–12% GU Tox gr3: 8–40% GU Tox gr 4:0–6% |
Small series and short follow up. |
BRFS, biochemical relapse free survival; CSS, cancer-specific survival; OS, overall survival, DFS, disease-free survival; EBRT, external radiotherapy; BT, brachytherapy; RP, radical prostatectomy; GI Tox, gastro-intestinal toxicity; GU Tox, genitourinary toxicity.
Fig. 1.
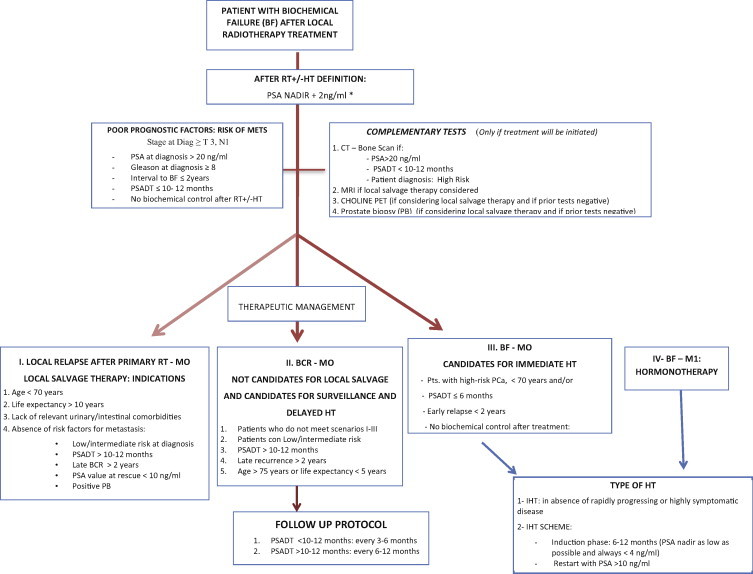
Imaging, treatment and follow-up algorithm for patients with biochemical failure following primary radiotherapy.
2.4. Treatment of M0 patients that present biochemical relapse and are not candidates for local salvage therapy
To date, the role of ADT in BCR – treatment initiation, regimen, and duration – remain controversial due to the lack of randomized studies to assess survival in patients who undergo ADT after BCR. This implies that, at present, there is no consensus for the management of such patients.22–24 Nevertheless, based on the available evidence, we can establish the following recommendations:
-
(1)In patients with biochemical failure alone, without any criteria of poor prognosis, and who do not wish to undergo or are not candidates for local salvage therapy, the best option is watchful waiting. The consensus among various researchers is that such patients present the following characteristics25–27:
-
1.Patients with low to intermediate-risk PCa.
-
2.PSADT ≥10–12 months.
-
3.Late failure – progression-free interval >48 months.
-
4.Age >75 years; life expectancy <5 years.
-
1.
-
(2)
Patients with BCR alone who are potential candidates for immediate ADT are those with the following characteristics:
-
1.
Young (age <70 years).
-
2.
High-risk PCa at diagnosis (primarily Gleason 8–10).
-
3.
PSADT <6 months.
-
4.
Clinical parameters suggestive of early relapse, with a progression-free interval of <2 years.
-
5.
No biochemical control after initial local treatment.
-
6.
PSA >10 ng/ml when BCR is detected.
In the current “PSA era”, the definitive 12-year results of the EORTC 30891 study,28 carried out in patients with locally-advanced M0 disease without local treatment, show that immediate ADT appears to primarily benefit patients with high-risk disease. In other patients, it appears safe to delay ADT initiation.
2.5. Treatment options in M1 patients with biochemical relapse
Most authors agree that ADT should be initiated immediately in patients with BCR and symptomatic metastasis given that this approach offers the greatest cost-effectiveness according to Bayoumi et al.26 However, in asymptomatic patients, the evidence is less clear and for that reason some authors prefer to delay ADT until symptoms appear, particularly given that the presumed objective of hormone therapy is overall survival. The uncertainty in these cases arises from the lack of randomized studies with sufficient statistical power to show a benefit in overall survival with the immediate use of ADT.
A Cochrane Library review27 of four randomized studies in the pre-PSA era concluded that, although the evidence is limited, the published data nevertheless seemed to suggest that the use of immediate ADT in advanced PCa slows disease progression and reduces related complications. Moreover, ADT can provide a small but statistically significant improvement in overall survival at 10 years.
The American Society of Clinical Oncology (ASCO) and European Society for Medical Oncology (ESMO) guidelines do not make any recommendations in this regard for patients with asymptomatic metastatic cancer. For this reason, in asymptomatic (or minimally symptomatic) M1 patients, one valid option might be to closely monitor informed patients (assuming the main goal is survival), with immediate initiation of hormonal treatment in symptomatic patients who have been informed of the risks and benefits of such therapy.
2.6. Continuous or intermittent ADT
Once hormonal treatment has been prescribed, the next decision is whether administration should be intermittent or continuous. The negative impact of ADT on the quality of life, together with its inability to completely eliminate clonogenic cells (with the attendant risk of developing resistance), have led to the design of non-inferiority studies to compare continuous ADT to intermittent hormonotherapy (IHT) in the two patient profiles described above – that is, in M0 patients with BCR and poor prognosis following radical radiotherapy and in patients with M1 disease.
In M0 patients with biochemical failure, the randomized Canadian study carried out by Crook et al.29 on 1386 patients (mean follow up: 7 years) concluded that intermittent ADT should be the standard treatment in this patient cohort because, despite the higher cancer-related mortality rate in the intermittent group (41% vs. 34%), the continuous group had a higher other-cause mortality rate (32.5% vs. 40%). As a result, the overall mortality rate in both groups was similar. Nevertheless, the role of predictive factors such as age, Gleason score and PSA kinetics in the selection of patients for intermittent therapy remain to be defined.
In M1 patients, most evidence comes from six randomized trials and a recent meta-analysis of eight randomized trials.30,31 The results show a small and non-significant decrease in cause-specific mortality, overall mortality, and disease progression with the use of IHT, but a significant reduction in the emergence of hot-flushes. The authors of that meta-analysis conclude that IHT is a reasonable option for well-informed patients who want to avoid these side-effects. However, due to differences in the methodologies and study variables among the various trials, it is difficult to establish definitive conclusions.
In the context of currently available evidence, it seems reasonable to recommend IHT in the context of biochemical failure in M0 and M1 patients whose disease is not progressing rapidly and is not highly symptomatic. However, the advantages and disadvantages of this therapy should be discussed with the patient prior to initiating IHT.
The proposed treatment regimen for IHT would include an induction period ranging from 6 to 12 months (mean: 9 months) to obtain the lowest possible PSA nadir (in all cases <4 ng/ml), and then restart ADT when PSA values rise above ≥10 ng/ml.
2.7. Follow up
Follow up in non-castration resistant patients with BCR should focus on detecting metastatic disease or in evaluating the metastasis. Patients with PSADT <6 months are at a greater risk for early metastasis. In those patients, follow up should be performed every 3–6 months with PSA determination, bone scans, and thoraco-abdominal CT. In patients with PSADT >12 months, follow up should be performed every 6–12 months.
3. Chapter II. Management of patients with disease progression after first-line hormonal therapy
-
1.
Definition of progression criteria for first-line hormonal therapy.
-
2.
Imaging tests. Physical examination.
-
3.
Treatment options.
3.1. Definition of progression criteria in first-line hormonal therapy
Biochemical progression is defined as at least three consecutive increases (with at least one week between each assessment) in the PSA plasma concentration starting from the nadir and resulting in two increases of 50% above nadir with at least 2 ng/ml detected in one of the PSA determinations. Clinical progression is defined as the emergence of disease at any level in M0 patients; in patients with metastatic disease, progression is defined according to the RECIST criteria32:
-
-
Bone progression: Defined as the appearance of two or more new lesions; ambiguous results should be confirmed by other imaging tests (CT or MRI).
-
-
Nodal progression: Only lymph nodes ≥1.5 cm in diameter should be considered involved and these should be evaluated to monitor changes in size; in M1 patients, radiological progression is defined as the emergence of two or more bone lesions on the bone scintigraphy, or the appearance of soft tissue lesions (RECIST criteria).
3.2. Imaging tests. Physical examination
At present, we do not have level I evidence that would allow us to establish recommendations for follow up after initiating ADT. For this reason, diagnostic procedures should only be performed during follow up if an effective therapeutic strategy exists if disease progression is detected.
Following the 2014 EAU4 recommendations, we can consider the following proposed treatment regimen. In asymptomatic M0 and M1 patients, the following is recommended:
-
-
Assessment of PSA and testosterone levels, with physical examination and appropriate anamnesis of symptoms and clinical signs every 3–6 months.
-
-
Monitor levels of creatinine, haemoglobin, alkaline phosphatase, and liver function every 3–6 months.
-
-
Bone densitometry. Basal and annual tests with T-scores from 1 to 2.5; every two years if the T-score <1.
-
-
In M0 patients who show a good treatment response and tolerance and who maintain PSA levels <4 ng/ml, follow up consultations could be scheduled every 6–12 months. In M1 cases, every 3–6 months.
-
-
In patients who develop progression during hormonal treatment, the imagining studies should be selected on a case-by-case basis. The use of routine thoraco-abdominal CT, MRI, and bone scintigraphy is not recommended in asymptomatic patients with PSA <20 ng/ml, since such tests cannot detect metastatic disease. In patients with PSA >4 ng/ml and factors of poor prognosis, a choline PET-CT scan has a disease detection rate of up to 82% and could be considered.
3.3. Treatment options
In patients who develop BCR following first-line hormonal therapy, treatment with LHRH analogues should be maintained continuously according to evidence from several published series that found better survival when testicular androgen suppression is maintained. This recommendation is based on the initial data from Manni33 as well as other, less conclusive data from Taylor34 and Hussain.35 Treatmentalgorithmdetails are summarized in figure 2.
-
•
Bicalutamide: Currently, first-line HT usually involves treatment with LHRH analogues alone, while second-line HT involves the addition of bicalutamide (50 mg dose), which yields a response rate of around 20% (decrease in PSA levels of at least 50%).36 Switching antiandrogens (flutamide for bicalutamide and vice versa) is considered second-line HT. With this approach, it is possible to achieve a decrease in PSA of up to 50%, with response duration of more than 6 months.37
-
•
Withdrawal of antiandrogens: If the first-line treatment is a complete hormone blockage (LHRH analogues plus antiandrogen), withdrawal of the antiandrogen can induce a response in approximately 30% of patients (reduction of PSA >50%) with a median response duration of 4 months.37–39
-
•
Ketoconazole: The adrenal glands secrete approximately 10% of the circulating androgens. This secretion can be inhibited by ketoconazole, which achieves PSA response rates of 25% that have a median duration of 4 months. The use of ketoconazole in conjunction with the simultaneous withdrawal of antiandrogens produces a significant increase in response rates (32% vs. 11%) and a longer PSA progression-free interval (8.6 vs. 5.9 months) compared to the withdrawal of the antiandrogen alone.40,41 However, due to the potential for significant liver toxicity with this drug, the European and Spanish Medicines agencies (EME; AEMPS)42 recently contraindicated the systemic use of Ketoconazole.
-
•
Corticosteroids: Corticosteroids inhibit adrenal androgens, which account for 10% of the total androgens. The use of corticosteroids yields a PSA response in up to 25% of patients, with a median duration of 4 months. The most commonly-used corticosteroid drugs and doses are dexamethasone (0.5–2 mg/day) and prednisone (5–10 mg/day).43,44 In one study, patients with progressive castration-resistant prostate cancer (CRPC) received LHRH analogues and oral dexamethasone (0.5 mg daily). The median pretreatment PSA level was 83 ng/ml. The median time to PSA progression for the entire cohort was 7.4 (range, 1–28) months. Low-dose dexamethasone could become the corticosteroid of choice in the management of CRPC43; studies have reported PSA response rates of 50% or greater in 20% of patients for prednisone or hydrocortisone, and in up to 60% for dexamethasone.44 Low doses of corticosteroids, such as prednisone 5–10 mg per day, can be used if metastatic prostate cancer progresses despite androgen suppression.45 Several studies reported good results with glucocorticoid therapy using low-dose dexamethasone (1.5–2.0 mg/day) or prednisolone (10 mg/day).43–45
Fig. 2.
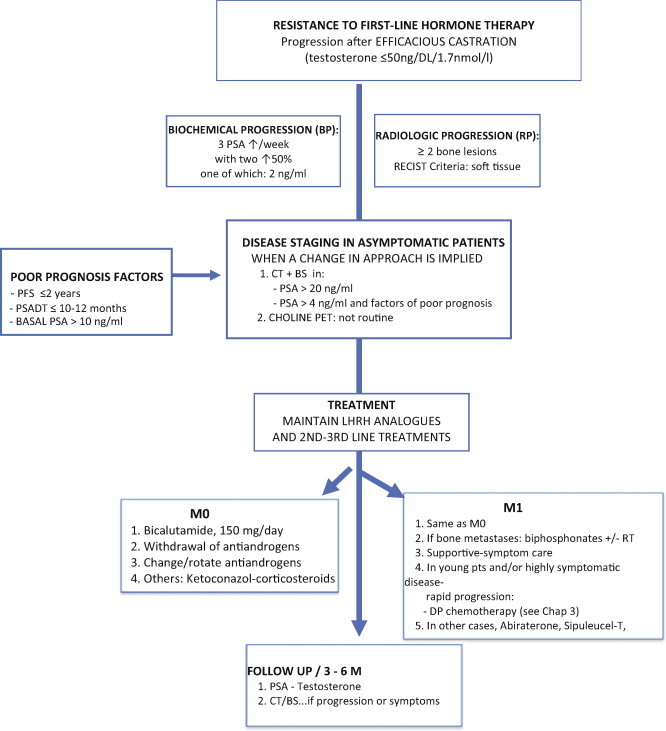
Imaging, treatment and follow-up algorithm for patients with resistance to first-line hormone therapy.
In summary, we can state that after a failure of first-line HT, the various second-line hormonal therapies produce overall PSA response rates in approximately 25% of patients, with a median duration ranging from 4 to 6 months.
4. Chapter III. Management of patients with castration-resistant prostate cancer
-
1.
Definition of castration resistance.
-
2.
Types of patients.
-
3.
Follow up and natural history of CRPC without metastasis.
-
4.
Follow up and natural history of CRPC with metastasis. Asymptomatic or minimally symptomatic patients. Symptomatic patients.
4.1. Definition of castration resistance
According to the 2014 EAU guidelines,4 CRPC is defined as tumour progression after an efficacious castration therapy (plasma testosterone levels ≤50 ng/dL [1.7 nmol/L]). Biochemical progression is defined as at least three successive increases in the plasma PSA numbers starting with the last nadir, in tests carried out with at least one week between each test, resulting in two increases of 50% above the nadir with a minimum of 2 ng/ml in one of these analyses. Before a patient receiving complete androgen blockade (CAB) can be classified as castration-resistant, the antiandrogen must be withdrawn previously (at least 4 weeks for flutamide and at least 6 weeks for bicalutamide). Radiological progression is defined as the appearance of two or more bone lesions on the bone scintigraphy or lesions in the soft tissue according to the RECIST criteria,32 with lymph nodes greater or equal to 2 cm in size.
4.2. Types of patients
CRPC is considered an incurable disease, with a median survival of less than 2 years and symptoms of metastatic involvement (usually bone metastases) are usually present. CRPC is a highly complex clinical situation in terms of tumour biology and the type of patient. From a clinical standpoint, the patient profile in CRPC is highly variable, ranging from completely asymptomatic patients whose only manifestation of progression is an elevated PSA, to other highly symptomatic cases with multiorgan involvement and a notable worsening of the patient's general condition.46 Although somewhat controversial, most authors recommend maintaining ADT with LHRH-agonists in patients with CRPC even when progression occurs; the Prostate Cancer Working Group-2 (PCWG2) document also recommends the same approach.47
4.3. Follow up and natural history of non-metastatic CRPC
In this subgroup of patients, there are no established criteria for the appropriate frequency and type of follow up, nor has consensus been reached regarding the value of administering active treatment or maintaining ADT. The natural history of the disease in such patients is disease progression, including the development of distant metastasis. The following factors are considered predictors of disease progression4,46,48:
-
-
PSA doubling time: this is considered the most important factor, with a linear relation between a decrease in PSADT and cause-specific mortality in this group of patients. A doubling time ≤10–12 months is considered a risk factor for the development of metastasis.
-
-
The progression-free interval until criteria for castration resistance are met: if this interval is >18–24 months, metastasis typically occurs later.
-
-
Baseline PSA at the start of ADT (progression and metastasis-free survival are worse if the PSA >10 ng/ml).
-
-
PSA nadir occurring during ADT.
Svatek49 used the aforementioned four factors to construct a nomogram, which has proven to have the greatest predictive value to date in this patient group (Fig. 1). In general, median survival in these patients is 45–50 months and approximately 57% will die of prostate cancer. In addition, approximately 20% of these patients will develop bone metastases in the first 2 years. The median metastatic-free survival time is approximately 30 months.
The 2014 EAU guidelines4 indicate that follow up in these patients should be individualized (evidence grade, C). However, a close reading of the EAU follow up recommendations leads to the following conclusions:
-
-
Since these are patients on ADT, a clinical examination with PSA and testosterone determination should be performed every 3–6 months. The physical examination should include an adequate assessment of the patient's clinical signs and symptoms.
-
-
The use of imaging tests in patients with stable disease (PSA doubling time >10 months) is not recommended.
-
-
If the doubling time is short (PSADT <8 months), the patient and PSA level should be monitored more closely (every 1–3 months) and imaging tests such as choline PET-CT should be ordered to rule out the presence of distant metastasis before symptoms emerge.
Although PSA kinetics can help to predict radiological progression, the absence of treatments with a proven efficacy in slowing or reducing the appearance of metastasis makes this patient group particularly suitable for clinical trials of new drugs and for the validation of imaging techniques such as choline PET-CT.
In summary, the management of patients with CRPC in whom the only manifestation of progression is an elevated PSA value and who do not present objective metastatic disease or associated symptoms is highly challenging.46 On one hand, the available data regarding the most appropriate treatment in these patients is scant, given that most published studies of CRPC have evaluated patients with clinically-evident metastatic disease. On the other hand, such patients can continue to have elevated PSA levels for months and even years (during which they often request treatment due to their elevated PSA levels), but still lack any clinical evidence that would clearly justify the use of ADT. Therefore, we should not rush to initiate treatments that could lead to increased toxicity that would worsen the quality of life.46,47,49
Table 2 summarizes the main studies currently underway in this area and the preliminary results thereof. Until the results from these and other future trials of similar design (http://clinicaltrials.gov/ct2/show/NCT00757692) are reported, it will not be possible to confirm whether an increase in PSA levels alone is sufficient to justify initiation of systemic treatment; the same uncertainty applies to selecting from among the various options the ideal treatment sequence and timing. For this reason, once all second-line hormonal therapies have been tried, the current recommendation is that such patients be included in clinical trials, or at least followed closely, until early evidence of clinical or radiological progression emerges. Exceptionally, there is a subgroup of patients (>75 years, life expectancy <5 years, and multiple associated co-morbidities) who are not candidates for aggressive systemic treatments and/or clinical trials. In these patients, both the follow up and complementary tests should be individualized. Follow up and treatmentalgorithmdetails are described in figure 3.
Table 2.
Drugs under investigation in patients with CRPC, with and without metastasis.
| CRPC without metastasis | |
|---|---|
| - VANDETANIB ± BICALUTAMIDE - ZIBOTENTAN vs. PLACEBO - Enzalutamide-MDV3100 vs. PLACEBO (PROSPER) - ORTERONEL |
Tyrosine-kinase inhibitor, antagonist of vascular endothelial growth factor and epidermal growth factor. Endothelin A inhibitor. Androgen receptor antagonist. Selective 17–20 lyase-inhibitor. |
| CRPC with metastasis | ||
|---|---|---|
| Closed phase 3 studies | -DOCETAXEL/PREDNISONA ± CALCITRIOL - DOCETAXEL/PREDNISONA ± BEVACIZUMAB |
ASCENT-2: no differences in OS CALGB 90401: no differences in OS |
| New Drugs | - LENALIDOMIDE - AFLIBERCEPT - AAFTERENTAN - ZIBOTENTAN - DASATINIB - CURTISEN |
Angiogenesis inhibitor Angiogenesis inhibitor Endothelin A inhibitor Endothelin A inhibitor Tyrosine kinase inhibitor Clusterin inhibitor |
Fig. 3.
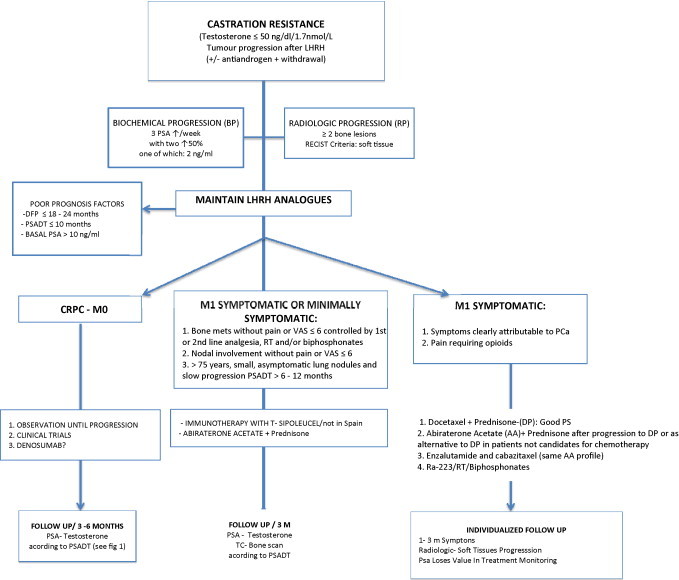
Imaging, treatment and follow-up algorithm for patients with castration-resistance prostate cancer.
4.4. Follow up and natural history of metastatic CRPC
Although the presence of metastasis implies incurability, there are two very different patient profiles in metastatic CRPC. The first patient profile includes asymptomatic or minimally symptomatic patients with a low rate of PSA progression, few bone metastases, and/or limited nodal involvement; in such patients, the tumour burden is low, and the prognosis is, therefore, better. In contrast, in the second patient type, patients are symptomatic, have rapid PSA progression, show the presence of visceral metastasis, and a significant increase in bone metastasis. In this second group, the median survival – even with treatment – is less than 12 months.4
In the absence of approved treatments to prevent metastatic progression in CRPC, the aim of current trials is to assess the early treatment of asymptomatic or minimally symptomatic patients with a low tumour burden with the aim of extending clinical progression-free survival and overall survival and to improve the quality of life while delaying initiation of highly toxic treatments such as cytotoxic chemotherapy. In these patients, the objective of new drug trials is not – as the PCWG2 document recommends47 – to measure PSA response but rather to assess the ability of these drugs to delay the emergence of complications and/or pain secondary to the metastasis; of course, another aim of such trials is to assess the drug's impact on overall survival and quality of life. It is essential that response to these drugs be measured according to widely-accepted objective criteria such as those defined in RECIST.32
4.4.1. Asymptomatic or minimally-symptomatic patients
This group includes the following:
-
-
Patients with nodal involvement alone, asymptomatic or with mild/moderate pain (Visual Analogue Scale (VAS) ≤6) controlled with first or second-line analgesia, without oedema (no inferior vena cava syndrome) or evidence of spinal compression.
-
-
Patients with bone metastasis without pain or mild/moderate pain (VAS ≤ 6) that is controlled with first or second-line analgesics, or patients who have shown a good analgesic response to palliative RT and/or to bisphosphonates.
-
-
Patients >75 years with metastasis in the form of small pulmonary nodules without any related clinical signs/symptoms and slow progression (PSADT >10–12 months).
The aim of treatment in the patients described above is to prevent clinical and radiological progression. Two drugs have been approved for this indication, both with level I evidence:
-
-
Sipuleucel-T Immunotherapy: indicated in patients with an Eastern Cooperative Oncology Group (ECOG) performance status ranging from 0 to 1, liver metastasis, and with a life expectancy >6 months. In the IMPACT 301 trial, Sipuleucel-T reduced mortality by 22% in this patient group (mean survival, 25.8 months vs. 21.7 in the placebo group). The toxicity profile includes the emergence of flu-like illness and headache. This drug is not currently commercialized in Spain.50
-
-
Abiraterone: indicated in patients without visceral metastasis. The dose is 1000 mg/day combined with prednisone 10 mg every 24 h. In the COU-AA-302 trial, this drug significantly improved radiological progression-free survival (HR 0.43; p < 0.0001), showing as well a benefit and trend towards improved overall survival (HR: 0.75; p < 0.097). Abiraterone also delayed initiation of chemotherapy (HR: 0.58; p < 0.0001), symptom emergence or worsening, and time to opioid initiation and worsening of ECOG status and PSA progression. Its toxicity profile is quite manageable, with grade 3/4 toxicity rates <6%. The side effects that need to be monitored are arterial hypertension, hypokaliemia, elevation of transaminases, and the presence of oedemas. According to the medical prospectus, this drug is indicated for “treatment of castration resistant prostate cancer in adult men that is asymptomatic or mildly symptomatic after failure of ADT in which chemotherapy is not yet clinically indicated”.51
-
-
Docetaxel with prednisone is also used in this patient profile, and, although the prospectus indicates its use in metastatic CRPC, most studies and clinical guidelines refer to patients with evident metastatic disease (that is to say, symptomatic patients).
The recommended follow up in these patients is a clinical assessment one month after treatment initiation to assess drug safety, in addition to radiological evaluation (CT and bone scintigraphy), blood tests, and clinical evaluation every 3 months.3
Both the European and American guidelines52 suggest the following alternative treatments to patients not considered candidates for the aforementioned treatments: first-generation antiandrogens, participation in clinical trials, or observation.
Finally, many drugs (tasquinimod, cabozantinib, enzalutamide, among others) are currently being evaluated in this group of patients prior to chemotherapy. Of these, enzalutamide has shown promising results in this patient group (level of evidence: IIA), with a toxicity profile that includes asthenia, diarrhoea, hot flushes, headaches, and seizures. This drug is not commercialized in Spain at present.
4.4.2. Symptomatic patients
The combination of docetaxel (75 mg/m2 every three weeks) and prednisone (5 mg every 12 h), which was one of the arms of the TAX 327 study,53 is the standard treatment in this patient group. Median OS improved by 2 months while mortality risk decreased by 24% with this treatment compared to the mitoxantrone/prednisone combination; moreover, 45% of patients had a PSA decrease >50%, 35% had improved pain, and 22%, better quality of life. The most significant toxic effect was haematological (neutropenia), and, less frequently, asthenia and diarrhoea. The benefit of docetaxel was evident in all the subgroups analyzed. In patients treated with combined docetaxel/prednisone (DP), a PSA increase alone should not be the only criterion to stop treatment. In fact, if no signs of clinical or radiological progression are present, the patient should continue with chemotherapy.53
The latest AUA guidelines52 for patients with mCRPC suggest that patients be classified into two groups according to their performance status (PS). The guidelines also provide criteria to define “symptomatic” patients:
-
-
Symptoms that are clearly attributable to the metastatic disease and not to other co-existing clinical conditions.
-
-
If patients have pain (VAS > 7), they must use continuous or regular opioid analgesics.
Although the DP combination is the standard treatment (level of evidence: I),4,52,54 after publication of the AFFIRM study and COU-AA-301,55 enzalutamide or the combination of abiraterone and prednisone in symptomatic patients could also be offered (level of evidence, grade B) as an alternative to chemotherapy in patients deemed unfit for chemotherapy due to their clinical condition; alternatively, abiraterone/prednisone and enzalutamide could be offered as standard treatment (level of evidence, grade A), if the patient has previously undergone docetaxel-based chemotherapy.56 Patients in the ALSYMPCA trial had no visceral metastases and may have previously received docetaxel. Six intravenous injections of Ra-223 (an alpha emitter) at a dose of 50 kBq per kg were administered every 4 weeks. All end-points of the study were confirmed with a significant prolongation of time to first symptomatic skeletal event (15.6 vs. 9.8 months) and increase OS by 3.8 months vs. placebo. Also, haematological toxicity >3, which might influence the administration of the chemotherapy, was <15%. These results have led to Ra-223 to be considered by the NCCN 20152 as a category 1 option in patients with painful bone metastases in CPRC regardless of the time of administration of chemotherapy. Ra-223 can be used with biphosphonates or denosumab. Consequently, in this group of patients, it is important to consider palliative RT and radioisotopes, both of which are efficacious analgesic treatments.
In symptomatic mCRPC patients who have previously received chemotherapy but still continue to present a good PS, abiraterone combined with prednisone is the treatment of choice, with an improvement of 4.6 months in OS and decreased bone events compared to placebo. In this same group of patients, enzalutamide57 is another treatment alternative. The toxicity profile of enzalutamide differs from combined abiraterone/prednisone, but with comparable results in terms of OS (Table 3).
Table 3.
Results of phase III trials in patients with mCRPC and new drug combinations.
| Study | Control arm | Active drug arm | Months difference in OS | |
|---|---|---|---|---|
| Ra 223 | ALSYMPCA: CRPC with bone metastases and symptoms | SUPPORT TREATMENT + PLACEBO 307 patients |
Ra 223 + SUPPORT TREATMENT 614 patients |
11.2 vs. 14 3.8 months |
| MDV 3100 | AFFIRM: CRPC in progression after chemotherapy PREVAIL: mCRPC IN CHEMOTHERAPY NAIVE |
PLACEBO ± CORTICOSTEROIDS 399 patients PLACEBO: 845 patients |
MDV3100± CORTICOSTEROIDS 800 patients MDV3100 872 patients |
13.6 vs. 18.4 4.8 months Enzalutamide reduced the risk of death by 29%. Study unblinded in 2013 |
| ABIRATERONE | COU-AA-301: CRPC with metastasis, asymptomatic or minimally symptomatic prior to chemotherapy | PLACEBO + CORTICOSTEROIDS 542 patients |
ABIRATERONE + CORTICOSTEROIDS 546 patients |
30.1 vs. 35.3 5.2 months |
| ABIRATERONE | COU-AA-301: CRPC with metastasis after chemotherapy | PLACEBO + CORTICOSTEROIDS 398 patients |
ABIRATERONE + CORTICOSTEROIDS 797 patients |
11.2 vs. 15.8 months 4.6 months |
| CABAZITAXEL | TROPIC: CRPC with metastasis after chemotherapy | MITOXANTRONE+ CORTICOSTEROIDS 377 patients |
CABAZITAXEL + CORTICOSTEROIDS 378 patients |
12.7 vs. 15.1 2.4 months |
| SIPULEUCEL-T | IMPACT 301: CRPC with metastasis prior to chemotherapy | PLACEBO 171 patients |
SIPULEUCEL T 341 patients |
21.7 vs. 25.8 4.1 months |
Finally, in selected cases, it is possible to restart chemotherapy with docetaxel every 2 or 3 weeks if the previous drugs fail or are not available, or if there was an initial response to DP but treatment was suspended due to toxicity.58 Although a decrease in PSA has been described in 60% of cases, the time to progression is less than 6 months.57
According to the 2015 NCCN guidelines,3 the decision to initiate second line treatment (abiraterone, enzalutamide or cabazitaxel)59 following DP should be based on the efficacy, safety and tolerability of these agents, but especially on the potential benefit in a particular patient. It is also necessary to determine if the patient has previously taken any of these agents. Notably, several studies have shown that abiraterone after chemotherapy is efficacious in patients who received abiraterone prior to docetaxel. No data are available regarding the efficacy of the sequential use of these agents in mCRPC, nor have any trials been conducted to compare these three drugs. Similarly, to date, no predictive models or biomarkers are yet available to identify which patient subgroup would benefit most from each of the drugs. The choice should, therefore, be based on clinical criteria (e.g., cabazitaxel should not be used in patients with liver or renal insufficiency or severe prior hematologic toxicity), patient preferences, and prior treatments. Treatment response should be closely monitored via clinical examinations, PSA determination, and imaging tests; treatment should only be stopped when intolerance, severe toxicity, or clinical progression is observed. The sequential use of this treatment is only reasonable as long as the patient continues to be a candidate for systemic treatment – in other words, while he maintains a good PS.
The final subgroup includes patients with symptomatic metastasis and poor PS. Typically, such patients are excluded from new drug trials and, therefore, it is not possible to extrapolate the results of such trials to this population. However, in clinical practice, if the physician considers it appropriate for a particular patient, it is possible to implement palliative management with support treatment, RT, radioisotopes, and, in selected cases, new hormonal treatments such as abiraterone + prednisone or enzalutamide, with appropriate monitoring and control of symptoms. Chemotherapy and immunotherapy are contraindicated in this patient group.50
Treatment response in this patient subgroup should be monitored individually according to symptoms (symptom management should be the main objective of palliative treatments). As in all the previous cases, the presence of visceral metastases and a short PSA doubling time (<3 months) are considered predictors of poor prognosis.25,44,49
Ethical standards
The manuscript does not contain clinical studies or patient data.
Conflict of interest
None declared.
Financial disclosure
None declared.
Acknowledgement
The authors would like to thank Bradley Londres for his assistance in translating and editing the text.
References
- 1.Roach M., III, Hanks G., Thames H., Jr. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys. 2006;65(July (4)):965–974. doi: 10.1016/j.ijrobp.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 2.Polkinghorn W.R., Zelefsky M.J. Improving outcomes in high-risk prostate cancer with radiotherapy. Rep Pract Oncol Radiother. 2013;18(6):333–337. doi: 10.1016/j.rpor.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.2015. NCCN Clinical Practice Guidelines for Prostate Cancer [Internet] http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site [cited 11.02.15] [Google Scholar]
- 4.Heidenreich A., Bastian P.J., Bellmunt J. EAU guidelines on prostate cancer Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65(February (2)):467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Keto C.J., Aronson W.J., Terris M.K. Detectable prostate-specific antigen Nadir during androgen-deprivation therapy predicts adverse prostate cancer-specific outcomes: results from the SEARCH database. Eur Urol. 2014;65(March (3)):620–627. doi: 10.1016/j.eururo.2012.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punnen S., Cooperberg M.R., D’Amico A.V. Management of biochemical recurrence after primary treatment of prostate cancer: a systematic review of the literature. Eur Urol. 2013;64(December (6)):905–915. doi: 10.1016/j.eururo.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 7.McMahon C.J., Bloch B.N., Lenkinski R.E., Rofsky N.M. Dynamic contrast-enhanced MR imaging in the evaluation of patients with prostate cancer. Magn Reson Imaging Clin N Am. 2009;17(May (2)):363–383. doi: 10.1016/j.mric.2009.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Kim C.K., Park B.K., Kim B. Diffusion-weighted MRI at 3 T for the evaluation of prostate cancer. Am J Roentgenol. 2010;194(June (6)):1461–1469. doi: 10.2214/AJR.09.3654. [DOI] [PubMed] [Google Scholar]
- 9.Yakar D., Hambrock T., Huisman H. Feasibility of 3 T dynamic contrast-enhanced magnetic resonance-guided biopsy in localizing local recurrence of prostate cancer after external beam radiation therapy. Invest Radiol. 2010;45(March (3)):121–125. doi: 10.1097/RLI.0b013e3181c7bcda. [DOI] [PubMed] [Google Scholar]
- 10.Umbehr M.H., Müntener M., Hany T., Sulser T., Bachmann L.M. The role of 11C-choline and 18F-fluorocholine positron emission tomography (PET) and PET/CT in prostate cancer: a systematic review and meta-analysis. Eur Urol. 2013;64(July (1)):106–117. doi: 10.1016/j.eururo.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 11.Ceci F., Castellucci P., Graziani T. 11C-choline PET/CT detects the site of relapse in the majority of prostate cancer patients showing biochemical recurrence after EBRT. Eur J Nucl Med Mol Imaging. 2014;41(May (5)):878–886. doi: 10.1007/s00259-013-2655-9. [DOI] [PubMed] [Google Scholar]
- 12.Evangelista L., Zattoni F., Guttilla A. Choline PET or PET/CT and biochemical relapse of prostate cancer: a systematic review and meta-analysis. Clin Nucl Med. 2013;38(May (5)):305–314. doi: 10.1097/RLU.0b013e3182867f3c. [DOI] [PubMed] [Google Scholar]
- 13.Panebianco V., Sciarra A., Lisi D. Prostate cancer: 1HMRS-DCEMR at 3 T versus [(18)F]choline PET/CT in the detection of local prostate cancer recurrence in men with biochemical progression after radical retropubic prostatectomy (RRP) Eur J Radiol. 2012;81(April (4)):700–708. doi: 10.1016/j.ejrad.2011.01.095. [DOI] [PubMed] [Google Scholar]
- 14.Rybalov M., Breeuwsma A.J., Leliveld A.M., Pruim J., Dierckx R.A., de Jong I.J. Impact of total PSA doubling time and PSA velocity on detection rates of 11C-Choline positron emission tomography in recurrent prostate cancer. World J Urol. 2013;31(April (2)):319–323. doi: 10.1007/s00345-012-0908-z. [DOI] [PubMed] [Google Scholar]
- 15.Greco C., Cascini G.L., Tamburrini O. Is there a role for positron emission tomography imaging in the early evaluation of prostate cancer relapse? Prostate Cancer Prostatic Dis. 2008;11(2):121–128. doi: 10.1038/sj.pcan.4501028. [DOI] [PubMed] [Google Scholar]
- 16.Daskivich T.J., Chamie K., Kwan L. Improved prediction of long-term, other cause mortality in men with prostate cancer. J Urol. 2011;186(November (5)):1868–1873. doi: 10.1016/j.juro.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Gomez-Veiga F., Mariño A., Alvarez L. Brachytherapy for the treatment of recurrent prostate cancer after radiotherapy or radical prostatectomy. BJU Int. 2012;109(February (Suppl. 1)):17–21. doi: 10.1111/j.1464-410X.2011.10826.x. [DOI] [PubMed] [Google Scholar]
- 18.Izawa J.I., Madsen L.T., Scott S.M. Salvage cryotherapy for recurrent prostate cancer after radiotherapy: variables affecting patient outcome. J Clin Oncol. 2002;20(June (11)):2664–2671. doi: 10.1200/JCO.2002.06.086. [DOI] [PubMed] [Google Scholar]
- 19.Ismail M., Ahmed S., Kastner C., Davies J. Salvage cryotherapy for recurrent prostate cancer after radiation failure: a prospective case series of the first 100 patients. BJU Int. 2007;100(October (4)):760–764. doi: 10.1111/j.1464-410X.2007.07045.x. [DOI] [PubMed] [Google Scholar]
- 20.Darwish O.M., Raj G.V. Management of biochemical recurrence after primary localized therapy for prostate cancer. Front Oncol. 2012;2:48. doi: 10.3389/fonc.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisters L.L., Leibovici D., Blute M. Locally recurrent prostate cancer after initial radiation therapy: a comparison of salvage radical prostatectomy versus cryotherapy. J Urol. 2009;182(August (2)):517–525. doi: 10.1016/j.juro.2009.04.006. [discussion 525-7] [DOI] [PubMed] [Google Scholar]
- 22.Pinover W.H., Horwitz E.M., Hanlon A.L., Uzzo R.G., Hanks G.E. Validation of a treatment policy for patients with prostate specific antigen failure after three-dimensional conformal prostate radiation therapy. Cancer. 2003;97(February (4)):1127–1133. doi: 10.1002/cncr.11166. [DOI] [PubMed] [Google Scholar]
- 23.Zelefsky M.J., Ben-Porat L., Scher H.I. Outcome predictors for the increasing PSA state after definitive external-beam radiotherapy for prostate cancer. J Clin Oncol. 2005;23(February (4)):826–831. doi: 10.1200/JCO.2005.02.111. [DOI] [PubMed] [Google Scholar]
- 24.Mydin A.R., Dunne M.T., Finn M.A., Armstrong J.G. Early salvage hormonal therapy for biochemical failure improved survival in prostate cancer patients after neoadjuvant hormonal therapy plus radiation therapy—a secondary analysis of irish clinical oncology research group 97-01. Int J Radiat Oncol Biol Phys. 2013;85(January (1)):101–108. doi: 10.1016/j.ijrobp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Studer U.E., Whelan P., Wimpissinger F. Differences in time to disease progression do not predict for cancer-specific survival in patients receiving immediate or deferred androgen-deprivation therapy for prostate cancer: final results of EORTC randomized Trial 30891 with 12 years of follow-up. Eur Urol. 2014;66(5):829–838. doi: 10.1016/j.eururo.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 26.Bayoumi A.M., Brown A.D., Garber A.M. Cost-effectiveness of androgen suppression therapies in advanced prostate cancer. J Natl Cancer Inst. 2000;92(November (21)):1731–1739. doi: 10.1093/jnci/92.21.1731. [DOI] [PubMed] [Google Scholar]
- 27.Nair B., Wilt T., MacDonald R., Rutks I. Early versus deferred androgen suppression in the treatment of advanced prostatic cancer. Cochrane Database Syst Rev. 2002;(1):CD003506. doi: 10.1002/14651858.CD003506. [DOI] [PubMed] [Google Scholar]
- 28.Studer U.E., Whelan P., Albrecht W. Immediate or deferred androgen deprivation for patients with prostate cancer not suitable for local treatment with curative intent: European Organisation for Research and Treatment of Cancer (EORTC) Trial 30891. J Clin Oncol. 2006;24(April (12)):1868–1876. doi: 10.1200/JCO.2005.04.7423. [DOI] [PubMed] [Google Scholar]
- 29.Crook J.M., O’Callaghan C.J., Duncan G. Intermittent androgen suppression for rising PSA level after radiotherapy. N Engl J Med. 2012;367(September (10)):895–903. doi: 10.1056/NEJMoa1201546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sciarra A., Abrahamsson P.A., Brausi M. Intermittent androgen-deprivation therapy in prostate cancer: a critical review focused on phase 3 trials. Eur Urol. 2013;64(November (5)):722–730. doi: 10.1016/j.eururo.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Tsai H.-T., Penson D.F., Makambi K.H., Lynch J.H., Van Den Eeden S.K., Potosky A.L. Efficacy of intermittent androgen deprivation therapy vs. conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013;82(August (2)):327–333. doi: 10.1016/j.urology.2013.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisenhauer E.A., Therasse P., Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(January (2)):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 33.Manni A., Bartholomew M., Caplan R. Androgen priming and chemotherapy in advanced prostate cancer: evaluation of determinants of clinical outcome. J Clin Oncol. 1988;6(September (9)):1456–1466. doi: 10.1200/JCO.1988.6.9.1456. [DOI] [PubMed] [Google Scholar]
- 34.Taylor C.D., Elson P., Trump D.L. Importance of continued testicular suppression in hormone-refractory prostate cancer. J Clin Oncol. 1993;11(November (11)):2167–2172. doi: 10.1200/JCO.1993.11.11.2167. [DOI] [PubMed] [Google Scholar]
- 35.Hussain M., Wolf M., Marshall E., Crawford E.D., Eisenberger M. Effects of continued androgen-deprivation therapy and other prognostic factors on response and survival in phase II chemotherapy trials for hormone-refractory prostate cancer: a Southwest Oncology Group report. J Clin Oncol. 1994;12(September (9)):1868–1875. doi: 10.1200/JCO.1994.12.9.1868. [DOI] [PubMed] [Google Scholar]
- 36.Kucuk O., Fisher E., Moinpour C.M. Phase II trial of bicalutamide in patients with advanced prostate cancer in whom conventional hormonal therapy failed: a Southwest Oncology Group study (SWOG 9235) Urology. 2001;58(July (1)):53–58. doi: 10.1016/s0090-4295(01)01010-x. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki H., Okihara K., Miyake H. Alternative nonsteroidal antiandrogen therapy for advanced prostate cancer that relapsed after initial maximum androgen blockade. J Urol. 2008;180(September (3)):921–927. doi: 10.1016/j.juro.2008.05.045. [DOI] [PubMed] [Google Scholar]
- 38.Sartor A.O., Tangen C.M., Hussain M.H.A. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426) Cancer. 2008;112(June (11)):2393–2400. doi: 10.1002/cncr.23473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scher H.I., Kelly W.K. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J Clin Oncol. 1993;11(August (8)):1566–1572. doi: 10.1200/JCO.1993.11.8.1566. [DOI] [PubMed] [Google Scholar]
- 40.Small E.J., Halabi S., Dawson N.A. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583) J Clin Oncol. 2004;22(March (6)):1025–1033. doi: 10.1200/JCO.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 41.Mahler C., Verhelst J., Denis L. Ketoconazole and liarozole in the treatment of advanced prostatic cancer. Cancer. 1993;71(February (Suppl. 3)):1068–1073. doi: 10.1002/1097-0142(19930201)71:3+<1068::aid-cncr2820711427>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 42.http://www.aemps.gob.es/informa/notasInformativas/medicamentosUsoHumano/seguridad/2013/NI-MUH_FV_21-2013-ketoconazol.htm.
- 43.Venkitaraman R., Thomas K., Huddart R.A., Horwich A., Dearnaley D.P., Parker C.C. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 2008;101(February (4)):440–443. doi: 10.1111/j.1464-410X.2007.07261.x. [DOI] [PubMed] [Google Scholar]
- 44.Kantoff P.W., Halabi S., Conaway M. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J Clin Oncol. 1999;17(August (8)):2506–2513. doi: 10.1200/JCO.1999.17.8.2506. [DOI] [PubMed] [Google Scholar]
- 45.Second-line treatment of metastatic prostate cancer Prednisone and radiotherapy for symptom relief. Prescrire Int. 2013;22(March (136)):74–78. [PubMed] [Google Scholar]
- 46.Arija J.A.A., Beca R.G., López C.L., Domínguez P.S., Lovelle A.S., Gilarranz Y.J. Treatment of the patient with castration-resistant biochemical progression of prostate cancer. Arch Esp Urol. 2012;65(February (1)):185–192. [PubMed] [Google Scholar]
- 47.Scher H.I., Halabi S., Tannock I. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26(March (7)):1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith M.R., Kabbinavar F., Saad F. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(May (13)):2918–2925. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 49.Svatek R., Karakiewicz P.I., Shulman M., Karam J., Perrotte P., Benaim E. Pre-treatment nomogram for disease-specific survival of patients with chemotherapy-naive androgen independent prostate cancer. Eur Urol. 2006;49(April (4)):666–674. doi: 10.1016/j.eururo.2005.11.032. [DOI] [PubMed] [Google Scholar]
- 50.Kantoff P.W., Higano C.S., Shore N.D. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 51.Ryan C.J., Smith M.R., Bono J.S.D. Interim analysis (IA) results of COU-AA-302, a randomized, phase III study of abiraterone acetate (AA) in chemotherapy-naive patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) J Clin Oncol [Internet] 2012;30(Suppl.) available from: http://meetinglibrary.asco.org/content/95300-114 [abstr LBA4518, cited 23.06.14] [Google Scholar]
- 52.Cookson M.S., Roth B.J., Dahm P. Castration-resistant prostate cancer: AUA Guideline. J Urol. 2013;190(August (2)):429–438. doi: 10.1016/j.juro.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Tannock I.F., de Wit R., Berry W.R. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(October (15)):1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 54.Mohler J., Armstrong A., Bahnson R.R. 2015. NCCN clinical practice guidelines for prostate cancer [Internet] available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site [cited 11.02.15] Version I. [Google Scholar]
- 55.De Bono J.S., Logothetis C.J., Molina A. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(May (21)):1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scher H.I., Fizazi K., Saad F. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. doi: 10.1056/NEJMoa1207506. [DOI] [PubMed] [Google Scholar]
- 57.Beer T.M., Armstrong A.J., Rathkopf D.E. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(June (5)):424–433. doi: 10.1056/NEJMoa1405095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kellokumpu-Lehtinen P.-L., Harmenberg U., Joensuu T. 2-Weekly versus 3-weekly docetaxel to treat castration-resistant advanced prostate cancer: a randomised, phase 3 trial. Lancet Oncol. 2013;14(February (2)):117–124. doi: 10.1016/S1470-2045(12)70537-5. [DOI] [PubMed] [Google Scholar]
- 59.Pouessel D., Oudard S., Gravis G., Priou F., Shen L., Culine S. Cabazitaxel for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: the TROPIC study in France. Bull Cancer (Paris) 2012;99(August (7–8)):731–741. doi: 10.1684/bdc.2012.1608. [DOI] [PubMed] [Google Scholar]


