Abstract
Aim
In this study we compared three different methods of evaluation of dose distribution.
Background
The aim of treatment planning is to prepare the treatment plan which the criteria are defined according to the international recommendations.
Materials and methods
For three groups of patients, for lung, breast and prostate, treated radically in Brzozow with external beams the treatment plans were prepared. For each patient the metrics of dose distribution in the PTV defined according to the ICRU Reports 50, 83 and according to the Nordic Association of Clinical were calculated. Also Homogeneity Index defined by Yoon was used in this work. Additionally for similar group of patients treated in Warsaw the same calculations were performed. Correlations between the standard deviations and: (1) the differences between the maximum and minimum doses, and (2) the differences between near maximum and near minimum doses normalized to median dose and (3) to prescribed dose were calculated.
Results
There was a very strong correlation between the standard deviation and the difference between the near-maximum and near-minimum doses for all locations regardless the prescription. Also good correlation was observed for the standard deviation and the difference between the maximum and minimum doses for patients treated in Brzozow.
Conclusions
The standard deviation may be estimated by the Homogeneity Index, however the relationship should be established for each location and each center separately.
Keywords: ICRU Report 50, ICRU Report 83, Nordic Association of Clinical Physics Report, Doses distribution homogeneity
1. Introduction
The aim of radiotherapy is to deliver the prescribed dose to the tumor while trying to spare the surrounding normal tissues as much as possible.1,2 In order to reach this aim, very sophisticated treatment techniques are used.3 Nevertheless, quite simple rules concerning treatment planning are still followed. In most cases, because the distribution of clonogenic cells is unknown, the planners tend to achieve a homogenous dose distribution within the Planning Target Volume (PTV). Due to inherent characteristics of the treatment beams, this goal is unachievable. Therefore, in practice, some heterogeneity of the dose distribution is present and has to be accepted. There are several recommendations which formulate requirements concerning dose distribution uniformity and dose prescription. According to the ICRU Report 50, the uniformity of dose distribution is described in terms of the maximum and minimum dose within the Planning Target Volume (PTV).4 The maximum dose in the PTV should not exceed 107% of the dose at the ICRU Reference Point and the minimum should not be smaller than 95% of dose at the ICRU Reference Point. This Report, published in 1993, is still used in many radiotherapy centers. Another dose prescription method was proposed by the Nordic Association of Clinical Physics Report.5 According to this Report the homogeneity of dose distribution should be evaluated by analyzing the standard deviation of dose distribution within the PTV. It is desirable to achieve dose distribution with standard deviation smaller than 3% of the prescribed dose. These two reports were published in the era of 3 dimensional conformal radiotherapy (3DCRT). In 2010, a new ICRU Report, intentionally written for Intensity Modulated Radiotherapy and for complicated 3DCRT plans, was published.6 In this ICRU Report (number 83), the authors propose to use the so called near minimum and near maximum doses instead of the minimum and maximum doses in the PTV as a measure of the uniformity of dose distribution. The near minimum D98% and near maximum doses D2% are doses received by 98% and 2% of the PTV volume. In the ICRU Report 83, there is no recommendation concerning D2% and D98%. The treatment team is responsible for accepting each treatment plan according to internal protocol. Based on these definitions, the homogeneity index HI is defined as:
A few years earlier Yoon introduced very similar Homogeneity Index metric.7 In his idea, the HI is defined as:
where Dpr is the prescribed dose.
The direct association of dose distribution and treatment outcome is highly desired.8 All these concepts of dose prescription and uniformity of dose distribution in the PTV only implicitly allow to quantify the correlation between the dose delivered to the target and treatment outcome. In the literature, there are several proposals to find the direct mathematical correlation between dose distribution and treatment outcome. The general idea was to reduce the dose distribution in the target to a single dose value which would be directly related to the probability of the tumor control.9
In this study, we analyze what the relationships are between the four different indices of dose distribution uniformity, i.e., between the difference of the maximum and minimum doses (DMM), the standard deviation (StDev), and the homogeneity indexes (HI, HIYoon).
2. Materials and methods
The study was performed for three groups of patients treated radically with external beams in Brzozow and Warsaw in the period of 2012–2013. There were patients with breast, lung and prostate cancer. There were 10 patients in each group, 30 patients in Brzozow and 30 patients in Warsaw, 60 patients altogether. A similar procedure of treatment plan preparation was applied in each group. However, different policy concerning the evaluation of dose distribution homogeneity was used. In Brzozow, the difference between the maximum and minimum dose was minimized (ICRU 50 Report), while in Warsaw the aim was to keep the standard deviation below 3% of the prescribed dose (Nordic Association of Clinical Physics Report). The CT examination was performed in treatment position (Somatom Open, Siemens) with 3–5 mm slice thickness in a spiral mode. For each patient, the Clinical Target Volume and organs at risk were delineated. In Brzozow, the treatment planning was performed with Xio (Elekta, version 4.70.00) treatment planning system, in Warsaw with Eclipse (Varian, version 10.0.39). The contouring and treatment plans were prepared according to the ICRU 50. In both centers, the dose was prescribed to the ICRU Reference Point. In XiO, the superpositions algorithm, and in Eclipse the Analytic Anisotropic Algorithm were used for dose distribution calculation. The calculation grid was 0.25 cm.
A short description of Brzozow patients and treatment plans is as follows:
Breast patients: Tis – T2N0M0, the CTV: breast gland, CTV-PTV margin: 0.5 cm, tangential field technique with photons of 4 and 6 MV; bolus of 0.5 cm.
Lung patients: T2N1M0 – T4N3M0 patients, CTV encompassing the tumor, and in some cases the regional lymph nodes, CTV-PTV margin: 1.0 cm, 3 field technique with 6 MV photons.
Prostate: T1c – T3bN0M0 patients, CTV consisted of the prostate gland and regional lymph nodes with margin of 0.8 cm, 3 field technique (AP field and two wedge lateral fields) or box technique with photons of 15 MV.
A short description of Warsaw patients and treatment plans is as follows:
Breast patients: T1N0M0 –T 2N0M0, the CTV: breast gland, CTV-PTV margin: 0.5 cm, tangential field technique with photons of 6 MV; in the planning process the external 0.5 cm of the gland was excluded from the PTV.
Lung patients: T2N1M0 – T4N3M0 patients, CTV encompassing the tumor, and in some cases the regional lymph nodes, CTV-PTV margin: 1.0 cm, 3 or 4 field technique with 6 MV photons.
Prostate: T1c – T3bN0M0 patients, CTV consisted of the prostate gland and in some patients regional lymph nodes with margins of cranio-caudal and anterio-posterior 0.7 cm, left-right 0.4 cm; 3 field technique (AP field and two wedge lateral fields) with photons of 15 MV.
In all cases, the shape of the fields was conformed automatically to the PTV with a Multileaf Collimator (in majority of cases 0.6 cm margin was used). For this work, the clinically accepted plans were used. In Brzozow, the dose was always prescribed to the ICRU Reference Point and for the breast group the total dose of 50 Gy, 2 Gy per fraction was administered, for lung patients 68 Gy, 2 Gy per fraction, and for prostate group 76 Gy, 2 Gy per fraction. In Warsaw, the dose was prescribed to the mean dose to the PTV and for the breast group the total dose of 45 Gy, 2.25 Gy per fraction was administered, for lung patients 58.8 Gy, 2.8 Gy per fraction, and for prostate group 65 Gy, 2.6 Gy per fraction. All dose distribution indices were relative ones so the fractionation and prescription did influence results.
In both centers for each patient the following metrics in the PTV were obtained: maximum dose (Dmax); the maximum dose in single calculation point, minimum dose (Dmin); according to ICRU Report 50, standard deviation of dose distribution (σ); in percent of mean dose delivered to the PTV, near minimum dose (D98%); according to ICRU Report 83, near maximum dose (D2%); according to ICRU Report 83, median dose (D50%); according to ICRU Report 83.
To find the relationships between each pairs of dose distributions homogeneity indices, the correlation was obtained.
3. Results
Figs. 1–3 show the correlation between the differences of maximum and minimum doses, and standard deviations, and between differences of maximum and minimum doses and homogeneity indexes according to ICRU83, and Yoon for breast, lung and prostate, respectively, for Brzozow patients. The excellent correlation between almost all these pairs of statistics was obtained (R2 > 0.62). The good correlation (R2 = 0.4453) was obtained between the standard deviation and both Homogeneity Indexes. For this group of data, there is a very small difference between the Homogeneity Index calculated according to ICRU and Homogeneity Index according to Yoon. Figs. 4–6 show the correlation between the differences of maximum and minimum doses and standard deviations, and between differences of maximum and minimum doses and homogeneity indexes according to ICRU83, and Yoon for breast, lung and prostate, respectively, for the Warsaw patients. In this case, also a very good correlation between standard deviation and both homogeneity indexes was obtained (R2 > 0.73), except the correlation between the standard deviation and the differences between the maximum and minimum doses. For breast and lung patients, there is no correlation between the standard deviation and the differences between the maximum and minimum doses. For prostate patients, the correlation between the standard deviations and the differences between the maximum and minimum doses is good (R2 = 0.4694).
Fig. 1.
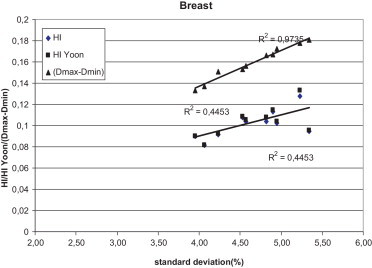
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for breast patients, for plans prepared in Brzozow.
Fig. 2.
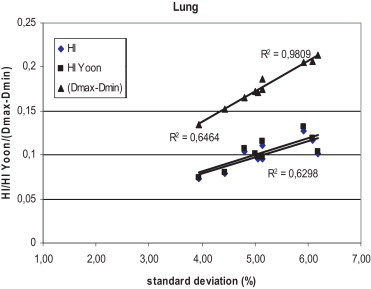
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for lung patients, for plans prepared in Brzozow.
Fig. 3.
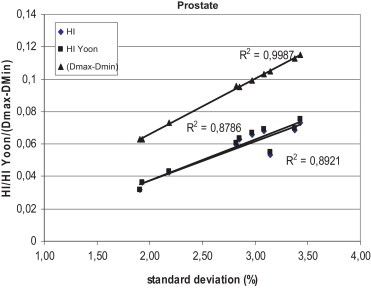
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for prostate patients, for plans prepared in Brzozow.
Fig. 4.
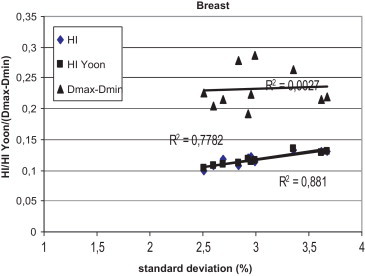
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for breast patients, for plans prepared in Warsaw.
Fig. 5.
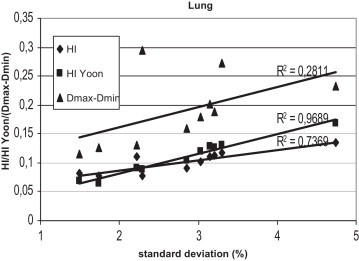
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for lung patients, for plans prepared in Warsaw.
Fig. 6.
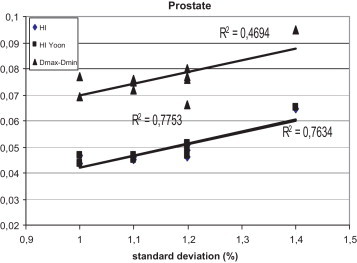
The correlation between the standard deviation and the homogeneity index according to ICRU 83 and according to Yoon for prostate patients, for plans prepared in Warsaw.
4. Discussion
The aim of treatment planning is to prepare the treatment plan which fulfills the criteria defined individually in each hospital. These criteria are defined usually according to the international recommendations. Dose prescription methods may be different in different hospitals. In some hospitals the treatment dose is prescribed to the ICRU Reference Point, in other hospitals, to the mean dose delivered to the PTV. The dose may also be prescribed to the median dose, as it is recommended by the ICRU 83. In consequence, numerically the same total prescribed dose may lead to different treatment outcome (different equivalent dose). Another source of differences in treatment outcome may result from a different dose distribution within the PTV. However, all reports recommend preparing the plan in which the dose distribution within the PTV is as uniform as possible but a satisfactory dose distribution may not always be achieved. In this study, we compared three different methods of the evaluation of dose distribution which are recommended by international bodies. In the ICRU Report 50, the inhomogeneity is expressed in terms of the difference between the maximum and minimum doses; in the ICRU Report 83, the Homogeneity Index is proposed; and the Nordic Association of Clinical Physicist recommends to evaluate the dose distribution homogeneity in terms of the standard deviation of doses in the PTV. The Homogeneity Index is described in terms of the so called near maximum and near minimum doses which were proposed instead of the maximum and minimum doses. Additionally, we performed the same comparison using the Homogeneity Index proposed by Yoon.7 Using the clinical data from two radiotherapy departments, where different policies concerning dose distribution homogeneity evaluation are used, we analyzed the relationships between these three homogeneity dose distribution indices.
We found that there is a very good correlation between standard deviations and between the standard deviation and the HI and HIYoon, no matter which recommendations were used in the process of treatment planning. In Brzozow, a good correlation was also obtained between the standard deviations and differences between the maximum and minimum doses. In Warsaw, there is no correlation between the standard deviations and the differences between the maximum and minimum doses for breast and lung patients, and good correlation for breast patients. In Brzozow, the homogeneity of the dose distribution is evaluated in terms of the difference between the maximum and minimum dose (MMD), i.e., the ICRU 50 recommendations are followed. In Warsaw, in the planning process, the standard deviation is minimized. It is very likely that these results reflect the different treatment planning policy. In Warsaw, the planners try to keep the standard deviation as small as possible, while in Brzozow the planning is driven by the ICRU 50 recommendations. In Warsaw, the standard deviation should be smaller than 3% of the prescribed dose. In Brzozow, the MMD is minimized. In Brzozow, the MMD is larger than 20% in only 3 cases. In Warsaw, the MMD exceeds 20% for 11 patients. On the other hand, in Warsaw there were only 3 plans with the standard deviation larger than 3% (percentages in relation to the prescribed dose). In Brzozow, the standard deviation was larger than 3% in 21 cases. If the HI were used for the evaluation of the dose distribution homogeneity, then much better dose distributions would be prepared in Brzozow. In this center the HI was larger than 0.2 in only 3 cases versus 11 cases in Warsaw. This analysis shows clearly that using different indices of dose distribution may lead to different conclusions concerning the evaluation of dose distribution. This seems to be especially important in clinical trials where integrity of clinical data is of special importance and when we want to compare our results of treatment planning with others. The correlations did not depend on the definition of homogeneity index. Both definitions according to the ICRU and Yoon lead to similar results.
What are advantages and disadvantages of each of these three methods of evaluation of dose distribution homogeneity? The only one which is not fully subjective metrics is the standard deviation. Brahme showed that there is a dependence between the homogeneity of dose distribution in the PTV and the treatment outcome.10,11 Under assumption that the dose variation within the target is small and that the density of clonogenic cells in the target is constant, the loss in tumor control probability is proportional to the square of the standard deviation, which may be described quantitatively by the formula:
where TCP(Dr) – tumor control probability for the actual dose distribution in the PTV, Dmean – the mean dose, γ – normalized dose response gradient, TCP(Dmean) – tumor control probability for Dmean, σ – standard deviation of dose distribution in the PTV.
If the normalized dose response gradient is about 2, and the relative standard deviation of the mean dose in the PTV is about 3%, then the loss in the TCP is about 4 percent points.
While Brahme describes the influence of inhomogeneities of the dose distribution on the treatment outcome in terms of changes of the TCP, Niemierko established a direct relation between the so called Effective Uniform Dose (EUD) and the dose distribution.12 In principle, the EUD was defined as the equivalent dose, which if distributed uniformly across the target, would lead to the same TCP as the actual dose distribution. The dependence of the EUD on the standard deviation will be addressed in another paper.
Most often, the homogeneity of dose distribution in the PTV is evaluated with the maximum and minimum doses in the PTV. Gradually, this policy is being replaced with the method proposed by the ICRU 83, namely the Homogeneity Index. These changes come from the obvious observation that, clinically, not only the value of minimum dose is important but also the volume of target which absorbs doses smaller than the prescribed one. It is also well known that the uncertainty of the minimum dose calculation is much larger than the calculation of dose to any other point of CTV. The point were minimum dose is obtained is often located in a high-gradient region at the edge of the PTV. This makes it highly sensitive to the resolution of the calculation, the algorithm used by the treatment planning system and the accuracy of contour delineation.13,14
Our result is also important from a practical point of view. There are treatment planning systems which do not provide the standard deviation (e.g. the XiO, Nucletron). We have shown that the Homogeneity Index can be used as a surrogate of the standard deviation. However, in each radiotherapy center the correleation between the standard deviation and the Homogeneity Index may be described by different mathematical formula and should be obtained separately for each location.
5. Conclusions
There is a very good correlation between the standard deviations and the Homogeneity Indexes regardless if the ICRU 83 or Yoon definitions are used. The mathematical formula describing the dependence of each pair of dose distribution indices should be defined for each location and each center separately.
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Marzena Mrozowska, Email: marzena.mrozowska5@gmail.com.
Paweł Kukołowicz, Email: pawel.kukolowicz@gmail.com.
References
- 1.Malicki J. The importance of accurate treatment planning, delivery, and dose verification. Rep Pract Oncol Radiother. 2012;17(March–April):63–118. doi: 10.1016/j.rpor.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fenoglietto P., Servagi-Vernat S., Azria D., Giraud P. Arcthérapie volumétrique modulée: ultime évolution de la radiothérapie conformationnelle? Cancer/Radiothérapie. 2012;16(5–6):398–440. doi: 10.1016/j.canrad.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 3.Skórska M., Piotrowski T., Kaźmierska J., Adamska K. A dosimetric comparison of IMRT versus helical tomotherapy for brain tumors. Phys Med. 2014;30:497–502. doi: 10.1016/j.ejmp.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 4.ICRU . Oxford University Press; Oxford, United Kingdom: 1993. International Commission on Radiation Units and Measurements Prescribing, recording and reporting photon beam therapy. ICRU Report 50. [Google Scholar]
- 5.Aaltonen P., Brahme A., Lax I. Specification of dose delivery in radiation therapy. Recommendation by the Nordic Association of Clinical Physics (NACP) Acta Oncol. 1997;36(Suppl. 10):1–32. [PubMed] [Google Scholar]
- 6.ICRU International Commission on Radiation Units and Measurements Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT). ICRU Report 83. J ICRU. 2010;10:1–106. [Google Scholar]
- 7.Yooun M., Park S.Y., Shin D. A new homogeneity index based on statistical analysis of the dose-volume histogram. J Appl Clin Med Phys. 2007;8:9–17. doi: 10.1120/jacmp.v8i2.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau D., Autret D., Krhili S. La radiothérapie avec modulation d’intensité rotationnelle apporte-t-elle un avantage dosimétrique dans le traitement du cancer bronchique localement évolué? Cancer/Radiothérapie. 2012;16(7):619–626. doi: 10.1016/j.canrad.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Voyant C., Julian D., Roustit R., Biffi K., Lantieri C. Biological effects and equivalent doses in radiotherapy: a software solution. Rep Pract Oncol Radiother. 2014;19(January–February):47–55. doi: 10.1016/j.rpor.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brahme A. Which parameters of the dose distribution are best related to the radiation response of tumours and normal tissues. Proceedings of the interregional seminars for Europe, the Middle East and Africa Organized by the IAEA Leuven; Leuven; 1991. pp. 37–58. [Google Scholar]
- 11.Brahme A. Precision requirements in radiation therapy. Acta Oncol. 1984;23:379–391. doi: 10.3109/02841868409136037. [DOI] [PubMed] [Google Scholar]
- 12.Niemierko A. Reporting and analyzing dose distributions: a concept of equivalent uniform dose. Med Phys. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 13.Knoos T., Wieslander T., Cozzi E.L. Comparison of dose calculation algorithms for treatment planning in external photon beam therapy for clinical situations. Phys Med Biol. 2006;51:5785–5807. doi: 10.1088/0031-9155/51/22/005. [DOI] [PubMed] [Google Scholar]
- 14.Cilla S., Digesu C., Macchia G. Clinical implications of different calculation algorithms in breast radiotherapy: a comparison between pencil beam and collapsed cone convolution. Phys Med. 2014;30:473–481. doi: 10.1016/j.ejmp.2014.01.002. [DOI] [PubMed] [Google Scholar]


